Abstract
Parathyroid hormone (PTH)-related peptide (PTHrP) controls the pace of pre- and post-natal growth plate development by activating the PTH1R in chondrocytes, while PTH maintains mineral and skeletal homeostasis by modulating calciotropic activities in kidneys, gut, and bone. The extracellular calcium-sensing receptor (CaSR) is a member of family C G protein-coupled receptor, which regulates mineral and skeletal homeostasis by controlling PTH secretion in parathyroid glands and Ca2+ excretion in kidneys. Recent studies showed the expression of CaSR in chondrocytes, osteoblasts, and osteoclasts and confirmed its non-redundant roles in modulating the recruitment, proliferation, survival, and differentiation of the cells. This review emphasizes the actions of CaSR and PTH1R signaling responses in cartilage and bone and discusses how these two signaling cascades interact to control growth plate development and maintain skeletal metabolism in physiological and pathological conditions. Lastly, novel therapeutic regimens that exploit interrelationship between the CaSR and PTH1R are proposed to produce more robust osteoanabolism.
Keywords: PTH1R, PTH, calcium-sensing receptor, osteoporosis, rickets, growth plate
1. Introduction
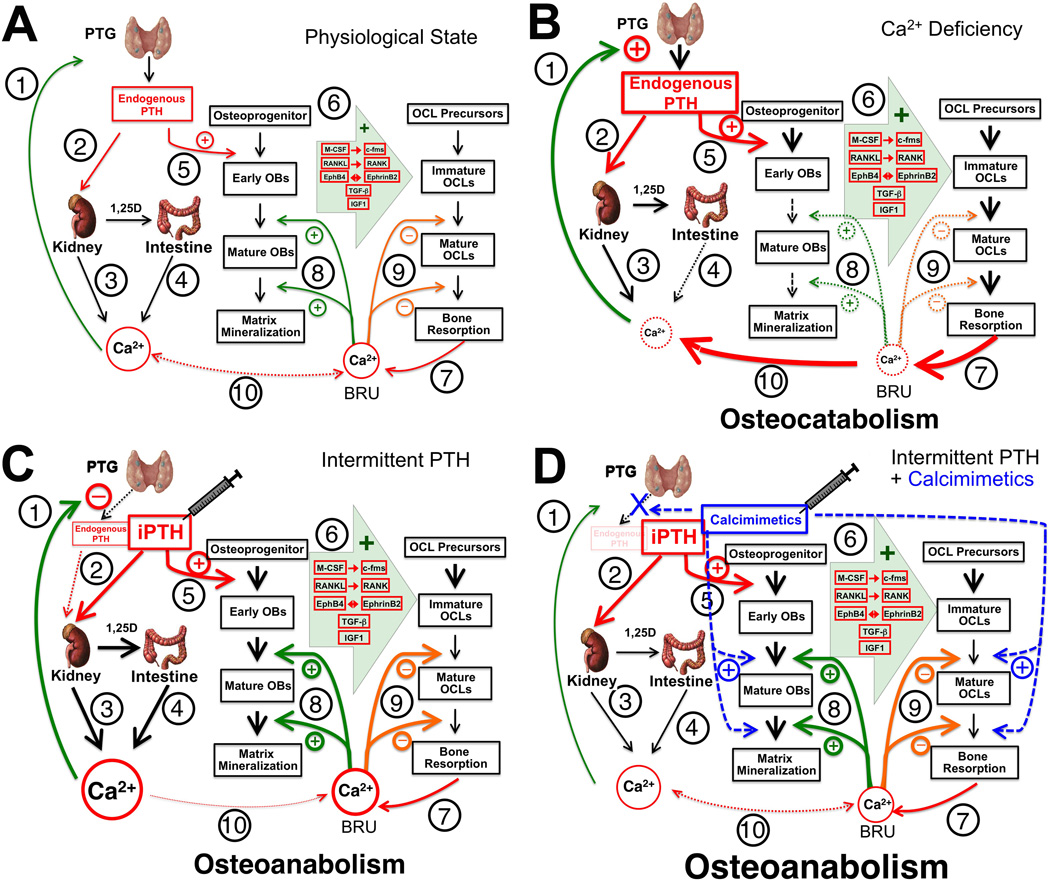
Maintaining normal Ca2+ homeostasis is essential for all cellular functions in our body. Land vertebrates develop large bony skeleton to store excess Ca2+ in the form of hydroxyapatite [Ca10(PO4)6(OH)2] and releases it to meet systemic demands at the time of Ca2+ deficiency. The parathyroid gland (PTG) also evolves to coordinate the calciotropic activities in the skeleton with those in the gut and kidney, by secreting the parathyroid hormone (PTH). The current working model for the regulation of serum Ca2+ concentration (sCa2+) emphasizes: (i) the ability of parathyroid cell (PTC) to respond to subtle changes in sCa2+ that promptly alter PTH secretion (Figure 1A, ➀); (ii) the ability of PTH to activate its receptor, PTH1R (➁), in the kidney to promote Ca2+ reabsorption (➂) and stimulate 1,25dihydroxyvitamin D (1,25D) production to increase intestinal Ca2+ absorption (➃); (iii) the ability of PTH to enhance bone turnover (➄-➈, see detailed descriptions in Section 8 Actions of PTH and PTH1R in bone) to release Ca2+ into the circulation (➉); and (iv) the negative feedback of increasing sCa2+ and serum 1,25D (s1,25D) to suppress PTH secretion by activating the extracellular Ca2+-sensing receptor (CaSR) and vitamin D receptor (VDR) (➀) to close this regulatory loop [1–3]. Defects at any point in this pathway disturb mineral balance and produce endocrine and skeletal dysfunction.
Figure 1.
Schemas for the actions of PTH/PTH1R and Ca2+/CaSR signaling in the regulation of mineral and skeletal metabolism under (A) a physiological state and (B) Ca2+ deficiency and its responses to (C) iPTH or (D) combined iPTH and calcimimetics treatment. See the text for detailed descriptions.
Skeletal development begins in the embryo and continues throughout adolescence until a peak bone mass is attained in early adulthood. The mature skeleton is then maintained by continuous bone turnover (or remodeling) through balanced bone-forming activities of osteoblasts (OBs) and bone-resorbing activities of osteoclasts (OCLs) (Figure 1A, ➅-➈) in the bone-remodeling units (BRUs). Excessive bone resorption due to aging, post-menopause, use of glucocorticoids, and metabolic diseases, like hyperparathyroidism (HPT), produces osteoporotic skeleton with increased risk of fracture [4–13].
Ca2+ availability critically impacts skeletal development and bone turnover [14]. Ca2+ deficiency produces rickets and osteomalacia, characterized by inadequate cartilage or bone matrix mineralization, in patients [15–18]. Supplementation of the diet with Ca2+ and vitamin D, and in some cases with Ca2+ alone, completely heals those cartilage and bone defects [14, 15, 19–21]. Similarly, rachitic changes in bone and cartilage in VDR knockout (KO) mice are prevented by a high Ca2+ diet [22–24]. The above observations underscore the importance of adequate Ca2+ supply to normal cartilage and bone development. Although Ca2+ could contribute passively to bone mineralization as an essential substrate, recent discoveries of CaSR in chondrocytes, OBs, and OCLs have prompted investigations for direct Ca2+ actions on those cells as a critical “growth factor”.
Inactivating mutations in the CASR gene reduce the responsiveness of PTC to changes in sCa2+ and produce familial hypocalciuric hypercalcemia (FHH) and neonatal severe HPT (NSHPT) in patients, who show elevated serum PTH (sPTH), s1,25D, and sCa2+ levels and in the severest forms a growth-retarded and under-mineralized skeleton [25–28]. Skeletal defects in NSHPT patients are likely caused by aberrant PTH secretion and the associated mineral and hormonal disturbances. Direct effects of mutant CaSR in chondrocyte and bone cell, however, cannot be ruled out [29–38].
Prolonged elevation of sPTH produces catabolic effects on bone [39–42], but once-daily (or intermittent) injections of supra-physiological doses of PTH1–34 or PTH1–84 increase trabecular bone mass in normal and osteoporotic animal models and in osteoporosis patients [43–47]. The anabolic effect of intermittent PTH (iPTH) appears to rely on its ability to promote bone-forming activities of OBs to a greater extent than the bone-resorbing activities of OCLs at the beginning of the treatment -- creating a so-called “anabolic window”, but its underlying cellular and molecular mechanisms remain unclear.
In the past decades, investigations using genetically manipulated mouse models and cell cultures have confirmed essential roles for the CaSR and PTH1R signaling in controlling pre-and post-natal skeletal development and maintenance of adult skeleton. However, perspectives on how these two signaling pathways interact in cartilage and bone are lacking. In light of two recent review articles that provide comprehensive updates on general systemic and local actions of the CaSR and PTH on bone and mineral metabolism [30, 48], this review emphasizes the interplay between the CaSR and PTH1R signaling in chondrocytes, OBs, and OCLs and proposes novel regimens exploiting this receptor interaction to enhance osteoanabolism for treatment of skeletal disease.
2. Endochondral bone formation
In vertebrates, all weight-bearing axial and appendicular skeletons are formed by endochondral bone formation that begins in early embryos. This process starts with the condensation of mesenchymal progenitors and their commitment to the chondrocytic lineage to form cartilaginous anlagen that later becomes a growth plate (GP). In the GP, chondrocytes proliferate, mature, and hypertrophy sequentially within single cell columns and then begin to deposit Ca2+/phosphate-containing minerals into the surrounding matrix after they reach terminal differentiation (Figure 2A). Within this mineralized matrix, the terminally differentiated chondrocytes produce matrix metalloproteinases to remodel surrounding matrix [49–51] and release growth factors to induce vascular invasion and promote OB differentiation at the chondro-osseous junction. It was originally proposed, mainly based on histological observations, that terminally differentiated hypertrophic chondrocytes in the GP undergo cell death and OBs arise from the osteoprogenitors delivered by the invading vasculature to replace the dying chondrocytes and produce new bone. This classic scheme of chondro-to-osteo transition has just undergone a significant paradigm shift [52–54]. By using protein-based fluorescent probes to label chondrocytes in vivo and by following the fate of the labeled cells in the bone using time-lapse cell/tissue imaging, it has been clearly shown that the majority of hypertrophic chondrocytes can trans-differentiate directly into OBs in the GP during endochondral bone formation or in the healing callus of fractured bone [53, 55–57]. GPs exist throughout adolescence to support longitudinal bone growth by repeating the above cell differentiation programs until the chondroprogenitor pool is exhausted at the time of GP closure in early adulthood. Aberrant acceleration or delay in chondrocyte differentiation produces disorganized GPs and impede bone growth [58].
