Abstract
Despite increasing competition from percutaneous interventions and other novel methods of non-surgical coronary revascularization, coronary artery bypass grafting (CABG) remains one of the most definitive and durable treatments for coronary artery disease, especially for those patients with extensive and diffuse disease. In recent years the CABG procedure itself has undergone innovation and evolution. This review article provides a brief historical perspective on the procedure, and examines the current state of modern variations including off-pump, limited-access, and robotic-assisted CABG.
Keywords: Coronary artery bypass grafting, Coronary artery disease, Surgery
Coronary artery bypass grafting (CABG) is arguably one of the most important and most studied surgical procedures in the history of medicine. Although no other operation has prolonged more lives, provided more relief of symptoms, and been more thoroughly studied, the future of traditional CABG is increasingly threatened by percutaneous alternatives and novel methods of surgical revascularization. Despite this evolution, CABG remains the most durable means of treating patients with coronary artery disease (CAD). This is especially true for patients with extensive and diffuse CAD. Because of the evolving technology, one must constantly assess the risks and benefits of these new techniques and compare them with standard, tested methods. This paper outlines the history and current state of the modern CABG procedures.
Development of CABG
Carrel, over a century ago, realized the connection between angina pectoris and stenoses of the coronary arteries.1 In a canine model, Carrel interposed a carotid artery segment between the descending thoracic aorta and the left coronary artery. Carrel received the Nobel Prize in Physiology or Medicine for this work on vascular techniques in 1912,1 but the lack of technology to support the heart hindered the development of surgical revascularization until the middle of the 20th century.
Montreal surgeon Vineberg, in the 1940s, implanted the internal thoracic arteries into the left ventricular myocardium in patients with angina.2 The Vineberg procedure was met with variable success, although it was eventually realized that the technique offered little or no benefit over a non-operative approach. This demonstrated the strong influence of the placebo effect.3 In 1958 Longmire et al reported on coronary artery endarterectomy, without the use of cardiopulmonary bypass (CPB).4 Longmire was likely the first to perform a direct internal thoracic artery–coronary artery anastomosis as a consequence of damage to the right coronary artery during one of his endarterectomy procedures.
Around the same time, a number of surgeons were reporting on their early experience with CABG. In 1962 Sabiston performed the first planned saphenous vein bypass operation at Duke University.5 In 1964 Kolesov grafted the left internal mammary artery (LIMA) to the left anterior descending (LAD) artery, without CPB,6 and Garrett et al reported on aortocoronary saphenous vein grafting.7 Favaloro began to routinely use reversed saphenous veins for aorto-coronary grafting.8 LIMA grafting to the LAD is credited to Green et al in 1968.9 The use of radial artery grafts was described by Carpentier et al in 1973, but was met with poor results.10 The use of this graft conduit was revived by Acar in 1989,10,11 and in recent years has been shown to be quite effective.12
In the 1970s CABG expanded as a treatment for patients with CAD. Several randomized trials, namely the Coronary Artery Surgery Study (CASS),13 the Veteran’s Administration Coronary Artery Bypass Trial,14 and the European Coronary Artery Bypass Trial,15 were conducted and would define the indications and confirm the efficacy of CABG.
CPB
The development of the heart–lung machine by Gibbon16 was the most important advance in the expanded role of CABG, and of cardiac surgery in general. CPB, and the utilization of cardioplegia, provided a motionless, blood-free surgical field, which enabled the precision and reproducibility necessary for direct coronary anastomosis. Despite the obvious benefits of CPB there was an immediate awareness of its inflammatory and other adverse effects. As the blood cells and humoral factors come into contact with the artificial surfaces of the components of the heart– lung machine, they are activated, resulting in a number of hematological and systemic inflammatory changes.17 These mechanisms, in addition to hemodilution, nearly always result to some extent in coagulopathy, edema, and end-organ dysfunction, including neurocognitive changes. The magnitude of this systemic inflammatory response, in some cases, can be severe and can result in extensive organ injury.
Efforts have been directed at reducing systemic inflammation associated with CPB. These efforts include decreasing the surface area and modifying the surface composition of the CPB circuit to minimize activation of neutrophils and other blood components, minimizing hemodilution by decreasing the volume of the priming solution needed to institute CPB and pre-treatment of patients with anti-inflammatory drugs.
Multivessel Off-Pump Coronary Artery Bypass
Off-pump CABG (off-pump CABG) dates back to the 1970s.18 Off-pump CABG, however, only recently gained widespread acceptance and entered the mainstream of clinical practice, propelled by a greater awareness of the potential morbidity of CPB and aortic manipulation and facilitated by improvements in surgical tools and techniques. Off-pump CABG is part of the procedural armamentarium of a growing proportion of surgeons worldwide.
The relative merit of off-pump CABG, when compared to conventional CABG with CPB, remains uncertain. Few topics in cardiac surgery have given rise to more debate and controversy. As a result, adoption of the off-pump CABG technique has been sporadic. Current US estimates (based on industry reports) suggest that between 18% and 25% of the 300,000 annual CABG procedures are performed off pump. This percentage seems to be much greater in Japan, China and parts of Europe, where reports suggest that the majority of multivessel CABG procedures are performed without CPB. The reasons for these regional differences in off-pump CABG preference are not certain. There is a perception by many performing a high proportion of off-pump CABG that the procedure is actually much better for the patient than the on-pump approach and that it is less expensive. In the USA a modest number of off-pump CABG procedures are performed by a minority of heart surgeons. That minority, however, performs predominantly off-pump CABG. The vast majority of heart surgeons in the USA perform <5% of cases without CPB.
