Abstract
The hepatitis C virus (HCV) infects ~200 million people worldwide. The majority of infected individuals develop persistent infection, resulting in chronic inflammation and liver disease, including cirrhosis and hepatocellular carcinoma. HCV’s ability to establish persistent infection is partly due to its ability to evade the immune response through multiple mechanisms, including suppression of natural killer (NK) cells. NK cells control HCV replication during the early phase of infection and regulate the progression to chronic disease. In particular, IFN-γ produced by NK cells limits viral replication in hepatocytes and is important for the initiation of adaptive immune responses. However, NK cell function is significantly impaired in chronic HCV patients. The cellular and molecular mechanisms responsible for impaired NK cell function in HCV infection are not well defined. Here, we analyzed the interaction of human NK cells with CD33+ PBMCs that were exposed to HCV. We found that NK cells co-cultured with HCV-conditioned CD33+ PBMCs produced lower amounts of IFN-γ, with no effect on granzyme B production or cell viability. Importantly, this suppression of NK cell-derived IFN-γ production was mediated by CD33+CD11bloHLA-DRlo myeloid derived suppressor cells (MDSCs) via an arginase-1-dependent inhibition of mTOR activation. Suppression of IFN-γ production was reversed by L-arginine supplementation, consistent with increased MDSC arginase-1 activity. These novel results identify the induction of MDSCs in HCV infection as a potent immune evasion strategy that suppresses anti-viral NK cell responses, further indicating that blockade of MDSCs may be a potential therapeutic approach to ameliorate chronic viral infections in the liver.
Keywords: Liver, HCV, MDSCs, NK cells, IFN-γ, Arginase-1, mTOR, chronic liver disease
Introduction
Hepatitis C virus (HCV), the causative agent of hepatitis C, infects over 200 million people worldwide. The majority of infected patients are unable to clear the infection (1, 2), and consequently develop liver fibrosis, cirrhosis and hepatocellular carcinoma (3). The discovery and use of viral protease and polymerase inhibitors have dramatically improved treatment outcomes and prognoses for HCV patients. However, the high cost of these inhibitors precludes benefit to many patients infected with HCV. Furthermore, there is no vaccine available to prevent HCV infection and the subsequent spread of virus. As such, there is a continued need to find alternative, more cost-effective prophylactic and therapeutic treatments.
One of the main challenges to developing a vaccine against HCV is the virus’s ability to evade immune responses (4, 5). HCV infection dysregulates both innate and adaptive immunity by hampering interferon (IFN) production, skewing the differentiation of CD4 T cells towards unfavorable Th2, Th17, and Treg subsets, and impairing the function of cytotoxic CD8 T cells (6-8). HCV is also known to suppress the function of natural kill (NK) cells (9), which play an important role in viral clearance as they comprise 20-30% of hepatic lymphocytes in humans (10, 11). Indeed, NK cells are key players in orchestrating effective immune responses, as they can directly lyse infected cells (12) and crosstalk with Kupffer cells and dendritic cells through production of IFN-γ (13, 14), leading to regulation of T cell responses (15, 16). Notably, production of IFN-γ by NK cells correlates with their expression of CD56. In healthy individuals, CD56high NK cells produce cytokines, while CD56low NK cells are both cytotoxic and capable of producing cytokines (17, 18). In contrast, chronic HCV patients have a subset of CD56− NK cells that are impaired in both IFN-γ production and cytotoxicity through an unknown mechanism (16, 19). Given the central role of NK cells in regulating adaptive immune responses, it is important to understand how NK cell functions are impaired during HCV infection as it may aid in the design of vaccines against the virus.
Myeloid derived suppressor cells (MDSCs) are a heterogeneous population defined by their ability to suppress proinflammatory immune responses. While the generation of MDSCs was originally described in tumor development, the immunosuppressive function of MDSCs has been reported in a variety of pathological conditions, including viral infections (20). MDSCs suppress the effector function of target cells through a number of mechanisms including the production of reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), and arginase-1 (Arg-1). We previously reported that HCV infection generates CD33+CDllb+HLA-DRlo/− MDSCs, which effectively suppress T cell responses through the production of ROS (21). However, despite the pivotal role of NK cells in controlling HCV infection through inhibition of viral replication and regulation of adaptive immunity, it is not known whether MDSCs generated during HCV infection regulate the effector function of NK cells.
In recent years, there have been a number of reports demonstrating the importance of metabolic pathways to immune cell function (22-24). The mTOR (mammalian target of rapamycin) pathway is central to both cellular metabolism and immune activation as it integrates environmental cues such as nutrient availability with the growth, proliferation, and production of effector cytokines in immune cells (25-27). Signaling through the PI3K-Akt axis stimulates the serine-threonine kinase activity of the mTOR complex, which activates protein production via phosphorylation of 4EBP1 (28). 4EBP1 is inactivated upon phosphorylation and released from the elongation initiation factor 4E (eIF4E) (29), allowing recruitment of ribosomes to the 5’ cap of mRNAs to initiate protein translation (30). Amino acids play a major role in triggering mTOR activation (31-33) given that protein synthesis is one of the primary outcomes of mTOR signaling (34). In particular, the amino acid L-arginine can induce the phosphorylation and activation of mTOR (31, 35). As L-arginine availability is reduced by the production of arginase-1 by MDSCs, we hypothesized that inhibition of mTOR activation may be a key mechanism by which MDSCs regulate NK cell function.
