Abstract
Background
While fungal exposures are assumed to provoke wheeze through irritant or allergenic mechanisms, little is known about the differential effects of indoor and outdoor fungi on early-life wheeze.
Methods
In a Boston prospective birth-cohort of 499 at-risk infants, culturable fungi in bedroom air and dust and outdoor air were measured at age 2–3 months. Wheeze was determined using bimonthly telephone-questionnaires. Odds ratios were estimated for an interquartile increase in fungal natural log-transformed concentrations, adjusting for predictors of wheeze and potential confounders.
Results
Increased odds of ‘any wheeze’ (≥1 versus 0 episodes) by age one were positively associated with indoor dust Alternaria [odds ratio (OR)=1.83; 95% confidence interval (CI), 1.07–3.14], Penicillium [OR=1.18; (0.98–1.43)] and Cladosporium [OR=1.47; (1.16–1.85)], indoor air Penicillium [OR=1.26; (0.92–1.74)], and outdoor air Cladosporium [OR=1.68; (1.04–2.72)]. In contrast, indoor dust yeasts were protective [OR=0.78; (0.66–0.93)]. ‘Frequent wheeze’ (≥2 versus <2 episodes) by age one was borderline associated with dust yeasts [OR=0.86; (0.70–1.04)] and indoor air yeasts [OR=1.53; (0.93–2.53)]. Alternaria concentration was associated with any wheeze for children with maternal mold sensitization [OR=9.16; (1.37–61.22)], but not for those without maternal mold sensitization [OR=1.32; (0.79–2.20)].
Conclusions
While wheeze rates were higher with exposures to fungal taxa considered to be irritant or allergenic in sensitive subjects, yeasts in the home had a strong protective association with wheeze in infancy. Molecular microbiologic studies may elucidate specific components of innate microbiologic stimulants that lead to contrasting effects on wheeze development.
Keywords: Yeast, Fungi, Wheeze, Asthma, Infants, Housing
INTRODUCTION
Fungi have indoor and outdoor sources (1–3). While fungi are a known risk for irritant or allergy-induced wheeze (4–7), little is known about the differential responses of children to infant exposure to individual indoor and outdoor airborne and dustborne fungal taxa. Morever, a cross-sectional study suggested that certain indoor fungi (Penicillium and Eurotium) may be protective (8).
Infancy is a vulnerable period when exposures may influence immune development, allergy, and wheeze (9). We hypothesized that transient elevations in early-life (age 2–3 months) fungal exposures are associated with wheeze in children by age one. We assessed whether associations were independent of reports of dampness or other known predictors of wheeze (10, 11). Finally, we investigated sources of vulnerability, focusing on maternal asthma or sensitization.
METHODS
Study Protocol
In a prospective Boston birth-cohort study, 499 infants (including six sets of twins) born to women with history of asthma and allergies were recruited between 1994 and 1996 (10). The study was approved by the Institutional Review Board of Boston’s Brigham and Women’s Hospital.
Early-life fungal exposure assessment when infants were aged 2–3 months has been described previously (12). Air samples were collected at 45 lpm using a Burkard culture-plate sampler with dichloran-glycerol (DG18) culture media. Indoor air was sampled 1–1.5m above the bedroom floor demarcated for dust collection. Outdoor air was collected 3m from the main entrance, but could not be collected if temperatures were <2.2 °C because culture plates would freeze. Positive-hole corrections scaled counts to an estimate of colony-forming units (CFUs) that would have been observed without co-impaction events (13). After air sampling, dust was collected from the bedroom floor, using a canister vacuum cleaner (The Eureka Co., Bloomington, IN). Dust suspensions were spread-plated on DG18 media. Fungal colonies were identified to genus-level using morphological methods (14), and reported as CFUs per gram of dust. In the subset with winter visits, fungi were measured again when infants were 8–9 months-old. Mean annual fungal concentrations were calculated as the average of the levels measured at the 2–3 and 8–9 month home visits.
Definition of Fungal Predictors
Fungal distributions were highly skewed and were represented as natural logarithm (ln)-transformed, continuous variables (adding 1 CFU-per-unit for values below the limit-of-detection). We classified exposures as: (1) individual genera; (2) ‘total fungi’ [sum of all detectable fungi from each medium (out-/indoor air and dust)]; (3) ‘high fungal exposure’ (binary variable, >75th percentile of any detectable genera); and (4) an ordinal count of genera that were >75th percentile.
Definition of Other Predictors
We adjusted for other predictors of wheeze, details of their ascertainment being reported elsewhere (10, 11). These included birthweight, self-reported physician-diagnosed maternal asthma, winter-birth, and maternal smoking during pregnancy. We did not adjust for environmental tobacco smoke, as it was rarely (<1%) reported. We also assessed whether results were independent of early-life exposures to cockroach allergen (≥2 U/g max Bla g 1 or 2 in living room dust), dust mite allergen (≥10µg/g Der p or f 1 baby’s bed), and endotoxin (≥ median of measured samples = 81.3 EU/mg, in living room dust).
Previously we found early-life fungi associated with increased risk of lower respiratory illness (LRI; physician-diagnosed croup, bronchitis, bronchiolitis, or pneumonia) by age one (12). LRI is associated with wheeze and may be on the pathway between fungal exposures and wheeze. We evaluated the timing between the first episode of wheeze and LRI.
Maternal sensitization ascertainment by serum IgE has been described previously (15). Mothers were classified with ‘mold atopy’ if sensitized to Alternaria or Aspergillus species, and ‘any atopy’ if sensitized to ≥1 allergen (mold, cat, dog, ragweed, ryegrass, cockroach, or dust-mite), or total IgE ≥200 IU/mL (15).
Outcome Definition
Outcome ascertainment has been described elsewhere (10). Bimonthly telephone questionnaires were administered to the child’s primary caregiver, asking “Since we last spoke with you on (date given), has your child had symptoms of wheeze?”. We defined ‘any wheeze’ as ≥1 report by age one versus no report. ‘Frequent wheeze’ was defined as ≥2 reports compared with <2.