The well-documented low mortality and morbidity in the majority of both off-pump CABG and conventional CABG patients compounds the difficulty in comparing these procedures.19,20 Although prospective, randomized trials comparing results after off-pump CABG and conventional CABG have been performed,21 a trial of sufficient size to show a statistical difference has only recently been performed. Surprisingly, that study showed little difference between the two methods.22 Several large, retrospective multicenter series have reported lower incidences of death, stroke, IABP requirement, postoperative transfusion, time on ventilator, and length of stay for off-pump CABG as compared to conventional CABG operations at the same institutions19,23 and to national database statistics. The impact of selection bias, however, in determining operative technique for any given patient in these series may be a confounding variable. Similar results suggesting superiority of the off-pump CABG procedure have been reported when propensity scores were used in an attempt to decrease the impact of selection bias in an unselected group of patients24 and in high-risk subgroups.25 Propensity scoring decreases dissimilarity between the 2 groups being compared with respect to major cardiac and non-cardiac morbidities. The results, however, do not take into account intraoperative findings, such as small, calcified, or diffusely atherosclerotic coronary arteries, intramyocardial or intra-adipose coronary arteries, poor bypass conduit quality, or other conditions that make revascularization technically more demanding and, at many centers, increase the likelihood of the procedure being performed with CPB. As a result, technically difficult revascularizations are more prevalent in the conventional CABG group.
Initial criticism aimed at off-pump CABG centered on concerns regarding adequacy of revascularization and quality and reproducibility of the coronary anastomoses. Initial reports did show a small but significant decrease in the average number of grafts per patient when off-pump CABG was performed as compared to conventional CABG.26 The development of immobilizers and techniques for lateral and inferior wall exposure allowed skilled off-pump CABG surgeons to perform bypasses to all areas of the heart. More recent series reported little difference in the average number of grafts constructed.25,27 Although a few publications have suggested that off-pump CABG is associated with decreased graft patency,25 the studies have generally been performed at centers that were relatively early in their off-pump CABG experience. There are now several well-documented series that have demonstrated excellent graft patency after off-pump CABG.21,28 The recently completed VA trial, however, did demonstrate a slightly but significantly reduced graft patency with off-pump CAB vs on-pump CABG.22
Although controversy remains, there is some evidence that off-pump CABG is associated with decreased operative risk in some high-risk groups. This has been reported in several retrospective, non-randomized studies,19,29 as well as in studies that looked specifically at older patients with reduced ejection fractions and at patients with renal failure.30,31 Other retrospective studies that examined this same topic, however, fail to show any difference between the two methods.32–34
A tailored left thoracotomy approach has been reported for reoperative grafting of the lateral wall in patients with patent LIMA– LAD grafts.35 Using this method a graft can be constructed from the descending thoracic aorta to lateral wall branches, avoiding the challenges associated with repeat sternotomy and LIMA-graft mobilization. Long-term patency data for this type of graft, however, are currently not available. Similarly, lower sternotomy and upper abdominal incisions have been described for constructing grafts to the inferior wall with the right gastroepiploic artery without CPB in the reoperative situation.36
Although no consensus exists, it is clear to most surgeons that there are many patients for whom off-pump CABG would be technically difficult or unwise (due to a deep intramuscular LAD; small, diffusely diseased coronaries; need for an extensive endarterectomy; or active ischemia associated with hemodynamic compromise). In contrast, some patients probably benefit from off-pump CABG, such as those in whom conventional CABG would expose them to unnecessary risk (due to presence of an atheromatous ascending aorta or systemic process that might be exacerbated by CPB). Therefore, familiarity with the techniques and the ability to perform off-pump CABG when necessary is important for the coronary surgeon. A study commissioned by the American Heart Association concluded that a quality operation can be performed with either off-pump or on-pump CABG, and that the outcome depends more on factors such as the quality of the surgeon, the quality of the institution, and a systematic approach to patient care, rather than the type of CABG operation.37 Exercising clinical judgment in selecting the appropriate approach for surgical revascularization in each patient is essential.
Neurocognitive Dysfunction After On-Pump vs Off-Pump CABG
Numerous published reports have demonstrated the new onset of subtle neurocognitive dysfunction in some patients after CABG.38,39 Depending on the sensitivity of the tests used, the incidence has been reported to be between 5% and 60%. Specific deficits include short-term memory loss, reduced ability to perform simple calculations, and disturbances in personality and mood.38,39 Many factors related to CPB have been implicated, including systemic inflammatory response, alterations in cerebral blood flow, and microemboli, either related to the CPB circuit or to cannulation and cross-clamping.39,40 Although some studies have shown that off-pump CABG is associated with a reduced incidence of neurocognitive dysfunction as compared to that of conventional CABG with CPB,21,40 most studies have shown no significant difference.41–43 This failure of off-pump CABG to favorably affect postoperative neurocognitive dysfunction has been largely attributed to the partial occlusion aortic clamp and to microemboli liberated during application and removal of the clamp. Although off-pump CABG obviates the need for placement of a perfusion catheter for CPB or a cross-clamp for cardiac arrest, the side-biting partial occlusion clamp, routinely used at many centers during construction of proximal anastomoses, arguably is equally or more traumatic.44 Avoiding aortic manipulation, using composite grafts arising from the in situ LIMA, and off-pump CABG have been shown to be important factors in avoiding postoperative stroke.45 The development of new proximal anastomotic devices and other novel techniques for proximal anastomosis46 may improve the incidence of neurocognitive dysfunction after off-pump CABG, but this is yet to be determined.