Here, we show that HCV-induced MDSCs suppress NK cell IFN-γ production by reducing the bioavailability of L-arginine via arginase-1. The suppression of NK cell IFN-γ production is due to a block in protein translation as there was no difference in the ability of NK cells to transcribe IFNG gene. The defect in translation of IFN-γ transcript appears likely due to a deficiency in mTOR activation, as NK cells exposed to HCV-induced MDSCs displayed decreased phosphorylation of mTOR and its substrates.
Materials and Methods
Cell lines and virus
Huh7.5.1 were grown in DMEM containing 10% FBS, penicillin/streptomycin (100μg/mL), L-glutamine (2mM), and 1x NEAA and infected with the JFH-1 strain of HCV at an m.o.i. of 0.1 for 5 days. JFH-1 was kindly provided by Dr. Wakita (Tokyo Metropolitan Institute) and grown as previously described (6).
CD33+ cells and NK cell co-cultures
Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors (Virginia Blood Services, Richmond, VA) using Sepmate™-50 (Stemcell Technologies) and frozen in 90% FBS/10% Dimethyl Sulfoxide (DMSO). CD45+, CD33+, or NK cells were purified from cell mixtures using EasySep selection kits (Stemcell Technologies). CD45+ cells were purified from co-culture of PBMCs with uninfected/infected Huh7.5.1 cells after 7 days and stained for MDSC markers by flow cytometry. In parallel experiments, CD33+ cells were obtained from co-culture of PBMCs and uninfected/infected Huh7.5.1 cells and were subsequently co-cultured for 2 days with autologous NK cells in RPMI1640 containing 10% FBS, penicillin/streptomycin (10μg/mL), and L-glutamine (2mM) at a ratio of 1:2. Purity of autologous NK cells was confirmed via flow cytometry as >82% CD56+ cells and <2.5% CD3+ cells. NK cells were stimulated with IL-12 (10ng/mL, PeproTech), IL-18 (10ng/mL, R&D Systems), and IL-2 (4μg/mL, eBioscience). The ROS scavenger catalase (100U/mL, Sigma-Aldrich, St. Louis, MO), L NG-monomethyl-L-arginineacetate (500 μM, Sigma-Aldrich), or N(ω)-hydroxy-nor-L-arginine (500μM, Cayman Chemicals, Ann Arbor, MI) was added during the 2-day co-culture of CD33+ cells and NK cells.
ELISA
IFN-γ and granzyme B in culture supernatants were measured using IFN-γ ready-set-go ELISA kit (eBioscience) and Granzyme B Platinum ELISA kit (eBioscience), respectively.
Flow cytometry for MDSCs
For identifying MDSCs, CD45+ cells magnetically sorted from the co-culture of PBMCs with uninfected/infected Huh7.5.1 cells were blocked with FcR blocking reagent (Miltenyi) and stained with the live/dead marker DAPI (Life Technologies), anti-CD33, -CD11b, and -HLA-DR (all from BD Pharmigen). For detecting intracellular arginase-1 production, CD33+ cells were magnetically sorted from co-cultures with NK cells and stained for MDSC surface markers. The cells were then fixed and permeabilized by Cytofix/Cytoperm (BD biosciences) and stained with the MDSC markers described above and anti-Arginase-1 (R&D Systems). Aqua live/dead stain (Life Technologies) was included to analyze cell viability. All stained cells were run on BD FACSCantoII (BD Biosciences) and analyzed using FlowJo software.
Flow cytometry for NK cells
Following co-culture with mock/HCV-conditioned CD33+ cells, NK cells were magnetically sorted and replated in fresh media containing IL-12 (10ng/mL) and IL-18 (10ng/mL) in the presence of Golgi Plug (eBioscience) for 5 hours. After blocking Fc receptor using the FcR blocking reagent (Miltenyi), the cells were stained with Aqua Live/Dead (Life Technologies), anti-CD56, -CD16, and -CD33 (all from BD Pharmigen). The cells were then permeabilized with Cytofix/Cytoperm (BD Biosciences) and stained with anti-IFN-γ (BD Pharmingen). For intracellular mTOR staining, NK cells were recovered following co-culture with mock- or HCV-conditioned CD33 cells separated by a 0.45μm transwell insert and restimulated with IL-12 (10ng/mL) and IL-18 (10ng/mL) for 2 days. The recovered cells were fixed in Cytofix (BD Biosciences), permeabilized using BD Phosflow Perm Buffer (III), and stained with rat anti-mTOR (R&D systems) and mouse anti-phospho-mTOR (BD Phosflow™), or mouse anti-phospho-4EBP1 (pT69) (BD Phosflow™). All cells were run on BD FACSCantoII (BD Biosciences) and analyzed using FlowJo software.
qRT-PCR
RNA was extracted from magnetically sorted NK cells using GenElute™ Mammalian Total RNA Miniprep Kit (Sigma-Aldrich). cDNA was made using the High Capacity RNA-to-cDNA kit (Applied Biosystems) and qRT-PCR was performed using Fast SYBR® Green master mix (Applied Biosystems). Gene expression was quantified on the StepOne Real Time PCR system (Applied Biosystems). Results were first normalized to GAPDH and then set relative to mock-conditioned controls. The following primers were purchased from Eurofins MWG Operon: IFNG forward 5’-TCGGTAACTGACTTGAATGTCCA-3’ and reverse 5’-TCGCTTCCCTGTTTTAGCTGC-3’, GAPDH forward 5’-TGCACCACCAACTGCTTAGC-3’ and reverse 5’-GCATGGACTGTGGTCATGAG-3’.