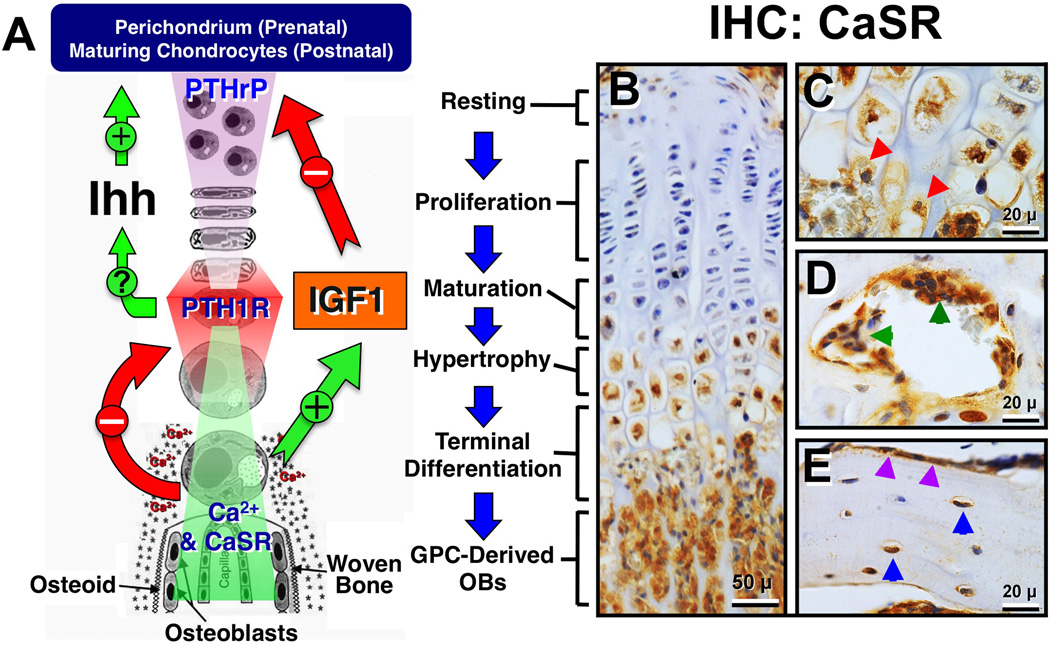
Figure 2.
(A) A schema for growth plate chondrocyte differentiation and its regulation by PTHrP/PTH1R/Ihh, IGF1/IGF1R, and Ca2+/CaSR signaling pathways. See the text for detailed descriptions. (B–E) Immunohistochemical (IHC) detection of CaSR protein in (B) mouse growth plate and primary spongiosa; (C) chondro-osseous junction; (D) resorbing pits in the secondary spongiosa, and (E) cortical bone of the tibia. Red, greed, purple, blue arrowheads depict terminally differentiated chondrocyte being released from cartilage matrix in (C), osteoclasts in (D), and bone-lining OBs and osteocytes in (E), respectively.
3. PTHrP and PTH1R in endochondral bone formation
Many transcription [58–69] and autocrine/paracrine factors [56, 70–83] were found to induce the commitment of progenitors to the chondrocytic lineage and to pace their differentiation [49, 56, 76, 84, 85]. Among them, the parathyroid hormone–related protein/Indian hedgehog (PTHrP/Ihh) feedback loop is the best-established pathway that prevents aberrant acceleration of chondrocyte differentiation and early closure of the GP [58, 86, 87]. According to the current model, PTHrP produced by perichondral cells in embryonic skeleton or by maturing/prehypertrophic chondrocytes in postnatal GPs diffuses into the proliferation zone where it activates the PTH1R and downstream signaling cascades to sustain the proliferative activities of the cells and delay their further differentiation [88] (Figure 2A). When chondrocytes eventually mature, they increase the production of Ihh to simulate its receptor Patched in the neighboring cells and increase PTHrP production via mechanisms that remain to be determined, thus constituting a feedback loop to slow down cell differentiation [87, 89] (Figure 2A). Pth1r, Pthrp, and Ihh gene KOs in mice all led to accelerated chondrocyte differentiation, early GP closure, and dwarfism [87]. Transgenic mice overexpressing PTHrP specifically in chondrocytes also presented short-limbed dwarfism, but their growth plates were composed exclusively of proliferating cells and lacked endochondral ossification [90], confirming the role of PTHrP/PTH1R signaling in preventing an early entry of proliferating chondrocyte into terminal differentiation.
Several elegant investigations explored signaling events underlying the actions of PTHrP on cartilage development with emphases on its ability to activate different heterotrimeric GTP-binding proteins (G-proteins) and multiple down-stream effectors in chondrocytes [86]. In fibroblastic COS-7 cells expressing exogenous PTH1Rs, binding of PTHrP to the receptor stimulated Gs-mediated cAMP synthesis as well as Gq-mediated intracellular Ca2+ releases, indicating the multifaceted actions of PTHrP/PTH1R signaling [91]. To determine the impact of Gs-mediated signaling responses on GP development, chimeric mice with GPs comprising mixed populations of normal and Gsα-deficient chondrocytes were studied [92]. In the chimeric GPs, Gsα-deficient chondrocytes appeared to stop proliferating and become hypertrophic prematurely [91] -- phenotypes similar to those of chondrocyte-specific PTH1R KO mice [93, 94]. It was, therefore, concluded that PTHrP activates Gs-mediated signaling responses to sustain chondrocyte proliferation [87].
In cultured chondrocytes, pharmacological stimulation of Gq-coupled protein kinase C (PKC) and mitogen-activated protein kinase kinase (MEK) pathway suppressed proliferation, enhanced cell hypertrophy, and increased expression of type X collagen [95, 96], supporting a role for Gq-mediated signaling in promoting chondrocyte terminal differentiation. But there has been no report on the study of mice with targeted ablation of Gαq specifically in chondrocytes to clearly define its impact on GP development in vivo. Instead, studies of mice with a knock-in of an engineered Pth1r mutant gene, which encodes a mutant PTH1R that retains the ability to activate Gs, but not Gq, signaling pathway, showed delayed GP ossification and increased chondrocyte proliferation [97]. Though, the effects were modest, likely due to the relatively restricted expression of PTH1R in the proliferation zone. Nevertheless, the investigators of the study concluded that the PTH1R-mediatd Gs and Gq signaling cascades constitute a “Yin-Yang” relationship to control the pace of cell differentiation [86, 87]. However, the fact that chondrocyte-specific PTH1R KO mice presented profoundly accelerated chondrocyte differentiation and early GP closure indicates the existence of other, perhaps Gq-coupled, mediators that can promote the terminal differentiation of chondrocyte and engage in a “tug-of-war” relationship with the PTHrP/PTH1R/Ihh feedback loop to control the pace of GP development. Recent studies suggest that Ca2+ and its receptor, CaSR, constitute a critical signaling pathway that instigates such “pro-differentiation” activities in chondrocytes.
4. Signaling transduction of the CaSR
The CaSR is a member of family C G-protein coupled receptor (GPCR), which consists of a large extracellular domain (ECD; ≈450–600 amino acids) for ligand binding, a seven-transmembrane domain (7-TMD) for G protein coupling, and a long intracellular C-terminal tail (≈250 amino acids) for recruitment of signaling molecules and for receptor binding to cytoskeletons [98, 99]. Members of family C GPCRs function exclusively in the form of multimeric complex [100, 101]. The CaSR can form homodimers [100, 101] or heterodimerize with other members of family C GPCRs, including metabotropic glutamate receptors [102] and type B gamma-aminobutyric acid receptors (GABABR1 and GABABR2) [103, 104]. Like other GPCRs, the CaSR activates multiple downstream signaling cascades by coupling to 3 major groups of G proteins, Gq/11, Gi/o and G12/13 [99]. Through coupling to the Gq/11, the CaSR activated different subtypes (β, γ, δ, ε, ζ, η) of phospholipase C (PLC) to cleave the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG), which activates protein kinase C, and inositol 1,4,5-trisphosphate (IP3), which releases Ca2+ from intracellular stores by binding to IP3 receptors in the stores [99]. This signaling cascade has been demonstrated in most of the cell systems tested, including parathyroid cells [105, 106], keratinocytes [107], chondrocytes, osteoblasts [29], and transformed cells expressing the CaSR exogenously [108], to regulate diverse cell functions ranging from PTH secretion [109], osteoblast migration [35], and cell growth, survival, and differentiation [33]. By coupling to the pertussis toxin-sensitive Gi/o, the CaSR suppressed adenylyl cyclase activities and cAMP production in PTCs [110] and OBs [29]. Activation of Gi/o also activated the extracellular-signal-regulated kinases (ERK1/2) in PTCs [111, 112], OBs [113, 114], and HEK-293 cells expressing exogenous CaSRs [115–117]. Through the activation of G12/13, the CaSR enhanced Wnt3a-βcatenin signaling to promote osteoblast differentiation [118], but inhibited osteoclastogenesis by suppressing the expression of the receptor activator of nuclear factor kappa-B ligand (RANKL) and increasing osteoprotegerin (OPG) expression [119]. The CaSR could also activate phospholipase D though coupling to G12/13 in Madin-Darby canine kidney cells [120].
5. CaSR in chondrocyte differentiation and cartilage development
Ca2+ deficiency produced rickets in childhood [18] and in VDR and Cyp27b1 KO mice [24, 121] by delaying chondrocyte differentiation and blocking matrix mineralization in their GPs. The ability of dietary Ca2+ supplements to reverse the GP defects [21] signifies the importance of Ca2+ availability to GP development. The expression of CaSR first appears in maturing chondrocytes in the GP and increases in hypertrophic chondrocytes (Figure 2B) [29] including those being released from the cartilage matrix at the chondro-osseous junction (Figure 2C, red arrowheads) and adjacent OBs [29]. This expression pattern supports a role for the CaSR in mediating the terminal differentiation of hypertrophic chondrocytes and their transformation into osteoblastic lineage, according to the newly established paradigm [52–54].
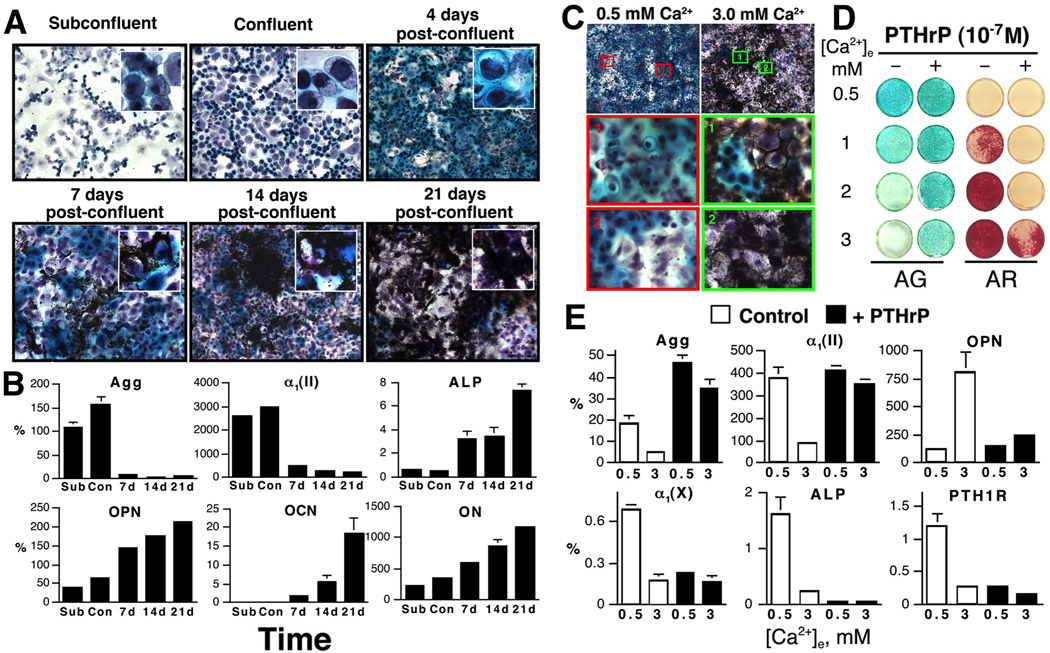
Direct actions of Ca2+ and CaSR on chondrocyte differentiation have been confirmed by studies of primary cells cultured from cartilage of different species, chondrogenic cell lines, and metatarsal bone rudiments explants. Chondrocytes in a high-density culture exhibited spontaneous differentiation that recapitulates keep steps of chondrogenesis as seen in vivo (Figure 3A) [29, 103, 122–124]. For example, mouse GP chondrocytes proliferated robustly and produced a proteoglycans (PG)-rich matrix immediately after plating (Figure 3A). Mineral deposition appeared to start in the matrix surrounding the hypertrophic chondrocytes (Figure 3A, 7-day post-confluence, insert). As mineral deposition increased in the cultures, PG accumulation declined and the cells lost their chondrocytic morphology (Figure 3A, 21-day post-confluence, insert). Along with those morphological changes, RNA levels for early differentiation markers -- aggrecan (Agg) and type II collagen α1 subunit [α1(II)] -- were highest in early cultures and decreased in later cultures (by >90%), while the expression of late differentiation markers -- alkaline phosphatase (ALP) and type X collagen α1 subunit [α1(X)], and putative OB markers --osteopontin (OPN), osteocalcin (OCN) and osteonectin (ON) increased with time of culture (Figure 3B). The above changes in cell morphology, gene expression, and matrix protein synthesis recapitulate steps of chondrogenesis and indicate time-dependent transformation of the cultured chondrocytes into the osteoblastic lineage.
