Abstract
Environmental factors acting during development of an individual may influence future health and disease susceptibility. Stressors, including altered diet, psychosocial stress, immune challenge, during gestation can have negative consequences on the intrauterine environment and increase disease susceptibility of the developing fetus. The long-term effects on offspring have been observed in humans and include greater susceptibility to psychiatric disease, such as depression and anxiety disorders, and adverse metabolic conditions including obesity, diabetes and cardiovascular disease. Studies in my laboratory use rodent models and incorporate a multilevel approach to determine the behavioral, physiological, and neurobiological correlates of disease development as a consequence of early life stressors. The road I took in developing this research program was a rather circuitous one and navigating that path would not have been possible without the many mentors, colleagues, fellows and students who provided critical support. Although my name appears on the plaque of the Alan N. Epstein Research Award, I share this with all those I had the privilege of working with along that road, as briefly summarized in this article.
Keywords: Assisted reproductive techniques, Somatic cell nuclear transfer, Social stress, Maternal stress, Maternal diet, Developmental origins of health and disease
1. Introduction
I am the “Lucky 13th” recipient of the Alan N. Epstein Research Award since it was established in 2002 by the Epstein family in his memory. Luck and new opportunities are what helped me through my journey with the assistance of many mentors and colleagues along the way. It is an honor to receive this award from my colleagues in SSIB. It is particularly special for me because I am a part of Alan Epstein’s scientific family tree through two of my mentors, Randall Sakai and Tim Moran. Although I never met him personally, I feel I have learned so much about who Alan Epstein was, what he believed in, and what he stood for through the SSIB membership and that training has helped me build my career in science.
As Randall Sakai noted in his introduction to my lecture, the field of accounting was where it all started for me. I earned a Bachelor’s of Business Administration from the University of Hawaii at Manoa (UH) in accounting and worked at a large public accounting firm for 3 years prior to deciding that I did not like the path I was on and could not picture myself in the shoes of my supervisors and senior managers. One career alternative was to enter the private sector. I planned a career transition that included heading back to school for a Master’s of Public Health degree in Health Care Administration to integrate my business background with my interest in health care. Then a series of events happened to fall into place at just the right times leading me along what I call the “road less traveled” to where I am today. I want to take this opportunity to share some of my experiences along that circuitous road, both personal and scientific. I consider myself privileged to have had outstanding mentors and colleagues helping me along that road and to whom I am forever indebted for their guidance, support, and confidence in me, including many among the SSIB membership.
2. University of Hawaii
2.1. Bob and Caroline Blanchard
I was first exposed to scientific research in Bob and Caroline Blanchard’s Behavioral Neurobiology lab at UH. As a student in the School of Public Health at UH, I had the opportunity to work in a research lab for a semester to earn academic credit. In the Blanchard lab I joined a post-doctoral fellow, Mark Hebert, and a visiting scientist from France, Denis Ardid, on their project to study the role of the amygdala on pain sensitivity and perception in rats. I watched rats on video tapes and scored their behavior for the entire semester. Every day I showed up, there were more videos to score. I have to admit that at the time, I was not attracted to scientific research at all. My goal was to finish scoring rat videos, earn my credits, and to move on to something “more interesting”. But that all changed once we compiled and analyzed the data that resulted from my hours of video scoring. Mark and Denis analyzed the data with me alongside them, explained why the experiment was done, and what the data meant. They made the point that we were studying something no one else had ever done and were very excited about our results, which they published and included me as a co-author [1]. I never thought about it in that way; that they had the freedom to ask questions that no one else had ever answered and to study it in a way that no one else had ever done before.
Just as I was about to leave the lab, a paid part-time student position became available and I decided to give research another chance. The Blanchard’s were collaborating with Ryuzo Yanagimachi in the Department of Anatomy and Reproductive Biology and he had a funded R01 to test the cognitive function and emotionality of mice produced by assisted reproductive techniques (ART) which are commonly used in humans to circumvent problems with infertility. Dr. Yanagimachi, or “Yana” as he is fondly called by his students and colleagues, made groundbreaking advances in reproductive biology throughout his amazing 55-year scientific career, of which nearly 50 years (and still counting!) have been at UH [2]. Yana’s work in in vitro fertilization (IVF) using animals (mice, hamsters and guinea pigs) laid the important, scientific foundation that ultimately allowed Patrick Steptoe and Robert Edwards of the UK to succeed in producing the first human “test tube” baby, Louise Brown, using IVF in 1978 [3]. This work earned Edwards (Steptoe has already passed away) the 2010 Nobel Prize in Physiology or Medicine. Since then, assisted reproductive techniques (ART) of various forms have helped infertile couples have children of their own.
In 1997 when I began working with Yana, the first children born by ART were just entering young adulthood (the first IVF baby Louise Brown was only 19 years old) and were about to start having children of their own. There was no evidence at the time that ARTs increased obstetric risk or frequency of adverse consequences for babies. However, there was no comprehensive longitudinal follow-up of development of the children and the numbers of subjects that were observed was still quite low. The question remained whether there would be long-term consequences of ART use that would emerge later in life and whether any of the potential deficits would be passed on to the next generation. Many of the more drastic forms of ART involved bypassing several natural biological processes such as sperm maturation, interactions with the female reproductive tract, sperm capacitation and interaction with the egg, and sperm membrane fusion with the egg. It was unknown how omission of some or all of those natural events affected early development of the embryo and resulting offspring. I had no experience with designing experiments or testing behavior in mice, but Bob, Caroline and Yana gave me a chance, one that I was lucky to have and I am grateful for.