Arginase assay
CD33+ cells were co-cultured with autologous NK cells separated by a 0.45μm transwell insert in complete media in the absence of phenol red. The cells were supplemented with IL-2 (4μg/mL) and stimulated with IL-12 (10ng/mL) and IL-18 (10ng/mL) for 48h. The CD33+ cells were recovered by magnetic sorting and the arginase activity assay (Sigma-Aldrich) was performed according to manufacturer’s instructions.
Sucrose purified JFH-1 virus
Sucrose purified JFH-1 virus was obtained from Dr. Lucy Golden-Mason. Sucrose density-gradient ultracentrifugation purification and concentration of virus was performed on pooled supernatants of JFH1 (Takaji Wakita, National Institute of Infectious Diseases, Japan) infected Huh7.5.1 cells (Francis Chisari, Scripps Research Institute, La Jolla, CA) and multiplicity of infection (m.o.i) determined by titration on Huh7.5.1 cells as previously described (36). Purified virions were used to infect Huh7.5.1 cells at m.o.i.s of 0.01, 0.1, and 1.0 for 5 days before the addition of PBMCs. After 7 days, CD45+ cells were obtained by magnetic selection (Stemcell Technology) and stained for MDSC markers as shown above. In addition, CD33+ cells were selected from the co-culture and cultured with autologous NK cells and stimulated for 2 days. The resulting cell culture media was obtained and tested for IFN-γ by ELISA (eBioscience).
Statistical analysis
Experimental results were analyzed for statistical significance using Wilcoxon matched pairs test, two-tailed paired t test, Mann-Whitney test, or Kruskal-Wallis test (One-way ANOVA) with Dunn’s post-test, as appropriate. p values of <0.05 were considered significant and are indicated in the figures.
Results
NK cells exhibit impaired IFN-γ production upon co-culture with HCV-conditioned myeloid cells
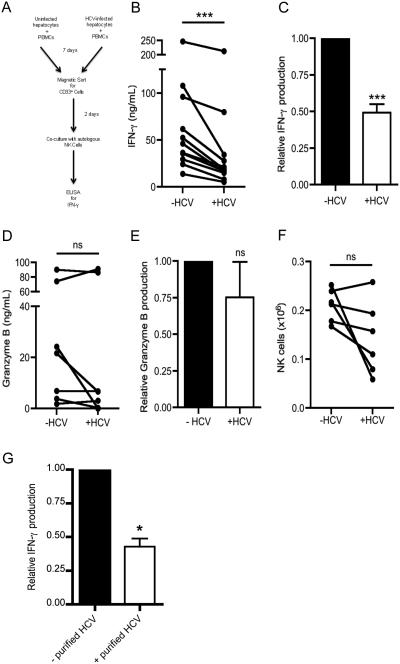
NK cells play a pivotal role in limiting virus replication via direct killing of infected cells and production of IFN-γ, which in turn augments anti-viral adaptive immunity . While NK cells from patients with acute HCV infection have intact effector function, those from chronically infected patients produce significantly lower amounts of IFN-γ (37). MDSCs are known to dampen immune responses of lymphocytes in acute and chronic viral infections (20). We therefore investigated whether myeloid cells exposed to HCV infection suppress NK effector function. To this end, we cultured peripheral blood mononuclear cells (PBMCs) with the hepatocyte cell line Huh7.5.1 uninfected or infected with HCV. Following 7 days of co-culture, we magnetically sorted CD33+ (myeloid) cells with several wash steps (hereafter referred to as uninfected-conditioned CD33+ cells or HCV-conditioned CD33+ cells) and added them to NK cells from autologous donors (See Fig. 1A). After 48 hours of stimulation with IL-12 and IL-18, NK cells co-cultured with HCV-conditioned CD33+ cells produced less IFN-γ (Fig. 1B-C). In contrast, there was no difference in granzyme B production between NK cells co-cultured with HCV-conditioned CD33+ cells and those cultured with uninfected-conditioned CD33+ cells (Fig. 1D-E). The decrease in IFN-γ production was not due to a loss of cell viability as the number of NK cells was comparable upon co-culture with uninfected- or HCV-conditioned CD33+ cells (Fig. 1F). Lastly, we confirmed that NK cells were the primary source of IFN-γ in the co-culture as only a negligible proportion of CD33+ myeloid cells stained for IFN-γ (Supplemental Fig. 1A). To verify that the JFH-1 virus is responsible for the MDSC-mediated suppression, sucrose gradient-purified virions were used to infect Huh cells. This too yielded the suppression of NK cell IFN-γ production (Fig. 1G). Together, these results show that HCV-conditioned myeloid cells specifically interfere with IFN-γ production by NK cells.
Figure 1. NK Cells display impaired IFN-γ production when co-cultured with HCV-conditioned myeloid cells.
