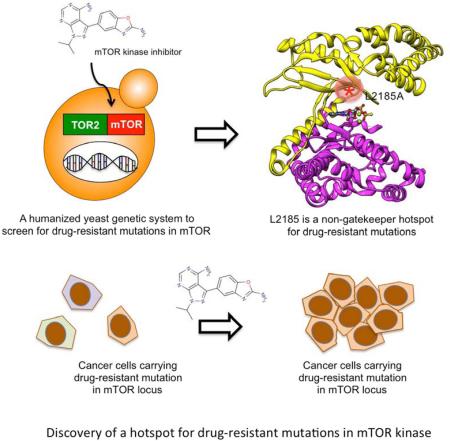
SUMMARY
Protein kinases are therapeutic targets for human cancer. However, “gatekeeper” mutations in tyrosine kinases cause acquired clinical resistance, limiting long-term treatment benefits. mTOR is a key cancer driver and drug target. Numerous small molecule mTOR kinase inhibitors have been developed, with some already in human clinical trials. Given our clinical experience with targeted therapeutics, acquired drug resistance in mTOR is thought likely but not yet documented. Herein, we describe identification of a hotspot (L2185) for drug-resistant mutations, which is distinct from the “gatekeeper” site, and a chemical scaffold refractory to drug-resistant mutations. We also provide new insights into mTOR kinase structure and function. The hotspot mutations are potentially useful as surrogate biomarkers for acquired drug resistance in ongoing clinical trials and future treatments, and to facilitate design of the next generation of mTOR-targeted drugs. Our study provides a foundation for further research on mTOR kinase function and targeting.
Graphical Abstract
INTRODUCTION
mTOR is a highly conserved serine/threonine protein kinase belonging to the PI3K-related kinase (PIKK) family (Wullschleger et al., 2006). mTOR forms two distinct kinase complexes, mTORC1 and mTORC2. mTORC1 controls cell growth and metabolism, in response to diverse cellular signals, including nutrients, growth factors and cytokines (Ma and Blenis, 2009). mTORC2 phosphorylates AKT at Ser473 and promotes cell survival (Sarbassov et al., 2005). Recent advances in cancer genomic sequencing have revealed cancer mutations frequently target mTOR pathway, resulting in hyperactivation of mTOR signaling that drives uncontrolled cancer growth, metabolism and survival (Wood et al., 2007). mTOR is an established molecular target for cancer therapy, because cancer cells tend to be addicted to aberrant mTOR signaling and mTOR inhibition is well tolerated (Bjornsti and Houghton, 2004; Guertin and Sabatini, 2007; Tsang et al., 2007).
Rapamycin is a macrolide natural product and a highly specific mTOR inhibitor. It forms a complex with FKBP12, which binds to the FRB domain of mTOR (Chen et al., 1995; Zheng et al., 1995). Two rapamycin analogs (rapalogs), temsirolimus and everolimus, are FDA -approved drugs for treatment of advanced renal cell and mammary carcinomas. However, rapamycin only partially inhibits TOR functions (Zheng et al., 1995), which is due to selective binding of FKBP12-rapamycin complex to mTORC1, but not mTORC2 (Loewith et al., 2002). Moreover, the clinical efficacy of rapalogs is limited with low overall objective response (Zhang et al., 2011b). Another shortcoming of rapalogs is that they lead to activation of the IRS1-PI3K-Akt negative feedback loop, sustaining survival of rapalog-treated cancer cells (O'Reilly et al., 2006; Sun et al., 2005). For these reasons, it is increasingly recognized that the therapeutic potential of rapalogs are limited.
The clinical success of ATP-competitive tyrosine kinase inhibitors (TKIs), such as imatinib and gefitinib, illustrates the value of targeting kinases as an effective anti-cancer strategy (Zhang et al., 2009). We previously found that mTOR kinase domain is required for both rapamycin-sensitive and rapamycin-insensitive aspects of cell growth and survival (Zheng et al., 1995), suggesting that TOR kinase domain is a more potent site for mTOR targeting. Subsequent studies lent support to this notion and revealed that the rapamycin-insensitive function is mTORC2-related (Loewith et al., 2002; Sarbassov et al., 2004). These findings provide a key rationale for developing ATP-competitive mTOR inhibitors for cancer therapy (Feldman et al., 2009; Guertin and Sabatini, 2009; Thoreen et al., 2009). In addition to selective mTOR kinase inhibitors such as PP242, OSI-027 and WYE-354, dual mTOR/PI3K inhibitors such as BEZ235 and Torin2 have been developed, which have additional advantage of preventing activation of the IRS1-PI3K-AKT negative feedback loop. Indeed, mTOR kinase inhibitors display superior anti-tumor effects compared with rapalogs in preclinical cancer models and are well tolerated with excellent toxicological profiles (Zhang et al., 2011a).
Since mTOR kinase inhibitors were described in 2008, numerous mTOR kinase targeting agents have been developed and entered into human clinical trials for cancer treatment (Zhang et al., 2011a). The remarkable speed with which human clinical trials have been initiated and the sheer number of different compounds being tested in patients underscore their therapeutic potential. Despite early promising results, major challenges remain. A comprehensive, mechanistic understanding of these small molecule inhibitors is lacking. Previous clinical experience with BCR-ABL and EGFR small molecule inhibitors shows that binding site drug-resistant mutations represent a major limiting factor for clinical efficacy (Zhang et al., 2009). In vitro mutagenesis screens have identified resistance mutations in ABL and EGFR kinases that faithfully recapitulate clinical observations (Azam et al., 2003; Engelman et al., 2006). The present study is aimed at developing a simple method and applying it to understand mTOR kinase function and drug-resistant mutations.
RESULTS
A S. cerevisiae System for Studying Chemical Inhibition of mTOR Kinase
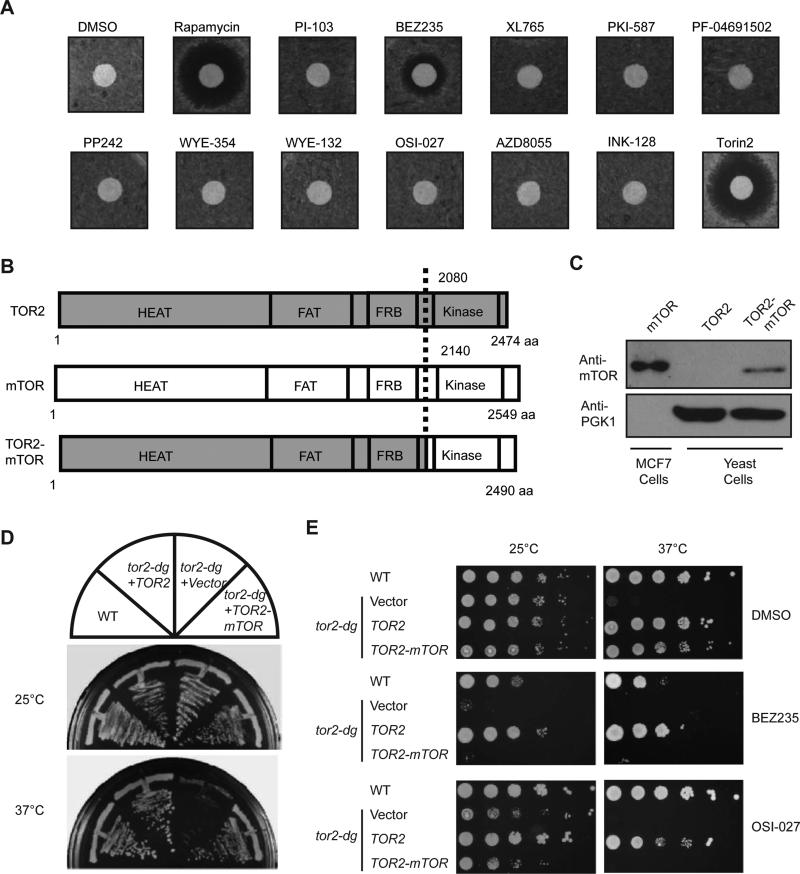
TOR is structurally and functionally conserved between humans and yeast. However, among a large panel of structurally diverse mTOR kinase inhibitors, only Torin2, and to a lesser degree, BEZ235, inhibit yeast growth (Figures 1A and S1), which is consistent an earlier observation (Liu et al., 2012). Poor sensitivity to mTOR inhibitors could be due to low permeability of yeast cells or insensitivity of yeast TOR kinase to these particular small molecules. To distinguish the two possibilities, we engineered a TOR2-mTOR fusion in which yeast TOR2 kinase domain is swapped in frame with mTOR kinase domain (Figure 1B). When expressed under the control of the TOR2 native promoter in a centromeric plasmid (Figure 1C), TOR2-mTOR fusion gene suppresses the temperature sensitivity of tor2-dg strain (Figures 1D and 1E), which carries a genomic TOR2 gene fused with degron, a tag rendering heat-inducible degradation of tagged proteins (Dohmen and Varshavsky, 2005), indicating that mTOR kinase domain complements the essential function of TOR2 kinase in yeast.