Yasuyuki Kimura from Fukushima Medical University in Japan was a visiting scientist with Yana and he produced 5 generations of mice using an ART known as round spermatid injection (ROSI), which involved injection of immature sperm directly into an egg thus bypassing many processes that normally occur during fertilization. Within a year we were able to complete behavioral tests on 5 generations of mice produced by ROSI. I found no gross abnormalities in growth through 10 weeks of age, fertility, or spatial learning and memory due to ROSI. Although the data were negative, Yana always emphasized that all data are important and teaches us something. He encouraged me to write up the data and it was my first publication as lead author [4]. Looking back now, these projects with the Yanagimachi lab are what ignited my interest in developmental biology and how changes in the early life environment can have long-term effects on offspring.
2.2. Ryuzo Yanagimachi and “Team Yana”
Yana was an unsung hero on the University of Hawaii campus at the time. In the same year that I started working with Yana, he was awarded Japan’s International Prize for Biology from the Emperor of Japan for his research. It is considered by some to be Japan’s equivalent of the Nobel Prize. While Yana was recognized for his research around the world, Yana’s laboratory was in a converted warehouse, a non-descript building tucked away in the back of the campus behind the food services operation. Yana was coming up on the end of his current R01 and he was planning on retiring after having had over 30 years of continuous funding from the NIH. I would have gone back to continuing in my studies in public health and health administration when luck struck again.
Yana had seven “Team Yana Rules” which he posted prominently in the laboratory. Rule number 7 reads: “The most important asset in this laboratory is not the most expensive equipment, but your brain. Machine(s) cannot dream. You can.”
One of Yana’s post doctoral fellows, Teruhiko Wakayama (“Teru”, now a Professor at Yamanashi University, Japan) had a dream of cloning a mouse from an adult somatic cell [5]. Earlier in the year, Ian Wilmut and colleagues in Scotland cloned a sheep, “Dolly” [6], but there was question about whether she was a true clone and replication of that feat was proving to be difficult. Teru used a slightly different technique (later dubbed the “Honolulu Technique”) and succeeded in cloning a mouse from an adult cumulus cell. Not only that, he replicated that over 6 generations of mice – clones of clones of clones…. All of this work was done in Yana’s converted warehouse laboratory at UH and began with a post doc’s dream [7]. Not even Yana believed it could be done. With international attention, new sources of funding, and Teru’s zest for accomplishing what may seem impossible to others, Yana postponed his retirement and set back to work in the laboratory. Teru and Yana agreed that behavioral and physiological phenotyping of the cloned mice was necessary and turned to me to do this. By now, I realized the allure of basic research that cannot necessarily be taught, only experienced. I jumped at the opportunity and as it turned out, this was to be the “tipping point” that changed the course of my career…again.
Producing a live organism from a fully differentiated somatic cell was thought to be impossible until 1997 when Dolly the sheep was cloned from a cell from the mammary gland of her donor. Mammalian somatic cell cloning has potential applications in basic and clinical research. It presented a novel technique to ask questions about genomic reprogramming, embryonic development and cancer development. In the clinic, it could be possible to generate cells, tissues, or even whole organs from a patient’s own cells and would circumvent problems with transplant rejection. Commercially, some proposed that somatic cell cloning would allow for mass production of genetically altered livestock for pharmaceutical production or human consumption. However, the long-term consequences of the technology were unknown. Clones are genetically identical to their cell donors, but are they phenotypically the same? The likelihood of adverse effects resulting from adult somatic cell cloning was even higher than with the ROSI mice I previously studied with Yana and Yasu. Cloning is far from a natural method of reproduction in mammals and it was still unknown whether bypassing meiosis and all natural fertilization events would result in serious adverse effects in full term offspring. In sum, we found that attainment of preweaning developmental milestones was delayed in cloned mice in 3 of 10 measures. However, tests of learning and memory, activity levels and motor function were not affected in adult clones compared to controls and serial cloning over 6 generations did not appear to have any significant effect on those measures [7, 8]. An important finding in our study, however, was that mammalian somatic cell cloning produced mice that had increased body weight as adults which lead to my next adventure.
3. Introduction to ingestive behavior, obesity research and SSIB
Yana, Teru and I did not think to follow up on the body weight finding ourselves simply because we did not have the expertise to do it and honestly, at the time, I did not realize the significance of it. In fact, this manuscript would end right here were it not for the events that happened next. Yana called a colleague, Ed Leiter, a well-known diabetes researcher at Jackson Laboratory (JAX), to inquire about phenotyping the mice and they agreed that the mice would be sacrificed at UH and tissues sent to JAX. In the meantime, Randall Sakai was visiting UH working with the Blanchard lab on a separate collaboration studying the neurobiological effects of chronic social stress in rats. He was wrapping up an experiment in Hawaii and Bob Blanchard suggested I show him my clone mouse data. I was unaware of Randall’s background in ingestive behavior research or that he was in the process of moving from the University of Pennsylvania to join Steve Woods, Randy Seeley, David D’Alessio and others at the University of Cincinnati’s (UC) new Obesity Research Center and together turning UC into a new hub for ingestive behavior and obesity research. In the end, Dr. Leiter at JAX told Yana that, “the group at UC had the resources and expertise to do a much better study”. He passed the project on and wished us well with an offer to help in whatever way JAX could. Yana gave us his blessing too and I soon found myself on a plane with the live mice headed from Honolulu to Cincinnati, OH for 3 weeks to work with Randall, Steve, Randy and their laboratories. This was a lesson in data sharing, collegiality, honesty, and most of all, working with others to make science happen.