Statistical Analysis
We compared outdoor and indoor air fungi and calculated Spearman correlations due to residual skewed distributions after ln-transformation. We compared unadjusted and adjusted logistic models, beginning with indoor air and dust using the full dataset. Odds ratios were scaled to an interquartile increase in ln-transformed concentration of the specific taxon. With fewer outdoor winter measures, we performed subset analyses on infants with measurable outdoor fungi, thereby maintaining constant sample-size across models: (1) indoor air and dust jointly in the same model; (2) outdoor air alone; (3) outdoor air and indoor dust jointly; and (4) outdoor and indoor air and indoor dust jointly. Sensitivity analyses excluding potential outliers (the highest two/three fungal concentrations) and also with generalized estimating equations to account for correlation in the twins were performed.
Fungi were included together in final models, checking correlations (r ≥ 0.8) to avoid colinearity. Models were validated using the Lasso approach for model shrinkage, to reduce the probability of chance findings (16). We also fit models for the binary ‘high exposure’ and the ordinal ‘count’ of fungal genera >75th percentile.
We used Wald and likelihood ratio tests to examine whether maternal sensitization or asthma modified the association between fungi and wheeze.
We used SAS 9.1 (SAS Institute, Inc, Cary, NC), except R 2.6.1 for the Lasso (17).
RESULTS
Cohort Characteristics
Of the 499 children, 211 (42%) and 96 (19%) experienced ‘any wheeze’ and ‘frequent wheeze’, respectively. Most (86%) wheeze episodes occurred after the early-life fungal assessments. Although 27% experienced ≥1 episode of LRI, 97% of this 27% experienced their first wheeze prior to their first LRI. Population characteristics have been described elsewhere (11–13). Children included 47% girls, 75% Caucasians, and 9% low-income families (<$30k/year). Forty-four percent reported visible dampness in the year preceding their age 2–3 month home visit.
Characteristics of Indoor versus Outdoor Air and Dust Fungi
Among the subset with 2 visits, total outdoor air (r=0.82, p<0.01, n=111), indoor air (r=0.78, p<0.01, n=195), and dust fungi (r=0.87, p<0.01, n=81) measured at visit 1 were highly correlated with respective mean annual concentrations. The following results focus on exposures at visit 1.
Alternaria, Aureobasidium, Cladosporium, nonsporulating, and total fungal concentrations were greater in outdoor air, while Aspergillus, Penicillium, and yeasts were greater indoors (Table 1). All distributions were positively-skewed. Correlation-coefficients (Supplemental Material, Tables 1a and 1b) ranged from −0.30 to 0.51, with the exception of outdoor~indoor air Cladosporium (0.74) and nonsporulating fungi (0.66). For total fungi, levels in air and dust were highest in summer/fall and lowest in winter/ spring. However, individual taxa in dust did not vary significantly by season (Figure 1). Outdoor air Alternaria, Cladosporium, and Penicillium were highest in the summer/fall, whereas outdoor air yeasts were lowest; this was generally reflected in the indoor air patterns of the specific taxa.
Table 1. Distributions of fungal exposures.
Statistics calculated for all measured samples, including values below limit of detection
| Fungi | # samples |
# detectable |
geometric mean |
median | 75th percentile (ln*) | max | IQR (ln-transformed*) |
|
|---|---|---|---|---|---|---|---|---|
| Alternaria | ||||||||
| outdoor air (CFU/m3) | 384 | 139 | 3.0 | 0.0 | 11.1 (2.5) | 1977.8 | 2.5 | |
| indoor air (CFU/m3) | 494 | 124 | 2.0 | 0.0 | 11.1 (2.5) | 122.2 | 2.5 | |
| bedroom floor dust (CFU/g) | 412 | 236 | 90.0 | 454.5 | 3846.2 (8.3) | 363636.4 | 8.3 | |
| Aureobasidium | ||||||||
| outdoor air (CFU/m3) | 384 | 113 | 2.5 | 0.0 | 11.1 (2.5) | 266.7 | 2.5 | |
| indoor air (CFU/m3) | 494 | 88 | 1.6 | 0.0 | 0.0 (0.0) | 266.7 | 0.0 | |
| bedroom floor dust (CFU/g) | 412 | 329 | 812.4 | 2857.1 | 10172.4 (9.2) | 295000.0 | 3.1 | |
| Cladosporium | ||||||||
| outdoor air (CFU/m3) | 384 | 344 | 99.5 | 161.1 | 466.7 (6.1) | 10088.9 | 2.6 | |
| indoor air (CFU/m3) | 494 | 384 | 33.1 | 44.4 | 211.1 (5.4) | 9877.8 | 2.9 | |
| bedroom floor dust (CFU/g) | 412 | 322 | 812.4 | 3779.8 | 8485.7 (9.0) | 3240000.0 | 2.9 | |
| Aspergillus | ||||||||
| outdoor air (CFU/m3) | 384 | 131 | 2.7 | 0.0 | 11.1 (2.5) | 1100.0 | 2.5 | |
| indoor air (CFU/m3) | 494 | 311 | 9.0 | 11.1 | 33.3 (3.5) | 866.7 | 3.5 | |
| bedroom floor dust (CFU/g) | 412 | 374 | 2697.3 | 4791.3 | 14264.2 (9.6) | 11175000.0 | 2.4 | |
| Penicillium | ||||||||
| outdoor air (CFU/m3) | 384 | 287 | 18.2 | 22.2 | 66.7 (4.2) | 1033.3 | 4.2 | |
| indoor air (CFU/m3) | 494 | 423 | 30.0 | 33.3 | 88.9 (4.5) | 10400.0 | 2.0 | |
| bedroom floor dust (CFU/g) | 412 | 368 | 1998.2 | 3846.2 | 9523.8 (9.2) | 1619047.6 | 2.1 | |
| Yeasts | ||||||||
| outdoor air (CFU/m3) | 384 | 120 | 2.7 | 0.0 | 11.1 (2.5) | 300.0 | 2.5 | |
| indoor air (CFU/m3) | 494 | 172 | 3.0 | 0.0 | 11.1 (2.5) | 700.0 | 2.5 | |
| bedroom floor dust (CFU/g) | 412 | 367 | 4023.9 | 8496.2 | 25000.0 (10.1) | 2630434.8 | 2.2 | |
| Zygomycetes | ||||||||
| bedroom floor dust (CFU/g) | 412 | 57 | 3.0 | 0.0 | 0.0 (0.0) | 342105.3 | 0.0 | |
| Total | ||||||||
| outdoor air (CFU/m3) | 384 | 368 | 445.9 | 533.3 | 972.2 (6.9) | 10411.1 | 1.5 | |
| indoor air (CFU/m3) | 494 | 368 | 244.7 | 300.0 | 588.9 (6.4) | 10400.0 | 1.8 | |
| bedroom floor dust (CFU/g) | 412 | 412 | 59874.1 | 71633.3 | 137416.7 (11.8) | 11225000.0 | 1.7 | |
Abbreviations: IQR=inter-quartile range
ln-transformed=ln (untransformed concentration+1); addition of 1 CFU for values below the limit of detection
Figure 1.