Figure 1. Developing a yeast system to assay for mTOR kinase inhibition.
(A) Wild type (WT) yeast cells were spread onto YPD plates and tested for sensitivity to structurally diverse mTOR kinase inhibitors by disc halo assay. Rapamycin was used as a positive control.
(B) The N-terminus of TOR2 (1-2080 aa) was fused in frame with mTOR kinase domain (2140-2549 aa). The TOR2-mTOR fusion is expressed under the control of TOR2 promoter in a centromeric plasmid.
(C) Yeast strain expressing WT TOR2 or TOR2-mTOR fusion was analyzed for expression by immunoblot with an antibody specific for mTOR kinase domain. PGK1 was used as a loading control and extracts from MCF7 breast cells were used as a positive control for mTOR.
(D) TOR2-mTOR fusion was expressed in tor2-dg and tested for its ability to complement TOR2 function by growth at permissive and restrictive temperatures.
(E) tor2-dg cells expressing TOR2 or TOR2-mTOR were serially diluted by 10-fold and tested for drug sensitivity on plates containing BEZ235 and OSI-027.
We next tested sensitivity of tor2-dg cells expressing TOR2-mTOR to mTOR kinase inhibitors. Similar to wild type (WT) cells, tor2-dg cells expressing TOR2 are poorly inhibited by mTOR kinase inhibitors BEZ235 and OSI-027 (Figure 1E). In contrast, tor2-dg strain expressing TOR2-mTOR is highly sensitive to these drugs (Figure 1E). This observation indicates that TOR2 is intrinsically resistant to mTOR kinase inhibitors, and that swapping TOR2 kinase domain with mTOR kinase domain renders yeast sensitivity to mTOR inhibitors.
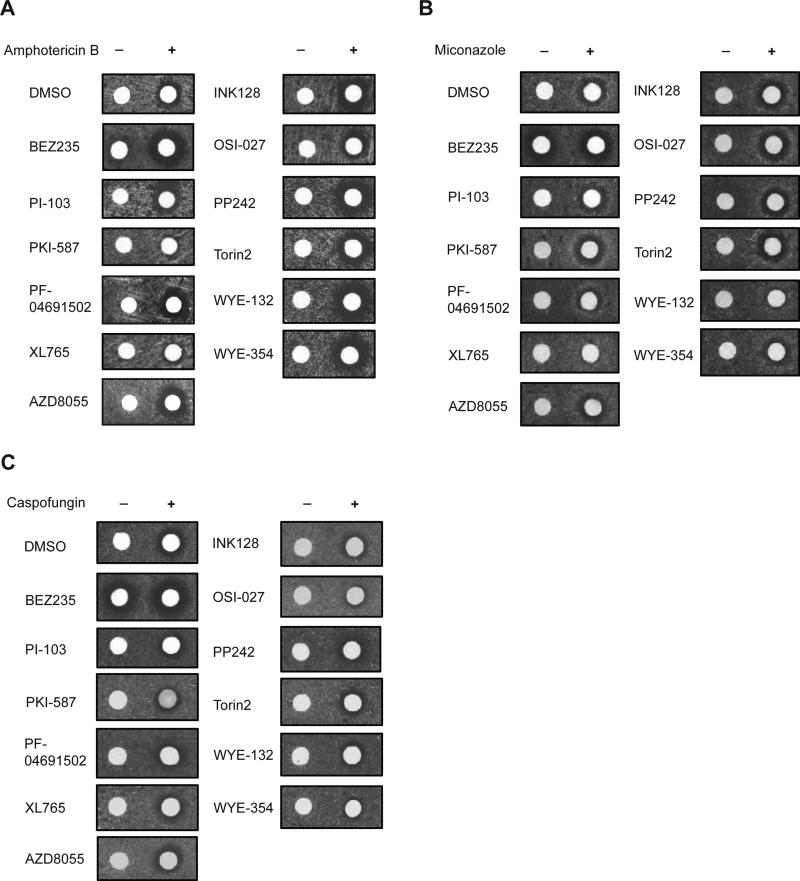
Even with TOR2-mTOR, tor2-dg strain remains resistant to majority of mTOR kinase inhibitors (Figure 2A). Yeast is known to be poorly permeable to small drug molecules (Emter et al., 2002; Simon and Bedalov, 2004). Deletion of ERG6, PDR1, and PDR3 has been used to enhance yeast cell permeability to organic compounds (Gray et al., 1998). However, yeast cells do not appear to tolerate erg6Δ in the tor2-dg background (data not shown). To explore an alternative method, we tested three different classes of antifungal drugs known to disrupt yeast cell wall or membrane structures. Amphotericin B, an amphipathic molecule that forms channel-like structures in the fungal membrane (Ghannoum and Rice, 1999), increases yeast sensitivity to most of mTOR kinase inhibitors (Figure 2A). In contrast, miconazole and caspofungin, antifungal drugs that disrupt ergosterol-containing yeast membrane and cell wall, respectively, fail to do so (Figures 2B and 2C). Amphotericin B was used thereafter to facilitate our studies of mTOR kinase inhibitors in yeast.
Figure 2. Enhancement of yeast cell permeability to structurally diverse mTOR kinase inhibitors by amphotericin B.
(A) tor2-dg cells expressing TOR2-mTOR were spread on synthetic complete (SC)-leucine plate and tested for drug sensitivity by disc halo assay using filter discs containing different mTOR kinase inhibitors supplemented with the drug carrier DMSO or amphotericin B (10 μM).
(B) Similar to Figure 2A except the filter discs were supplemented with miconazole 50 μM).
(C) Similar to Figure 2A except the filter discs were supplemented with caspofungin 50 μM).
Mutational Analysis of ‘Gatekeeper’ Residue in mTOR Kinase
Non-small cell lung cancer (NSCLC) patients responding to initial erlotinib treatment typically relapse within six months. In 50% cases, it is due to a single missense mutation, T790M, at the gatekeeper site of EGFR (Bell et al., 2005; Kobayashi et al., 2005; Pao et al., 2005). The larger methionine at this position constrains erlotinib binding, causing drug resistance while retaining the kinase's catalytic activity. A similar gatekeeper mutation (T315I) in the ABL kinase domain renders resistance of chronic myeloid leukemia (CML) to imatinib (Gorre et al., 2001). These observations suggest the gatekeeper residue contributes to resistance to ATP-competitive kinase inhibitors.