Our studies at UC showed that the cloned mice were more sensitive to the anorexic effect of exogenous leptin and MTII, a melanocortin-3/4 agonist. The clones had greater adiposity than age-matched controls and were hyperleptinemic and hyperinsulinemic. We mated cloned male and female mice, both of which were obese, and found that the offspring were similar in weight to the control mice. Thus, the obese phenotype was not genetically passed on through the germline to offspring suggesting that an epigenetic (an alteration not attributable to changes in DNA sequence) mechanism was responsible for the altered phenotype in the cloned mice [9].
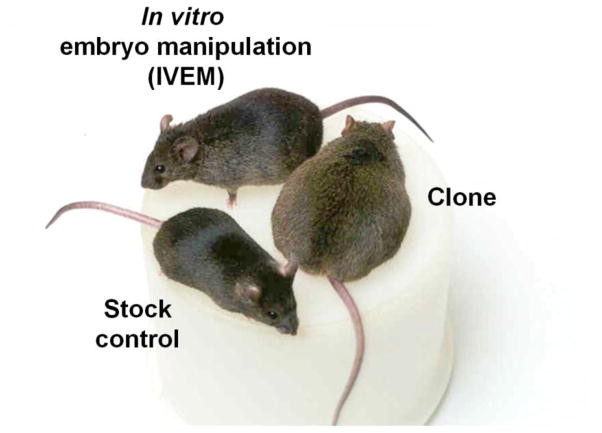
Yukiko Yamazaki and Hidenori Akustu, post docs in Yana’s lab, helped generate additional cloned mice and critical control groups for the project. An important finding was that the in vitro embryo manipulated (IVEM) control group also displayed increased body weight and adiposity compared to control mice from a commercial supplier. The IVEM group was generated to control for all the mechanical manipulations and in vitro culture exposure that the cloned embryos experienced. The IVEM mice were derived from an embryo that was naturally fertilized in vivo, collected at the 2-cell stage (~day 2 after mating), exposed to mechanical handling and in vitro culture media until it reached the blastocyst stage of development (~day 4), transferred into a surrogate dam, and delivered by caesarian section on E19.5. The IVEM mice displayed a phenotype that fell between the cloned mice and commercially purchased control mice suggesting that mechanical stress, in vitro culture conditions, or both could contribute to the obese phenotype [9, 10] (Figure 1). In other words, the early embryonic environment had significant long-term consequences on resulting offspring that only emerged in early adulthood. These data have greater implications for offspring produced by ART. Millions of children worldwide, approximately 1–3% of all births (1.5% in the U.S. [11]), were born using some form of ART and these data suggest that there may be long-term metabolic consequences for the children that require medical follow-up. Indeed, recent epidemiological data suggest that children born by ARTs, such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), exhibit early metabolic differences compared to spontaneously conceived children, including greater adiposity, higher blood pressure and hyperglycemia [12–15].
Figure 1.
Cloned mouse and representative mice from two control groups. Cloned mice are obese (pictured on the right) compared to representatives of control groups, commercial stock mouse (bottom left) and in vitro embryo manipulated (IVEM) mouse (top left). Adapted from [9].
I presented the cloned mouse data at the SSIB Annual Meeting in Dublin, Ireland in 2000. It was my first trip to Europe punctuated by spending 10 hours at Charles de Galle airport in Paris because the Air France Concord crashed and barely making it at midnight to pick up keys to my dorm room at Trinity College due to the delay. All that happened in addition to having to give my first scientific talk at my first scientific meeting the very next day. That meeting was my introduction to SSIB and I certainly will never forget it. Not because of the events leading up to the meeting, but because I learned that SSIB was more than just a scientific society because of its membership. Randall introduced me to so many people who I now count as close colleagues and, more importantly, as friends. That is where my network started, even before I attended a day of graduate school classes. I am fortunate to have been introduced to networking early and I have come to realize throughout my career that building a network of colleagues and continuously nurturing that network is so important, more so than continuously toiling at the lab bench.
4. University of Cincinnati
I switched programs and completed a Master of Science in Biomedical Sciences at UH, left the comfort of island paradise in Hawaii and traded the Pacific Ocean for the Ohio River. The next stop on my journey was the University of Cincinnati (UC) in Cincinnati, OH. The Obesity Research Center at UC had just been established and was gaining momentum having assembled an impressive team of investigators with Sakai, Woods, Seeley, D’Alessio, Patrick Tso, and Jim Herman. It was a wonderful place to do science, particularly with my growing interests in stress and metabolism. Given the collaborations among the Blanchard, Yanagimachi and Sakai labs, it was a natural transition and I decided that I would do my Ph.D. in Neuroscience in Randall’s neuroendocrinology laboratory. He impressed me with his guidance, mentorship, and enthusiasm through all aspects of the cloned mouse project even with 4,500 miles separating our labs and it was clear that he would serve as an outstanding graduate advisor.