Most surgeons agree that off-pump CABG is well suited for revascularization in patients with significant atheromas or calcification of the ascending aorta. In this setting, free grafts constructed from either the right internal mammary artery or the radial artery can be attached to the side of the in situ LIMA and used to perform multivessel off-pump CABG without manipulating the aorta. Occasionally, the innominate, subclavian, or axillary arteries can be used as a site for a proximal anastomosis if they are free of atheromatous plaque.47 Free grafts can also be attached to a small, disease-free area of the aorta with an automated anastomotic device that obviates the need for a side-biting clamp.
Limited Access Coronary Artery Bypass
Efforts to minimize the invasiveness of CABG have focused on decreasing the trauma associated with surgical access. Although median sternotomy is generally well tolerated, patients must refrain from heavy lifting for approximately 2 months after surgery to allow the sternum to heal. Although the incidence of wound complications is generally low, sternal wound infections can be serious and life-threatening. Moreover, the musculoskeletal trauma associated with spreading the halves of the sternum is associated with a systemic inflammatory response that is synergistic with CPB in causing morbidity.48
Minimally Invasive Direct Coronary Artery Bypass (MIDCAB)
In 1995 Benetti et al introduced the MIDCAB procedure,49 which was rapidly adopted by multiple centers in the USA and Europe.50 The procedure is performed through a small anterior lateral thoracotomy and often consists of a single-vessel off-pump bypass using the LIMA to bypass the LAD. Many early series documented satisfactory results, with lengths of stay shorter than those for standard CABG, decreased resource utilization, earlier return to full activity, reduced requirements for transfusion, and excellent graft patency.50–52 Some reports suggested a reduced incidence of postoperative atrial fibrillation,53 but this has not been substantiated by other studies.54,55 Although thoracoscopic LIMA mobilization was described in the initial report,49 most US centers elected to use direct vision and aggressive chest wall retraction to avoid the learning curve associated with videoscopic instrumentation and visualization. The aggressive chest wall retraction required for LIMA mobilization often results in significant early postoperative pain. These factors contributed to the decrease in popularity of the traditional MIDCAB procedure. Nevertheless, several centers skilled in MIDCAB continue to perform the procedure in large numbers and report excellent results.52,56,57 Other groups have championed video-assisted MIDCAB, in which the LIMA is mobilized with conventional videoscopic instruments; the LIMA is then anastomosed to the LAD through a small thoracotomy.58,59 This approach mitigates some of the shortcomings of a standard MIDCAB, but it is associated with a considerable learning curve. Evaluating the position of the heart and the location of the LAD before the chest wall is incised allows the accurate placement of the chest incision, which further reduces the need for retraction.
Robotic-Assisted CABG
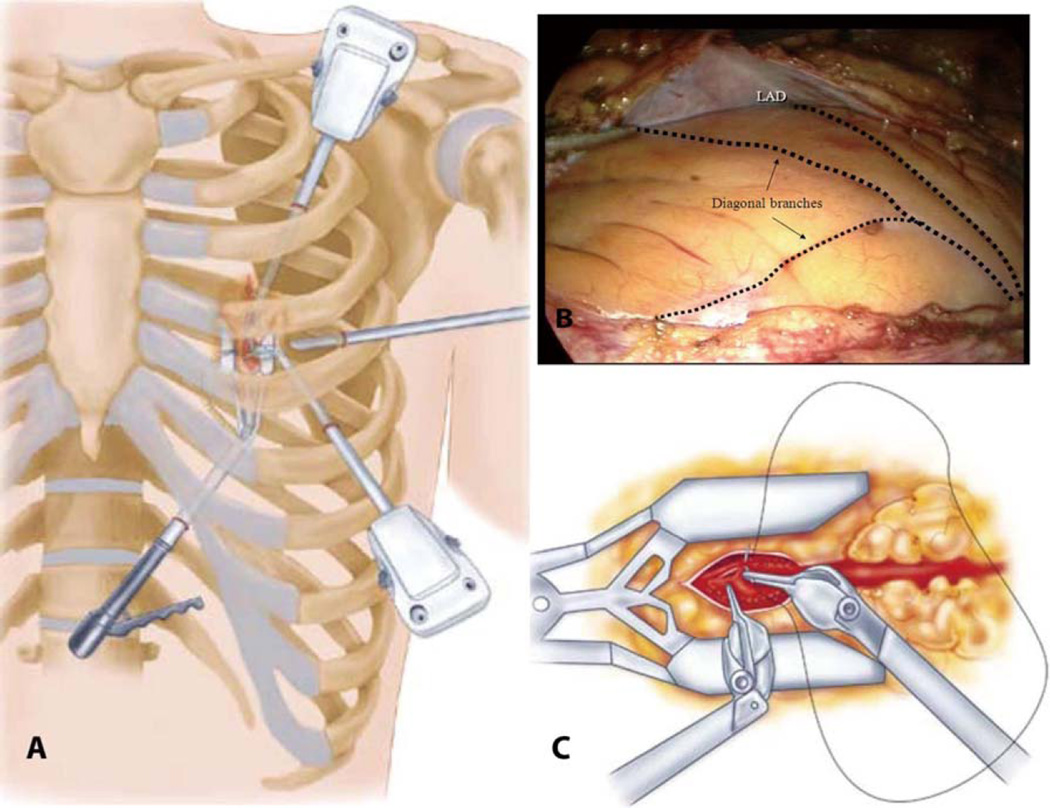
The surgical robot is an elegant microprocessor-controlled, electromechanical instrument that allows the surgeon to remotely manipulate fully articulating videoscopic instruments by way of master– slave servos and microprocessor control. These long, thin instruments, which can be inserted into the closed chest through half-inch incisions, are designed to allow multiple degrees of freedom and can precisely emulate the surgeon’s movements at the control console (Figure).60 A clear benefit to the robotic approach over other methods, however, has not been demonstrated.