Figure 3.
(A) PG accumulation and mineral deposition by Alcian green and von Kossa staining, respectively, in mouse GPCs cultured for different times. Cultures were counterstained with hematoxylin. Insets: high-power (100×) views. (B) RNA levels, assessed by q-PCR, for Agg, and a1(II), ALP, OPN, OCN, and ON, in mouse GPCs cultured for various times [subconfluent (Sub), confluent (Con), 7, 14, and 21 days post-confluence]. The level of gene is expressed as “%” of L19 expression. (C) Effects of different [Ca2+]e on proteoglycans (PG) accumulation, mineralization in mouse GPCs cultured at 0.5 or 3.0 mM Ca2+ for 14 days after confluence and viewed at 20× and 100× in upper and 2 lower panels, respectively. (D) PG accumulation and mineral accumulation assessed by Alcian green and Alizarin red staining, respectively, and (E) RNA expression assessed by qPCR in mouse GPCs cultured at different [Ca2+]e (0.5 to 3.0 mM) in the absence (−) or presence (+) of 10−7 M PTHrP for 14 days. RNA levels are presented as the percentage of ribosomal L19 gene expression.
Changes in [Ca2+]e profoundly impacted the differentiation of cultured chondrocytes. In tibio-tarsal chondrocytes cultured from chicken embryos, high [Ca2+]e increased the expression of α1(X) [125]. In mouse GP chondrocyte cultures, raising [Ca2+]e dose-dependently suppressed PG accumulation, increased mineral accumulation (Figure 3C and 3D -PTHrP), reduced expression of chondrocyte markers [Agg, α1(II), and α1(X)], and increased expression of OPN (Figure 3E, Control) [29, 103, 122, 123]. Similar effects of high [Ca2+]e were seen in cultures of non-transformed chondrogenic RCJ3.1C5.18 (or C5.18) cells, cloned from fetal rat calvarias [29, 124, 126]. The ability of high [Ca2+]e to increase intracellular Ca2+ mobilization and promote terminal differentiation in C5.18 cells could be blocked by overexpression of a dominant-negative CaSR or anti-sense RNA in the cells [29, 124, 126]. In cultures of fetal rat metatarsal bone explants, administration of CaSR agonist (or calcimimetics) increased their longitudinal growth by enhancing chondrocyte differentiation in the GP [127]. The above studies confirm the actions of Ca2+ and CaSR in promoting chondrocyte differentiation and mineralizing functions, and perhaps to speed up their transformation to acquire osteogenic phenotypes.
A global CaSR KO (Exon5CaSR−/−) mouse model was generated by inserting a neomycin gene cassette into the exon 5 of the gene, which encodes 77 amino acids in the ECD of the receptor. The Exon5CaSR−/− mice manifested severe phenotypes of human disorder NSHPT -- HPT, hypercalcemia, hypocalciuria, hypophosphatemia, parathyroid hyperplasia, and failure to thrive and the mice died before 3–4 weeks of age. Analyses of their bones reveled severe rickets with delayed formation of secondary ossification center, expanded and disorganized growth plate, and impaired bone formation [36, 128]. Interestingly, the growth and skeletal defects and early death of the Exon5CaSR −/− mice could be rescued by preventing the development of HPT after breeding the mice with PTH−/− mice lacking PTH gene or Gcm2−/− mice lacking the development of PTG [129, 130]. The reversal of skeletal defects in the Exon5CaSR −/−;PTH−/−and Exon5CaSR −/−;Gcm2−/− double KO mice led to the conclusion that the CaSR is not essential for skeletal development [129, 130] and prompted searches for other Ca2+-sensing mechanism(s) [131, 132]. However, it was not realized at the time that the exon 5 gene-targeting strategy allowed an in-frame gene-splicing event to exclude the exon 5 along with the inserted neomycin cassette from the full-length transcript, producing a truncated CaSR lacking 77 amino acids in its ECD. This truncated receptor was expressed in the skin, growth plate, and bone of the Exon5CaSR −/− mice [122, 133] and is sufficient to render Ca2+-resposiveness in GPCs cultured from the mice [122].
To clearly define the role of CaSR in skeletal development, a floxed-CaSR mouse model was generated by flanking the exon 7 of the Casr gene with two loxP sites. The exon 7 encodes the entire 7-TM domain and the C-terminal tail, which are absolutely required for the coupling of the receptor to downstream signaling cascades [33]. The utility of this floxed-CaSR model was validated by the generation of PTC-specific CaSR KO mice (PTCCaSRΔflox/Δflox) through breeding the floxed-CaSR mice with PTH-Cre mice expressing Cre-recombinase under the control of PTH promoter [33]. Analyses of genomic DNA, RNA, and protein extracted from different tissues of the PTCCaSRΔflox/Δflox mice showed completely deletion of the exon 7 of the gene in PTGs, but not in other vital organs [33]. PTCCaSRΔflox/Δflox mice presented severe HPT hypercalcemia, skeletal and growth phenotypes, and early death as seen in the Exon5CaSR −/− mice, except that the PTCCaSRΔflox/Δflox mice developed hypercalciuria, but not hypocalciuria, due to the preservation of normal renal CaSR functions, which enhances Ca2+ excretion in response to hypercalcemia [33].
To determine the role of CaSR in GP development, the floxed-CaSR mice were bred with Col(II)-Cre mice, which express Cre recombinase under the control of α1(II) gene promoter [134]. Unexpectedly, the resulting CartCaSRΔflox/Δflox embryos died before embryonic day 13 (E13), with severely under-mineralized skeleton [33]. The cause for the early death of CartCaSRΔflox/Δflox embryos remains unclear [33]. As chondrogenesis also takes place during the development of heart valve [135–138], defective cardiac functions could have caused the death of CartCaSRΔflox/Δflox embryos. These observations also support the ability of exon5-less CaSR to sustain the development of Exon5CaSR −/− embryos. An additional mouse model was made to study the impact of CaSR function at later stages of GP development by breeding floxed-CaSR mice with Tam-Col(II)-Cre mice, which express tamoxifen-inducible Cre recombinase under the control of α1(II) gene promoter [139] to achieve time-dependent chondrocyte-specific CaSR gene ablation. The resulting Tam-CartCaSRflox/flox mice developed normally until adulthood in the absence of tamoxifen. Induction of CaSR KO in E18–19 embryos by a single maternal injection of tamoxifen profoundly ablated CaSR expression in the GPs of the newborn Tam-CartCaSRΔflox/Δflox mice, which presented short stature with expanded and under-mineralized GPs and delayed chondrocyte terminal differentiation as seen in rickets [33]. These in vivo studies confirm a non-redundant role for the CaSR in mediating chondrocyte differentiation and GP development.
6. Interplay between Ca2+/CaSR and PTHrP/PTH1R signaling in chondrocytes
Studies of cultured GP chondrocytes revealed a close interaction between PTHrP/PTH1R and Ca2+/CaSR signaling pathways in controlling the pace of chondrocyte differentiation. Raising [Ca2+]e profoundly inhibited PTH1R and PTHrP expression in cultures of mouse GP chondrocytes (Figure 3E, Control and unpublished observations) [123]. Conversely, treating those cultures with PTHrP(1–34) significantly blunted the ability of high [Ca2+]e to suppress PG accumulation and promote mineral deposition (Figure 3D; -PTHrP vs +PTHrP). In cells maintained at 0.5 mM Ca2+, treatment with PTHrP significantly increased Agg expression and markedly reduced the expression of α1(X) and ALP -- markers of maturing and hypertrophic chondrocytes (Figure 3E). Incubation with PTHrP also blocked the ability of high [Ca2+]e to inhibit Agg and α1(II) expression and to increase OPN RNA levels (Figure 3E), suggesting that increased PTHrP/PTH1R signaling can counteract the effects of high [Ca2+]e on cell differentiation.
In the GPs of Tam-CartCaSRΔflox/Δflox mice, the expression of IGF1 and IGF1R was profoundly reduced [33], suggesting that Ca2+/CaSR could promote chondrocyte differentiation at least in part by enhancing IGF1 signaling (Figure 2A). This scenario is supported by the ability of Igf1r gene knockdown to suppress the ability of high [Ca2+]e to promote terminal differentiation and matrix mineralization in cultured chondrocytes [33]. Furthermore, ablating the Igf1r gene specifically in GP chondrocytes in mice increased their expression of PTHrP, but not PTH1R [140], indicating a negative regulation of PTHrP expression by IGF1R signaling. These observations support a paradigm in which Ca2+/CaSR signaling counteracts PTHrP/PTH1R signaling by suppressing PTH1R expression independently of IGF1/IGF1R signaling and by inhibiting PTHrP expression via the IGF1R-dependent pathway to support normal progression of chondrocyte differentiation and growth plate development (Figure 2A).
7. Bone modeling and remodeling
At the end of chondrogenesis in the GP, vascular invasion recruits OCL precursors to the chondro-osseous junction where they differentiate and resorb mineralized cartilage matrix to facilitate the release of GP-derived OB precursors [53, 55–57]. The vasculature may also provide a migratory pathway for osterix-expressing osteoprogenitors from the periosteum to future bone sites [141, 142]. The relative contributions of various sources of OB precursors to overall bone development remain unclear.
In the primary spongiosa beneath the GP, osteoprogenitors progress though the stages of pre-OBs, committed OBs, mature OBs, and osteocytes, which are characterized by the expression of specific marker proteins, osterix (Osx), type I collagen [Col(I)], OCN, and dentin matrix protein 1 (DMP1), respectively. The immature OBs produce a large quantity of Col(I), which constitutes the majority of protein matrix (or osteoid), while mature OBs exert mineralizing functions to deposit Ca2+ and phosphate into the protein matrix to increase its mechanical strength. At the end of bone-forming activity, OBs, which are embedded in the mineralized matrix, become osteocytes, while others turn into inactive flattened bone-lining OBs. Upon stimulation by calcemic factors, like PTH, bone-lining OBs are reactivated and OCLs are recruited to the BRUs, which serve to liberate matrix Ca2+ to meet systemic demands of Ca2+ and repair micro damages of the bone.
8. Actions of PTH and PTH1R in bone
Comparison of the skeletal phenotypes in PTH−/− mice and PTH−/−;PTHrP−/− double KO mice indicated PTH-dependent bone-forming activities in the primary spongiosa of long bone [143]. Bone cell-specific PTH1R KO mouse models are, however, required to further define cell-autonomous actions of the receptor. Thus far, there is no report on study of mice with PTH1R KO at early stages of osteoblast differentiation. Mice with osteocyte-specific PTH1R KO showed increases in bone mineral density and trabecular and cortical bone volume and thickness, along with a low bone turnover state due to suppressed OB and OCL activities [144]. Interestingly, mice with osteocyte-specific overexpression of a constitutively active PTH1R also showed increased trabecular and cortical bone mass, but in the state of high bone turnover [145, 146]. These studies support a role for the osteocytic PTH1R in controlling bone turnover, but other factors are involved in balancing bone forming and resorbing activities and determining overall bone accrual.