4.1. Social stress in the new and improved Visible Burrow System (VBS)
Although Randall’s Ph.D. with Alan Epstein was in the salt appetite and fluid balance fields, he expanded his research to the neurobiology of stress as a post doc in Bruce McEwen’s laboratory at The Rockefeller University. Shortly after joining the McEwen lab, Randall initiated a collaboration project with Bob and Caroline Blanchard at UH to use the Visible Burrow System (VBS) model of social stress in rats to study the consequences of chronic stress in a more naturalistic environment rather than using restraint stress which was common in many stress labs [16]. In the VBS model, 4–5 male rats are housed with 2–3 female rats as a colony in a VBS which is an apparatus that is designed to mimic the rats’ natural living environment in underground burrows [17]. A dominance hierarchy quickly forms among the males in the colony resulting in one dominant and several subordinate rats. The VBS model of social stress is a powerful tool because of its ethological relevance and naturalistic qualities. Rats normally form social hierarchies within colonies in the wild and the resulting stress is instituted by the animals themselves without investigator influence. Together over the years, they documented alterations in hormone activity and regulation, metabolic activity, reproductive function, neurochemistry and neuronal morphology in subordinate animals and found that there are costs associated with being the dominant as well [18–23]. With Randall’s move to UC, he extended use of the VBS model from studies of aggression, defensive behavior, and HPA axis dysregulation to that of stress-induced metabolic consequences which was the focus of my Ph.D. dissertation.
Chronic subordination stress over a typical experiment lasting 14 days results in significant weight loss (~10–15% of body weight) among subordinate rats. Body weight of dominants was affected by VBS housing as well: the dominants typically lost a small but significant amount of weight in the VBS (~5%), and also fail to gain weight at the rate of the control animals housed with a female in standard cages [24]. The metabolic basis of the weight loss was not studied under these conditions and became the topic of my dissertation. I found that subordinates lost both adipose and lean tissue, whereas the weight loss in the dominant animals was attributable to loss of adipose tissue also [24]. When the rats were removed from the stress environment in the VBS, subordinates were hyperphagic and quickly regained their body weight, but did so primarily as visceral adipose tissue while dominant rats regained body weight as both lean and adipose tissue. Furthermore, when rats were subjected to repeated cycles of social stress and recovery periods their metabolic deficits progressively worsened indicating cumulative effects of stress for an individual over time [25, 26].
We hypothesized that the change in body composition was due to the different endocrine conditions that subordinate and dominant animals were in at the end of social housing in the VBS. That is, subordinates had low testosterone and high corticosterone levels and this state would promote adipose tissue deposition. In contrast, since dominant animals have testosterone levels similar to that of controls and corticosterone levels intermediate compared to that of controls and subordinates, dominants could regain weight as both lean and adipose tissue. Mary Nguyen, a fellow graduate student in the lab, found that by treating subordinates with 5α-dihydrotestosterone (DHT), a non-aromatizable, reduced form of testosterone, weight loss during chronic stress in the VBS was not affected, but DHT promoted weight gain as lean body mass during recovery [27]. Together, the metabolic changes occurring in VBS-housed animals suggested that chronic subordination stress may lead to a state that mimics metabolic syndrome in humans.
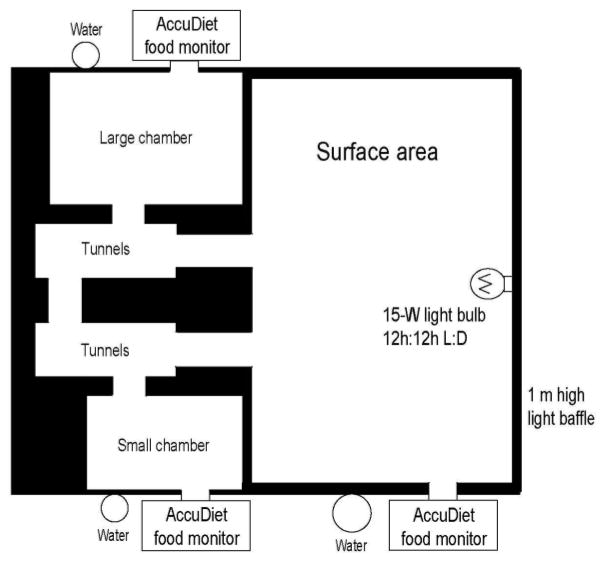
An important adaptation that Randall and I introduced to the VBS set up at UC was outfitting each apparatus with computerized monitoring systems to measure food intake and determine meal patterns for each animal, each uniquely identified by a subcutaneously implanted microchip, while group-housed during chronic stress (Figure 2). All animals decreased food intake upon introduction to the VBS; however, once the social hierarchy was established, dominants quickly returned to their basal level of intake, while the subordinates showed a continued suppression in both the number and size of meals despite having access to food and water in all areas of the VBS; the diurnal pattern of meal consumption was also shifted among the subordinates perhaps suggesting altered circadian rhythms as well [28, 29]. Here was an example of taking a well-established model, extending it to ask new questions and modifying and improving on what was already in existence rather than “re-inventing the wheel”.
Figure 2.
Diagram of a modified visible burrow system (VBS). AccuDiet food intake monitors with identification scanners are located at each feeding station. When a rat’s head enters the food access tunnel, its identification microchip (implanted subcutaneously on top of the head) is scanned and the time, duration and amount of food is recorded electronically [29].
4.2. Extension of cloned mouse and ART studies
Randall gave me the freedom to pursue other studies in addition to my Ph.D. dissertation project. I continued working on the cloned mouse and ART studies on the side since they were still experiments too risky for a graduate student Ph.D. project. He also encouraged me to collaborate with other graduate students and post docs in other labs which was an early lesson about how to do collaborative science which is so critical in the current research environment. No longer is science done by an individual investigator who can “do it all”.