Figure.
Robotic coronary artery bypass grafting. (A) A 30° scope, 2 instrument ports, and a sub-xyphoid endostabilizer are inserted into the left chest via four small incisions, (B) permitting visualization and manipulation of the left internal mammary artery (LIMA) and coronary vessels, including the left anterior descending coronary artery (LAD). (C) The arteriotomy, mobilization of the LIMA, and anastomosis are completed endoscopically. Reproduced with permission from Atlas of Cardiac Surgical Techniques (1st edn).60
Since the introduction of surgical robotics in the 1990s, there has been a progressive increase in utilization for thoracic surgical procedures. Although mitral valve and noncardiac thoracic procedures account for the majority of cases, there are increasing reports of robotic-assisted coronary revascularization procedures. These reports include robotic LIMA harvest followed by a traditional MIDCAB61 or left thoracotomy off-pump CABG,62 totally endoscopic coronary artery bypass (TECAB) on the arrested heart,63,64 and totally endoscopic bypass without CPB (OP-TECAB).64 Although most TECABs and OP-TECABs involve only a LIMA–LAD graft, recent reports described a series of multivessel revascularization procedures.63 These series have demonstrated that each of these methods of limited access off-pump coronary bypass is associated with a shorter hospital stay, less time on mechanical ventilation, fewer transfusions, and a more rapid return to full activity. The operative times are considerably longer than for open procedures, but improved time efficiency with experience is the norm. Also, questions related to graft patency and long-term results persist. Several earlier reports suggested a conversion to an open procedure in >50% of cases, but with increased experience, conversion in the ≤10% range is more common.64
Because of the added expense and difficulty with learning the technique, the routine use of surgical robotics in CABG surgery does not seem likely in the near future. The robot has and will continue to evolve. Improved video resolution, lower mass arms, the addition of a fourth telemanipulator, and the availability of an elegant robotic coronary stabilizer will likely increase its effectiveness and extend its application. Refinement of automated distal anastomotic devices may further increase the growth of robotic coronary revascularization surgery.
Hybrid MIDCAB Approach
Recently, reports have documented successful application of a hybrid strategy combining one of the above minimally invasive LIMA– LAD bypass procedures with catheter-based interventions on the circumflex or right coronary arteries for the treatment of multivessel disease. In most series the catheter-based interventions, which generally entail placement of a drug-eluting stent, were performed several days before or several days after the surgical revascularization,65 although a same-day hybrid approach has been described.61 In theory, hybrid procedures provide a complete revascularization while maintaining the survival benefit and angina relief of a LIMA–LAD graft and avoiding the morbidity of sternotomy. Before widespread adoption will occur, however, patency and outcome data are required. Although more labor and cost intensive than traditional CABG, improvements in the techniques and coordination between the surgeon and interventional cardiologists will probably increase the effectiveness and value of the hybrid approach.
Port-Access CABG
In 1996 Heartport™ introduced port-access cardiac surgery as a means of decreasing the trauma associated with surgical access. In port-access cardiac surgery, specialized catheters are inserted into the femoral artery and vein; these catheters allow for initiation of CPB, occlusion of the ascending aorta, administration of cardioplegia, and venting of the ascending aorta. Additional catheters introduced percutaneously into the left jugular vein allow for delivery of retrograde cardioplegia into the coronary sinus and venting of the pulmonary artery. A small chest incision can then be tailored for the desired procedure. In the late 1990s several series of multivessel CABG procedures performed through a left thoracotomy demonstrated acceptable results with port access institution of CPB and coronary revascularization and other types of cardiac operations.66,67 While this method became less popular for multivessel bypass, port access has remained an essential component of single-vessel TECAB, and it remains an enabling technique in performing limited access mitral and tricuspid procedures and repair of atrial septal defects. Recently, improved techniques and instruments have made multivessel coronary artery bypass a viable method. McGinn et al examined the feasibility and safety of minimally invasive surgical coronary bypass grafting (MICS CABG) using port access and a small incision approach.68 This is a novel approach to the surgical treatment of patients with coronary disease that may be performed with or without the use of CPB. During the operation an apical positioner and epicardial stabilizer are introduced into the chest through the subxyphoid and left 7th intercostal spaces, respectively. The left internal thoracic artery was used to graft the LAD artery, and radial artery or saphenous vein segments used to graft the lateral and inferior myocardial regions. There was a 7.6% utilization of CPB, a 3.8% conversion to sternotomy, and a 2.2% return to the operating room for bleeding. Perioperative mortality occurred in 1.3% of patients. The study demonstrated that MICS CABG is feasible and that the short-term outcomes are excellent. There were several limitations, however. First, there was no comparison to other techniques. It is questionable how universally this procedure can be applied. The low number of bypasses performed (just over 2 per patient) suggest that this is not an average or typical group of CABG patients, and that the patients were “cherry picked”. The authors admit that there is an extensive learning curve. Although the cosmetic result is obviously better compared to a standard sternotomy incision, that study does not demonstrate improved short- or long-term outcomes or prove better graft patency or survival, which would suggest an improvement over current techniques. MICS CABG, however, may potentially make multivessel minimally invasive surgery more effective and more widely available.