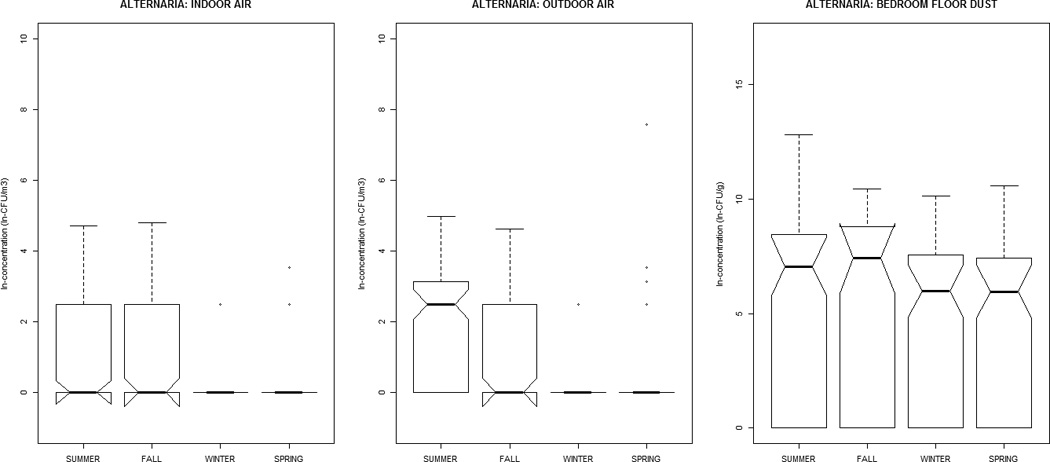
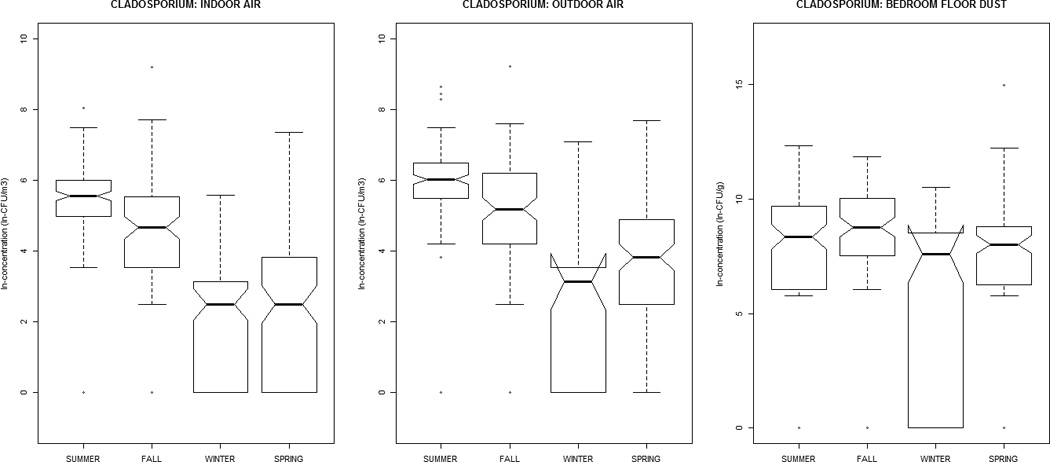
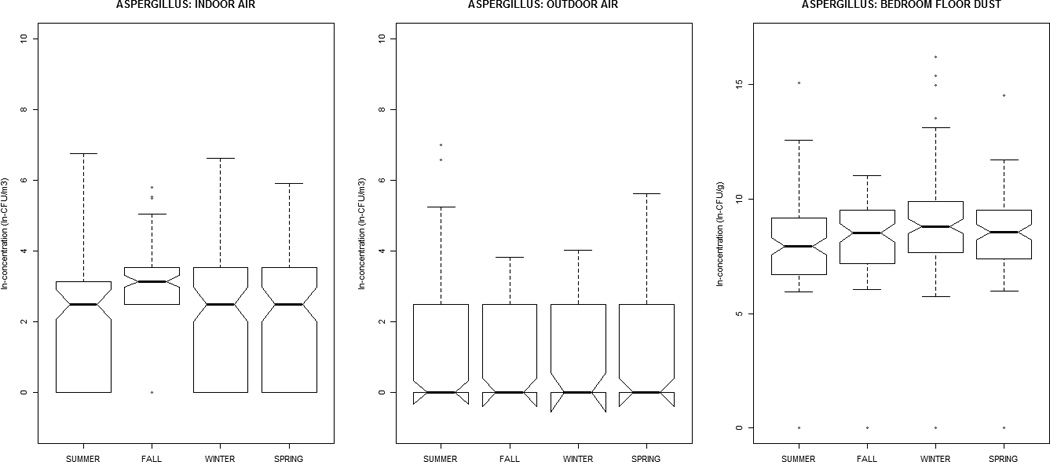
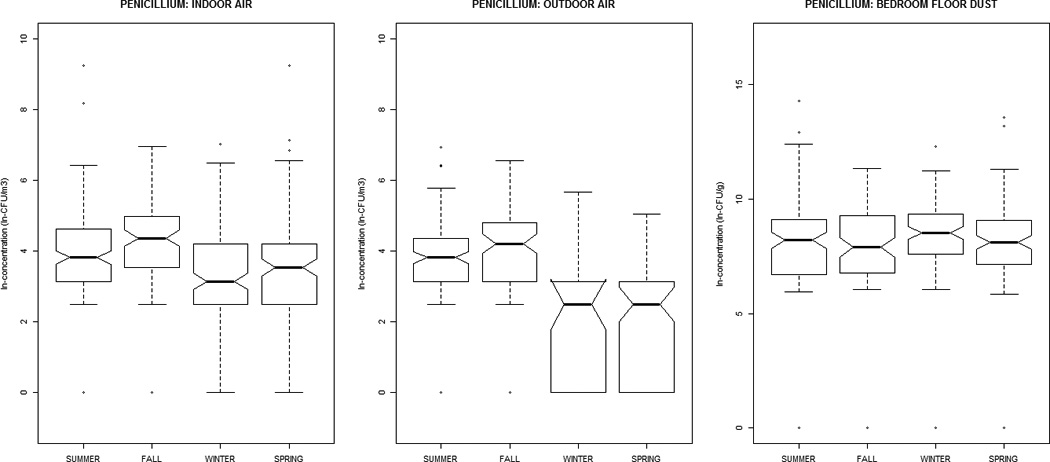
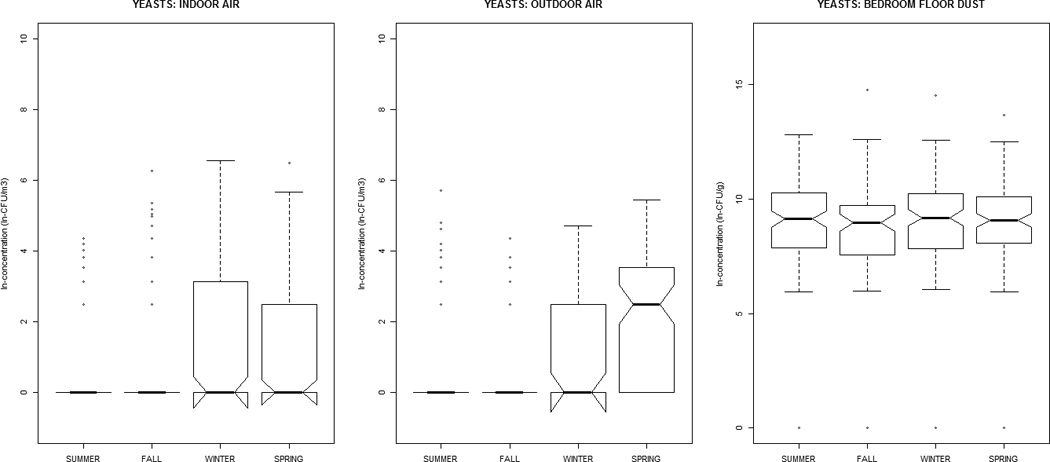
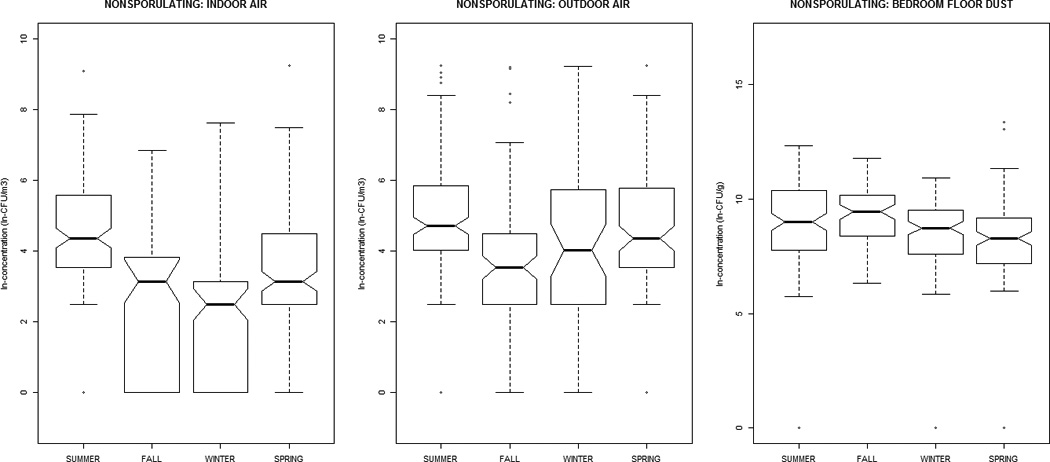
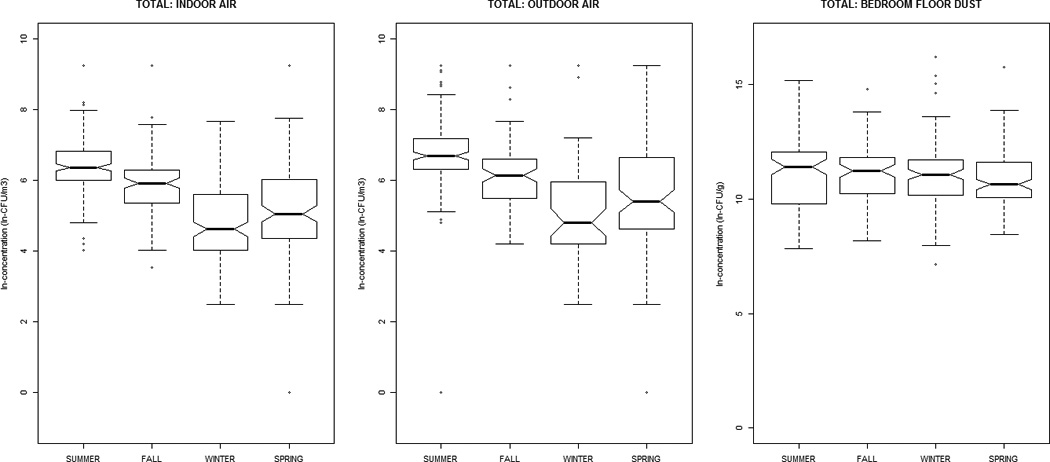
The distribution of airborne (indoor / outdoor) and dustborne (bedroom floor dust) fungal concentrations by sampling-season, at the homes of children (age 2–3 months) in the Epidemiology of Home Allergens and Asthma Study.
Fungal Exposure Predicts Wheeze
Elevated odds of ‘any wheeze’ occurred for increased dustborne Alternaria concentrations (OR=1.83; 95% CI, 1.07–3.14), and for both dustborne (OR=1.47; 95% CI, 1.16–1.85) and outdoor airborne (OR=1.68; 95% CI, 1.04–2.72) Cladosporium in models with other fungi included (Table 3). There was a trend towards increased risk with elevated indoor airborne (OR=1.26; 95% CI, 0.92–1.74) and dustborne (OR=1.18; 95% CI, 0.98–1.43) Penicillium. Yet, dustborne yeasts had protective associations (OR=0.78; 95% CI, 0.66–0.93). Findings were consistent considering genera separately (Table 2) or jointly (Table 3).