We extended the clone mouse studies and found that the altered metabolic phenotype was independent of the donor cell type, the mouse strain, or sex [30], thus indicating that there were features of the technique or to the process of reprogramming a differentiated, adult somatic cell to pluripotency that altered the adult phenotype. We eventually secured R01 funding to expand the project to include mice produced by ARTs, such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), in addition to the cloned mice since ART has greater translational relevance for humans. Karen Scott, a graduate student in Randall’s lab, headed a study that found altered glucose tolerance in mice born by IVF and ICSI as well as cloning prior to increases in body weight and adiposity [31]. An important caveat about all of our ART studies is that the mice used to produce offspring were not infertile, thus it is unclear whether the altered phenotypes would be greater in magnitude or if other adverse consequences would emerge with an underlying fertility deficit in the parent mice. Together the studies emphasize that investigation regarding the long-term effects of manipulations associated with ART is warranted.
The time came for me to consider who I was going to do my post-doctoral training with. Back at my first SSIB meeting in Dublin, Ireland in 2000, someone in the audience raised his hand and stood up tall when my presentation was open for discussion. This was the part that I had been dreading the most! He proceeded to compliment me for giving a nice presentation (even though, according to Gary Schwartz, I was speaking with a “Hawaiian English” accent throughout because I was so nervous!) and asked a very fair and thoughtful question for which I was glad I had an answer to. That was my introduction to Tim Moran. Over the course of my 5 years in grad school I grew to know and respect Tim not only as a rigorous and thoughtful scientist, but also as a highly-regarded mentor and it became clear as I neared the end of my graduate studies that he was who I wanted to train with as a post-doctoral fellow.
5. Johns Hopkins University
I moved further east to Baltimore, MD, this time trading the Ohio River for the Chesapeake Bay, and Cincinnati-style chili for Maryland’s blue crab; it was great to be living next to an ocean again! As I thought about what direction my research would go in, I realized that I had an opportunity to integrate the experiences I had at UH and UC into a new project with Tim Moran’s laboratory at Johns Hopkins University (JHU). From the outset, Tim made it clear that the main objective of my time as a post-doc was to develop a program of research of my own, in addition to contributing to other studies in the lab. This is a philosophy he steadfastly believes in and one that I am grateful for and pass on to my own trainees. A common theme underlying my previous research experience was that an organism’s environment, whether it is exposure to in vitro culture media as an embryo or chronic stress and HPA axis activation in adulthood, can have a significant and persistent influence on the physiology and behavior of an individual independent of genetics. I was very interested in how environmental perturbations during sensitive periods of development, particularly during intrauterine and early postnatal life, influence the long-term health of offspring.
Just as I joined Tim’s lab, NIH’s NIDDK announced that they were also very interested in the obese and diabetic intrauterine environment’s effect on offspring and allocated funds for grants specifically on this topic and Tim received one of those grants. Not only did it provide additional funds specifically for the type of studies that I wanted to do, but helping to write the grant alongside someone as successful as Tim is with NIH funding was one of the most beneficial training exercises I did as a post doc.
5.1. Kick start from a “kangaroo” and an RFA
As a new post doc, one of my first priorities was to secure my own funding so I started collecting data for an NIH F32 post-doc fellowship proposal. Then one Sunday morning a few months after I started in the lab, a story in the Baltimore Sun newspaper caught my eye. It described a brand new funding initiative issued by the NIH that was aimed at helping facilitate post doctoral trainees’ transition from fellow to independent investigator, the K99/R00 Pathway to Independence Award (a.k.a. “kangaroo” award). I was one of the first awardees of the K99/R00 award which is what helped to kick start my post-doctoral research and set the foundation for my current program of research. I learned that opportunities can present themselves in the most unexpected places and that being ready to capitalize on them, and actually pursuing them, at any time is essential.
5.2. Prenatal stress influences on offspring phenotype
I met Jim Koenig, who was a Professor of Psychiatry at the University of Maryland, at a the Neurobiology of Stress Workshop that he organized in the summer of 2005, right before I was to move to Johns Hopkins. He gave a talk about his research investigating the influence of prenatal stress on the susceptibility of rat offspring to schizophrenia-like behavior [32]. I was intrigued by his observation that the rat pups from dams that were stressed during the 3rd week of gestation were heavier than those from control rat dams. As soon as I moved to Baltimore, Tim and I visited Jim in his lab at the Maryland Psychiatric Research Center. Jim was not planning to follow up on the body weight phenotype in the prenatal stress model and fully supported me in setting up the rat model in Tim’s lab to extend those findings as my own project. This meeting was the start of a collaborative and mentoring relationship with Jim that continues today. Having multiple mentors has been extremely helpful throughout my career. All of them were outstanding in their own ways and being able to collect input from different points of view and drawing from different experiences has been invaluable throughout my training and career.