Graft Selection and Patency
Multiple long-term angiographic studies have described the patency rates of different types of grafts, and risk-factors associated with their premature closure. It is unquestionable that the internal mammary artery graft to the LAD artery graft markedly improves the long-term quality of CABG with regard to survival and relief of symptoms. Many studies have demonstrated better clinical outcomes with the increased use of arterial grafts.69 These, however, have nearly always been retrospective analyses subject to considerable selection bias, making definitive conclusions questionable. Several methods have attempted to improve vein graft patency. The Prevent IV trial examined the effects of a gene transcription factor decoy to minimize intimal hyperplasia. Despite a good theory, the results were negative.70 In a new attempt, vein graft patency after application of an external support and reduction of lumen diameter has been examined and demonstrated improved patency in preliminary studies.71 The CASCADE study demonstrated a >95% vein graft patency at 1 year, if care was taken in using only good-quality vein. In addition, the study examined the additive effects of clopidogril with aspirin on graft patency. The addition of clopidogrel did not improve either arterial or vein graft patency after 1 year.72
Conclusion
CABG remains a durable method of coronary revascularization. Its future is assured, but efforts should be directed at further development and research in order to evaluate the role of such factors as complete arterial grafting, modified vein grafts, minimally invasive techniques, secondary prevention programs, and adjunct pharmacotherapeutic strategies in achieving a more effective and less morbid operation. Regardless of the modification, an objective assessment of the results needs to be made in order to assure optimal surgical or percutaneous revascularization and quality of care.
References
- 1.Carrel A. On the experimental surgery of the thoracic aorta and the heart. Ann Surg. 1910;52:83. doi: 10.1097/00000658-191007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vineberg A. Restoration of coronary circulation by anastomosis. Can Med Assoc J. 1946;55:117–119. [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsner J, Moseley P, Mills N, Bower PJ. Long-term follow-up of internal mammary artery myocardial implantation. Ann Thorac Surg. 1977;23:118–121. doi: 10.1016/s0003-4975(10)64083-0. [DOI] [PubMed] [Google Scholar]
- 4.Longmire W, Cannon J, Kattus AA. Direct-vision coronary endarterectomy for angina pectoris. N Engl J Med. 1958;259:993–999. doi: 10.1056/NEJM195811202592101. [DOI] [PubMed] [Google Scholar]
- 5.Mueller RL, Rosengart TK, Isom OW. The history of surgery for ischemic heart disease. Ann Thorac Surg. 1997;63:869–878. doi: 10.1016/s0003-4975(96)01375-6. [DOI] [PubMed] [Google Scholar]
- 6.Kolesov V, Potashov L. Surgery of coronary arteries. Eksp Khir Anesteziol. 1965;10:3. [PubMed] [Google Scholar]
- 7.Garrett H, Dennis E, DeBakey M. Aortocoronary bypass with saphenous vein graft. Seven-year follow up. JAMA. 1973;223:792–794. [PubMed] [Google Scholar]
- 8.Favaloro RG. Saphenous vein graft in the surgical treatment of coronary artery disease: Operative technique. J Thorac Cardiovasc Surg. 1969;58:178. [PubMed] [Google Scholar]
- 9.Green GE, Spencer FC, Tice DA, Stertzer SH. Arterial and venous microsurgical bypass grafts for coronary artery disease. J Thorac Cardiovasc Surg. 1970;60:491–503. [PubMed] [Google Scholar]
- 10.Carpentier A, Guermonprez JL, Deloche A, Frechette C, DuBost C. The aorta-to-coronary radial artery bypass graft: A technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–121. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 11.Acar C, Jebara VA, Portoghese M, Beyssen B, Pagny JY, Grare P, et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54:652–659. doi: 10.1016/0003-4975(92)91007-v. discussion 659 – 660. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi J. Radial artery as a graft for coronary artery bypass grafting. Circ J. 2009;73:1178–1183. doi: 10.1253/circj.cj-09-0322. [DOI] [PubMed] [Google Scholar]
- 13.Coronary artery surgery study (CASS) A randomized trial of coronary artery bypass surgery: Survival data. Circulation. 1983;68:939–950. doi: 10.1161/01.cir.68.5.939. [DOI] [PubMed] [Google Scholar]
- 14.Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. N Engl J Med. 1984;311:1333–1339. doi: 10.1056/NEJM198411223112102. [DOI] [PubMed] [Google Scholar]
- 15.Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319:332–337. doi: 10.1056/NEJM198808113190603. [DOI] [PubMed] [Google Scholar]
- 16.Gibbon JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–185. [PubMed] [Google Scholar]