Direct actions of PTH on OBs were deduced from in vitro studies using cultures of bone marrow-derived osteoprogenitors, osteoblasts/osteocytes released from bone fragments, and osteogenic cell lines, and in vivo studies of mice injected with PTH [44–47]. Those studies together support the scheme that PTH increases osteoblastic activities by recruiting osteoprogenitors and sustaining the proliferation and survival of the committed OBs (Figure 1A, ➄). Although, iPTH could increase bone accrual and more importantly bone mineralization in vivo [44–47], PTH actually inhibits terminal differentiation of primary OBs or OB-like cell lines and their mineralizing functions in culture [147–151]. This paradox between in vivo and in vitro observations suggests that iPTH in vivo must produce other changes in bone microenvironment that are needed to promote terminal differentiation of the newly recruited OBs by iPTH. This effect is not recapitulated in OB cultures, likely due to the absence of OCL activity.
In vivo, PTH enhances osteoclastic activities by increasing osteoblastic expression of RANKL, macrophage colony stimulating factor (M-CSF), and other cytokines to recruit osteoclast precursors and promote their growth and differentiation through activation of RANK, c-fms (M-CSF receptor), and other signaling pathways in the cells (Figure 1A, ➅) [152–155]. The above PTH actions on OBs are mediated by cell-autonomous responses as well as by locally produced growth factors and cytokines, including IGF1 [155–157], fibroblast growth factor-2 (FGF-2) [158, 159], Wnt signaling-related agonists and antagonists [160–163], periostin [164], and sympathetic tone [165]. These sequential effects of PTH on OBs and then OCLs provide not only a cellular basis for iPTH to increase bone turnover rate, but also a time window to produce anabolic effects before its catabolic effects catch up [44–47].
The importance of OCL activity in producing osteoanabolism is shown by studies of antiresorptives, such as bisphosphonates and the humanized monoclonal antibody denosumab, which binds to and neutralizes the activity of RANKL and therefore suppresses osteoclastogenesis and bone resorption [166–170]. Antiresorptive agents not only block bone resorption, but also impede bone-forming activities in the BRUs. The exact mechanisms for coupling bone resorption to formation remain unclear. It is proposed that OCLs interact with OBs directly through binding of their cell-surface receptors (e.g., the RANK/RANKL and Ephrin/Eph systems) [152–155] to promote mutual cell differentiation and functions. Alternatively, OCLs actively release growth factors (e.g., IGF1 and TGFβ) [171, 172] and other constituents (e.g., Ca2+) from the matrix that may serve as anabolic signals to recruit osteoprogenitors and promote their differentiation. It has been shown that local [Ca2+] can rise to >40 mM at sites of active resorption [173] and that Ca2+ can function as a strong anabolic signal for OB recruitment, growth, survival, and differentiation [29, 31, 33, 174–178]. Based on this coupling mechanism, inhibition of bone resorption by antiresorptives is expected to limit local Ca2+ availability and thereby slow down OB differentiation and bone formation. On the other hand, increasing bone turnover by iPTH is anticipated to increase local [Ca2+] bathing the OBs and promote their differentiation (Figure 1A, ➇).
9. Actions of Ca2+ and CaSR in bone
9.1 Osteoblastogenesis
Studies of primary OBs and osteocytes and osteoblastic cell lines in culture demonstrated the ability of extracellular Ca2+ to stimulate acute signaling responses and enhance the migration, proliferation, survival, expression of terminal differentiation markers, and mineralizing functions of the cells by activating the CaSR [29, 32, 33, 35, 37, 174, 178–181]. In bone, the CaSR was found in active OBs, inactive bone-lining cells, and osteocytes ([29] and Figure 2B, 2E). As seen in cultured chondrocytes, CaSR activation with specific agonists stimulated Gq-mediated PLC activity, increased production of IP3, elevated [Ca2+]i, and opened Ca2+-dependent K+ channels to promote chemotaxis and cell proliferation in cultures of osteoblastic MC3T3-E1 [35, 182]. In calvarial OBs, CaSR activation (i) stimulated ERK 1/2 and downstream Akt and glycogen synthase kinase 3β (GSK3β) signaling cascades to promote cell growth, survival, and matrix mineralization [178, 180] and (ii) activated PLC and store-operated Ca2+ entry to increase [Ca2+]i to support cell proliferation [183]. The above observations are just few of many studies demonstrating the multifaceted actions of the CaSR in mediating OB proliferation, survival, and terminal differentiation.
While the above in vitro studies support a role for the CaSR in OB differentiation, its role in vivo had been controversial. This was due to the ability of concurrent Pth or Gcm2 gene KO to rescue the skeletal defects in the global Exon5CaSR−/− mice [184]. It was concluded at the time that the development of HPT due to defective CaSRs in PTCs was the main cause for skeletal defects observed in the Exon5CaSR−/− mice [129, 130]. Again, follow-up studies revealed the expression of the truncated exon 5-less CaSR in the cartilage and bone of the Exon5CaSR−/− mice. This truncated CaSR appeared to be sufficient to support overall skeletal development. The latter notion was further supported by studies of mice with OB-specific ablation of the exon 7 of the Casr gene KO [185].
Deletion of the exon 7 of Casr at the early stage of OB differentiation in vivo by crossing the floxed-CaSR mice with mice expressing Cre under the control of two different versions (2.3 and 3.6 kb) of the Col(I)-α1 gene promoter produced the OBCaSRΔflox/Δflox mice [185], which died before 3–4 weeks of age with severely blunted growth. µCT images reveled their severely under-mineralized skeletons with multiple unhealed bone fractures [33, 175]. Histomorphometric analyses of the CaSR-deficient bones showed reduced bone formation rates and bone volume and large quantifies of unmineralized osteoid deposited in both trabecular and cortical bone. Gene expression profiling showed profoundly reduced expression of OB differentiation markers, but increased expression of IL-10 gene -- an inducer of cell apoptosis. The up-regulation of the latter gene was consistent with an increased number of apoptotic OBs and osteocytes in the bones of the KO mice [33, 175]. The above skeletal defects were presented in the presence of lower serum PTH levels, further supporting cell-autonomous effects of the gene KO. These data together confirm an essential role for the CaSR in mediating OB proliferation, survival, and mineralizing functions.
Mice with transgenic overexpression of a constitutively active CaSR mutant cDNA under the control of a 3.5-kb OCN gene promoter were also made to examine the impact of the CaSR in mature OBs [176, 177]. The transgenic mice displayed mild osteopenia due to increased number and activity of osteoclast as a result of increased RANKL expression, supporting a role for the CaSR in mediating the coupling between osteoblastic and osteoclastic activities.
9.2 Osteoclastogenesis
OCLs responded to changes in [Ca2+]e in culture [186–189]. CaSR expression has been detected in monocytes and macrophages freshly isolated from human bone marrow [190], and in osteoclasts cultured from bone marrow and spleen [37, 38]. In situ hybridization and immunohistochemistry (Figure 2C, green arrowheads) confirmed the expression of CaSR mRNA and protein, respectively, in bone marrow cells and osteoclasts in resorbing pits [29]. High [Ca2+]e and/or CaSR agonists stimulated PLC, elevated [Ca2+]i [187–189, 191], and enhanced the translocation of nuclear factor NF-κB [189] in cultured OCLs. Some of those signaling responses were blunted in OCLs cultured from Exon5CaSR−/− mice [189]. High [Ca2+]e also inhibited the differentiation [37], secretion of acid phosphatase [188], and bone-resorbing functions in cultured OCLs [38] and increased apoptosis of mature OCLs [189]. The above in vitro data support the scheme that CaSR activation inhibits bone-resorbing activities by suppressing differentiation and secretory function of OCL and promoting cell apoptosis. Interestingly, allosteric activator of the CaSR (e.g., cinacalcet HCl) at the concentration, which suppresses PTH section in PTCs, had no effect on resorbing functions of human OCLs in culture [192]. Similarly, the inhibitory effect of the allosteric inhibitor of the CaSR, NPS 2143, on resorbing functions of cultured human OCLs could be seen only at concentrations that are 250 fold higher than those required to block the CaSR in PTCs [193]. These data indicate different pharmacological profiles of the CaSR in OCLs vs PTCs. As decreasing pH renders the CaSR a right-shifted Ca2+ set-point [194], so the CaSR in OCLs are predicted to be less responsive to its ligand (i.e., Ca2+) and perhaps its modulators (calcimimetics and calcilytics) in actively resorbing pits. By using a combination H+ and Ca2+ double-barreled electrode, Silver et al. showed that the pH reached a lower limit of 4.7 and the [Ca2+]e rose to a maximum of 40 mM in the erosion sites of the bone. It is plausible that the OCL CaSRs could be operational under such high [Ca2+]e environments despite the low pH. Future in vitro studies of OCL cultures with better controls of [Ca2+]e and pH that mimic in vivo conditions and in vivo studies of OCL-specific CaSR KO mice are required to clearly define the CaSR actions in OCLs.
10. Interplay between Ca2+/CaSR and PTHrP/PTH1R signaling in bone
Based on the studies reviewed above, we propose the following models for the regulation of mineral and bone metabolism by the interactions between Ca2+/CaSR and PTHrP/PTH1R signaling. Under a physiological state (Figure 1A), a normal sCa2+ level (➀) maintains a steady supply of PTH from PTCs (➁) to support basal Ca2+ reabsorption in the kidney (➂) and Ca2+ absorption in the gut (via stimulation of renal 1,25D production) (➃), together maintaining Ca2+ homeostasis at the level that meets the systemic demand. This level of PTH also supports steady bone forming activities by recruiting OB progenitors, activating bone-lining OBs, and sustaining their survival to maintain an adequate number of bone-forming cells (Figure 1A, ➄) [44, 157, 158, 195–200]. Through production of growth factors and/or direct physical interactions, the OBs aid in the recruitment of osteoclast precursors and their survival and differentiation (➅) in the BRUs. The resulting bone resorbing activities liberate matrix Ca2+ into fluid bathing OBs and OCLs (➆). Under conditions of Ca2+ sufficiency, low systemic demand of Ca2+ (➉) allows retention of the liberated Ca2+ to increase local [Ca2+] that stimulates OB maturation and their mineralizing functions to redeposit the Ca2+ into newly formed matrices (➇). The increasing [Ca2+]e also feeds back to OCLs to prevent their further expansion and aberrant bone resorption (➈). These balanced bone-forming and bone-resorbing activities sustain a steady bone turnover rate to continuously remodel the skeleton without bone loss.
In conditions of chronic Ca2+ deficiency (Figure 1B), e.g., insufficient Ca2+ and/or vitamin D intakes, reduced sCa2+ levels increase PTH secretion (➀) in PTGs to enhance (➁) renal Ca2+ reabsorption (➂) and 1,25D production to increase intestinal Ca2+ absorption in an attempt to restore sCa2+ levels to normal. But inadequate intestinal Ca2+ intakes (➃) prevent such normalization and lead to chronic hypocalcemia and sustained elevation of sPTH, which drastically increases bone turnover rates (➄-➆). As a result, excessive bone resorption releases a large amount of Ca2+ (➆), which is shunted into the circulation (➉) to further meet the systemic demands of Ca2+. Consequently, the decreasing [Ca2+]e in the BRUs retards the maturation and differentiation of the OBs as well as their mineralizing functions (➇), leading to accumulation of unmineralized osteoid and osteomalacia. Inability of low [Ca2+]e to check on the osteoclastogenesis (➈) further increases bone resorption and exacerbates the catabolic effects of chronic HPT.