I established the prenatal stress paradigm in Tim’s lab with Jim’s input and my initial studies showed that offspring were predisposed to diet-induced obesity in adulthood if exposed to prenatal stress in utero. Maternal consumption of a high fat diet during gestation resulted in a similar phenotype in offspring [33, 34]. However, contrary to what we hypothesized, the two early life manipulations did not have additive or synergistic effects under the experimental conditions that we tested. We continue to use these models in the lab and extended the studies to address other questions related to stress neurobiology and energy homoeostasis and the mechanisms through which these systems may be persistently altered as a result of early life experiences. This was not necessarily a new concept. Others had proposed the idea of the developmental origins of health and disease decades earlier [35–37]. However, despite the wealth of evidence for developmental “programming” effects of environmental factors such as maternal stress or maternal nutrition, the exact mechanisms through which early life events have permanent effects on offspring remain poorly understood.
5.3. Maternal diet and offspring metabolic phenotype
We found that offspring of rat dams fed high-fat diet are heavier by the time they are 7 days old and become obese and have impaired glucose tolerance by the time they are weaned at 21 days of age [34]. Ryan Purcell was a research technician in the lab and took on a project to analyze maternal behavior, milk composition, and the pups’ ingestive behavior independent of their mother’s presence. He found that those dams fed a high fat diet nursed longer and their milk was higher in fat and protein content, thus was more energy dense. In addition, the rat pups that were nursed by moms fed high fat diet spent more time nursing and consumed more milk in a defined period of time in an independent ingestion test [38]. The offspring continued to consume more calories through adulthood when provided with either standard chow or high fat diet and preferred to consume palatable high fat diet when given a choice [39].
Bo Sun, a graduate student in the lab, took offspring from chow fed dams and high fat diet fed dams and cross-fostered all of the offspring resulting in 4 groups of pups: CHOW-CHOW, CHOW-HF, HF-CHOW and HF-HF as defined by the dams’ diet during gestation and lactation. We used this paradigm in order to determine the relative contribution of the prenatal versus the postnatal maternal diets to the offspring’s phenotype. Bo found that offspring cross-fostered to dams consuming high fat diet during the postnatal period significantly reduced leptin-induced pSTAT3 levels in the arcuate nucleus likely contributing to hyperphagia, impaired glucose tolerance and obesity observed in offspring. This finding was independent of the offspring’s prenatal diet, thus suggesting that the postnatal diet had a greater influence in determining offspring’s metabolic phenotype [39]. As a follow up to these studies, Lin Song, another graduate student in the lab, has undertaken studies to determine how development of specific hypothalamic neurocircuits is affected by exposure to maternal HF diet during the gestation or lactation period.
The associations between consumption of a high-fat or ‘Western’ diet and metabolic disorders such as obesity, diabetes, and cardiovascular disease have long been recognized and a growing body of literature suggests that diets high in fat can also have a profound impact on cognitive function (reviewed in [40]). Furthermore, the effects of maternal obesity and consumption of high fat or high energy diets are shown to affect developing offspring as well. Zac Cordner, an MD/PhD student in the lab, has documented using the maternal diet cross-fostering strategy that high fat diet consumption during the prenatal or postnatal or both periods has persistent long-term effects on cognitive function and reward behavior of offspring [41].
In the current environment of escalating obesity and consumption of high fat diets, our findings have implications for the health of future generations and potential preventative strategies are actively being investigated. Studies by other investigators, including those by Barry Levin and Sheng Bi, previously demonstrated that exercise during the early adolescent period had persistent beneficial effects against hyperphagia and other metabolic deficits in genetic models of obesity [42, 43]. We were interested in determining whether early life exercise would have long-term effects on obesity prevention in offspring from dams fed high fat diet throughout gestation and lactation. Indeed, exercise did rescue decreased leptin sensitivity, although it did not prevent greater weight gain in the offspring of high fat fed dams [44].
5.4. Exploring epigenetic mechanisms
The term “epigenetics” literally translates to “above the genome.” This term was used by developmental biologist Conrad Waddington in the 1940s to refer to the “interactions of genes with their environment which bring the phenotype into being”. Waddington did not have a molecular mechanism for how genetics was linked to developmental biology, but his ideas set the stage for what we know today. The field of epigenetics and epigenetic modifications in mediating neural function has received much attention in recent years. We know today that epigenetic modifications are mitotically heritable chemical alterations of DNA that are not mediated by changes in the primary DNA sequence, can influence gene expression, and are maintained during cell division. The molecular mechanisms responsible for epigenetic regulation of gene expression include histone modifications (such as acetylation and methylation), non-coding RNAs (microRNA and long non-coding RNA) and DNA methylation (addition of a methyl group to cytosine-guanine dinucleotides, CpGs). However, much is still unknown about epigenetic regulation of gene expression and the list of epigenetic mediators is certain to grow as we learn more.
I had the opportunity to incorporate molecular epigenetic assays into our animal studies to discover potential mechanisms that underlie the observed alterations in behavioral and physiological phenotypes in offspring from our models. As I put my K99 proposal together, Tim introduced me to Jimmy Potash, a clinical faculty member in the Hopkins Psychiatry Department and a psychiatric geneticist. He was working with Andy Feinberg, a pioneer in cancer epigenetics and who was developing novel, cutting-edge technologies to assess epigenetic modifications across the genome. Jimmy became a mentor to me in the clinical and genetic/epigenetic realms, which nicely complemented the expertise of the other mentors for my project.