- 17.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: Mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 18.Laborde F, Abdelmeguid I, Piwnica A. Aortocoronary bypass without extracorporeal circulation: Why and when? Eur J Cardiothorac Surg. 1989;3:152–154. doi: 10.1016/1010-7940(89)90094-8. [DOI] [PubMed] [Google Scholar]
- 19.Arom KV, Flavin TF, Emery RW, Kshettry VR, Janey PA, Petersen RJ. Safety and efficacy of off-pump coronary artery bypass grafting. Ann Thorac Surg. 2000;69:704–710. doi: 10.1016/s0003-4975(99)01510-6. [DOI] [PubMed] [Google Scholar]
- 20.Cleveland JC, Shroyer AL, Chen AY, Peterson E, Grover FL. Off-pump coronary artery bypass grafting decreases risk-adjusted mortality and morbidity. Ann Thorac Surg. 2001;72:1282–1288. doi: 10.1016/s0003-4975(01)03006-5. [DOI] [PubMed] [Google Scholar]
- 21.Al-Ruzzeh S, George S, Bustami M, Wray J, Ilsley CD, Athansiou T, et al. Effect of off-pump coronary artery bypass surgery on clinical, angiographic, neurocognitive, and quality of life outcomes: Randomised controlled trial. BMJ. 2006;332:1365. doi: 10.1136/bmj.38852.479907.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361:1827–1837. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 23.Cartier R, Brann S, Dagenais F, Martineau R, Couturier A. Systematic off-pump coronary artery revascularization in multivessel disease: Experience of three hundred cases. J Thorac Cardiovasc Surg. 2000;119:221–229. doi: 10.1016/S0022-5223(00)70176-0. [DOI] [PubMed] [Google Scholar]
- 24.Magee MJ, Jablonski KA, Stamou SC, Pfister AJ, Dewey TM, Dullum MK, et al. Elimination of cardiopulmonary bypass improves early survival for multivessel coronary artery bypass patients. Ann Thorac Surg. 2002;73:1196–1202. doi: 10.1016/s0003-4975(01)03587-1. [DOI] [PubMed] [Google Scholar]
- 25.Khan NE, De Sousza A, Mister R, Flather M, Clague J, Davies S, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21–28. doi: 10.1056/NEJMoa031282. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez F, Cohn WE, Baribeau YR, Tryzelaar JF, Charlesworth DC, Clough RA, et al. In-hospital outcomes of off-pump versus on-pump coronary artery bypass procedures: A multicenter experience. Ann Thorac Surg. 2001;72:1526–1533. doi: 10.1016/s0003-4975(01)03202-7. [DOI] [PubMed] [Google Scholar]
- 27.Calafiore AM, Di Mauro M, Canosa C, Di Giammarco G, Iaco AL, Contini M. Early and late outcome of myocardial revascularization with and without cardiopulmonary bypass in high risk patients (EuroSCORE > or = 6) Eur J Cardiothorac Surg. 2003;23:360–367. doi: 10.1016/s1010-7940(02)00800-x. [DOI] [PubMed] [Google Scholar]
- 28.Puskas JD, Williams WH, Duke PG, Staples GR, Glas KE, Marshall JJ, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: A prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 29.Stamou SC, Corso PJ. Coronary revascularization without cardiopulmonary bypass in high-risk patients: A route to the future. Ann Thorac Surg. 2001;71:1056–1061. doi: 10.1016/s0003-4975(00)02325-0. [DOI] [PubMed] [Google Scholar]
- 30.Stamou SC, Dangas G, Dullum MK, Pfister AJ, Boyce SW, Bafi AS, et al. Beating heart surgery in octogenarians: Perioperative outcome and comparison with younger age groups. Ann Thorac Surg. 2000;69:1140–1145. doi: 10.1016/s0003-4975(99)01430-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Boyce SW, Hill PC, Sun X, Lee A, Haile E, et al. Off-pump coronary artery bypass grafting improves in-hospital mortality in patients with dialysis-dependent renal failure. Cardiovasc Revasc Med. 2009;10:12–16. doi: 10.1016/j.carrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Linde J, Moller C, Hughes P, Steinbruchei D. Off-pump versus on-pump CABG in high-risk patients: Short- and mid-term outcome. Scand Cardiovasc J. 2006;40:209–213. doi: 10.1080/14017430600669874. [DOI] [PubMed] [Google Scholar]
- 33.Tugtekin S, Kappert U, Alexiou K, Wilbring M, Nagpal AD, Matschke K. Coronary artery bypass grafting in octogenarians: Outcome with and without extracorporeal circulation. Thorac Cardiovasc Surg. 2007;55:407–411. doi: 10.1055/s-2007-965380. [DOI] [PubMed] [Google Scholar]
- 34.Youn YN, Chang BC, Hong YS, Kwak YL, Yoo KJ. Early and midterm impacts of cardiopulmonary bypass on coronary artery bypass grafting in patients with poor left ventricular dysfunction: A propensity score analysis. Circ J. 2007;71:1387–1394. doi: 10.1253/circj.71.1387. [DOI] [PubMed] [Google Scholar]
- 35.Fonger JD, Doty JR, Sussman MS, Salomon NW. Lateral MIDCAB grafting via limited posterior thoracotomy. Eur J Cardiothorac Surg. 1997;12:399–404. doi: 10.1016/s1010-7940(97)00177-2. [DOI] [PubMed] [Google Scholar]
- 36.Abraham R, Ricci M, Salerno T, Kerr P. A minimally invasive alternative approach for reoperative grafting of the right coronary artery. J Card Surg. 2002;17:289–291. doi: 10.1111/j.1540-8191.2001.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 37.Sellke FW, DiMaio JM, Caplan LR, Ferguson TB, Gardner TJ, Hiratzka LF, et al. Comparing on-pump and off-pump coronary artery bypass grafting: Numerous studies but few conclusions: A scientific statement from the American Heart Association council on cardiovascular surgery and anesthesia in collaboration with the interdisciplinary working group on quality of care and outcomes research. Circulation. 2005;111:2858–2864. doi: 10.1161/CIRCULATIONAHA.105.165030. [DOI] [PubMed] [Google Scholar]