Table 3. Associations of early-life fungi with any wheeze by age one.
Analyses evaluate associations of all listed fungal genera jointly#
| ANY WHEEZE (≥1 vs 0 episodes) | |||||||
|---|---|---|---|---|---|---|---|
| Fungal genera | Full Dataset (n=407) | Subset with Outdoor Air Measures (n=316) |
|||||
| Indoor Air & Dust | Outdoor & Indoor Air & Dust |
||||||
| OR† | 95% CI | OR† | 95% CI | OR† | 95% CI | ||
| Alternaria | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 0.90 | (0.56–1.43) | |
| Indoor air (ln-CFU/m3) | 1.09 | (0.69–1.71) | 0.91 | (0.56–1.47) | 0.87 | (0.52–1.47) | |
| Bedroom floor dust (ln-CFU/g) | 1.45 | (0.92–2.29) | 1.84* | (1.08–3.12) | 1.83* | (1.07–3.14) | |
| Cladosporium | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.68* | (1.04–2.72) | |
| Indoor air (ln-CFU/m3) | 0.79 | (0.56–1.11) | 1.09 | (0.72–1.67) | 0.85 | (0.51–1.41) | |
| Bedroom floor dust (ln-CFU/g) | 1.28* | (1.07–1.54) | 1.41* | (1.13–1.76) | 1.47* | (1.16–1.85) | |
| Aspergillus | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.17 | (0.77–1.79) | |
| Indoor air (ln-CFU/m3) | 1.04 | (0.68–1.59) | 0.98 | (0.60–1.60) | 1.01 | (0.60–1.68) | |
| Bedroom floor dust (ln-CFU/g) | 0.97 | (0.81–1.17) | 0.96 | (0.78–1.18) | 0.98 | (0.80–1.21) | |
| Penicillium | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 0.68 | (0.34–1.37) | |
| Indoor air (ln-CFU/m3) | 1.10 | (0.85–1.43) | 1.21 | (0.89–1.64) | 1.26 | (0.92–1.74) | |
| Bedroom floor dust (ln-CFU/g) | 1.18* | (1.00–1.39) | 1.19 | (0.99–1.44) | 1.18 | (0.98–1.43) | |
| Yeasts | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.03 | (0.66–1.61) | |
| Indoor air (ln-CFU/m3) | 1.19 | (0.86–1.64) | 1.13 | (0.77–1.66) | 1.12 | (0.74–1.69) | |
| Bedroom floor dust (ln-CFU/g) | 0.84* | (0.73–0.97) | 0.78* | (0.66–0.92) | 0.78* | (0.66–0.93) | |
Abbreviations: OR=odds ratio; CI=confidence interval.
Models adjust for winter birth (yes/no), low birthweight quartile (yes/no), maternal smoking during pregnancy (yes/no), and maternal history of physician-diagnosed asthma (yes/no).
NOTE - associations are also independent of gender, black ethnicity, low family income, day-care attendance in the first year of life, maternal atopy, building type, living room dust endotoxin (≥median=81.3 EU/mg) and cockroach allergen (Bla g 1 or 2≥0.05 U/g [≡2ng/g]), dust-mite allergen (≥10µg/g Der p or f 1 baby’s bed), lower respiratory illness by age one (croup, bronchitis, bronchiolitis, or pneumonia), and visible reports of mold, water damage, or dampness in the home, at any time over the year before birth (results not shown)
Odds ratio for an inter-quartile increase in the ln-transformed fungal concentration.
p≤0.05.
Table 2. Associations of early-life fungi with any wheeze by age one.
Analyses evaluate associations of each fungal genus separately
| ANY WHEEZE (≥1 vs 0 episodes) | |||||||
|---|---|---|---|---|---|---|---|
| Fungal genera | Full Dataset (n=407) | Subset with Outdoor Air Measures (n=316) |
|||||
| Indoor Air & Dust | Outdoor & Indoor Air & Dust |
||||||
| OR† | 95% CI | OR† | 95% CI | OR† | 95% CI | ||
| Alternaria | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.07 | (0.71–1.61) | |
| Indoor air (ln-CFU/m3) | 1.01 | (0.67–1.50) | 1.01 | (0.66–1.55) | 0.98 | (0.61–1.56) | |
| Bedroom floor dust (ln-CFU/g) | 1.47* | (0.95–2.28) | 1.84* | (1.12–3.02) | 1.84* | (1.12–3.02) | |
| Cladosporium | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.46* | (0.97–2.21) | |
| Indoor air (ln-CFU/m3) | 0.80 | (0.59–1.08) | 1.04 | (0.71–1.52) | 0.80 | (0.50–1.29) | |
| Bedroom floor dust (ln-CFU/g) | 1.28* | (1.08–1.52) | 1.40* | (1.14–1.73) | 1.43* | (1.16–1.77) | |
| Aspergillus | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.07 | (0.73–1.58) | |
| Indoor air (ln-CFU/m3) | 1.03 | (0.69–1.54) | 0.97 | (0.62–1.53) | 0.96 | (0.60–1.52) | |
| Bedroom floor dust (ln-CFU/g) | 1.03 | (0.87–1.23) | 1.04 | (0.86–1.26) | 1.04 | (0.86–1.26) | |
| Penicillium | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 0.91 | (0.50–1.65) | |
| Indoor air (ln-CFU/m3) | 1.05 | (0.82–1.33) | 1.15 | (0.87–1.52) | 1.17 | (0.87–1.56) | |
| Bedroom floor dust (ln-CFU/g) | 1.19* | (1.02–1.39) | 1.21* | (1.02–1.44) | 1.21* | (1.01–1.43) | |
| Yeasts | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 0.99 | (0.66–1.49) | |
| Indoor air (ln-CFU/m3) | 1.16 | (0.85–1.57) | 1.02 | (0.71–1.45) | 1.02 | (0.70–1.49) | |
| Bedroom floor dust (ln-CFU/g) | 0.89* | (0.78–1.03) | 0.85* | (0.72–0.99) | 0.85* | (0.72–0.99) | |
| Total | |||||||
| Outdoor air (ln-CFU/m3) | - | - | - | - | 1.15 | (0.84–1.56) | |
| Indoor air (ln-CFU/m3) | 1.06 | (0.77–1.45) | 1.25 | (0.84–1.86) | 1.13 | (0.72–1.79) | |
| Bedroom floor dust (ln-CFU/g) | 1.07 | (0.82–1.39) | 1.04 | (0.77–1.41) | 1.05 | (0.77–1.42) | |
Abbreviations: OR=odds ratio; CI=confidence interval.