My introduction to epigenetics began with a colloboration with Richard Lee, then a post doc with Jimmy. It is well-documented that chronic stress and exposure to glucocorticoids have adverse behavioral effects including anxiety and depression, as well as metabolic consequences such as obesity and insulin resistance. Richard and I worked on a study to determine how adolescent exposure to chronic glucocorticoids could alter epigenetic modifications on HPA axis thus leading to behavioral deficits. We found that 4 week treatment of adolescent mice resulted in anxiety-like behavior that was associated with persistent changes in expression and DNA methylation of Fkbp5, a chaperone protein involved in HPA axis regulation. To begin to determine whether corticosterone had a causal role in the observed changes in gene expression and DNA methylation, Richard treated mouse hippocampal neurons in vitro with corticosterone. The results confirmed that expression of Fkbp5 increased and DNA methylation decreased supporting a potential causal role for glucocorticoid-induced epigenetic alterations in producing changes in gene expression and, ultimately, behavior [45].
With our prenatal stress studies, Gretha Boersma, a post doc in the lab, found that prenatal stress in rats alters gene expression potentially leading to altered stress-coping styles of the offspring which predispose them to metabolic disorders in adulthood [46]. She focused on brain derived neurotrophic factor (BDNF) as a potential mediator behind altered behavior in prenatally stressed offspring. BDNF is critical in supporting brain development and she found lower Bdnf gene expression in prenatal stress offspring in the hippocampus and amygdala, areas of the brain important for HPA axis regulation, cognitive function and emotionality. Using bisulfite pyrosequencing, a method to quantify methylation at individual CpG resolution, she measured DNA methylation of BDNF since this has been implicated in regulation of Bdnf gene expression. DNA methylation, specifically of Exon 4, of the BDNF gene is higher in both the hippocampus and amygdala of prenatal stress offspring corresponding to the decreased expression specifically in these brain regions [47]. The data suggest that DNA methylation and mRNA expression of Bdnf may contribute to the offspring phenotype resulting from prenatal stress exposure since Bdnf plays a major role in neuronal development and previous studies have shown that prenatal stress affects neuronal development during this specific early postnatal period.
Clearly, using a candidate gene strategy to determine the epigenetic contribution to behavior is tedious, in part because very limited regions of DNA can be assayed at one time. Expanding on our collaboration with Richard Lee and other colleagues in psychiatric bioinformatics at JHU, we have helped develop custom-designed genome-wide platforms for use in mouse and rat [48, 49]. We look forward to reporting findings using these powerful assays. We recognize that there is still much more research to do in determining the exact contribution that epigenetics has to metabolic and other brain disorders and this continues to be a challenging, yet stimulating area of investigation that has the potential to provide critical insights about the link between the environment and an organism’s physiology and behavior.
6. Final perspectives and acknowledgements
In closing, a phrase from Jake Shimabukuro’s “Hula Girl” soundtrack sums up my journey well,
“Imagine living out a dream, a dream that wasn’t really yours. But through the journey you have learned, you must have had that dream before.” [50]
As with any challenging journey there are bound to be periods when you ask yourself, “why am I doing this and is it really worth it?” I certainly did, many times. My answer was, obviously, yes and it is because of the great people I worked with in the lab, my mentors who provided support, guidance, and many words of wisdom just when I needed it, and the many colleagues I knew I could count on for help and advice. I share this award with so many people who have supported me along this journey. Many thanks to my family who provided unconditional love and support, even though I chose the road less traveled and moved further east and away from Hawaii with every step of my career. I could not have navigated the path without the guidance and support of many mentors along the way, in particular, Bob & Caroline Blanchard, Ryuzo Yanagimachi, Randall Sakai, and Tim Moran. I thank the individuals who nominated me and provided letters of support for me for the Epstein Award. I extend heartfelt gratitude to the Epstein family for generously endowing the Alan N. Epstein Research Award to recognize early career investigators in this field and to keep Alan’s legacy alive (Figure 3). I have a deep appreciation for the SSIB membership who, for the last 14 years, provided me with an environment that is collegial and nurturing, but scientifically critical and challenging for SSIB-lings like myself to learn, grow, and mature in. Finally, thank you to the funding agencies who provided financial support for these and other studies: NIH NIDDK (T32DK-59803; R01DK066596 and R01DK068273 to R.R.S.; R01DK077623 to T.H.M.), NINDS (F31NS047791), NIMH (T32MH-15330), NICHD (K99/R00HD055030), NIMH (R21MH097150), the Albert J. Ryan Foundation, the Brain and Behavior Research Foundation (NARSAD), and the Maryland Nutrition and Obesity Research Center.
Figure 3.
Alan Epstein. Alan (left) is pictured here with his favorite species to study, the rat. He also studied the pigeon, monkey, opposum, and extended his work into sheep and humans. Unfortunately, I never got to meet Alan in person. However, through my mentors and the SSIB I learned about who Alan was, what he represented, and recognize the impact that he has had on my entire career.
Figure 4.
Highlights.
“The most important asset in this laboratory is not the most expensive equipment, but your brain. Machine(s) cannot dream. You can.” -- Dr. Ryuzo Yanagimachi
Develop your network early and nurture it continuously.
Don’t reinvent the wheel, improve on it to ask new and important questions.
Collaborative science is critical as no scientist can be an island.