(A-F) PBMCs were cultured with Huh7.5.1 cells uninfected or infected with HCV for 7 days, after which CD33+ cells were positively selected by magnetic separation. Autologous NK cells were purified by negative selection and co-cultured with CD33+ cells at a 2:1 ratio in the presence of IL-2/IL-12/IL-18 for 2 days. Culture supernatants were used for (B) IFN-γ and (D) granzyme B quantification by ELISA. Relative (C) IFN-γ and (E) granzyme B production was calculated by normalizing to values of NK cells co-cultured with mock-conditioned CD33+ cells. (F) Cell viability of NK cells recovered after co-culture was assessed by flow cytometry. NK cells were gated on live cells, singlets, forward and side scatter, and CD33− cells to determine the numbers of NK cells. (G) PBMCs were cultured with Huh7.5.1 cells uninfected or infected with purified HCV virions for 7 days, after which CD33+ cells were positively selected by magnetic separation. Autologous NK cells were purified by negative selection and co-cultured with CD33+ cells at a 2:1 ratio in the presence of IL-2/IL-12/IL-18 for 2 days. Culture supernatants were used for IFN-γ quantification by ELISA and normalized to the uninfected control. Results are the mean or representative of 6-11 independent experiments. ns denotes not significant, ***p<0.001, (B, D, F) Wilcoxon signed rank test or (C, E, G) two-tailed paired t test.
Reduction in NK cell IFN-γ production is post-transcriptionally regulated
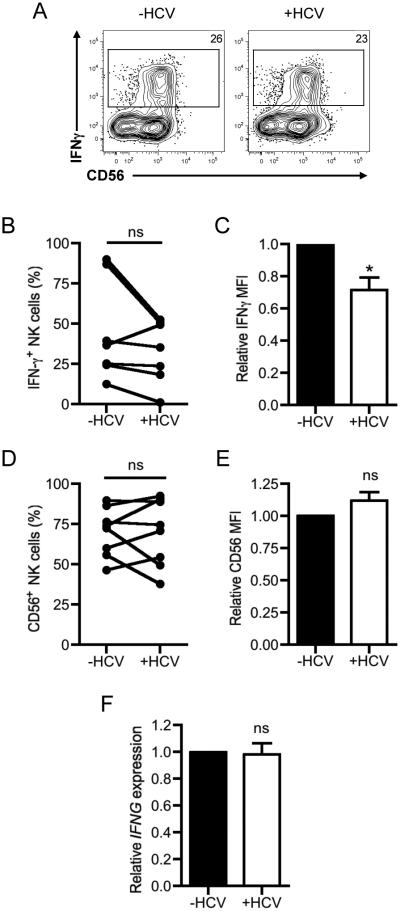
Since the defect in IFN-γ production was not due to differences in the number of total NK cells, the reduction in NK cell IFN-γ secretion following co-culture with HCV-conditioned myeloid cells likely reflected a defect in overall NK cell activation or a selective deficiency in IFN-γ production. To explore these possibilities, we first assessed the ability of NK cells to produce IFN-γ at the single cell level by intracellular cytokine staining. As shown in Fig. 2A-B, the frequency of IFN-γ producing NK cells were comparable upon co-culture with uninfected- or HCV-conditioned CD33+ cells. However, the mean fluorescence intensity (MFI) of the IFN-γ-producing NK cells was reduced in NK cells that encountered HCV-conditioned CD33+ cells, indicating that these cells were producing less IFN-γ on a per cell basis (Fig. 2C). However, there was no significant difference in the expression of the human NK differentiation marker CD56 in total NK cells when cultured with HCV-conditioned myeloid cells (Fig. 2A, D-E). These results suggest that the differentiation status of NK cells was not altered in the presence of HCV-conditioned CD33+ cells. To further investigate the molecular basis of the decrease in IFN-γ production by NK cells, we assessed the transcriptional status of the IFNG gene. Quantitative PCR analysis revealed that IFNG mRNA was found in similar levels in NK cells co-cultured with mock- or HCV-conditioned CD33+ cells (Fig. 2F). Thus, the deficit in IFN-γ production by NK cells was likely due to regulation of post-transcriptional events by HCV-conditioned CD33+ cells.
Figure 2. Decrease in IFN-γ production is independent of IFNG transcription and differentiation status of NK cells.
NK cells were co-cultured with mock- or HCV-conditioned CD33+ cells in the presence of IL-2/IL-12/IL-18 for 2 days. The cells were then harvested by detachin treatment and incubated with IL-12/IL-18 in the presence of GolgiPlug for 5h and stained for surface markers and intracellular expression of IFN-γ. NK cells were gated on live cells, singlets, forward and side scatter, and CD33− cells. (A) Representative dot plots of IFN-γ and CD56 expression in NK cells co-cultured with mock- or HCV-conditioned CD33+ cells. (B) Frequency of IFN-γ+ NK cells (C) Relative mean fluorescence intensity (MFI) of IFN-γ in NK cells. (D) Frequency and (E) relative MFI of CD56+ NK cells was calculated from cell treated as described in (A). (F) Mock- or HCV-conditioned CD33+ cells were separated from NK cells with a 0.45μm transwell insert and stimulated with IL2/IL12/IL18. After 2 days, NK cells were recovered, lysed and the expression of IFNG was assessed relative to GAPDH expression by qRT-PCR. IFNG expression was normalized to NK cells co-cultured with mock-conditioned CD33+ cells. Results are the mean or representative of 6-8 independent experiments. ns denotes not significant, *p<0.05, (B-D) two-tailed paired t test or (E) Wilcoxon signed rank test.