- 38.Boodhwani M, Rubens FD, Wozny D, Rodriguez R, Alsefaou A, Hendry PJ, et al. Predictors of early neurocognitive deficits in low-risk patients undergoing on-pump coronary artery bypass surgery. Circulation. 2006;114(Suppl):I-461–I-466. doi: 10.1161/CIRCULATIONAHA.105.001354. [DOI] [PubMed] [Google Scholar]
- 39.Newman MF, Mathew JP, Grocott HP, Mackensen GB, Monk T, Welsh-Bohmer KA, et al. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. doi: 10.1016/S0140-6736(06)69254-4. [DOI] [PubMed] [Google Scholar]
- 40.Diegeler A, Hirsch R, Schneider F, Schilling LO, Falk V, Rauch T, et al. Neuromonitoring and neurocognitive outcome in off-pump versus conventional coronary bypass operation. Ann Thorac Surg. 2000;69:1162–1166. doi: 10.1016/s0003-4975(99)01574-x. [DOI] [PubMed] [Google Scholar]
- 41.Motallebzadeh R, Bland JM, Markus HS, Kaski JC, Jahangiri M. Neurocognitive function and cerebral emboli: Randomized study of on-pump versus off-pump coronary artery bypass surgery. Ann Thorac Surg. 2007;83:475–482. doi: 10.1016/j.athoracsur.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Stroobant N, van Nooten G, De Bacquer D, Van Belleghem Y, Vingerhoets G. Neuropsychological functioning 3–5 years after coronary artery bypass grafting: Does the pump make a difference? Eur J Cardiothorac Surg. 2008;34:396–401. doi: 10.1016/j.ejcts.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Van Dijk D, Jansen EW, Hijman R, Nierich AP, Diephuis JC, Moons KG, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: A randomized trial. JAMA. 2002;287:1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 44.Hammon JW, Stump DA, Butterworth JF, Moody DM, Rorie K, Deal DD, et al. Coronary artery bypass grafting with single cross-clamp results in fewer persistent neuropsychological deficits than multiple clamp or off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;84:1174–1178. doi: 10.1016/j.athoracsur.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi J, Tagusari O, Bando K, Niwaya K, Nakajima H, Ishida M, et al. Total arterial off-pump coronary revascularization with only internal thoracic artery and composite radial artery grafts. Heart Surg Forum. 2002;6:30–37. doi: 10.1532/hsf.969. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Chen X, Shi K, Xu M, Wang L, Jiang Y. Novel surgical method of proximal anastomosis in off-pump coronary artery bypass grafting. Circ J. 2009;73:1342–1343. doi: 10.1253/circj.cj-09-0055. [DOI] [PubMed] [Google Scholar]
- 47.Bonatti J, Coulson AS, Bakhshay SA, Posch L, Sloan TJ. The subclavian and axillary arteries as inflow vessels for coronary artery bypass grafts: Combined experience from three cardiac surgery centers. Heart Surg Forum. 2000;3:307–311. [PubMed] [Google Scholar]
- 48.Gu YJ, Mariani MA, Boonstra PW, Grandjean JG, van Oeveren W. Complement activation in coronary artery bypass grafting patients without cardiopulmonary bypass: The role of tissue injury by surgical incision. Chest. 1999;116:892–898. doi: 10.1378/chest.116.4.892. [DOI] [PubMed] [Google Scholar]
- 49.Benetti FJ, Ballester C, Sani G, Doonstra P, Grandjean J. Video assisted coronary bypass surgery. J Card Surg. 1995;10:620–625. doi: 10.1111/j.1540-8191.1995.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 50.Greenspun HG, Adourian UA, Fonger JD, Fan JS. Minimally invasive direct coronary artery bypass (MIDCAB): Surgical techniques and anesthetic considerations. J Cardiothorac Vasc Anesth. 1996;10:507–509. doi: 10.1016/s1053-0770(05)80013-8. [DOI] [PubMed] [Google Scholar]
- 51.Detter C, Reichenspurner H, Boehm DH, Thalhammer M, Raptis P, Schutz A, et al. Minimally invasive direct coronary artery bypass grafting (MIDCAB) and off-pump coronary artery bypass grafting (OPCAB): Two techniques for beating heart surgery. Heart Surg Forum. 2002;5:157–162. [PubMed] [Google Scholar]
- 52.Subramanian VA, Patel NU. Current status of MIDCAB procedure. Curr Opin Cardiol. 2001;16:268–270. doi: 10.1097/00001573-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 53.d’Amato TA, Savage EB, Wiechmann RJ, Sakert T, Benckart DH, Magovern JA. Reduced incidence of atrial fibrillation with minimally invasive direct coronary artery bypass. Ann Thorac Surg. 2000;70:2013–2016. doi: 10.1016/s0003-4975(00)02134-2. [DOI] [PubMed] [Google Scholar]
- 54.Cohn WE, Sirois CA, Johnson RG. Atrial fibrillation after minimally invasive coronary artery bypass grafting: A retrospective, matched study. J Thorac Cardiovasc Surg. 1999;117:298–301. doi: 10.1016/S0022-5223(99)70426-5. [DOI] [PubMed] [Google Scholar]