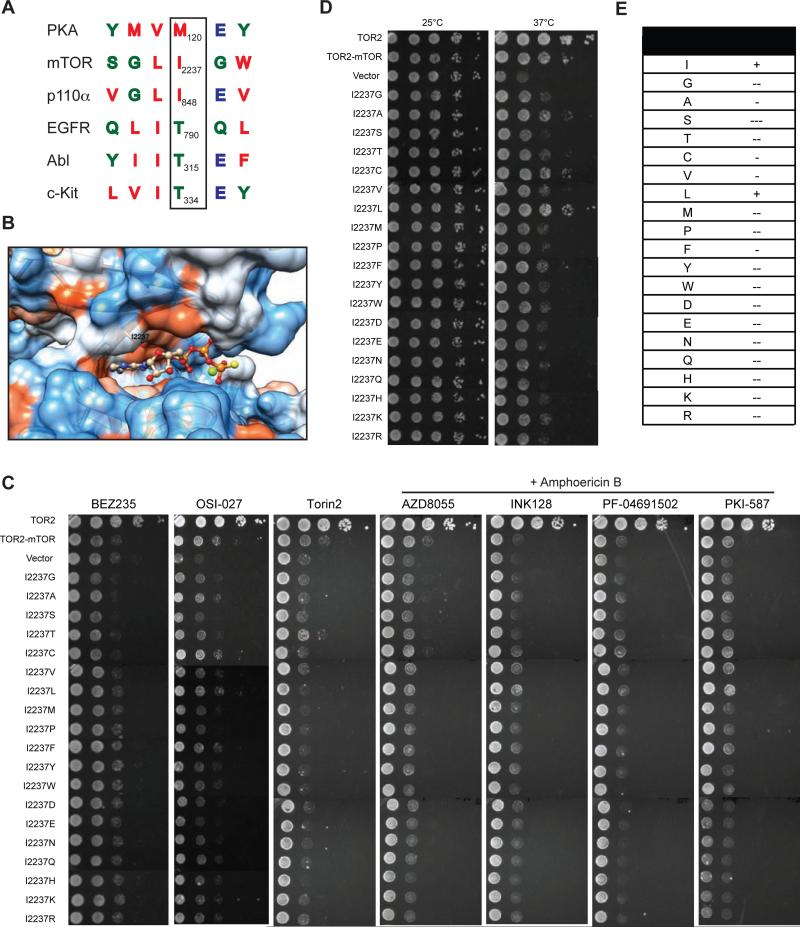
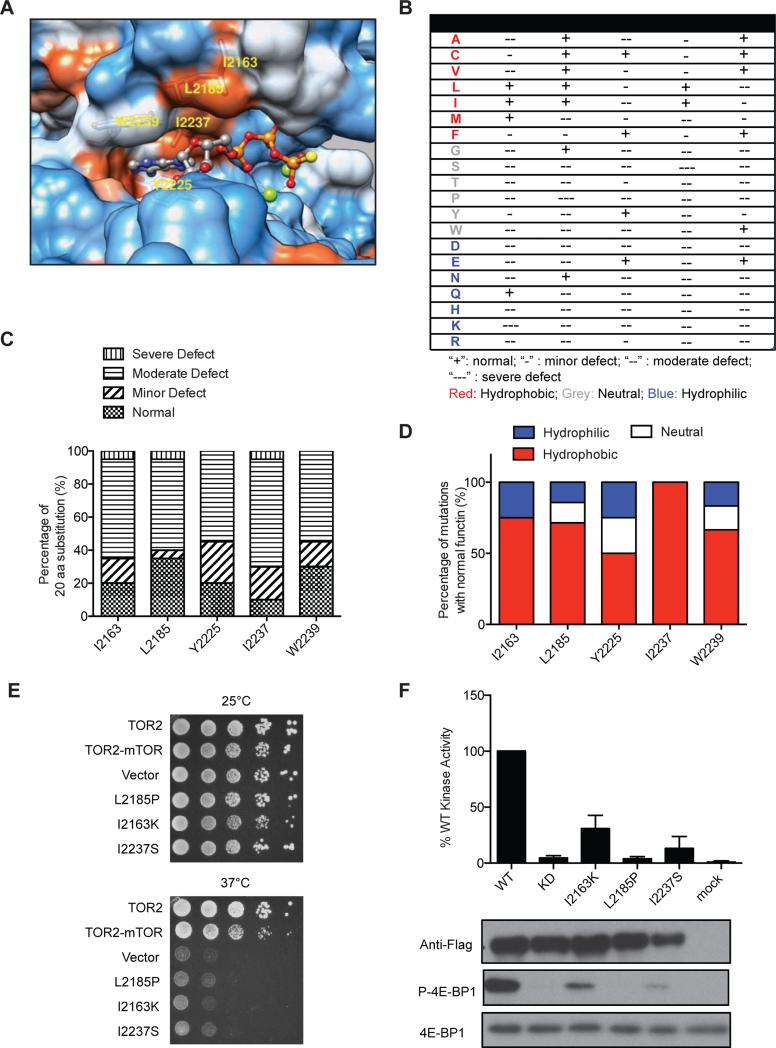
Based on sequence alignment, the mTOR gatekeeper residue is predicted to be I2237 (Sturgill and Hall, 2009). It is located within the hydrophobic pocket of N-lobe of the kinase domain (Figures 3A, S2 and 3B). In contrast to the conserved threonine gatekeeper residue in tyrosine kinases, both mTOR and PI3Kα have a relatively bulky isoleucine at this position (Figure 3A) (Vogt, 2008; Zunder et al., 2008). To evaluate the significance of the gatekeeper site, we performed saturation mutagenesis of I2237 in TOR2-mTOR fusion. Resulting TOR2-mTOR mutants were assayed for their sensitivity to chemically diverse mTOR kinase inhibitors. However, none of the mutations exhibit discernible drug resistance (Figure 3C). Strikingly, only the I2237L mutation fully preserves mTOR kinase function (Figures. 3D and 3E), suggesting that mTOR's gatekeeper position does not tolerate any substitution except the highly conserved leucine, which explains the lack of drug resistant gatekeeper mutations. A similar phenomenon was also observed with another atypical kinase, PI3Kα (Zunder et al., 2008), suggesting that mTOR and PI3K are similar with respect to the function of the gatekeeper residue.
Figure 3. Mutational analysis of the gatekeeper residue in mTOR kinase.
(A) Sequence alignment of the gatekeeper site for PKA, c-Kit, EGFR, ABL, p110-PI3Kα, and mTOR. Arrowhead marks the gatekeeper residue.
(B) Electrostatic model of the ATP-binding pocket of mTOR kinase (PDB ID code 4JSP). The I2237 position is as indicated. Atom is colored as follows: N, blue; O, red; P, orange; S, yellow; Mg+2, green. Surface representation is as follows: hydrophobic residue, red; neutral residue, white; hydrophilic residue, blue.
(C) tor2-dg cells expressing WT or mutant TOR2-mTOR were serially diluted by 10-fold and assayed for drug sensitivity on SC-leucine plates containing BEZ235, OSI-027, or Torin2, or AZD8055, BEZ235, INK128, PF-04691502 or PKI-587 in the presence of amphotericin B.
(D) tor2-dg cells expressing WT or mutant TOR2-mTOR were serially diluted by 10-fold and assayed for cell growth at different temperatures. Vector and TOR2 plasmids were used as a negative and positive control, respectively.
(E) Summary of gatekeeper mutations and their effects on mTOR kinase function. “+”: normal function; “−”: minor defect; “−−”: moderate defect, and “−−−”: severe defect.
Identification of A Non-Gatekeeper Hotspot for Drug-Resistant Mutations in mTOR Kinase Domain
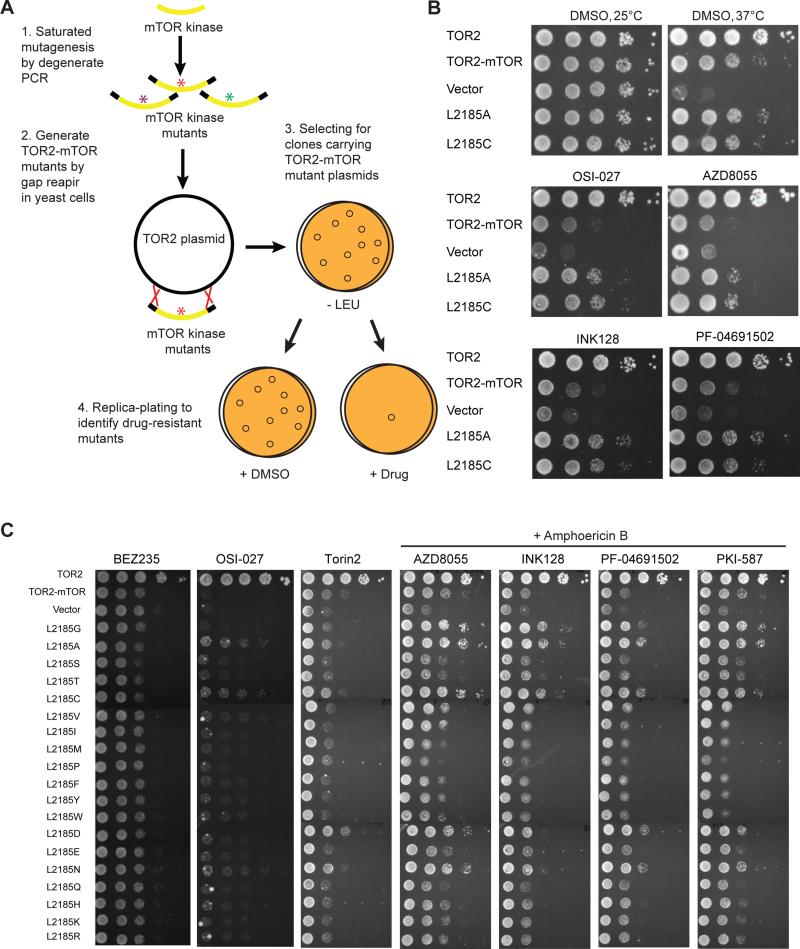
Yeast is a powerful model organism for genetic screens. We employed our yeast system and the following strategy to identify drug resistant mutations in mTOR kinase domain (Figure 4A). In this scheme, mTOR kinase mutants are generated through error-prone PCR amplification, and are recombined with a gapped TOR2 plasmid by ‘gap-repair’ to create TOR2-mTOR fusions in frame through homologous recombination in yeast cells. ‘Gap-repair’ is an efficient method to generate a library of mutant clones (Martzen et al., 1999; Uetz et al., 2000). TOR2-mTOR mutants are then replica-plated onto OSI-027-containing plates to screen for drug resistant mutations, which leads to isolation of drug-resistant clones carrying L2185A and L2185C mutations. Interestingly, These mutations also confer resistance to AZD8055, INK128, and PF-04691502 (Figure 4B), suggesting that L2185 is important for binding of structurally diverse mTOR kinase inhibitors.