HCV-conditioned MDSCs suppress NK cell IFN-γ production via arginase-1
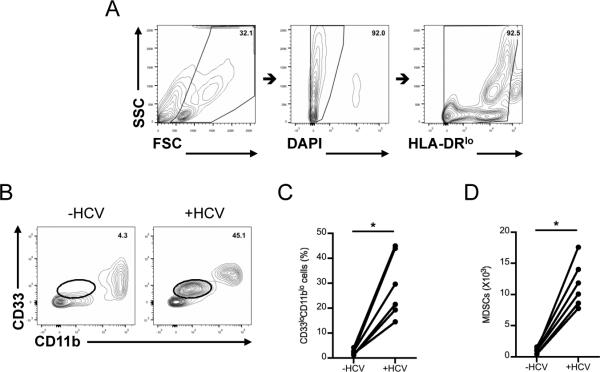
We next sought to identify the molecular determinants originating from HCV-conditioned CD33+ cells that enabled regulation of IFN-γ production by NK cells. We have previously demonstrated that PBMCs co-cultured with HCV-infected hepatocytes exhibit immunosuppressive functions characteristic of myeloid derived suppressor cells (MDSCs) (21). Given that HCV-conditioned CD33+ cells potently inhibited NK cell effector function, we investigated whether the CD33+ population included MDSCs with immunosuppressive capabilities. Indeed, co-culture of PBMCs with HCV-infected hepatocytes produced a distinct population of CD33+CD11bloHLA-DRlo cells (Fig. 3A-B), as described previously by us (4). Importantly, this population of MDSCs was minimally represented among PBMCs cultured with uninfected hepatocytes (Fig. 3C-D). To verify HCV infection in hepatocytes, we determined the quantity of HCV RNA and the level of core protein by qRT-PCR and Western blot analysis, respectively (Supplemental Fig. 2A-B) as well as the ability of sucrose purified virus to infect hepatocytes (Supplemental Fig. 2C). We further examined the relationship between viral dose and the frequency of MDSC by assessing MDSC accumulation after the co-culture of PBMC with various m.o.i. of JFH1 virus (Supplemental Fig. 2D), suggesting the increased frequency of MDSC detectable in high virus dose. In addition, there is a trend where chronic HCV patients with high virus titer (>800,000IU/mL) have more MDSCs than patients with low virus titer (Supplemental Fig. 2E), while there is no correlation between the frequency of MDSC and ALT level (data not shown). These results suggest that HCV is capable of inducing MDSC, which plays a role in controlling infection rather than hepatic inflammation.
Figure 3. HCV-conditioned CD33+ cells include MDSCs.
PBMCs were cultured with Huh7.5.1 cells uninfected or infected with HCV for 7 days after which CD33+ cells were positively selected by magnetic separation. (A and B) MDSCs were defined as CD33+CD11bloHLA-DRlo gated on forward and side scatter, live cells, HLA-DRlo, CD33 and CD11b. (C) Frequency and (D) number of MDSCs was calculated from the gating strategy described in A and B. Results are the mean or representative of 6 independent experiments. *p<0.05, (C and D) Wilcoxon signed rank test.
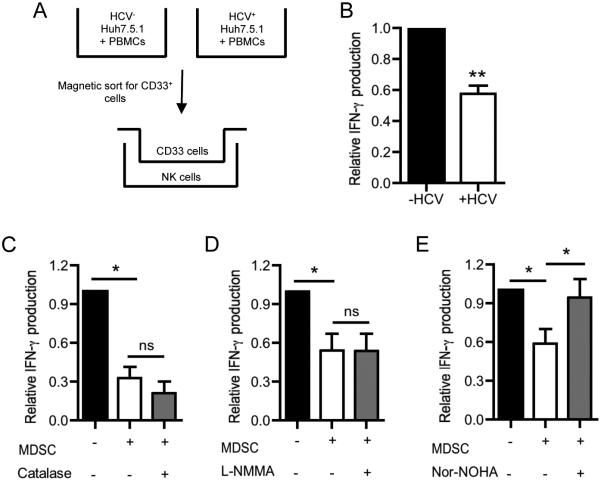
MDSCs are known to inhibit immune cells through contact-dependent and contact-independent mechanisms (38). Therefore, we determined if the crosstalk between CD33+ cells and NK cells required cell-cell contact by employing 0.45μm transwell inserts to physically separate the two populations during co-culture (Fig. 4A). HCV-dependent suppression of NK cell IFN-γ production occurred even in the absence of cell contact between myeloid and NK cells (Fig. 4B). Given these findings, we sought to identify soluble immunosuppressive mediators produced by HCV-conditioned MDSCs.
Figure 4. HCV-induced MDSCs suppress NK cell IFN-γ production via contact-independent production of arginase-1.
(A) Mock- or HCV-conditioned CD33+ cells were co-cultured with NK cells in the presence of a 0.45μm transwell insert. (B) After 2 days in culture, IFN-γ production by NK cells was assessed by intracellular staining. (C-E) Mock- or HCV-conditioned CD33+ cells were co-cultured with IL-12/IL-18 stimulated NK cells for 2 days in the presence or absence of (C) Catalase which decomposes ROS, (D) L-NMMA which inhibits iNOS, or E) nor-NOHA which inhibits arginase-1. IFN-γ release into culture supernatants was assessed by ELISA. Results are the mean or representative of 6-8 independent experiments. ns denotes not significant, *p<0.05, **p<0.01, (B) Wilcoxon signed rank test, (B) two-tailed paired t test, or (C-E) Kruskal-Wallis test with Dunn’s post-test.