- 55.Hravnak M, Hoffman LA, Saul MI, Zullo TG, Cuneo JF, Whitman GR, et al. Atrial fibrillation: Prevalence after minimally invasive direct and standard coronary artery bypass. Ann Thorac Surg. 2001;71:1491–1495. doi: 10.1016/s0003-4975(01)02477-8. [DOI] [PubMed] [Google Scholar]
- 56.Holzhey DM, Jacobs S, Mochalski M, Walther T, Thiele H, Mohr FW, et al. Seven-year follow-up after minimally invasive direct coronary artery bypass: Experience with more than 1300 patients. Ann Thorac Surg. 2007;83:108–114. doi: 10.1016/j.athoracsur.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs S, Holzhey DM, Falk V, Garbade J, Walther T, Mohr FW. High-risk patients with multivessel disease: Is there a role for incomplete myocardial revascularization via minimally invasive direct coronary artery bypass grafting? Heart Surg Forum. 2007;10:E459–E462. doi: 10.1532/HSF98.20061193. [DOI] [PubMed] [Google Scholar]
- 58.Vassiliades TA, Reddy VS, Puskas JD, Guyton RA. Long-term results of the endoscopic atraumatic coronary artery bypass. Ann Thorac Surg. 2007;83:979–984. doi: 10.1016/j.athoracsur.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 59.West D, Flather M, Pepper J, Trimlett R, Yap J, De Souza A. Improved recovery after the endoscopic atraumatic coronary artery bypass procedure compared with sternotomy for off-pump bypass of the left internal thoracic artery to the left anterior descending coronary artery: A case-matched study. Heart Surg Forum. 2004;7:E546–E550. doi: 10.1532/HSF98.20041037. [DOI] [PubMed] [Google Scholar]
- 60.Sellke FW, Ruel M. Atlas of cardiac surgical techniques. 1st. Philadelphia, PA: Elsevier; 2010. [Google Scholar]
- 61.Kiaii B, McClure RS, Stewart P, Rayman R, Swinamer SA, Suematsu Y, et al. Simultaneous integrated coronary artery revascularization with long-term angiographic follow-up. J Thorac Cardiovasc Surg. 2008;136:702–708. doi: 10.1016/j.jtcvs.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava S, Gadasalli S, Agusala M, Kolluru R, Naidu J, Shroff M, et al. Use of bilateral internal thoracic arteries in CABG through lateral thoracotomy with robotic assistance in 150 patients. Ann Thorac Surg. 2006;81:800–806. doi: 10.1016/j.athoracsur.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 63.Bonatti J, Schachner T, Bonaros N, Ohlinger A, Rutzier E, Feuchtner G, et al. Robotic totally endoscopic double-vessel bypass grafting: A further step toward closed-chest surgical treatment of multivessel coronary artery disease. Heart Surg Forum. 2007;10:E239–E242. doi: 10.1532/HSF98.20070702. [DOI] [PubMed] [Google Scholar]
- 64.de Canniere D, Wimmer-Greinecker G, Cichon R, Gulielmos V, Van Praet F, Seshadri-Kreaden U, et al. Feasibility, safety, and efficacy of totally endoscopic coronary artery bypass grafting: Multicenter European experience. J Thorac Cardiovasc Surg. 2007;134:710–716. doi: 10.1016/j.jtcvs.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 65.Katz MR, van Praet F, De Canniere D, Murphy D, Siwek L, Seshadri-Kreaden U, et al. Integrated coronary revascularization: Percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation. 2006;114(Suppl):I-473–I-476. doi: 10.1161/CIRCULATIONAHA.105.001537. [DOI] [PubMed] [Google Scholar]
- 66.Groh MA, Sutherland SE, Burton HG, Johnson AM, Ely SW. Port-access coronary artery bypass grafting: Technique and comparative results. Ann Thorac Surg. 1999;68:1506–1508. doi: 10.1016/s0003-4975(99)00949-2. [DOI] [PubMed] [Google Scholar]
- 67.Grossi EA, Groh MA, Lefrak EA, Ribakove GH, Albus RA, Galloway AC, et al. Results of a prospective multicenter study on port-access coronary bypass grafting. Ann Thorac Surg. 1999;68:1475–1477. doi: 10.1016/s0003-4975(99)00959-5. [DOI] [PubMed] [Google Scholar]
- 68.McGinn JTJ, Usman S, Lapierre H, Pothula VR, Mesana TG, Ruel M. Minimally invasive coronary artery bypass grafting: Dual-center experience in 450 consecutive patients. Circulation. 2009;120(Suppl):S78–S84. doi: 10.1161/CIRCULATIONAHA.108.840041. [DOI] [PubMed] [Google Scholar]
- 69.Lytle BW, Blackstone EH, Loop FD, Houghtaling PL, Arnold JH, Akhrass R, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg. 1999;117:855–872. doi: 10.1016/S0022-5223(99)70365-X. [DOI] [PubMed] [Google Scholar]
- 70.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TBJ, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 71.Zilla P, Human P, Wolf M, Lichtenberg W, Rafiee N, Bezuidenhout D, et al. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. J Thorac Cardiovasc Surg. 2008;136:717–725. doi: 10.1016/j.jtcvs.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 72.Kulik A, Le May M, Wells GA, Mesana TG, Ruel M. The clopidogrel after surgery for coronary artery disease (CASCADE) randomized controlled trial: Clopidogrel and aspirin versus aspirin alone after coronary bypass surgery [ NCT00228423] Curr Control Trials Cardiovasc Med. 2005;6:15. doi: 10.1186/1468-6708-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]