Figure 4. Identification of a hotspot for drug-resistant mutations in mTOR kinase domain.
(A) Scheme of a yeast-based screen for drug-resistant mutations in mTOR kinase domain. mTOR kinase domain is amplified by error-prone PCR to generate randomized mutations, which is then recombined in frame into the TOR2-mTOR plasmid by gap-repair in tor2-dg cells, and is selected on SC-leucine minus plates. Replica plating is then made onto SC-leucine plates containing DMSO or mTOR kinase inhibitor for selection of drug resistant clones.
(B) tor2-dg cells expressing WT or mutant TOR2-mTOR were serially diluted by 10-fold and assayed for sensitivity to different mTOR kinase inhibitors in the presence of amphotericin B. Vector and TOR2 were used as controls. Drug resistant assay was performed at 37°C in the presence of amphotericin B (except OSI-027).
(C) Systematic mutational analysis of L2185 on drug resistance. tor2-dg cells expressing WT or mutant TOR2-mTOR carrying all possible mutations at L2185 were serially diluted by 10-fold and tested for sensitivity to different mTOR kinase inhibitors at 37°C. AZD8055, BEZ235, INK128, PF-04691502 and PKI-587 were supplemented with amphotericin B.
To systematically evaluate mutational effect at this position, we performed saturation mutagenesis of L2185 and systematically investigated for the potential of each point mutation to confer drug resistance (Figure 4C). The result shows that L2185A and L2185C are the most important mutations, conferring resistance to OSI-027, AZD8055, INK128, PF-04691502, and PKI-587. In addition, L2185D and L2185N are moderately resistant to AZD8055, PF-04691502, and PKI-587; and L2185G to AZD8055, INK128, PF-04691502, and PKI-587. The differential effect of L2185 mutations on different mTOR kinase inhibitors likely reflects structural diversity of the tested compounds and distinct requirements at position 2185. Of note, L2185A and L2185C mutants remain sensitive to BEZ235 and Torin2, two compounds with closely related chemical structures, suggesting that a common structural scaffold(s) renders these agents less susceptible to the binding site mutations.
L2185A Mutation Confers Drug-Resistance in Colorectal and Lung Cancers
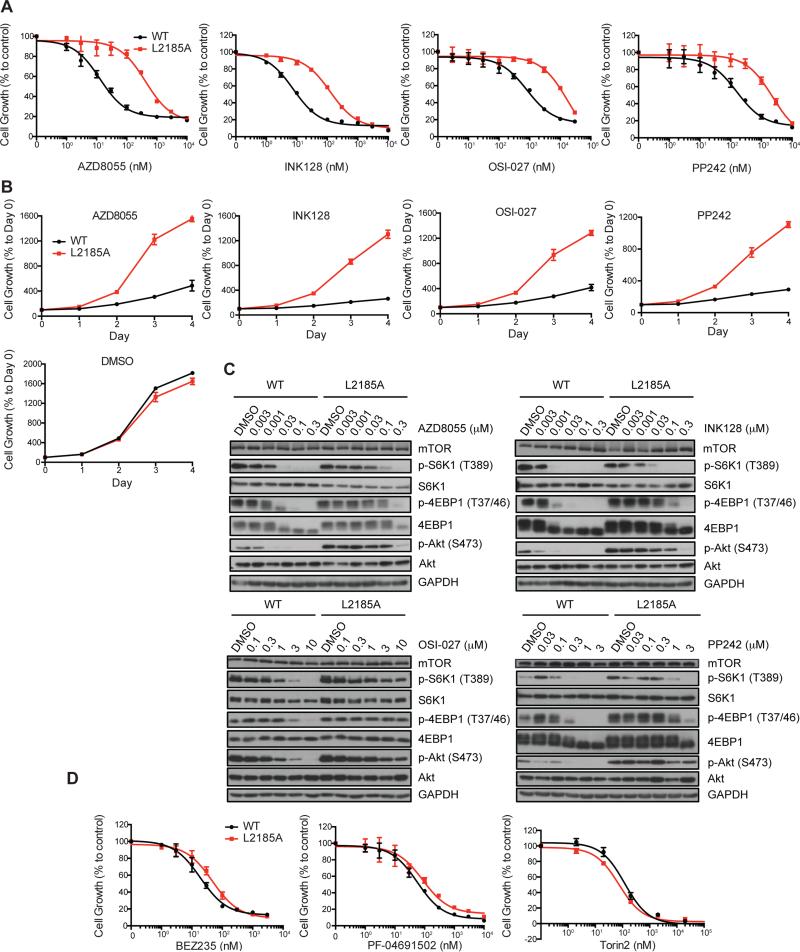
To evaluate drug-resistant mutations identified with our yeast system in human cancer models, we transiently expressed Flag-mTOR(L2185A) in HEK293T cells and found that it confers resistance to OSI-027 and INK128 (Figure S3A). To test the significance of our findings in more physiologically relevant cancer models, we used the CRISPR/Cas9 genome editing technology (Cong et al., 2013; Mali et al., 2013) to integrate L2185A into the mTOR locus of SW480 colorectal and H460 lung cancer cell lines (Figure S3B), representing cancer types with high mortality rates due to lack of efficacious targeted therapy. L2185A mutation indeed confers resistance to AZD8055, INK128, OSI-027, and PP242 in SW480 colorectal cancer cells (Figures 5A and 5B), which is accompanied by drug-resistant signaling by both mTORC1 and mTORC2 (Figure 5C). Independent mutant clones provide essentially the same phenotype (Figure S3C), indicating that off target effect is unlikely. Interestingly, we did not observe significant differences in drug resistance between heterozygous and homozygous mutants (Figures S3C and S3D), suggesting that the mutant allele is dominant. Notably, AKT(S473) phosphorylation is moderately more drug-resistant than S6K1(T389) phosphorylation in mTOR(L2185A) versus WT cells (Figure 5C), suggesting that L2185A mutation differentially affects drug-binding in two different mTOR complexes. Comparable results were obtained with H460 lung cancer cells with select compounds (Figures S3E and S3F), suggesting that drug-resistance by L2185A is not tumor type-specific.
Figure 5. L2185A mutation confers resistance to mTOR kinase inhibitors in colorectal cancer models.
(A) SW480 cells carrying homozygous WT mTOR or L2185A mutant alleles were treated with various concentrations of AZD8055, INK-128, OSI-027 and PP242 for 2 day. Growth of SW480 cells was measured by SRB assay. Data represent means ± SD in three independent experiments.
(B) SW480 cells carrying homozygous WT and L2185A mutant mTOR allele were treated with a single dose of AZD8055 (100 nM), INK-128 (100nM), OSI-027 (6,000 nM), and PP242 (2,000 nM) for different times. Cell growth was measured by SRB assay. The drug carrier DMSO was used as a control. Data represent means ± SD in three independent experiments.