MDSCs employ numerous soluble factors to inhibit host immune responses, including the production of reactive oxygen species (ROS), nitric oxide synthase (NOS), and arginase-1. ROS regulates immune responses through activation of apoptosis in immune cells (39), while nitric oxide produced by NOS nitrosylates and dissociates protein complexes involved in immune activation (40, 41). Arginase-1 depletes local supplies of L-arginine and can cause inefficient proliferation of activated lymphocytes (42). Considering their potent immunosuppressive effects, we investigated if ROS, NOS, or arginase-1 may be responsible for dampening NK cell IFN-γ production using pharmacologic inhibitors of each of these factors during co-culture of NK cells with CD33+ cells: catalase scavenges ROS, L-NG-monomethyl L-arginineacetate (L-NMMA) inhibits NOS, and Nω-hydroxy-L-arginine (Nor-NOHA) inhibits arginase-1. Addition of catalase and L-NMMA failed to reverse the suppressive effect of HCV-conditioned CD33+ cells on NK cell IFN-γ production (Fig. 4C-D). In contrast, Nor-NOHA restored IFN-γ production in NK cells exposed to HCV-conditioned CD33+ cells (Fig. 4E). Importantly, Nor-NOHA did not change IFN-γ production when added to NK cells co-cultured with mock-conditioned CD33+ cells (Supplemental Fig. 1B). HCV-induced CD33+ cells thus appear to be MDSCs that suppress NK cell IFN-γ production via arginase-1.
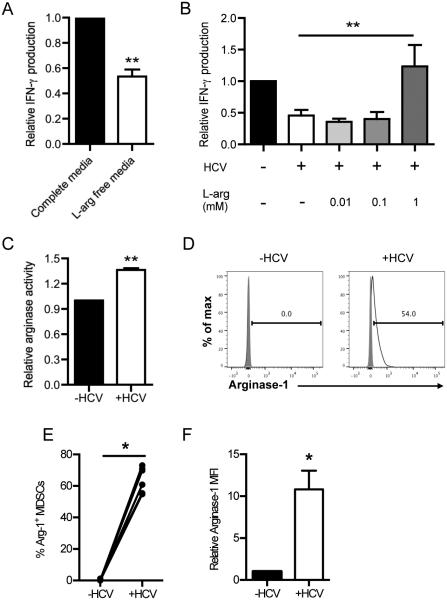
Inhibition of NK cell IFN-γ production by HCV-conditioned myeloid cells is reversed by L-arginine supplementation
Arginase-1 catabolizes L-arginine into urea and ornithine. Although L-arginine is nonessential in most individuals, it is possible that an acute loss of L-arginine due to arginase-1 activity could affect the function of local immune cells. Therefore, we cultured NK cells in complete media or media depleted of L-arginine. Consistent with the increase in IFN-γ production upon inhibition of arginase-1 (Fig. 4E), NK cells grown in complete media produced more IFN-γ than those grown in L-arginine-deficient conditions (Fig. 5A). We further confirmed the requirement for arginase-1 in suppressing NK cell IFN-γ production by replenishing L-arginine in L-arginine-depleted co-cultures of CD33+ cells and NK cells. Addition of 1 mM L-arginine, which approximates L-arginine levels found in standard RPMI formulations, reversed the inhibition of NK cell IFN-γ production (Fig. 5B). Notably, supplementing L-arginine in co-cultures of NK cells and uninfected-conditioned CD33+ cells did not change IFN-γ production (Supplemental Fig. 1C), verifying that L-arginine was specifically counteracting the enhanced arginase-1 activity of HCV-conditioned CD33+ cells. Indeed, direct assessment of arginase-1 function revealed that HCV-conditioned CD33+ cells had increased arginase-1 activity when compared to mock-conditioned CD33+ cells (Fig. 5C).
Figure 5. Increased arginase-1 expression in HCV-conditioned CD33+ cells depletes arginine levels required for NK cell IFN-γ production.
(A) NK cells were grown in complete media or L-arginine-deficient media and were stimulated with IL-12/IL-18 for 2 days. IFN-γ in the supernatant was measured by ELISA. (B) NK cells cultured with mock- or HCV-conditioned CD33+ cells were stimulated with IL-2/IL-12/IL-18 and supplemented with 0, 0.01, 0.1, or 1mM L-arginine for 2 days. IFN-γ in the supernatant was measured by ELISA. (C) Mock- or HCV-conditioned CD33+ cells were cultured with NK cells and separated a 0.45μm transwell insert. Following 2 days of co-culture, arginase activity in the CD33+ cells was determined using an arginase assay kit. (D) Mock- or HCV-conditioned CD33+ cells were cultured with NK cells for 2 days. CD33+ cells were then recovered by magnetic selection and intracellular arginase-1 expression was assessed on MDSCs gated on forward and side scatter, live cells, singlets, CD33+, HLA-DRlo and CD11blo. (E) Frequency of Arginase-1+ MDSCs and (F) MFI of Arginase-1 expression was quantified from the experiment described in D. Results are the mean or representative of 3-8 independent experiments. *p<0.05, **p<0.01, (A, C, F) two-tailed paired t test, (B) Kruskal-Wallis test with Dunn’s post-test. or (E) Wilcoxon signed rank test.
We next validated the increased arginase-1 activity by intracellular staining of arginase-1. As seen in Fig. 5D-F, both the frequency and level of arginase-1 expression was increased in HCV-conditioned CD33+ cells. Collectively, these results demonstrate that HCV-conditioned CD33+ cells suppress NK cell IFN-γ production via a contact-independent, arginase-1-dependent mechanism, which is distinct from the production of ROS that is used to suppress T cells (21). Arginase-1 produced by HCV-conditioned MDSCs is thus most likely responsible for reducing IFN-γ synthesis in NK cells.