11. Skeletal anabolism by targeting the PTH1R and CaSR in bone
Osteoporosis is a growing epidemic that afflicts aging men and women across the world [201]. iPTH is the only FDA-approved therapy that produces skeletal anabolism [43–47], but its dosing is limited to the lowest level that produces anabolic effects with an acceptable rate of hypercalcemia as an adverse effect[43, 45]. A better understanding of the mechanism underlying the anabolic effects of iPTH is needed to improve the therapy. Based on the current data, we propose that daily injections of supra-physiological doses of PTH1–34 (Figure 1C, ➁) in addition to the endogenous PTH1–84 transiently enhance calcemic activities in the kidney (➂) and the gut (➃) to a degree that can cause hypercalcemia and perhaps shunting of Ca2+ into the bone (➉). As a result, local [Ca2+]e is elevated in the BRUs and promotes the differentiation and mineralizing functions (➇) of the OBs recruited by the injected PTH (➄). Although the increased OB activity is also anticipated to promote osteoclastogenesis (➅) in the BRUs, this effect could be transient due to a short half-life of PTH (in minutes) and a negative feedback of the increasing [Ca2+] (➈) at least before the Ca2+ is redeposit into the matrix. The above events together give an anabolic window for a bone gain. We hypothesize that continuous infusion of PTH eventually allows osteoclastic activities to surpass the osteoblastic activities due to the enhancement of RANKL/RANK signaling, therefore producing catabolic effects on bone. According to this scheme, the efficacy of iPTH treatment will highly depend on the availability of Ca2+ and calcemic functions in the kidney and the intestine. This may explain for the considerably variable efficacies of the treatment in patients. This regulatory scheme also critically relies on a functional CaSR in OBs. Indeed, a blunted anabolic effect of iPTH was recently observed in the OBCaSRΔflox/Δflox mice [202]. The latter study also raises the possibility of targeting the CaSRs in OBs and OCLs to enhance skeletal anabolism.
We have proposed to co-inject calcimimetics to enhance the anabolic effects of iPTH. Calcimimetics are non-ionic allosteric CaSR agonists that are being used clinically to treat HPT and hypercalcemia by potentiating extracellular Ca2+-induced inhibition of PTH secretion and thereby suppressing the calciotropic actions in the kidney, intestine, and bone [203–206]. We theorize that transient activation of CaSR in PTCs with calcimimetics will dampen the secretion of endogenous PTH1–84, and therefore reduce its calciotropic activities in the kidney (➂) and gut (➃), therefore alleviating some of the adverse hypercalcemic effects in patients also receiving iPTH (Figure 1D). According to our working model, the injected PTH will continue to promote OB (➄) and then OCL activities (➅) in the BRUs. Although a smaller increase in sCa2+ level is anticipated to give a less increase in local [Ca2+]e in the BRUs (➉), when compared to iPTH treatment alone, the injected calcimimetic is anticipated to enhance the Ca2+-responsivenes of OB by shifting the Ca2+ set-point of the CaSR to the left and therefore promote the differentiation and functions of OBs (➇). The inhibitory actions of calcimimetics on OCL activities are anticipated to slow down bone resorption by inhibiting OCL recruitment, differentiation and survival (➈), therefore expanding the anabolic window. The actions of both agents together are expected to produce more robust anabolism with less or no hypercalcemia. Preliminary studies indeed showed that daily co-injections of a calcimimetic, (NPS-R568, 20 nmole/kg) with PTH1–34 (40–80 µg/kg) for 4–6 weeks in both adult (3 months old) male and aging (12 months old) female mice (i) completely prevented the development of hypercalcemia, (ii) produced anabolic effects on trabecular bone that was 2–3 fold more robust than that with iPTH treatment alone, and (iii) produced significant anabolic effects and increased bone strength at cortical sites, which were absent with iPTH treatment alone [207]. The ability of this combined PTH/calcimimetic treatment to address the issue of hypercalcemia may allow use of higher doses of PTH to build more bone mass perhaps over a shorter time-course to minimize possible risks of osteosarcoma and make treatment more effective and cost less. Cinacalcet, an orally active calcimimetic, is approved to treat hypercalcemia in patients with primary and secondary HPT and has been in clinical use for several years. Translation of this novel combination drug strategy to human disease therapy could be facilitated.
12. Conclusion
In cartilage and bone, close complementary interactions between CaSR and PTH1R signaling are required for smooth progression of chondrocyte, OB, and OCL differentiation. Regimens with combined pharmaceutics concurrently targeting these two receptors have the propensity of producing more robust anabolic bone effects than treatments with individual compound. However, the dosing of the compounds and timing (concurrent vs sequential) for the drug deliveries remain to be optimized. Based on their cDNA sequences, the CaSRs expressed in OBs and chondrocytes are identical to that cloned from the PTGs [29, 122]. Immunoblotting analyses, however, showed distinct glycosylation patterns of the receptor in chondrocytes and OBs compared to that in PTGs and in HEK-293 cells expressing CaSR cDNA [29]. This difference in post-translational modification could produce different pharmacological profiles of the receptor at different anatomical sites (e.g., OCLs in the resorbing pits), but this concept has not been formally addressed. In addition, the CaSR forms heteromeric complexes with type B γ-aminobutyric acid receptor (GABABR1 and R2) in OBs and chondrocytes [[103], and unpublished data]. In GABABR1-deficient chondrocytes, the ability of Ca2+ to stimulate acute signaling responses was reduced significantly [103]. Since GABABR1 and R2 are co-localized with the CaSR in many tissues at various levels [103, 104], it is plausible that different stoichiometric interactions among these receptors and perhaps with other members of family C GPCRs could produce receptor complexes with distinct pharmacological files in a cell-specific manner. These differences in receptor processing and complex formation provide opportunities for designs of tissue-specific compounds to enhance skeletal anabolism.
Acknowledgments
This work was supported by NIH grants RO1 AG021353 and AR067291 and by the Department of Veteran Affairs Program Project Award Program in Bone Disease BX001599 and Merit Review Grant BX001960.
Abbreviations
- 1,25D
1,25-dihydroxyvitamin D
- Agg
aggregan
- ALP
alkaline phosphatase
- BRU
bone remodeling unit
- CaSR
extracellular calcium-sensing receptor
- [Ca2+]e
extracellular calcium concentration
- CDM, Col(I)
type I collagen
- α1(I)
alpha 1 subunit of the type I collagen
- Col(II)
type II collagen
- α1(II)
alpha 1 subunit of the type II collagen
- DMP1
dentin matrix protieon-1
- HPT
hyperparathyroidism
- IGF1
insulin-like growth factor
- Ihh
Indian hedgehog
- KO
gene knockout
- Gcm2
glial cells missing homolog 2
- GP
growth plate
- GPC
growth plate chondrocyte
- iPTH
daily injection (or intermittent) PTH treatment
- M-CSF
macrophage colony-stimulating factor
- µCT
micro-computed tomography
- OB
osteoblast
- OCL
osteoclast
- OCN
osteocalcin
- ON
osteonectin
- OPG
osteoprotegerin
- OPN
osteopontin
- PG
proteoglycan
- PTC
parathyroid cell
- PTG
parathyroid gland
- PTH
parathyroid hormone
- PTH1R
parathyroid hormone 1 receptor
- PTHrP
parathyroid hormone-related protein
- RANK
receptor activator of nuclear factor kappa-B
- RANKL
RANK ligand
- Floxed-CaSR
control mice carrying loxP sequences flanking the exon 7 of the Casr gene
- Exon5CaSR−/−
mice with insertion a neomycin gene cassette into the exon 5 of the Casr gene
- CartCaSRΔflox/Δflox
mice with constitutive ablation of the exon 7 of the Casr gene targeted specifically to chondrocytes
- Tam-CartCaSRΔflox/Δflox
mice with tamoxifen-induced ablation of the exon 7 of the Casr gene targeted specifically to chondrocytes
- OBCaSRΔflox/Δflox
mice with constitutive osteoclast-specific ablation of the exon 7 of the Casr gene
- PTH−/−
mice with global deletion of Pth gene
- Gcm2−/−
mice with global deletion of Gcm2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
All authors have no conflict of interest
References
- 1.Civitelli R, Ziambaras K. Calcium and phosphate homeostasis: concerted interplay of new regulators. J Endocrinol Invest. 2011;34:3–7. [PubMed] [Google Scholar]
- 2.Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 3.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 1):S23–S30. doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 4.Makras P, Delaroudis S, Anastasilakis AD. Novel therapies for osteoporosis. Metabolism. 2015;64:1199–1214. doi: 10.1016/j.metabol.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickx G, Boudin E, Van Hul W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nat Rev Rheumatol. 2015;11:462–474. doi: 10.1038/nrrheum.2015.48. [DOI] [PubMed] [Google Scholar]
- 7.Andreopoulou P, Bockman RS. Management of postmenopausal osteoporosis. Annu Rev Med. 2014;66:329–342. doi: 10.1146/annurev-med-070313-022841. [DOI] [PubMed] [Google Scholar]
- 8.Chen JF, Yang KH, Zhang ZL, Chang HC, Chen Y, Sowa H, et al. A systematic review on the use of daily subcutaneous administration of teriparatide for treatment of patients with osteoporosis at high risk for fracture in Asia. Osteoporos Int. 2014;26:11–28. doi: 10.1007/s00198-014-2838-7. [DOI] [PubMed] [Google Scholar]
- 9.Khan TS, Fraser LA. Type 1 diabetes and osteoporosis: from molecular pathways to bone phenotype. J Osteoporos. 2015;2015:174186. doi: 10.1155/2015/174186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandeira F, Griz LH, Bandeira C, Pinho J, Lucena CS, Alencar C, et al. Prevalence of cortical osteoporosis in mild and severe primary hyperparathyroidism and its relationship with bone markers and vitamin D status. J Clin Densitom. 2009;12:195–199. doi: 10.1016/j.jocd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Yendt ER, Kovacs KA, Jones G. Secondary hyperparathyroidism in primary osteoporosis and osteopenia: optimizing calcium and vitamin D intakes to levels recommended by expert panels may not be sufficient for correction. Clin Endocrinol (Oxf) 2008;69:855–863. doi: 10.1111/j.1365-2265.2008.03261.x. [DOI] [PubMed] [Google Scholar]
- 12.Monchik JM, Gorgun E. Normocalcemic hyperparathyroidism in patients with osteoporosis. Surgery. 2004;136:1242–1246. doi: 10.1016/j.surg.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 13.Mazzuoli GF, D'Erasmo E, Pisani D. Primary hyperparathyroidism and osteoporosis. Aging (Milano) 1998;10:225–231. doi: 10.1007/BF03339656. [DOI] [PubMed] [Google Scholar]
- 14.Ray D, Goswami R, Gupta N, Tomar N, Singh N, Sreenivas V. Predisposition to vitamin D deficiency osteomalacia and rickets in females is linked to their 25(OH)D and calcium intake rather than vitamin D receptor gene polymorphism. Clin Endocrinol (Oxf) 2009;71:334–340. doi: 10.1111/j.1365-2265.2008.03500.x. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom WH. When you see rickets, consider calcium deficiency. J Pediatr. 1998;133:722–724. doi: 10.1016/s0022-3476(98)70138-6. [DOI] [PubMed] [Google Scholar]
- 16.Wharton B, Bishop N. Rickets. Lancet. 2003;362:1389–1400. doi: 10.1016/S0140-6736(03)14636-3. [DOI] [PubMed] [Google Scholar]