Odds ratio for an inter-quartile increase in the ln-transformed fungal concentration: exceptions; inter-quartile range for indoor airborne Aureobasidium & dustborne Zygomycetes=0.0 CFU/m3; neither Aureobasidium nor Zygomycetes were predictors of any wheeze by age one.
All models presented adjust for winter birth (yes/no), low birthweight quartile (yes/no), maternal smoking during pregnancy (yes/no), and maternal history of physician-diagnosed asthma (yes/no).
p≤0.1.
Estimates for ‘frequent wheeze’ risk were less precise than those for ‘any wheeze’ (Supplemental Material, Tables 2 and 3). Adjusting for other genera, indoor airborne yeasts were borderline associated with increased odds (OR=1.53; 95% CI, 0.93–2.53), whereas dustborne yeasts appeared borderline protective (OR=0.86; 95% CI, 0.70–1.04). There were suggestions of associations with dustborne Alternaria, Cladosporium), and Aspergillus.
Associations were independent of winter-birth, low birthweight, maternal smoking during pregnancy, and maternal asthma. Models that either adjusted for birth- or sampling-season categorically, yielded consistent results. Relationships were not confounded by gender, ethnicity, income, daycare attendance or LRI by age one, maternal atopy, building type, endotoxin, indoor allergens (cockroach and dust mite), and self-reported mold/dampness.
We obtained consistent results in sensitivity analyses that: 1) excluded extremely high fungal exposures; 2) accounted for the correlation among the twins; and 3) with the Lasso approach. Models treating fungi continuously were more precise than with binary fungi (>75th percentile; results not shown). Reported mold/dampness was not significantly associated with any wheeze (OR=1.27; 95% CI, 0.88–1.83) or frequent wheeze (OR=1.06; 95% CI, 0.67–1.69). Wheeze was not associated with binary ‘high fungal exposure’ (any fungi >75th percentile), ordinal ‘count’, or total fungal exposure variables.
Effect Modification by Maternal Mold Sensitization
Dustborne Alternaria was associated with higher odds of ‘any wheeze’ for children with mold-sensitized mothers [(OR=9.16; 95% CI, 1.37–61.22), n=46] than those with non-sensitized mothers [(OR=1.32; 95% CI, 0.79–2.20), n=315], (interaction p=0.05). However, associations were not modified by maternal ‘any atopy’ or asthma. Effect modification for ‘frequent wheeze’ was not observed.
DISCUSSION
While we found increased odds of wheeze in the first year of life with elevated infant fungal exposures in indoor dust (Alternaria, Penicillium and Cladosporium), indoor air (Penicillium), and outdoor air (Cladosporium), the odds of wheeze were reduced with elevated indoor dustborne yeast levels, even after adjusting for visible home dampness, endotoxin, or other known wheeze risk factors (10, 11).
How Do Fungi Modify Infant Wheeze Risk?
Fungi may be irritants, leading directly to airway inflammation, or may increase infection risk, leading to inflammation and bronchoconstriction (5). Early-life wheeze is usually induced by viral illnesses in vulnerable infants (18). While mold predicted increased risk of LRI (12), 97% children experienced their first wheeze prior to their first LRI, making LRI less likely to be on the pathway between early-life fungal exposures and wheeze expression.
Inhalant infant allergy is often undetectable. Nevertheless, the increased wheeze response to Alternaria of children with mold-sensitized mothers may be an early manifestation of allergic symptoms (19). We could not evaluate modification of infant wheeze risk by maternal sensitivity to other genera, as we only tested maternal IgE for Alternaria or Aspergillus.
Increased ambient basidiospore and ascospore concentrations during infancy were associated with increased wheeze odds at age 2 in a birth-cohort of 514 low-income, predominantly Mexican children in an agricultural region of California (20). However, in a subgroup of this population, there was no in-vitro evidence for a relative increase in Th2 expression.
The protective role of dustborne yeasts adds to the small but growing evidence that not all fungi or fungal products need be risk factors for sensitization or a Th2 bias (21). While unmeasured confounders may explain the protective role of yeasts, we adjusted for several known potential confounders and predictors of early-life wheeze. Yeasts are unicellular fungi that are phylogenetically diverse, whereas the species we found to be harmful in our study are multicellular fungi. Variability in pathogen-associated molecular patterns (PAMPs) within yeast species as well as between unicellular and multicellular fungi may differentially stimulate innate responses through variable pattern-recognition receptors (PRRs). Toll-like receptor 2 (TLR2) recognize PAMPs from fungi, thereby initiating signal transduction pathways that induce expression of genes that regulate innate and subsequent adaptive immune responses (22). Molecular microbiologic studies may elucidate specific components of innate microbiologic stimulants that lead to contrasting effects on wheeze development.
In a birth-cohort of 574 at-risk infants, high dust (1–3)-β-D-glucan concentrations (>60µg/g) appeared protective of recurrent wheezing by age one (23). Differential glucan levels between genera (24) and types of yeasts (25) may explain the variable associations with respiratory and allergic disease (26). Taxonomically-resolved exposure measures may clarify this inconsistency (27). The direction and magnitude of wheeze response may be related to the route, dose, and timing of exposure, and the child’s vulnerability factors (28). As gut immune responses are key to development of immune tolerance (29), ingestion of floor dust by infants and toddlers may result in protection, whereas aerosolized yeasts may be irritants. Moreover the yeasts that aerosolize may differ from yeasts in floor dust.
Method for Ascertainment of Mold Exposure May Influence Findings: No Gold Standard
Our study is one of the most comprehensive evaluations of early-life fungal effects on infant wheeze risk. Few studies have simultaneously measured fungi in outdoor air and indoor dust and air. Despite the potential health significance of exposures, cost considerations lead most studies to rely on visible mold. A Connecticut study of high-risk children measured indoor airborne, but not outdoor or dust culturable fungi (30). They reported increased age one wheeze risk for those 36 children with high Penicillium levels (30). Exposure to >75th percentile of indoor airborne Penicillium (but no other fungi) at age 3 months predicted increased age one wheeze risk in a smaller study of 103 at-risk infants from the low-income urban population of Syracuse, NY (31).