L-arginine depletion selectively affects IFN-γ production in NK cells
To further dissect our findings on arginase-1 in MDSC-mediated suppression of NK cell IFN-γ production, we assessed the effects of L-arginine deprivation on other NK cell functions. Accordingly, we evaluated cell viability and granzyme B production in NK cells cultured in the presence of the IL-2/IL-12/IL-18 stimulatory cocktail in L-arginine-deficient media. In contrast to the decrease in IFN-γ production seen in NK cells grown in L-arginine-deficient media (Fig. 5A), there was no difference in NK cell viability or granzyme B production (Supplemental Fig. 3A-B), suggesting that the absence of L-arginine does not result in a global insufficiency in NK activity. Instead, the availability of L-arginine regulates specific effector functions, namely IFN-γ production, in NK cells.
MDSC-mediated suppression of IFN-γ production is mediated by reduced mTOR signaling
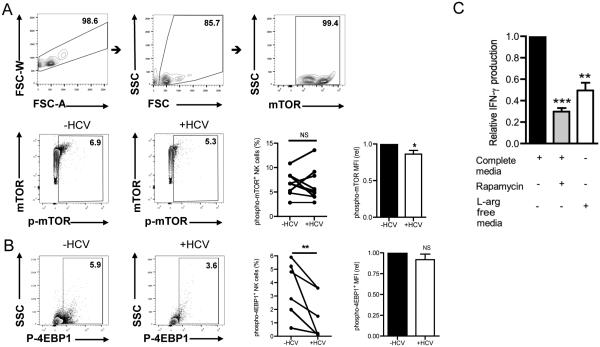
L-arginine and other amino acids are a primary stimulus for activating the mTOR pathway (43, 44). The mTOR pathway integrates complex environmental cues such as nutrient availability with protein translation and higher order cellular functions, including proliferation and cytokine production (45). Considering that the defect in NK cell IFN-γ production in our system was post-transcriptional, we reasoned that the mTOR pathway would be inefficiently activated in NK cells cultured with HCV-conditioned CD33+ cells. To test this hypothesis, we analyzed activation of the mTOR pathway in NK cells grown in L-arginine deficient media. As shown in Fig. 6A, NK cells that were cultured with HCV-conditioned CD33+ cells expressed reduced levels of phosphorylated mTOR when compared to those cultured with uninfected-conditioned CD33+ cells. Similarly, NK cells grown in L-arginine-free media also expressed reduced levels of phosphorylated mTOR compared to their counterparts grown in complete media (data not shown).
Figure 6. Suppression of NK cell IFN-γ production is mediated by reduced mTOR signaling.
NK cells were co-cultured with mock- or HCV-conditioned CD33+ cells separated by a 0.45 μm transwell insert. Following 2 days in culture, NK cells were recovered and phosphorylation of mTOR was assessed by flow cytometry. (A) Cells were gated on singlets, forward and side scatter, mTOR+ cells, and phospho-mTOR+. mTOR and phospho-mTOR positive cells were gated based on FMOs. MFI of phospho-mTOR expression was quantified from the experiment. (B) Activation of the mTOR substrate, 4EBP1, was assessed measuring the phosphorylation of 4EBP1 at residue T69 by flow cytometry. As above, cells were gated on singlets, forward and side scatter, and phosphor-4EBP1+ cells based on FMOs. The MFI of p-4EBP1 of the p-4EBP1+ cells was computed. (C) NK cells were grown in complete media with and without the mTOR inhibitor rapamycin or L-arginine deficient media for 2 days. IFN-γ in the supernatant was measured by ELISA. Results are representative or mean of 8-10 independent experiments. *p<0.01, **p<0.01, ns denotes not significant, (A, B) Wilcoxon signed rank test. **p<0.01, ***p<0.001, (C) Kruskal-Wallis test with Dunn’s post-test.
4EBP1, a downstream target of the mTOR complex, is a translational repressor that is inactivated upon phosphorylation. To further examine the effect of arginase-1 producing cells on the mTOR pathway in NK cells, we examined the phosphorylation status of 4EBP1 in NK cells co-cultured with HCV-conditioned CD33+ cells. Not surprisingly, a lower percentage of NK cells recovered after co-culture with HCV-conditioned CD33+ cells expressed phosphorylated 4EBP1 compared to those isolated after co-culture with uninfected-conditioned CD33+ cells (Fig. 6B). Complementing these results, phosphorylation of 4EBP1 was also decreased in NK cells grown in L-arginine-deficient media (data not shown). Lastly, we treated NK cells with the mTOR inhibitor, rapamycin, to verify the importance of the mTOR pathway to NK cell IFN-γ production. Indeed, IFN-γ production by NK cells treated with rapamycin was comparable to IFN-γ produced by NK cells grown in L-arginine-depleted media (Fig. 6C). Taken together, our results suggest that HCV-induced CD33+ MDSCs exhaust local supplies of L-arginine, causing insufficient mTOR activation, which likely decreases translation of IFN-γ transcript in NK cells.
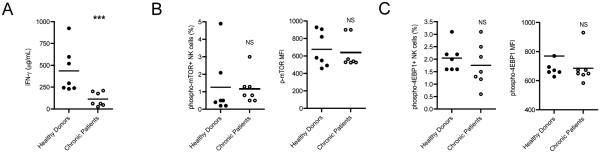
As we have established that NK cells deprived of arginine as a result of exposure to MDSCs have impaired IFN-γ production due to impairment in mTOR signaling, we sought to determine if NK cells from chronic HCV patients exhibited similar traits. NK cells obtained from chronic HCV patients exhibited impaired IFN-γ response compared to NK cells from healthy donors upon stimulation (Fig. 7A). Further analysis on mTOR activation by NK cells from chronic HCV patient revealed that there was a slight decrease in mTOR activation by chronic HCV patients’ NK cells compared to healthy individuals with no statistical significance (Fig. 7B). Moreover NK cells from chronic HCV patients tended to display lower levels of 4EBP1 activation (Fig. 7C), suggesting that the mTOR signaling pathway may be impaired in NK cells during chronic HCV patients.