(C) SW480 cells carrying homozygous WT and L2185A mutant mTOR were treated with various concentrations of INK-128, OSI-027, AZD8055 and PP242 for 1 hr. The effect on the level of P-S6K, S6K, P-4E-BP1, 4EB-P1, P-AKT and AKT was analyzed by immunoblot.
(D) SW480 cells carrying homozygous WT and L2185A mutant mTOR alleles were treated with various concentrations of BEZ235, PF-0691502 and Torin2 for 2 day. The growth of SW480 cells was measured by SRB assay. Data represent means ± SD in three independent experiments.
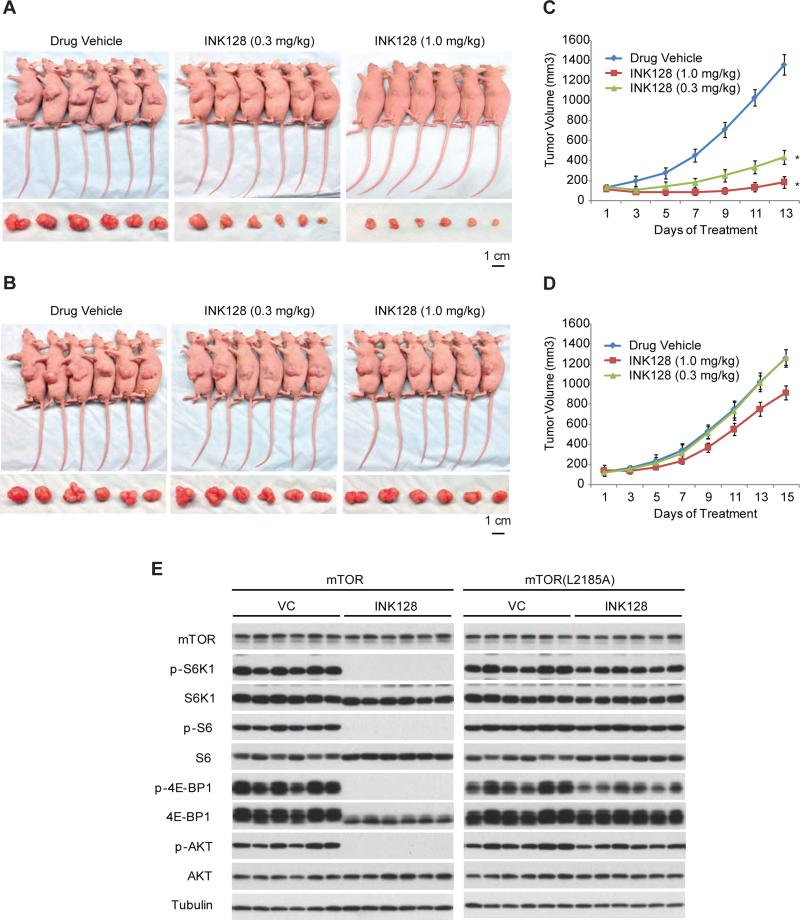
As seen in yeast, the L2185A mutant remains sensitive to BEZ235 and Torin2 in colorectal and lung cancer cells (Figures 5D and S3G). Curiously, although L2185A confers resistance to PF-04691502 in the yeast assay (Figure 4B), SW480 cells caType equation here.rrying this mutation are still sensitive to PF-04691502 (Figure 5G). Because PF-04691502 is an mTOR/PI3K dual inhibitor, the discrepancy between yeast and human cells may be attributed to inhibition of type I PI3Ks in colorectal cancer cells by PF-04691502, which is absent from yeast. To determine whether L2185A renders drug resistance in vivo, we generated xenograft tumors derived from SW480 cells carrying WT or L2185A mutant mTOR. When delivered via intraperitoneal injection, INK128 strongly attenuates growth of WT mTOR tumors, but has little or no effect on mTOR(L2185A) bearing tumors (Figures 6A-D). Similarly, L2185A renders xenograft tumors drug-resistance in mTORC1 and mTORC2 signaling in xenograft tumors, which is in contrast to the complete blockadge of mTOR signaling in WT mTOR tumors (Figure 6F). Together, these results demonstrate that L2185A confers drug resistance in vitro and in vivo.
Figure 6. L2185A mutation renders resistance of xenograft tumors to mTOR kinase inhibitors.
(A) Mice bearing xenograft tumors derived from SW480 cells expressing WT mTOR were administered with INK128 at 1 mg/kg or 0.3 mg/kg, once daily via intraperitoneal injection. Shown are representative animals and excised tumors after drug treatment.
(B) Same as Figure 6A except xenograft tumors were derived from SW480 carrying homozygous mTOR(L2185A) alleles.
(C) Tumor volume measurement for SW480 xenograft tumors expressing mTOR (WT) (expressed as means ± SD; n = 8, *P < 0.01, vs. vehicle control).
(D) Tumor volume measurement for SW480 xenograft tumors expressing mTOR(L2185A) mutant (expressed as means ± SD; n = 8).
(E) Tissue extracts from xenograft tumors at the end of treatment with or without 1 mg/kg INK128 were analyzed for the level of P-S6K, S6K, P-S6, S6, P-4E-BP1, 4E-BP1, P-AKT and AKT was analyzed by immunoblot. Six tumor samples from each animal group were shown with each lane representing an individual tumor sample.
Mutational Analysis of Conserved Hydrophobic Pocket
‘Hydrophobic spines’ within the active site are increasingly recognized to the binding of ATP and ATP-competitive inhibitors of protein kinases (Kornev et al., 2006). The recently published crystal structure of mTOR kinase domain provides a detailed three-dimensional view of mTOR's ATP-binding site (Yang et al., 2013). Several residues, including I2163, L2185, Y2225, I2237 and W2239, are highly conserved in PI3K and PI3K-related kinases (Figure S2). They appear to form an N-lobe-like hydrophobic pocket involved in binding of ATP and ATP-competitive mTOR inhibitors (Figure 7A). To understand their significance, we systematically mutated them and determined the effect of the substitutions with the yeast growth assay (Figure 7B). Most of the mutations cause loss of mTOR catalytic activity to different degrees, with only 10-35% retaining normal mTOR kinase function (Figure 7C). Interestingly, over 50% hydrophobic substitutions retain normal mTOR function (Figure 7D). Notably I2237 can only tolerate hydrophobic substitutions, underscoring the importance of this hydrophobic environment.
Figure 7. Saturation mutagenesis of highly conserved hydrophobic residues of mTOR kinase domain.
(A) Shown is hydrophobic surface representation of the ATP-binding pocket of mTOR kinase bound with an ATP molecule (PDB ID code 4JSP). ATP atoms are colored as follows: N, blue; O, red; P, orange; S, yellow; Mg+2, green. Protein surface representation is as follows: hydrophobic residue, red; neutral residue, white; hydrophilic residue, blue.
(B) Summary of the effect of mutations at different conserved hydrophobic residues in mTOR kinase domain. “+”: normal; “−”: minor defect; “−−”: moderate defect; “−−−”: severe defect.
(C) Stacked bar graph summarizes each category of mutations in terms of function as a percentage of total mutations.
(D) Stacked bar graph summarizing hydrophobic, neutral, and hydrophilic mutations as a percentage of total mutations with normal mTOR kinase function.
(E) Shown is yeast growth-based assay for several representative mutations with severe loss-of-function in mTOR kinase.
(F) WT and mutant Flag-mTOR were transiently expressed in HEK293T cells, immunoprecipitated, and assayed for mTOR kinase activity toward recombinant 4E-BP1 in vitro. Phosphorylation of 4E-BP1 was analyzed by immunoblot using a P-4E-BP1 specific antibody. Data represent means ± SD in three independent experiments.
To verify our yeast-based results, several loss-of-function mTOR mutants (Figure 7E) were expressed as Flag-tagged proteins in HEK293T cells and assayed for kinase activity in vitro using recombinant 4E-BP1 as a substrate (Figure 7F). Severe loss of kinase activity was confirmed, validating the yeast results. Hydrophobic interactions are known to be important for binding of ATP and TKIs in the ATP-binding pockets of protein tyrosine kinases (Zhang et al., 2009). Our results demonstrate that the hydrophobic environment of the ATP-binding pocket is also critical for catalytic function of mTOR, an atypical protein serine/threonine kinase.