Indoor and outdoor airborne, but not dustborne measurements were available for the Inner-City Asthma Study of mold-sensitive children (aged 5–11years) with asthma (6). Outdoor fungi predicted more asthma symptoms, whereas indoor fungi predicted more unscheduled emergency room or clinic visits in the past 2 weeks. As the investigators suggest, the short duration of air sampling, and lack of dust measures could have led to an underestimate of the role of indoor mold on asthma symptoms.
Compared to airborne fungi, bedroom dust fungi were most consistently linked to wheeze, likely because dust cultures represent a more time-integrated exposure than 1-minute air samples (1). There is no gold standard method to estimate mold exposure (5), and ideal studies use a combination of approaches. While concentrations vary temporally within homes (32), fungi in early-life were highly correlated with average annual concentrations. Moreover, our early-life measures represent a vulnerable period when exposures may influence immune and respiratory development (9).
Despite fungal seasonal variability, fungal-wheeze associations were independent of season; children born in winter had reduced wheeze risk (OR=0.58; 95% CI, 0.38–0.90), relative to other birth-seasons. Other seasonally variable in- utero or post-natal exposures (e.g., aeroallergens, vitamin D) may explain the role of birth-season in early-life wheeze (33).
Study Limitations
In addition to sampling time and taxonomic resolution limitations, our analytical technique required spores be culturable, but non-detectable culturable fungi may include non-viable spores and fungal fragments that carry allergens or irritants. Strongly hydrophilic (water activity, aw>0.9) fungi may be underrepresented due to the high solute concentration in the culture media used, DG18 (34) or because very few homes were severely water-damaged. In fact, hydrophilic fungi (e.g., Fusarium, Stachybotrys) were rarely recovered as their growth and survival relies on continuously wet materials (4).
While we had sufficient power to assess ‘any wheeze’, we lacked power to fully evaluate the risk of ‘frequent wheeze’, which is more predictive of future asthma (35). We recognize the number of fungi evaluated may lead to chance findings. However, we found consistent results with the Lasso method (16).
CONCLUSIONS
In one of the most comprehensive evaluations of early-life fungal health effects, our prospective birth-cohort has shown that while wheeze rates were higher with exposures to fungi usually considered to be irritant or allergenic in sensitive subjects, yeasts in the home had a strong protective association with wheeze in infancy. The influences of fungi on the risk of wheeze may vary by form, functional differences in genera, by timing, dose, or mode of exposure, or by inherited susceptibility factors. Molecular microbiologic studies may elucidate specific components of innate microbiologic stimulants that lead to contrasting effects on wheeze development. Such studies may result in groundbreaking findings as translational research that could contribute to development of new targeted pharmacologic or biotic primary or secondary asthma and allergy therapies.
Supplementary Material
Acknowledgments
The authors thank the research assistants (Jimmy Kamel, Doris Kwan, and Tara Webb), Dr Harriet Burge for her involvement in the study design and fungal measurements, Dr Hongshu Guan for his statistical review, and the families who have participated in this project. We also thank Professors Brent Coull and Petros Koutrakis for their support as part of Behrooz Behbod’s doctoral thesis committee. This manuscript has been supported by the following grants: R01 AI035786, EPA RD-83241601, AI-20565, and K99 HL109162 02. Dr Behbod’s doctorate has been supported by the Harvard–Cyprus Endowment Scholarship.
Abbreviations
- aw
water activity (or equilibrium relative humidity, %ERH) is the vapor pressure generated by moisture present in a hygroscopic product. It represents the moisture content on a surface that is available for fungal growth
- Bla g 1 or 2
Cockroach allergen from Blattella germanica
- bn
billion
- CFU
colony-forming unit
- CI
confidence interval
- D50
aerodynamic diameter cutpoint where 50%of the particles are captured. Above this cutpoint, a greater percentage is captured
- DG18
dichloran-glycerol culture media
- EPS-Pen/Asp
extracellular polysaccharides from Penicillium or Aspergillus
- EU
endotoxin unit
- IgE
immunoglobulin E
- Inc
incorporated company
- IQR
interquartile range
- lpm
liters per minute
- LRI
lower respiratory illness
- Ltd
limited company
- r
correlation coefficient
- SD
standard deviation
- Th1
type 1 T-helper cell
- Th2
type 2 T-helper cell
Footnotes
Authors Contributions:
Behrooz Behbod analysed and interpreted the data, and was responsible for writing this manuscript as first author.
Joanne Sordillo assisted with the analysis and interpretation of data, and critically revising for important intellectual content.
Elaine Hoffman assisted with the analysis and interpretation of data, and critically revising for important intellectual content.
Soma Datta assisted with acquisition of data, and critically revising for important intellectual content.
Michael Muilenberg assisted with the acquisition and interpretation of data, and critically revising for important intellectual content.
James Scott assisted with the interpretation of data, and critically revising for important intellectual content.
Ginger Chew assisted with the acquisition and interpretation of data, and critically revising for important intellectual content.
Thomas Platts-Mills assisted with the interpretation of data, and critically revising for important intellectual content.
Joel Schwartz assisted with the interpretation of data, and critically revising for important intellectual content.
Harriet Burge assisted with the design and acquisition of data, and critically revising for important intellectual content.
Diane Gold was responsible for the conception and design, acquisition, analysis and interpretation of data, and critically revising for important intellectual content.
All authors have given final approval of the version to be published.
Conflict of Interest:
All authors of this manuscript declare that they do not have a conflict of interest including relevant financial interests, activities, relationships and affiliation.