- 17.Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both? Am J Clin Nutr. 2004;80:1725S–1729S. doi: 10.1093/ajcn/80.6.1725S. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thacher T, Glew RH, Isichei C, Lawson JO, Scariano JK, Hollis BW, et al. Rickets in Nigerian children: response to calcium supplementation. J Trop Pediatr. 1999;45:202–207. doi: 10.1093/tropej/45.4.202. [DOI] [PubMed] [Google Scholar]
- 20.Clark SA, Boass A, Toverud SU. Effects of high dietary contents of calcium and phosphorus on mineral metabolism and growth of vitamin D-deficient suckling and weaned rats. Bone Miner. 1987;2:257–270. [PubMed] [Google Scholar]
- 21.Thacher TD. Calcium-deficiency rickets. Endocr Dev. 2003;6:105–125. doi: 10.1159/000072773. [DOI] [PubMed] [Google Scholar]
- 22.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 24.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 25.Brown EM. Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (CaSR) Biochem Pharmacol. 2010;80:297–307. doi: 10.1016/j.bcp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Pollak MR, Seidman CE, Brown EM. Three inherited disorders of calcium sensing. Medicine. 1996;75:115–123. doi: 10.1097/00005792-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hannan FM, Nesbit MA, Zhang C, Cranston T, Curley AJ, Harding B, et al. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21:2768–2778. doi: 10.1093/hmg/dds105. [DOI] [PubMed] [Google Scholar]
- 28.Marx SJ, Simonds WF, Agarwal SK, Burns AL, Weinstein LS, Cochran C, et al. Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J Bone Miner Res. 2002;17(Suppl 2):N37–N43. [PubMed] [Google Scholar]
- 29.Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt SA, et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology. 1999;140:5883–5893. doi: 10.1210/endo.140.12.7190. [DOI] [PubMed] [Google Scholar]
- 30.Goltzman D, Hendy GN. The calcium-sensing receptor in bone--mechanistic and therapeutic insights. Nat Rev Endocrinol. 2015;11:298–307. doi: 10.1038/nrendo.2015.30. [DOI] [PubMed] [Google Scholar]
- 31.Riccardi D, Brennan SC, Chang W. The extracellular calcium-sensing receptor, CaSR, in fetal development. Best Pract Res Clin Endocrinol Metab. 2013;27:443–453. doi: 10.1016/j.beem.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang N, Chen TH, Cheng Z, Li A, Santa Maria C, Tu C, et al. Calcium-sensing receptors (CaSRs) in mature osteoblasts regulate bone formation and maintenance of bone mass: studies in osteocalcin (OCN) conditional knockout mice. J Bone Miner Res. 2012;27 Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=51d4e88b-f79d-47e2-a15b-134f0c57b52e. [Google Scholar]
- 33.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Science signaling. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown EM, Lian JB. New insights in bone biology: unmasking skeletal effects of the extracellular calcium-sensing receptor. Science signaling. 2008;1:pe40. doi: 10.1126/scisignal.135pe40. [DOI] [PubMed] [Google Scholar]
- 35.Godwin SL, Soltoff SP. Calcium-sensing receptor-mediated activation of phospholipase C-gamma1 is downstream of phospholipase C-beta and protein kinase C in MC3T3-E1 osteoblasts. Bone. 2002;30:559–566. doi: 10.1016/s8756-3282(01)00700-1. [DOI] [PubMed] [Google Scholar]
- 36.Garner SC, Pi M, Tu Q, Quarles LD. Rickets in cation-sensing receptor-deficient mice: an unexpected skeletal phenotype. Endocrinology. 2001;142:3996–4005. doi: 10.1210/endo.142.9.8364. [DOI] [PubMed] [Google Scholar]
- 37.Kanatani M, Sugimoto T, Kanzawa M, Yano S, Chihara K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem Biophys Res Commun. 1999;261:144–148. doi: 10.1006/bbrc.1999.0932. [DOI] [PubMed] [Google Scholar]
- 38.Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun. 1998;245:419–422. doi: 10.1006/bbrc.1998.8448. [DOI] [PubMed] [Google Scholar]
- 39.Onyia JE, Helvering LM, Gelbert L, Wei T, Huang S, Chen P, et al. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. J Cell Biochem. 2005;95:403–418. doi: 10.1002/jcb.20438. [DOI] [PubMed] [Google Scholar]
- 40.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, et al. Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone. 2003;33:372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 42.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, et al. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 43.FDA. NDA#21-318: Statistical review and evaluation -- clinical studies. 2000 http://wwwfdagov/ohrms/dockets/ac/01/briefing/3761b2_04_statisticshtm.
- 44.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 46.Cusano NE, Bilezikian JP. Combination anabolic and antiresorptive therapy for osteoporosis. Endocrinol Metab Clin North Am. 2012;41:643–654. doi: 10.1016/j.ecl.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Aslan D, Andersen MD, Gede LB, de Franca TK, Jorgensen SR, Schwarz P, et al. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scandinavian journal of clinical and laboratory investigation. 2012;72:14–22. doi: 10.3109/00365513.2011.624631. [DOI] [PubMed] [Google Scholar]
- 48.Cianferotti L, Gomes AR, Fabbri S, Tanini A, Brandi ML. The calcium-sensing receptor in bone metabolism: from bench to bedside and back. Osteoporos Int. 2015;26:2055–2071. doi: 10.1007/s00198-015-3203-1. [DOI] [PubMed] [Google Scholar]
- 49.Malemud CJ. Matrix metalloproteinases: role in skeletal development and growth plate disorders. Front Biosci. 2006;11:1702–1715. doi: 10.2741/1916. [DOI] [PubMed] [Google Scholar]
- 50.Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, et al. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 51.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 52.Tsang KY, Chan D, Cheah KS. Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev Growth Differ. 2015;57:179–192. doi: 10.1111/dgd.12203. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeung Tsang K, Wa Tsang S, Chan D, Cheah KS. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res C Embryo Today. 2014;102:52–73. doi: 10.1002/bdrc.21060. [DOI] [PubMed] [Google Scholar]
- 55.Bahney CS, Hu DP, Miclau T, 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne) 2015;6:4. doi: 10.3389/fendo.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie Y, Zhou S, Chen H, Du X, Chen L. Recent research on the growth plate: Advances in fibroblast growth factor signaling in growth plate development and disorders. J Mol Endocrinol. 2014;53:T11–T34. doi: 10.1530/JME-14-0012. [DOI] [PubMed] [Google Scholar]
- 57.Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, et al. Chondrocytes Directly Transform into Bone Cells in Mandibular Condyle Growth. J Dent Res. 2015 doi: 10.1177/0022034515598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 59.Kronenberg H, Kobayashi T. Transcriptional regulation in development of bone. Endocrinology. 2004 doi: 10.1210/en.2004-1343. [DOI] [PubMed] [Google Scholar]
- 60.Gerstenfeld LC, Shapiro FD. Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem. 1996;62:1–9. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C1::AID-JCB1%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 61.Goltzman D, Hendy GN, White JH. Vitamin D and its receptor during late development. Biochim Biophys Acta. 2015;1849:171–180. doi: 10.1016/j.bbagrm.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 62.Huang W, Chung UI, Kronenberg HM, de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci U S A. 2001;98:160–165. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang W, Zhou X, Lefebvre V, de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasaki M, Le AX, Helms JA. Expression of indian hedgehog, bone morphogenetic protein 6 and gli during skeletal morphogenesis. Mech Dev. 1997;69:197–202. doi: 10.1016/s0925-4773(97)00145-7. [DOI] [PubMed] [Google Scholar]
- 65.Long F, Schipani E, Asahara H, Kronenberg H, Montminy M. The CREB family of activators is required for endochondral bone development. Development. 2001;128:541–550. doi: 10.1242/dev.128.4.541. [DOI] [PubMed] [Google Scholar]
- 66.McEwen DG, Green RP, Naski MC, Towler DA, Ornitz DM. Fibroblast growth factor receptor 3 gene transcription is suppressed by cyclic adenosine 3',5'-monophosphate Identification of a chondrocytic regulatory element. J Biol Chem. 1999;274:30934–30942. doi: 10.1074/jbc.274.43.30934. [DOI] [PubMed] [Google Scholar]
- 67.O'Keefe RJ, Loveys LS, Hicks DG, Reynolds PR, Crabb ID, Puzas JE, et al. Differential regulation of type-II and type-X collagen synthesis by parathyroid hormone-related protein in chick growth-plate chondrocytes. J Orthop Res. 1997;15:162–174. doi: 10.1002/jor.1100150203. [DOI] [PubMed] [Google Scholar]
- 68.Riemer S, Gebhard S, Beier F, Poschl E, von der Mark K. Role of c-fos in the regulation of type X collagen gene expression by PTH and PTHrP: localization of a PTH/PTHrP-responsive region in the human COL10A1 enhancer. J Cell Biochem. 2002;86:688–699. doi: 10.1002/jcb.10260. [DOI] [PubMed] [Google Scholar]
- 69.Xiao ZS, Hjelmeland AB, Quarles LD. Selective deficiency of the "bone-related" Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis. J Biol Chem. 2004;279:20307–20313. doi: 10.1074/jbc.M401109200. [DOI] [PubMed] [Google Scholar]
- 70.Amizuka N, Davidson D, Liu H, Valverde-Franco G, Chai S, Maeda T, et al. Signalling by fibroblast growth factor receptor 3 and parathyroid hormone-related peptide coordinate cartilage and bone development. Bone. 2004;34:13–25. doi: 10.1016/j.bone.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Barak-Shalom T, Schickler M, Knopov V, Shapira R, Hurwitz S, Pines M. Synthesis and phosphorylation of osteopontin by avian epiphyseal growth-plate chondrocytes as affected by differentiation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;111:49–59. doi: 10.1016/0742-8413(95)00021-x. [DOI] [PubMed] [Google Scholar]
- 72.Chung R, Xian CJ. Recent research on the growth plate: Mechanisms for growth plate injury repair and potential cell-based therapies for regeneration. J Mol Endocrinol. 2014;53:T45–T61. doi: 10.1530/JME-14-0062. [DOI] [PubMed] [Google Scholar]
- 73.Farquharson C, Jefferies D. Chondrocytes and longitudinal bone growth: the development of tibial dyschondroplasia. Poult Sci. 2000;79:994–1004. doi: 10.1093/ps/79.7.994. [DOI] [PubMed] [Google Scholar]
- 74.Hill DJ, Logan A. Peptide growth factors and their interactions during chondrogenesis. Prog Growth Factor Res. 1992;4:45–68. doi: 10.1016/0955-2235(92)90004-2. [DOI] [PubMed] [Google Scholar]
- 75.Isgaard J, Moller C, Isaksson OG, Nilsson A, Mathews LS, Norstedt G. Regulation of insulin-like growth factor messenger ribonucleic acid in rat growth plate by growth hormone. Endocrinology. 1988;122:1515–1520. doi: 10.1210/endo-122-4-1515. [DOI] [PubMed] [Google Scholar]
- 76.Michigami T. Current understanding on the molecular basis of chondrogenesis. Clin Pediatr Endocrinol. 2014;23:1–8. doi: 10.1297/cpe.23.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nilsson A, Isgaard J, Lindahl A, Dahlstrom A, Skottner A, Isaksson OG. Regulation by growth hormone of number of chondrocytes containing IGF-I in rat growth plate. Science. 1986;233:571–574. doi: 10.1126/science.3523759. [DOI] [PubMed] [Google Scholar]
- 78.Pacifici M, Shimo T, Gentili C, Kirsch T, Freeman TA, Enomoto-Iwamoto M, et al. Syndecan-3: a cell-surface heparan sulfate proteoglycan important for chondrocyte proliferation and function during limb skeletogenesis. J Bone Miner Metab. 2005;23:191–199. doi: 10.1007/s00774-004-0584-1. [DOI] [PubMed] [Google Scholar]
- 79.Reddi AH, Ma SS, Cunningham NS. Induction and maintenance of new bone formation by growth and differentiation factors. Ann Chir Gynaecol. 1988;77:189–192. [PubMed] [Google Scholar]
- 80.Smink JJ, Koster JG, Gresnigt MG, Rooman R, Koedam JA, Van Buul-Offers SC. IGF and IGF-binding protein expression in the growth plate of normal, dexamethasone-treated and human IGF-II transgenic mice. J Endocrinol. 2002;175:143–153. doi: 10.1677/joe.0.1750143. [DOI] [PubMed] [Google Scholar]