DISCUSSION
Small molecule kinase inhibitors are proven clinically effective against malignancies in which kinase targets are hyper-activated, driving uncontrolled growth and proliferation. However, tumors typically develop drug resistance within six months after initial treatment. A major mechanism underpinning acquired resistance to kinase inhibitors is binding site mutations (Gorre et al., 2001; Heinrich et al., 2003; Kobayashi et al., 2005). Thus, identification of resistant mutations is crucial for clinical diagnosis and development of new strategies to overcome resistant variants. To this end, we have developed a robust yeast tool to screen and study drug-resistant mutations in mTOR kinase domain. By simply measuring yeast growth, it enables the identification and analysis of residues in mTOR kinase domain crucial for mTOR functions and drug-resistance.
Unlike mammalian cells, yeast cells are poorly permeable to small molecules due to the unique cell wall and plasma membrane structures, which has been a major barrier for using yeast for drug research and screens (Emter et al., 2002; Simon and Bedalov, 2004). Yeast strains with deletion of ERG6 (alteration in membrane composition by inhibiting ergosterol biosynthesis), PDR1, and PDR3 (decrease in drug efflux) have been developed to improve drug permeability (Dunstan et al., 2002). However, the major drawback of erg6Δ strain is dramatically decreased plasmid transformation efficiency and sexual conjugation, which limit yeast as a useful tool for drug screening (Gaber et al., 1989). Here we found that the antifungal drug amphotericin B can enhance cell permeability to structurally diverse mTOR kinase inhibitors. Curiously, although miconazole, a potent inhibitor of ergosterol biosynthesis, fails to enhance drug sensitivity, suggesting that targeting this lipid pathway alone is an ineffective strategy. It will be interesting to determine if amphotericin B is broadly useful for different classes of small molecules, which could significantly expand the role of yeast as a general tool for drug discovery.
Gatekeeper residues are common locations for acquisition of TKI drug-resistance. Unlike most protein kinases that have a bulky gatekeeper residue (e.g., methionine), more than 40% tyrosine kinases utilize a threonine at this position. The presence of a small gatekeeper residue in the tyrosine kinases appears to make them more amenable to regulation. In PI3Ks and PIKKs, the gatekeeper is a bulky isoleucine residue (except for leucine in ATM). The presumptive mTOR gatekeeper residue, I2237, is located in the N-lobe hydrophobic pocket, where it is thought to engage in hydrophobic interaction with the adenine moiety of ATP. Strikingly, only substitution with leucine, methionine, or valine is tolerated at this position. Any other substitution causes a severe loss in mTOR kinase function. A similar phenomenon was observed with the isoleucine gatekeeper residue (I848) in p110-PI3Kα (Vogt, 2008; Zunder et al., 2008). Thus, the relatively bulky gatekeeper residue and the importance of gatekeeper residue in maintaining the hydrophobic pocket almost certainly limit its contribution to drug resistance in mTOR and PI3Kα.
The drug-resistant mutation hotspot L2185 is also part of N-lobe hydrophobic pocket. Because L2185 is further away from ATP than I2237, it appears more tolerant to substitution by smaller hydrophobic residues (e.g., alanine and cysteine), while creating an incipient cavity in the active site that destabilizes binding of mTOR inhibitors (e.g., AZD8055, INK128, OSI-027, and PP242) via loss of van der Waals contact(s) (Figures S4A and S4B). Therefore, unlike gatekeeper mutations in tyrosine kinases, where substitution of the smaller residue to a bulkier side chain constrains drug binding (Taylor and Kornev, 2011), mutation of L2185 of mTOR to a smaller residue such as alanine results in drug resistance by weakening drug binding.
It is remarkable that mutation of L2185 does not confer resistance to either Torin2 or BEZ235, both of which have three-ring fused heterocyclic structure. The distance between L2185 and the adenine-like tricyclic ring of Torin2 (3.9Å) is farther away than PP242 (3.4Å) (Figure S4C). Because hydrophobic interaction strength decreases rapidly with increasing separation, L2185 would appear to play a less significant role in stabilizing binding of Torin2 versus PP242. Thus, substitution of leucine with an alanine has less impact on Torin2 binding (as opposed to PP242). The tricyclic Torin2 ring is thought to stack with W2239 of mTOR and stabilize the drug binding (Yang et al., 2013). Such a stacking interaction may, therefore, mitigate any decrease in productive hydrophobic interactions caused by L2185 mutations and maintain the sensitivity of either Torin2 or BEZ235. This observation suggests that incorporation of chemotypes isostructural to the tricyclic ring of Torin2 would be advantageous in minimizing acquired drug resistance. Knowledge of “gatekeeper” mutations has aided discovery of second generation TKIs, such as bafetinib and dastinib, which appear less susceptible to drug-resistant mutations (Santos et al., 2010; Tokarski et al., 2006). Moreover, such inhibitors should be reserved for only L2185 mutant tumors. Our characterization of L2185 mutations may be useful in improving the design of mTOR kinase inhibitors and treatment strategy.
In addition to identifying drug-resistant mutations, our yeast system is useful for probing the structure and function of mTOR kinase domain. In a typical protein kinase catalytic domain, there are two hydrophobic pockets inside the active site critical for adenine binding (Liu and Gray, 2006). We found that a cluster of conserved hydrophobic residues in the N-lobe is critical for maintaining mTOR kinase function. In a previous study of Protein Kinase A (PKA) also in a S. cerevisiae system, most residues within the ATP binding pocket of PKA were tolerant to mutations (Kennedy et al., 2009). In contrast, the data herein show that mutation of conserved hydrophobic residues in mTOR active site is not well-tolerated, and caused substantial loss of catalytic function (Figure 7C). These distinctions likely reflect evolutionary differences in kinase regulation between atypical protein kinases (e.g. mTOR), and the canonical protein kinases (e.g., PKA).
Conserved residues of the hydrophobic core of the PKA catalytic domain have been extensively characterized by Taylor and co-workers (Kornev et al., 2006; Meharena et al., 2013; Taylor and Kornev, 2011). Three-dimensional alignment of the structures of PKA (PDB ID code 1ATP) and mTOR (4JSP) permitted presumptive identification of mTOR residues corresponding to the R- and C-Spines of PKA (Figures S4D and S4E). Our structural alignment documents that mTOR residues I2163 and L2185 (both characterized herein) correspond to PKA C-Spine residues V57 and A70, respectively (Figures S4D and 4E). We suggest, therefore, that mutation of either I2163 or L2185 impairs mTOR catalytic activity by disrupting the structure of the C-Spine of this atypical protein kinase. In PKA, three “Shell” residues [V104 (Sh1), M120 (Sh2), and M118 (Sh3)] stabilize the structure of the R-Spine (Meharena et al., Plos Biol, 2013). Within mTOR, these three residues correspond to Y2225 (Sh1), I2237 (Sh2), and G2235 (Sh3). Lack of conservation of these “Shell” resides between PKA and mTOR suggests that the R-spine of mTOR may not be as dynamic as its counterpart in PKA. The latter might fill the adenine pocket and prevent binding of ATP. It is also interesting to note that similar to RAF kinase, the equivalents of I2163 and L2185 can tolerate smaller hydrophobic residues but not phenylalanine (Hu et al., 2013; Hu et al., 2011; Shaw et al., 2014). Phenylalanine might fill the adenine pocket and prevent binding of ATP. Finally, the salt bridge between the C-and R-Spines [E91(OE2)--K72(NZ)=3.6 Å] in PKA corresponds to an analogous salt bridge in mTOR [E2190(OE1)--K2187(NZ)=2.8Å] (Figure S4F), which could control the catalytic activity as well as bridge the two spines as seen with PKA (Taylor and Kornev, 2011). While the importance of hydrophobic environment and hydrophobic structures are well studied in the canonical protein kinases, it is much less well understood in the atypical kinase such as mTOR. It would be of considerable interest to elucidate the function of hydrophobic residues in mTOR, which could help improve future design of mTOR kinase inhibitors.