REFERENCES
- 1.Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor GT, Walter M, Mitchell H, Kattan M, Morgan WJ, Gruchalla RS, et al. Airborne fungi in the homes of children with asthma in low-income urban communities: The Inner-City Asthma Study. J Allergy Clin Immunol. 2004;114(3):599–606. doi: 10.1016/j.jaci.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 3.Shelton BG, Kirkland KH, Flanders D, Morris GK. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl Environ Microbiol. 2002;68(4):1743–1753. doi: 10.1128/AEM.68.4.1743-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burge HA, Otten JAFungi. In: Bioaerosols: Assessment and Control. Macher J, Ammann HA, Burge HA, Milton DK, Morey PR, editors. Cincinnati: American Conference of Governmental Industrial Hygienists; 1999. pp. 19.1–19.13. [Google Scholar]
- 5.Institute of Medicine, Committee on the Assessment of Asthma and Indoor Air. Clearing the air: asthma and indoor air exposures. Washington DC: National Academy Press; 2000. [Google Scholar]
- 6.Pongracic JA, O’Connor GT, Muilenberg M, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J Allergy Clin Immunol. 2010;125(3):593–599. doi: 10.1016/j.jaci.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO guidelines for indoor air quality: dampness and mould. Copenhagen, Denmark: WHO Regional Office for Europe; 2009. [PubMed] [Google Scholar]
- 8.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WOCM, Braun-Fahrländer C, et al. Exposure to Environmental Microorganisms and Childhood Asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 9.Rylander R, Etzel R. Introduction and summary: workshop on children’s health and indoor mold exposure. Environ Health Perspect. 1999;107:465. doi: 10.1289/ehp.99107s3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills TAE, Weiss ST. Predictors of repeated wheeze in the first year of life. The relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160(1):227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163(2):322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 12.Stark PC, Burge HA, Ryan LM, Milton DK, Gold DR. Fungal Levels in the Home and Lower Respiratory Tract Illnesses in the First Year of Life. Am J Respir Crit Care Med. 2003;168:232–237. doi: 10.1164/rccm.200207-730OC. [DOI] [PubMed] [Google Scholar]
- 13.Andersen AA. New sampler for the collection, sizing and enumeration of viable airborne particles. J Bacteriol. 1958;76:471–484. doi: 10.1128/jb.76.5.471-484.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Arx JA. The genera of fungi sporulating in pure culture. Lehre, Germany: Verlag von J Cramer; 1970. [Google Scholar]
- 15.Lewis SA, Weiss ST, Platts-Mills TAE, Syring M, Gold DR. Association of specific allergen sensitization with socioeconomic factors and allergic disease in a population of Boston women. J Allergy Clin Immunol. 2001;107(4):615–622. doi: 10.1067/mai.2001.113523. [DOI] [PubMed] [Google Scholar]
- 16.Tibshirani R. The Lasso method for variable selection in the Cox model. Statist Med. 1997;16(4):385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Team, RDC . In: R: A language and environment for statistical computing. R Development Core Team, editor. Vienna, Austria: R Foundation for Statistical Computing; 2008. Available from http://www.r-project.org. [Google Scholar]
- 18.Lloyd CM, Robinson DS. Allergen-induced airway remodeling. Eur Respir J. 2007;29:1020–1032. doi: 10.1183/09031936.00150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portnoy JM, Barnes CS, Kennedy K. Importance of mold allergy in asthma. Curr Allergy Asthma Rep. 2008;8(1):71–78. doi: 10.1007/s11882-008-0013-y. [DOI] [PubMed] [Google Scholar]
- 20.Harley KG, Macher JM, Lipsett M, Duramad P, Holland NT, Prager SS, et al. Fungi and pollen exposure in the first months of life and risk of early childhood wheezing. Thorax. 2009;64:353–358. doi: 10.1136/thx.2007.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douwes J, van Strien R, Doekes G, Smit J, Kerkhof M, Gerritsen J, et al. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol. 2006;117(5):1067–1073. doi: 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117(5):979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 988. [DOI] [PubMed] [Google Scholar]
- 23.Iossifova YY, Reponen T, Bernstein DI, Levin L, Kalra H, Campo P, et al. House dust (1–3)-β-d-glucan and wheezing in infants. Allergy. 2007;62(5):504–513. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foto M, Plett J, Berghout J, Miller JD. Modification of the Limulus amebocyte lysate assay for the analysis of glucan in indoor environments. Anal Bioanal Chem. 2004;379(1):156–162. doi: 10.1007/s00216-004-2583-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim KS, Chang JE, Yun HS. Estimation of soluble β-glucan content of yeast cell wall by the sensitivity to Glucanex® 200G treatment. Enzyme and Microbial Technology. 2004;35(6–7):672–677. [Google Scholar]
- 26.Osborne M, Reponen T, Adhikari A, Cho SH, Grinshpun SA, Levin L, et al. Specific fungal exposures, allergic sensitization, and rhinitis in infants. Pediatric Allergy and Immunology. 2006;17(6):450–457. doi: 10.1111/j.1399-3038.2006.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol. 2012;130:639–644. doi: 10.1016/j.jaci.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura Y, Sumiyoshi M, Suzuki T, Suzuki T, Sakanaka M. Inhibitory effects of water-soluble low-molecular-weight β-(1,3-1,6)D-glucan purified from Aureobasidium pullulans GM-NH-1A1 strain on food allergic reactions in mice. International Immunopharmacology. 2007;7:963–972. doi: 10.1016/j.intimp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Noverra MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends in Microbiology. 2004;12(12):562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, et al. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect. 2002;110(12):A781–A786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford JA, Anagnost SE, Wang CJK, Hunt A, Anbar RD, et al. Indoor airborne fungi and wheeze in the first year of life among a cohort of infants at risk for asthma. J Exp Sci Env Epi. 2010;20:503–515. doi: 10.1038/jes.2009.27. [DOI] [PubMed] [Google Scholar]
- 32.Chew GL, Douwes J, Doekes G, Higgins KM, Van Strien R, Spithoven J, et al. Fungal extracellular polysaccharides, β(1→3)-glucans and culturable fungi in repeated sampling of house dust. Indoor Air. 2001;11:171–178. doi: 10.1034/j.1600-0668.2001.011003171.x. [DOI] [PubMed] [Google Scholar]
- 33.Gold DR, Bloomberg GR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, et al. Parental characteristics, somatic fetal growth, and birth-season influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol. 2009;124(5):1078–1087. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhoeff AP, van Reenen-Hoekstra ES, Samson RA, Brunekreef PJ, van Wijnen JH. Fungal propagules in house dust. I. Comparison of analytic methods and their value as estimators of potential exposure. Allergy. 1994;49:533–539. doi: 10.1111/j.1398-9995.1994.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 35.Ly NP, Gold DR, Weiss ST, Celedon JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132–e1138. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