- 81.van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- 82.Wu LN, Genge BR, Ishikawa Y, Wuthier RE. Modulation of cultured chicken growth plate chondrocytes by transforming growth factor-beta 1 and basic fibroblast growth factor. J Cell Biochem. 1992;49:181–198. doi: 10.1002/jcb.240490211. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida E, Noshiro M, Kawamoto T, Tsutsumi S, Kuruta Y, Kato Y. Direct inhibition of Indian hedgehog expression by parathyroid hormone (PTH)/PTH-related peptide and up-regulation by retinoic acid in growth plate chondrocyte cultures. Exp Cell Res. 2001;265:64–72. doi: 10.1006/excr.2001.5161. [DOI] [PubMed] [Google Scholar]
- 84.Schipani E, Mangiavini L, Merceron C. HIF-1alpha and growth plate development: what we really know. Bonekey Rep. 2015;4:730. doi: 10.1038/bonekey.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michigami T. Regulatory mechanisms for the development of growth plate cartilage. Cell Mol Life Sci. 2013;70:4213–4221. doi: 10.1007/s00018-013-1346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chagin AS, Kronenberg HM. Role of G-proteins in the differentiation of epiphyseal chondrocytes. J Mol Endocrinol. 2014;53:R39–R45. doi: 10.1530/JME-14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 88.Chung UI, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci U S A. 1998;95:13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 90.Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci U S A. 1996;93:10240–10245. doi: 10.1073/pnas.93.19.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Juppner H, Kronenberg HM, et al. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci U S A. 2004;101:14794–14799. doi: 10.1073/pnas.0405091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 94.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matta C, Mobasheri A. Regulation of chondrogenesis by protein kinase C: Emerging new roles in calcium signalling. Cell Signal. 2014;26:979–1000. doi: 10.1016/j.cellsig.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Nurminsky D, Magee C, Faverman L, Nurminskaya M. Regulation of chondrocyte differentiation by actin-severing protein adseverin. Dev Biol. 2007;302:427–437. doi: 10.1016/j.ydbio.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo J, Chung UI, Kondo H, Bringhurst FR, Kronenberg HM. The PTH/PTHrP receptor can delay chondrocyte hypertrophy in vivo without activating phospholipase C. Dev Cell. 2002;3:183–194. doi: 10.1016/s1534-5807(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 98.Chang W, Shoback D. Extracellular Ca2+-sensing receptors--an overview. Cell Calcium. 2004;35:183–196. doi: 10.1016/j.ceca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Conigrave AD, Ward DT. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab. 2013;27:315–331. doi: 10.1016/j.beem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Bai M. Dimerization of G-protein-coupled receptors: roles in signal transduction. Cell Signal. 2004;16:175–186. doi: 10.1016/s0898-6568(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 101.Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 102.Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 103.Cheng Z, Tu C, Rodriguez L, Chen TH, Dvorak MM, Margeta M, et al. Type B gamma-aminobutyric acid receptors modulate the function of the extracellular Ca2+-sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148:4984–4992. doi: 10.1210/en.2007-0653. [DOI] [PubMed] [Google Scholar]
- 104.Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, et al. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 105.Brown E, Enyedi P, LeBoff M, Rotberg J, Preston J, Chen C. High extracellular Ca2+ and Mg2+ stimulate accumulation of inositol phosphates in bovine parathyroid cells. FEBS Lett. 1987;218:113–118. doi: 10.1016/0014-5793(87)81029-3. [DOI] [PubMed] [Google Scholar]
- 106.Shoback D, Thatcher J, Leombruno R, Brown E. Effects of extracellular Ca++ and Mg++ on cytosolic Ca++ and PTH release in dispersed bovine parathyroid cells. Endocrinology. 1983;113:424–426. doi: 10.1210/endo-113-1-424. [DOI] [PubMed] [Google Scholar]
- 107.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 108.Chang W, Pratt S, Chen TH, Nemeth E, Huang Z, Shoback D. Coupling of calcium receptors to inositol phosphate and cyclic AMP generation in mammalian cells and Xenopus laevis oocytes and immunodetection of receptor protein by region-specific antipeptide antisera. J Bone Miner Res. 1998;13:570–580. doi: 10.1359/jbmr.1998.13.4.570. [DOI] [PubMed] [Google Scholar]
- 109.Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+ -sensing receptor. Mol Endocrinol. 2007;21:274–280. doi: 10.1210/me.2006-0110. [DOI] [PubMed] [Google Scholar]
- 110.Chen CJ, Barnett JV, Congo DA, Brown EM. Divalent cations suppress 3',5'-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology. 1989;124:233–239. doi: 10.1210/endo-124-1-233. [DOI] [PubMed] [Google Scholar]
- 111.Avlani VA, Ma W, Mun HC, Leach K, Delbridge L, Christopoulos A, et al. Calcium-sensing receptor-dependent activation of CREB phosphorylation in HEK293 cells and human parathyroid cells. Am J Physiol Endocrinol Metab. 304:E1097–E1104. doi: 10.1152/ajpendo.00054.2013. [DOI] [PubMed] [Google Scholar]
- 112.Kifor O, Diaz R, Butters R, Kifor I, Brown EM. The calcium-sensing receptor is localized in caveolin-rich plasma membrane domains of bovine parathyroid cells. J Biol Chem. 1998;273:21708–21713. doi: 10.1074/jbc.273.34.21708. [DOI] [PubMed] [Google Scholar]
- 113.Pi M, Quarles LD. Osteoblast calcium-sensing receptor has characteristics of ANF/7TM receptors. J Cell Biochem. 2005;95:1081–1092. doi: 10.1002/jcb.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fromigue O, Hay E, Barbara A, Petrel C, Traiffort E, Ruat M, et al. Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med. 2009;13:2189–2199. doi: 10.1111/j.1582-4934.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goolam MA, Ward JH, Avlani VA, Leach K, Christopoulos A, Conigrave AD. Roles of intraloops-2 and −3 and the proximal C-terminus in signalling pathway selection from the human calcium-sensing receptor. FEBS Lett. 2014;588:3340–3346. doi: 10.1016/j.febslet.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 116.Davey AE, Leach K, Valant C, Conigrave AD, Sexton PM, Christopoulos A. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology. 2012;153:1232–1241. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 117.Thomsen AR, Hvidtfeldt M, Brauner-Osborne H. Biased agonism of the calcium-sensing receptor. Cell Calcium. 2011;51:107–116. doi: 10.1016/j.ceca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 118.Rybchyn MS, Slater M, Conigrave AD, Mason RS. An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J Biol Chem. 2011;286:23771–23779. doi: 10.1074/jbc.M111.251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brennan TC, Rybchyn MS, Green W, Atwa S, Conigrave AD, Mason RS. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol. 2009;157:1291–1300. doi: 10.1111/j.1476-5381.2009.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang C, Hujer KM, Wu Z, Miller RT. The Ca2+-sensing receptor couples to Galpha12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol. 2004;286:C22–C30. doi: 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- 121.Goltzman D, Miao D, Panda DK, Hendy GN. Effects of calcium and of the Vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol. 2004;89– 90:485–489. doi: 10.1016/j.jsbmb.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez L, Tu C, Cheng Z, Chen TH, Bikle D, Shoback D, et al. Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology. 2005;146:5294–5303. doi: 10.1210/en.2005-0256. [DOI] [PubMed] [Google Scholar]
- 123.Rodriguez L, Cheng Z, Chen TH, Tu C, Chang W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology. 2005;146:4597–4608. doi: 10.1210/en.2005-0437. [DOI] [PubMed] [Google Scholar]
- 124.Chang W, Tu C, Bajra R, Komuves L, Miller S, Strewler G, et al. Calcium sensing in cultured chondrogenic RCJ3.1C5.18 cells. Endocrinology. 1999;140:1911–1919. doi: 10.1210/endo.140.4.6639. [DOI] [PubMed] [Google Scholar]
- 125.Bonen DK, Schmid TM. Elevated extracellular calcium concentrations induce type X collagen synthesis in chondrocyte cultures. J Cell Biol. 1991;115:1171–1178. doi: 10.1083/jcb.115.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chang W, Tu C, Pratt S, Chen TH, Shoback D. Extracellular Ca(2+)-sensing receptors modulate matrix production and mineralization in chondrogenic RCJ3.1C5.18 cells. Endocrinology. 2002;143:1467–1474. doi: 10.1210/endo.143.4.8709. [DOI] [PubMed] [Google Scholar]
- 127.Wu S, Palese T, Mishra OP, Delivoria-Papadopoulos M, De Luca F. Effects of Ca2+ sensing receptor activation in the growth plate. FASEB J. 2004;18:143–145. doi: 10.1096/fj.03-0294fje. [DOI] [PubMed] [Google Scholar]
- 128.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 129.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, et al. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111:1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pi M, Zhang L, Lei SF, Huang MZ, Zhu W, Zhang J, et al. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res. 2010;25:1092–1102. doi: 10.1359/jbmr.091037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pi M, Quarles LD. A novel cation-sensing mechanism in osteoblasts is a molecular target for strontium. J Bone Miner Res. 2004;19:862–869. doi: 10.1359/JBMR.040114. [DOI] [PubMed] [Google Scholar]
- 133.Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem. 1998;273:23344–23352. doi: 10.1074/jbc.273.36.23344. [DOI] [PubMed] [Google Scholar]
- 134.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- 135.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 137.Lopez D, Duran AC, Sans-Coma V. Formation of cartilage in cardiac semilunar valves of chick and quail. Ann Anat. 2000;182:349–359. doi: 10.1016/S0940-9602(00)80009-6. [DOI] [PubMed] [Google Scholar]
- 138.Swiderski RE, Daniels KJ, Jensen KL, Solursh M. Type II collagen is transiently expressed during avian cardiac valve morphogenesis. Dev Dyn. 1994;200:294–304. doi: 10.1002/aja.1002000404. [DOI] [PubMed] [Google Scholar]
- 139.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen MT, Mackem S, et al. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res. 2011;26:1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow evelopment. Dev Cell. 2014;29:340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest. 2002;109:1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saini V, Marengi DA, Barry KJ, Fulzele KS, Heiden E, Liu X, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288:20122–20134. doi: 10.1074/jbc.M112.441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res. 2011;26:1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, et al. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koh AJ, Beecher CA, Rosol TJ, McCauley LK. 3',5'-Cyclic adenosine monophosphate activation in osteoblastic cells: effects on parathyroid hormone-1 receptors and osteoblastic differentiation in vitro. Endocrinology. 1999;140:3154–3162. doi: 10.1210/endo.140.7.6872. [DOI] [PubMed] [Google Scholar]
- 149.Zerega B, Cermelli S, Bianco P, Cancedda R, Cancedda FD. Parathyroid hormone [PTH(1–34)] and parathyroid hormone-related protein [PTHrP(1–34)] promote reversion of hypertrophic chondrocytes to a prehypertrophic proliferating phenotype and prevent terminal differentiation of osteoblast-like cells. J Bone Miner Res. 1999;14:1281–1289. doi: 10.1359/jbmr.1999.14.8.1281. [DOI] [PubMed] [Google Scholar]