Unlike cancer-driving mutations, drug resistant mutations are not readily detectable until clinical resistance is developed. Because mTOR kinase inhibitors have not yet approved for human use, the clinical significance of the non-gatekeeper hotspot mutations remains to be determined. Nonetheless, Our findings can impact the field in several ways. First, the drug-resistant mutation profiles could provide guidance for monitoring the potential occurrence of drug-resistant mutations during human clinical trials. Second, our study provides valuable insights into the structure-function relationship of mTOR kinase. It provides insights into the mechanism of action for mTOR kinase inhibitors and drug resistance, which can help with design of future mTOR inhibitors. Finally, Drug-resistant mTOR mutants can be powerful tools for probing the physiological functions of mTOR kinase, as does the rapamycin-resistant mTOR mutants that have made much contributions to understanding of mTORC1.
EXPERIMENTAL PROCEDURES
Plasmids, Mutagenesis and Library Screen
The TOR2-mTOR hybrid plasmid was constructed by replacing the C-terminal region of TOR2 of pML40-TOR2 (Alarcon et al., 1996) (encoding for 2080-2474 aa) in frame with a DNA fragment corresponding to C-terminal domain of mTOR (encoding for 2140-2549 aa). To construct mutant TOR2-mTOR plasmids, mTOR C-terminal region was amplified by PCR and co-transformed into yeast with XmaI-digested pML40-TOR2 plasmid that excised the TOR2 C-terminal region. The resultant TOR2-mTOR fusion plasmids are constructed in reading frame by gap-repair in yeast.
The wild type and kinase-dead pCDNA3-Flag-mTOR plasmids were obtained from Addgene. The I2163K, L2185A, L2185C, L2185P and I2237S mutant plasmids were constructed by using overlap extension-PCR method for site-directed mutagenesis. For site-specific saturation mutagenesis, pUC18-mTOR kinase plasmid was constructed by insertion of mTOR C-terminal domain (aa 2140-2549) into pUC18 vector at the XmaI site. The pUC18-mTOR plasmid was mutagenized at the I2163, L2185, Y2225, I2237 and W2239 residues by degenerate PCR with NNK primers. The mutation library was constructed using QuikChange mutagenesis kit according to the manufacture's instruction (Agilent) and transformed into chemically competent E. coli. Plasmid DNA was extracted by miniprep kit (Promega) and the mutations were verified by sequencing.
Yeast Strains and Culture and Growth Assays
The tor2-dg temperature-sensitive strain was generated from the W303 background (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) as described previously (Dohmen and Varshavsky, 2005). For the halo assay, log phase W303 wild type or tor2-dg cells were spread evenly on YPD agarose plates. After drying, small sterile filter discs were placed on the surface and 5 μl of rapamycin (1 μM), BEZ235 (5 μM), PKI-587 (3 μM), other mTOR inhibitors (10 μM), or DMSO were applied to each disc. Plates were incubated at 30°C (wild type) or 37 °C (tor2-dg) for 3 day.
To enhance yeast cell permeability, log phase W303 tor2-dg cells were spread evenly on SD-Leu agarose plates. After drying, small sterile filter discs were placed on the surface and 5 μl of indicated mTOR inhibitors or DMSO were applied to each disc with or without adding 5 μl of amphotericin B (10 μM), miconazole (50 μM) or caspofungin (50 μM). Plates were incubated at 37°C for 3 day. Drug resistance profiles of TOR2-mTOR mutant cells were determined by the spotting assay. For this assay, 10-fold serial dilutions of cells were spotted on SD -leu plates in the presence of indicated mTOR inhibitors with or without amphotericin B (200 nM) and incubated at 37°C for 3 day.
Cancer Cell Lines and CRISPR/Cas9-Mediated Mutagenesis
Cancer cell lines were maintained in DMEM or RPMI 1640, supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen). CRISPR/cas9 technology was used to engineer mutant mTOR allele in SW480 colorectal and H460 lung cancer cells. Briefly, The target sequence for mTOR, GCTGCATCACACGCTCATCC was designed through the online tool at http://crispr.mit.edu, and cloned into pSPCas9(BB)-2A-GFP vector (PX458 in Addgene). Genomic mTOR mutation was engineered using a protocol as described (Ran et al., 2013) and was confirmed by targeted sequencing.
Immunological and Chemical Reagents, and Proliferation Assays
Antibodies against tubulin, S6K, phospho-S6K (Thr389), 4E-BP1, phospho-4E-BP1 (Thr37/46), Akt, phospho-Akt (Thr308), and phospho-Akt (Ser473) were purchased from Cell Signaling Technology. For mTOR inhibitors, PI-103, PP242, WYE-354, and WYE-132 were purchased from Chemdea; BEZ235 was purchased from LC Laboratories; XL765, PKI-587, PF-04691504, OSI-027, AZD8055, INK-128 and Torin2 were purchased from Selleck Chemicals. For drug sensitivity test, cancer cells were seeded in 96-well plates at a density of 2,000 cells per well. 24 hours later, different concentrations of mTOR inhibitors were added in quadruplicate. Cell growth was measured by the sulforhodamine B (SRB) assay as previously described (Vichai and Kirtikara, 2006).
In vitro mTOR kinase Assay
The mTOR kinase activity was assessed by in vitro kinase assay as described (Sancak et al., 2007). Briefly, pCDNA3-Flag-mTOR variants were transfected into HEK293T cells by calcium phosphate transfection. After 48 hrs, cells were washed with ice-cold PBS buffer and lysed with lysis buffer (40 mM HEPES [pH 7.4], 2 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 0.3% CHAPS, protease inhibitor cocktail [Roche], phosSTOP [Roche] and 1 mM PMSF [Sigma]). Cell lysates were incubated with anti-Flag M2 antibody (Sigma) for 1.5 hrs, which was followed by 1 hr incubation with Protein A/G PLUS-Agarose (Santa Cruz). The immunoprecipitates were washed twice with washing buffer (40 mM HEPES [pH 7.4], 150 mM NaCl, 2 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 0.3% CHAPS) and three times with IP buffer (25 mM HEPES [pH 7.4], 20 mM KCl). Kinase assay was performed in 15μl kinase buffer (25 mM HEPES [pH 7.4], 50 mM KCl, 10 mM MgCl2, 250 μM ATP) containing 150 ng of GST-4E-BP1 for 20 min at 30 °C. The kinase reaction was stopped by adding 30 μl 2-fold SDS sample buffer and incubated at 95 °C for 5 min. Phosphorylation of 4E-BP1 was analyzed by Western blot.
Xenograft Tumor Models
Female athymic NCr-nu/nu mice (4-6 weeks old) were obtained from Taconic Farms. They were injected subcutaneously into the left flank with 2×106 SW480 wild type or mutant cells to establish xenograft tumors. 3 day after injection, mice were randomly divided into 3 groups (8 animals per group). Group 1 was given 1 mg/kg INK128; group 2 was given 0.3 mg/kg INK128, and group 3 was given the vehicle used for administration (vehicle control, VC). INK128 was used according to previous studies, which were at much lower doses than the reported maximum tolerated doses (Gild et al., 2013; Hayman et al., 2014; Hsieh et al., 2012). INK128 was administered once daily via intraperitoneal injection (i.p.) with freshly prepared drug solution in 100 μl of PBS (final DMSO concentration = 0.33%) just before administration. Bidimensional tumor measurements were taken every 2 day and mice were weighed once weekly. Tumor volume was calculated by the following formula: tumor volume (mm3) = (shorter diameter2 × longer diameter)/2 and are presented as means ± SD (n = 8) (Zhang and Zheng, 2012). For analysis of signaling inhibition, tumor tissues were removed from the animals after administration of the last dose of drug, and immediately frozen in liquid nitrogen. Tissue extracts were prepared for analysis of mTOR signaling by Western blot. The animal studies were approved by Rutgers University Institutional Animal Care and Use Committee, and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Modeling of L2185A mTOR Kinase Domain
An atomic model of the L2185A mutant form of the mTOR kinase domain was generated via deletion of the Cγ, Cδ1, and Cδ2 atoms of residue 2185. Intratomic distances between the Cβ atom of the modeled mutant enzyme and PP242, Torin, and ATP were estimated directly by assuming that the position of the bound ligand was unaffected by the mutation.
Supplementary Material
Highlights.