Opportunities are only beneficial to those who take advantage of them.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert MA, et al. Amygdala lesions produce analgesia in a novel, ethologically relevant acute pain test. Physiol Behav. 1999;67(1):99–105. doi: 10.1016/s0031-9384(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 2.Yanagimachi R. Germ cell research: a personal perspective. Biol Reprod. 2009;80(2):204–18. doi: 10.1095/biolreprod.108.071993. [DOI] [PubMed] [Google Scholar]
- 3.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 4.Tamashiro KL, et al. Bypassing spermiogenesis for several generations does not have detrimental consequences on the fertility and neurobehavior of offspring: a study using the mouse. J Assist Reprod Genet. 1999;16(6):315–24. doi: 10.1023/A:1020406016312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakayama T, et al. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394(6691):369–74. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 6.Wilmut I, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 7.Wakayama T, et al. Cloning of mice to six generations. Nature. 2000;407(6802):318–9. doi: 10.1038/35030301. [DOI] [PubMed] [Google Scholar]
- 8.Tamashiro KL, et al. Postnatal growth and behavioral development of mice cloned from adult cumulus cells. Biol Reprod. 2000;63(1):328–34. doi: 10.1095/biolreprod63.1.328. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro KL, et al. Cloned mice have an obese phenotype not transmitted to their offspring. Nat Med. 2002;8(3):262–7. doi: 10.1038/nm0302-262. [DOI] [PubMed] [Google Scholar]
- 10.Wilmut I. Are there any normal cloned mammals? Nat Med. 2002;8(3):215–6. doi: 10.1038/nm0302-215. [DOI] [PubMed] [Google Scholar]
- 11.Sunderam S, et al. Assisted reproductive technology surveillance--United States, 2011. MMWR Surveill Summ. 2014;63(10):1–28. [PubMed] [Google Scholar]
- 12.Ceelen M, et al. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92(9):3417–23. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- 13.Ceelen M, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24(11):2788–95. doi: 10.1093/humrep/dep273. [DOI] [PubMed] [Google Scholar]
- 14.Ceelen M, et al. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93(5):1682–8. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 15.Ceelen M, et al. Growth and development of children born after in vitro fertilization. Fertil Steril. 2008;90(5):1662–73. doi: 10.1016/j.fertnstert.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard DC, et al. Subordination stress: behavioral, brain and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard DC, Blanchard RJ. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neurosci Biobehav Rev. 1990;14(4):455–62. doi: 10.1016/s0149-7634(05)80068-5. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard DC, et al. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 19.McKittrick CR, et al. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37(6):383–93. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 20.McKittrick CR, et al. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2):85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Hardy MP, et al. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67(6):1750–5. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- 22.Spencer RL, et al. Chronic social stress produces reductions in available splenic type II corticosteroid receptor binding and plasma corticosteroid binding globulin levels. Psychoneuroendocrinology. 1996;21(1):95–109. doi: 10.1016/0306-4530(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 23.Albeck DS, et al. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17(12):4895–903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamashiro KL, et al. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80(5):683–93. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Tamashiro KL, et al. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007;91(4):440–8. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamashiro KL, et al. Social stress and recovery: Implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1864–74. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen MM, et al. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology. 2007;148(12):6145–56. doi: 10.1210/en.2007-0471. [DOI] [PubMed] [Google Scholar]
- 28.Melhorn SJ, et al. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R813–22. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89(4):536–42. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Tamashiro KL, et al. Health consequences of cloning mice. In: Inui A, editor. Epigenetic Risks of Cloning. Taylor & Francis Books; 2006. pp. 1–16. [Google Scholar]
- 31.Scott KA, et al. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod. 2010;83(2):220–7. doi: 10.1095/biolreprod.109.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig JI, et al. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156(2):251–61. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Tamashiro KL, Moran TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav. 2010;100(5):560–6. doi: 10.1016/j.physbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamashiro KL, et al. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58(5):1116–25. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker DJ, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 36.Dubos R, Savage D, Schaedler R. Biological Freudianism. Lasting effects of early environmental influences. Pediatrics. 1966;38(5):789–800. [PubMed] [Google Scholar]
- 37.Wadhwa PD, et al. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–68. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell RH, et al. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104(3):474–9. doi: 10.1016/j.physbeh.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun B, et al. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes. 2012;61(11):2833–41. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangel A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci. 2013;16(12):1717–24. doi: 10.1038/nn.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordner ZA, et al. Society for the Study of Ingestive Behavior. Seattle, WA, USA: 2014. Maternal high-fat diet results in cognitive impairment and hippocampal gene expression changes in rat offspring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi S, et al. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146(4):1676–85. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- 43.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R290–301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 44.Sun B, et al. Early postweaning exercise improves central leptin sensitivity in offspring of rat dams fed high-fat diet during pregnancy and lactation. Am J Physiol Regul Integr Comp Physiol. 2013;305(9):R1076–84. doi: 10.1152/ajpregu.00566.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee RS, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151(9):4332–43. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boersma GJ, et al. Prenatal stress and stress coping style interact to predict metabolic risk in male rats. Endocrinology. 2014;155(4):1302–12. doi: 10.1210/en.2013-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boersma GJ, et al. Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics. 2014;9(3):437–47. doi: 10.4161/epi.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee RS, et al. Adaptation of the CHARM DNA methylation platform for the rat genome reveals novel brain region-specific differences. Epigenetics. 2011;6(11):1378–90. doi: 10.4161/epi.6.11.18072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hing B, et al. Adaptation of the targeted capture Methyl-Seq platform for the mouse genome identifies novel tissue-specific DNA methylation patterns of genes involved in neurodevelopment. Epigenetics. 2015:0. doi: 10.1080/15592294.2015.1045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimabukuro J. Gently Weeps. Hitchhike Records, Sony Music Distribution; 2006. Hula Girl. [Google Scholar]