- 150.van der Horst G, Farih-Sips H, Lowik CW, Karperien M. Multiple mechanisms are involved in inhibition of osteoblast differentiation by PTHrP and PTH in KS483 Cells. J Bone Miner Res. 2005;20:2233–2244. doi: 10.1359/JBMR.050821. [DOI] [PubMed] [Google Scholar]
- 151.Choudhary S, Huang H, Raisz L, Pilbeam C. Anabolic effects of PTH in cyclooxygenase-2 knockout osteoblasts in vitro. Biochem Biophys Res Commun. 2008;372:536–541. doi: 10.1016/j.bbrc.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Menendez A, Fong CT, Elalieh HZ, Chang W, Bikle DD. Ephrin B2/EphB4 mediates the actions of IGF-I signaling in regulating endochondral bone formation. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2196. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell adhesion & migration. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Greenfield EM. Anabolic effects of intermittent PTH on osteoblasts. Current molecular pharmacology. 2012;5:127–134. [PubMed] [Google Scholar]
- 155.Bikle DD, Wang Y. Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone. Current molecular pharmacology. 2012;5:135–142. [PMC free article] [PubMed] [Google Scholar]
- 156.Bikle DD. Growth hormone/insulin-like growth factor-1/PTH axis in bone. J Bone Miner Res. 2008;23:581–583. doi: 10.1359/jbmr.080111. [DOI] [PubMed] [Google Scholar]
- 157.Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, et al. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006;189:289–299. doi: 10.1677/joe.1.06657. [DOI] [PubMed] [Google Scholar]
- 158.Fei Y, Xiao L, Hurley MM. The impaired bone anabolic effect of PTH in the absence of endogenous FGF2 is partially due to reduced ATF4 expression. Biochem Biophys Res Commun. 2011;412:160–164. doi: 10.1016/j.bbrc.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fei Y, Hurley MM. Role of fibroblast growth factor 2 and Wnt signaling in anabolic effects of parathyroid hormone on bone formation. J Cell Physiol. 2012;227:3539–3545. doi: 10.1002/jcp.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bergenstock MK, Partridge NC. Parathyroid hormone stimulation of noncanonical Wnt signaling in bone. Ann N Y Acad Sci. 2007;1116:354–359. doi: 10.1196/annals.1402.047. [DOI] [PubMed] [Google Scholar]
- 161.Canalis E. Update in new anabolic therapies for osteoporosis. J Clin Endocrinol Metab. 2010;95:1496–1504. doi: 10.1210/jc.2009-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010;21:237–244. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 163.Lee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Curr Opin Nephrol Hypertens. 2009;18:298–302. doi: 10.1097/MNH.0b013e32832c2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bonnet N, Conway SJ, Ferrari SL. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc Natl Acad Sci U S A. 2012;109:15048–15053. doi: 10.1073/pnas.1203085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Ezura Y, et al. Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2012;109:7433–7438. doi: 10.1073/pnas.1109036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Josse R, Khan A, Ngui D, Shapiro M. Denosumab, a new pharmacotherapy option for postmenopausal osteoporosis. Curr Med Res Opin. 2013;29:205–216. doi: 10.1185/03007995.2013.763779. [DOI] [PubMed] [Google Scholar]
- 167.Sutton EE, Riche DM. Denosumab, a RANK ligand inhibitor, for postmenopausal women with osteoporosis. The Annals of pharmacotherapy. 2012;46:1000–1009. doi: 10.1345/aph.1Q543. [DOI] [PubMed] [Google Scholar]
- 168.Lewiecki EM, Bilezikian JP. Denosumab for the treatment of osteoporosis and cancer-related conditions. Clinical pharmacology and therapeutics. 2012;91:123–133. doi: 10.1038/clpt.2011.268. [DOI] [PubMed] [Google Scholar]
- 169.Johnson GL. Denosumab (prolia) for treatment of postmenopausal osteoporosis. American family physician. 2012;85:334–336. [PubMed] [Google Scholar]
- 170.Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: a review of preclinical and clinical data. Clinical therapeutics. 2012;34:521–536. doi: 10.1016/j.clinthera.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 171.Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J Clin Invest. 2014;124:466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Crane JL, Cao X. Function of matrix IGF-1 in coupling bone resorption and formation. Journal of molecular medicine. 2014;92:107–115. doi: 10.1007/s00109-013-1084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 174.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol. 2012;74:271–297. doi: 10.1146/annurev-physiol-020911-153318. [DOI] [PubMed] [Google Scholar]
- 175.Dvorak-Ewell MM, Chen TH, Liang N, Garvey C, Liu B, Tu C, et al. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26:2935–2947. doi: 10.1002/jbmr.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang W, Dvorak M, Shoback D. Assessing constitutive activity of extracellular calcium-sensing receptors in vitro and in bone. Methods Enzymol. 2010;484:253–266. doi: 10.1016/B978-0-12-381298-8.00013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dvorak MM, Chen TH, Orwoll B, Garvey C, Chang W, Bikle DD, et al. Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology. 2007;148:3156–3163. doi: 10.1210/en.2007-0147. [DOI] [PubMed] [Google Scholar]
- 178.Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A. 2004;101:5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone. 2010;46:571–576. doi: 10.1016/j.bone.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 180.Chattopadhyay N, Yano S, Tfelt-Hansen J, Rooney P, Kanuparthi D, Bandyopadhyay S, et al. Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology. 2004;145:3451–3462. doi: 10.1210/en.2003-1127. [DOI] [PubMed] [Google Scholar]
- 181.Marie PJ. The calcium-sensing receptor in bone cells: A potential therapeutic target in osteoporosis. Bone. 2009 doi: 10.1016/j.bone.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 182.Ye CP, Yamaguchi T, Chattopadhyay N, Sanders JL, Vassilev PM, Brown EM. Extracellular calcium-sensing-receptor (CaR)-mediated opening of an outward K(+) channel in murine MC3T3-E1 osteoblastic cells: evidence for expression of a functional CaR. Bone. 2000;27:21–27. doi: 10.1016/s8756-3282(00)00288-x. [DOI] [PubMed] [Google Scholar]
- 183.Hu F, Pan L, Zhang K, Xing F, Wang X, Lee I, et al. Elevation of extracellular Ca2+ induces store-operated calcium entry via calcium-sensing receptors: a pathway contributes to the proliferation of osteoblasts. PLoS One. 2014;9:e107217. doi: 10.1371/journal.pone.0107217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism [see comments] Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 185.Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, et al. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol. 2004;48:645–653. doi: 10.1387/ijdb.041816fl. [DOI] [PubMed] [Google Scholar]
- 186.Datta HK, MacIntyre I, Zaidi M. The effect of extracellular calcium elevation on morphology and function of isolated rat osteoclasts. Biosci Rep. 1989;9:747–751. doi: 10.1007/BF01114813. [DOI] [PubMed] [Google Scholar]
- 187.Malgaroli A, Meldolesi J, Zallone AZ, Teti A. Control of cytosolic free calcium in rat and chicken osteoclasts. The role of extracellular calcium and calcitonin. J Biol Chem. 1989;264:14342–14347. [PubMed] [Google Scholar]
- 188.Zaidi M, Kerby J, Huang CL, Alam T, Rathod H, Chambers TJ, et al. Divalent cations mimic the inhibitory effect of extracellular ionised calcium on bone resorption by isolated rat osteoclasts: further evidence for a "calcium receptor". J Cell Physiol. 1991;149:422–427. doi: 10.1002/jcp.1041490310. [DOI] [PubMed] [Google Scholar]
- 189.Mentaverri R, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, et al. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J. 2006;20:2562–2564. doi: 10.1096/fj.06-6304fje. [DOI] [PubMed] [Google Scholar]
- 190.House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res. 1997;12:1959–1970. doi: 10.1359/jbmr.1997.12.12.1959. [DOI] [PubMed] [Google Scholar]
- 191.Bennett BD, Alvarez U, Hruska KA. Receptor-operated osteoclast calcium sensing. Endocrinology. 2001;142:1968–1974. doi: 10.1210/endo.142.5.8125. [DOI] [PubMed] [Google Scholar]
- 192.Shalhoub V, Grisanti M, Padagas J, Scully S, Rattan A, Qi M, et al. In vitro studies with the calcimimetic, cinacalcet HCl, on normal human adult osteoblastic and osteoclastic cells. Crit Rev Eukaryot Gene Expr. 2003;13:89–106. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.30. [DOI] [PubMed] [Google Scholar]
- 193.Gowen M, Stroup GB, Dodds RA, James IE, Votta BJ, Smith BR, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004;279:37241–37249. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 195.Zhu J, Siclari VA, Liu F, Spatz JM, Chandra A, Divieti Pajevic P, et al. Amphiregulin-EGFR signaling mediates the migration of bone marrow mesenchymal progenitors toward PTH-stimulated osteoblasts and osteocytes. PLoS One. 2012;7:e50099. doi: 10.1371/journal.pone.0050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li JY, Adams J, Calvi LM, Lane TF, DiPaolo R, Weitzmann MN, et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood. 2012;120:4352–4362. doi: 10.1182/blood-2012-06-438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Alvarez MB, Childress P, Philip BK, Gerard-O'Riley R, Hanlon M, Herbert BS, et al. Immortalization and characterization of osteoblast cell lines generated from wild-type and Nmp4-null mouse bone marrow stromal cells using murine telomerase reverse transcriptase (mTERT) J Cell Physiol. 2012;227:1873–1882. doi: 10.1002/jcp.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Datta NS, Kolailat R, Fite A, Pettway G, Abou-Samra AB. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell Signal. 2010;22:457–466. doi: 10.1016/j.cellsig.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39:1361–1372. doi: 10.1016/j.bone.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 200.Kim SH, Jun S, Jang HS, Lim SK. Identification of parathyroid hormone-regulated proteins in mouse bone marrow cells by proteomics. Biochem Biophys Res Commun. 2005;330:423–429. doi: 10.1016/j.bbrc.2005.02.173. [DOI] [PubMed] [Google Scholar]
- 201.National Osteoporosis Foundation. What is Osteoporosis? 2014 http://noforg/articles/7. [Google Scholar]
- 202.Al-Dujaili SA, Koh AJ, Dang M, Mi X, Chang W, Ma P, et al. Calcium sensing receptor function supports osteoblast survival and acts as a co-factor in PTH anabolic actions in bone. J Cell Biochem. 2015 doi: 10.1002/jcb.25447. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wesseling-Perry K, Salusky IB. Phosphate binders, vitamin D and calcimimetics in the management of chronic kidney disease-mineral bone disorders (CKD-MBD) in children. Pediatr Nephrol. 2013;28:617–625. doi: 10.1007/s00467-012-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Verheyen N, Pilz S, Eller K, Kienreich K, Fahrleitner-Pammer A, Pieske B, et al. Cinacalcet hydrochloride for the treatment of hyperparathyroidism. Expert opinion on pharmacotherapy. 2013;14:793–806. doi: 10.1517/14656566.2013.777041. [DOI] [PubMed] [Google Scholar]
- 205.Palmer SC, Nistor I, Craig JC, Pellegrini F, Messa P, Tonelli M, et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS medicine. 2013;10:e1001436. doi: 10.1371/journal.pmed.1001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nemeth EF, Shoback D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab. 2013;27:373–384. doi: 10.1016/j.beem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 207.Santa Maria C, Li A, Cheng Z, Song F, Shoback D, Tu C-L, et al. Skeletal Anabolism By Concurrently Targeting the PTH1R and CaSR. ASBMR Annual Meeting; Seattle, USA. 2015. [Google Scholar]