L2185 of mTOR kinase is a hotspot for drug-resistant mutations
The “Gatekeeper’ residue does not confer drug resistance
A three-ring heterocyclic chemical structure is refractory to drug-resistance
C- and R-spines of mTOR kinase is crucial for its catalytic function
ACKNOLWEDGEMENT
We thank Dr. Huanwang Yang for assistance with structural modeling, and Dr. Maria Cardenas for providing reagent. Rutgers Cancer Institute of New Jersey Flow Cytometry and Bioinformatics Core Facilities were supported by NCI Cancer Center Support Grant P30-CA072720. This work was supported by NIH R01 grants CA123391 and CA166575 (X.F.Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
T.J.W., X.W.W., Y.J.Z. and L.H.M. designed and performed experiments, and prepared figures; J.E.K. and S.K.B. provided expertise in structural modeling of mTOR kinase; X.F.Z. conceived and directed the project, and designed experiments; T.J.W, S.K.B and X.F.Z. wrote the manuscript.
REFERENCES
- Alarcon CM, Cardenas ME, Heitman J. Mammalian RAFT1 kinase domain provides rapamycin-sensitive TOR function in yeast. Genes & development. 1996;10:279–288. doi: 10.1101/gad.10.3.279. [DOI] [PubMed] [Google Scholar]
- Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, Sharma SV, Brannigan BW, Mohapatra G, Settleman J, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- Bjornsti M-A, Houghton PJ. The tor pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. PNAS. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science (New York, NY) 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen RJ, Varshavsky A. Heat - Inducible Degron and the Making of Conditional Mutants. In: Raymond JD, editor. Methods in Enzymology. Academic Press; 2005. pp. 799–822. [DOI] [PubMed] [Google Scholar]
- Dunstan HM, Ludlow C, Goehle S, Cronk M, Szankasi P, Evans DR, Simon JA, Lamb JR. Cell-based assays for identification of novel double-strand break-inducing agents. Journal of the National Cancer Institute. 2002;94:88–94. doi: 10.1093/jnci/94.2.88. [DOI] [PubMed] [Google Scholar]
- Emter R, Heese-Peck A, Kralli A. ERG6 and PDR5 regulate small lipophilic drug accumulation in yeast cells via distinct mechanisms. FEBS Letters. 2002;521:57–61. doi: 10.1016/s0014-5793(02)02818-1. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Mukohara T, Zejnullahu K, Lifshits E, Borras AM, Gale CM, Naumov GN, Yeap BY, Jarrell E, Sun J, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. The Journal of clinical investigation. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS biology. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber RF, Copple DM, Kennedy BK, Vidal M, Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Molecular and cellular biology. 1989;9:3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum MA, Rice LB. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clinical Microbiology Reviews. 1999;12:501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gild ML, Landa I, Ryder M, Ghossein RA, Knauf JA, Fagin JA. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocrine-Related Cancer. 2013;20:659–667. doi: 10.1530/ERC-13-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science (New York, NY) 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Gray NS, Wodicka L, Thunnissen A-MWH, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, et al. Exploiting Chemical Libraries, Structure, and Genomics in the Search for Kinase Inhibitors. Science. 1998;281:533–538. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]
- Guertin D, Sabatini D. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- Hayman TJ, Wahba A, Rath BH, Bae H, Kramp T, Shankavaram UT, Camphausen K, Tofilon PJ. The ATP-Competitive mTOR Inhibitor INK128 Enhances In Vitro and In Vivo Radiosensitivity of Pancreatic Carcinoma Cells. Clinical Cancer Research. 2014;20:110–119. doi: 10.1158/1078-0432.CCR-13-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Stites EC, Yu H, Germino EA, Meharena HS, Stork PJ, Kornev AP, Taylor SS, Shaw AS. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell. 2013;154:1036–1046. doi: 10.1016/j.cell.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Yu H, Kornev AP, Zhao J, Filbert EL, Taylor SS, Shaw AS. Mutation that blocks ATP binding creates a pseudokinase stabilizing the scaffolding function of kinase suppressor of Ras, CRAF and BRAF. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6067–6072. doi: 10.1073/pnas.1102554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EJ, Yang J, Pillus L, Taylor SS, Ghosh G. Identifying critical non-catalytic residues that modulate protein kinase A activity. PloS one. 2009;4:e4746. doi: 10.1371/journal.pone.0004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR Mutation and Resistance of Non–Small-Cell Lung Cancer to Gefitinib. New England Journal of Medicine. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kornev AP, Haste NM, Taylor SS, Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17783–17788. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ren T, Fresques T, Oppliger W, Niles BJ, Hur W, Sabatini DM, Hall MN, Powers T, Gray NS. Selective ATP-competitive inhibitors of TOR suppress rapamycin-insensitive function of TORC2 in Saccharomyces cerevisiae. ACS chemical biology. 2012;7:982–987. doi: 10.1021/cb300058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nature chemical biology. 2006;2:358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo J, Bonenfant D, Oppliger W, Jenoe P, Hall M. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Ma X, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science (New York, NY) 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzen MR, McCraith SM, Spinelli SL, Torres FM, Fields S, Grayhack EJ, Phizicky EM. A Biochemical Genomics Approach for Identifying Genes by the Activity of Their Products. Science. 1999;286:1153–1155. doi: 10.1126/science.286.5442.1153. [DOI] [PubMed] [Google Scholar]
- Meharena HS, Chang P, Keshwani MM, Oruganty K, Nene AK, Kannan N, Taylor SS, Kornev AP. Deciphering the structural basis of eukaryotic protein kinase regulation. PLoS biology. 2013;11:e1001680. doi: 10.1371/journal.pbio.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature protocols. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Molecular cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Santos F, Kantarjian H, Cortes J, Quintas-Cardama A. Bafetinib, a dual Bcr-Abl/Lyn tyrosine kinase inhibitor for the potential treatment of leukemia. Curr Opin Investig Drugs. 2010;11:1450–1465. [PubMed] [Google Scholar]
- Sarbassov D, Ali S, Kim D, Guertin D, Latek R, Erdjument-Bromage H, Tempst P, Sabatini D. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Shaw AS, Kornev AP, Hu J, Ahuja LG, Taylor SS. Kinases and pseudokinases: lessons from RAF. Molecular and cellular biology. 2014;34:1538–1546. doi: 10.1128/MCB.00057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Bedalov A. Yeast as a model system for anticancer drug discovery. Nature reviews Cancer. 2004;4:481–492. doi: 10.1038/nrc1372. [DOI] [PubMed] [Google Scholar]
- Sturgill TW, Hall MN. Activating mutations in TOR are in similar structures as oncogenic mutations in PI3KCalpha. ACS chemical biology. 2009;4:999–1015. doi: 10.1021/cb900193e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer research. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends in biochemical sciences. 2011;36:65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski JS, Newitt JA, Chang CYJ, Cheng JD, Wittekind M, Kiefer SE, Kish K, Lee FYF, Borzillerri R, Lombardo LJ, et al. The Structure of Dasatinib (BMS-354825) Bound to Activated ABL Kinase Domain Elucidates Its Inhibitory Activity against Imatinib-Resistant ABL Mutants. Cancer Research. 2006;66:5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- Tsang C, Qi H, Liu L, Zheng X. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature protocols. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Vogt PK. Drug-Resistant Phosphatidylinositol 3-Kinase: Guidance for the Preemptive Strike. Cancer Cell. 2008;14:107–108. doi: 10.1016/j.ccr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The Genomic Landscapes of Human Breast and Colorectal Cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall M. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature reviews Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Duan Y, Zheng X. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011a;16:325–331. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zheng X. mTOR-independent 4E-BP1 phosphorylation is associated with cancer resistance to mTOR kinase inhibitors. Cell Cycle. 2012;11:594–603. doi: 10.4161/cc.11.3.19096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, Bao Y-J, Dai Q, Yang W-Y, Cheng P, Zhu L-M, Wang B-J, Jiang F-H. mTOR signaling is involved in indomethacin and nimesulide suppression of colorectal cancer cell growth via a COX-2 independent pathway. Ann Surg Oncol. 2011b;18:580–588. doi: 10.1245/s10434-010-1268-9. [DOI] [PubMed] [Google Scholar]
- Zheng X, Florentino D, Chen J, Crabtree G, Schreiber S. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82:121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Zunder ER, Knight ZA, Houseman BT, Apsel B, Shokat KM. Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110 alpha. Cancer cell. 2008;14:180–192. doi: 10.1016/j.ccr.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.