FIGURE 7. Chronic HCV patients’ NK cells display impaired IFN-γ production in response to IL-12/IL-18 stimulation.
PBMCs were obtained from chronic 7 HCV patients and 7 healthy donors and then frozen in 90% FBS/10% DMSO. NK cells were magnetically sorted from thawed PBMCs and cultured for 2 days with IL-2/IL-12/IL-18. (A) The cell media was collected, spun to remove cell bodies, and IFN-γ in the culture media was determined by ELISA. (B, C) More cells were recovered by detachin treatment, combined with the existing cell pellet, and then phosphorylation levels of mTOR and 4EBP1 were assessed by flow cytometry. (B) Cells were gated on singlets, forward and side scatter, mTOR+ cells. MFIs of phospho-mTOR and total mTOR expression were quantified from the experiment. mTOR and phosphor-mTOR positive cells were gated based on FMOs as shown in Fig. 6. (C) The level of phosphorylated 4EBP1 (pT69) was assessed. As above, cells were gated on singlets, forward and side scatter, and phosphor-4EBP1+ cells based on FMOs. The MFI of p-4EBP1 of the p-4EBP1+ cells was computed. ***p<0.001, ns denotes not significant, (A-C) Mann-Whitney Test.
Discussion
In this report, we demonstrate that HCV infection induces CD33+CD11bloHLA-DRlo MDSCs, which suppress NK cell IFN-γ production by depleting L-arginine via an arginase-1-dependent mechanism. The loss of L-arginine subsequently fails to drive efficient activation of the mTOR pathway, which is necessary for translating IFN-γ mRNA into secreted protein. In contrast, L-arginine deprivation or the presence of HCV-conditioned CD33+ cells does not affect granzyme B production or NK cell viability. These results underscore the multitude of immune evasion strategies employed by HCV and identify a specific target that can be manipulated in chronic viral infections in the liver.
MDSCs have been reported to suppress other immune cells through a variety of mechanisms. We previously reported that extracellular HCV core protein triggers the generation of MDSCs (21). These MDSCs upregulated NADPH oxidase to increase ROS production, which subsequently suppressed CD4 and CD8 T cell IFN-γ production. As NK cells are also a significant source of IFN-γ during the early phase of viral infections, it was important to identify the influence of HCV-induced MDSCs on NK cell effector function. Indeed, the decrease in NK cell IFN-γ production by HCV-induced CD33+ cells (Fig. 1B) reiterates the potent immunoregulatory role of MDSCs in inhibiting IFN-γ production during HCV infection. In contrast to ROS-dependent suppression of T cells, however, the mechanism of suppression of NK cells required increased levels of arginase-1 in HCV-conditioned MDSCs (Fig. 4). It is worthwhile to point out that arginase-1 activity is increased in HCV-infected hepatocytes (46), however in our system, the contribution of hepatocyte arginase-1 has been eliminated because they have been eliminated from the culture with NK cells. Furthermore, it has been reported that the presence of arginase-1-producing MDSCs in HCV patients is associated with a poor prognosis and patients undergoing anti-viral therapy have fewer circulating arginase-1 producing myeloid cells (47). These observations suggest that L-arginine availability likely decreases over the course of infection. It is therefore tempting to speculate whether the difference in mechanism of suppression between T cells and NK cells reflects differences in nutrient utilization by responding immune cells over the duration of HCV infection; perhaps early-responders such as NK cells utilize L-arginine to a greater extent than late-arriving T cells. Conversely, a recent study reported the presence of arginase-1 producing MDSCs that dampened T cell responses in hepatitis B virus infection (48). Differences in pathogens may thus also regulate the mechanisms and target cells of MDSC-mediated suppression in chronic viral infections. Consequently, MDSCs generated in vivo during chronic viral infections probably change their suppression mechanisms to target various immune populations at specific stages of infection with distinct pathogens. Nevertheless, HCV-induced MDSCs must play an important role in disease progression as two reports have shown that increased MDSC frequencies in treatment-naïve HCV patients compared to healthy individuals and patients undergoing anti-viral treatment, and that the MDSC frequencies correlated positively to HCV RNA loads (47, 49).
Arginase-1 converts L-arginine into ornithine and urea, thus reducing local levels of L-arginine. As L-arginine can directly regulate NK cell phenotype and function, the acute loss of L-arginine can profoundly affect NK cell responses (50). The effect on NK cell responses likely corresponds to the number of MDSCs and hence arginase-1 in the system (Supplemental Fig. 4). Specifically, granulocyte-derived arginase was shown to decrease NK cell IFN-γ production with little to no effect on IFN-γ transcription, NK cell viability, or granule release (51). Our results expand on these findings and identify a defect in mTOR activation as a potential mechanism by which arginase-1 activity impairs NK cell IFN-γ production. It is interesting that the percentages of cells expressing phosphor-mTOR are not significantly different, but the MFIs are, and on the other hand, the percentages of cells expressing phosphor-4EBP1 are different but the MFI isn’t. We attribute these observations to the fact that most cells would express some level of phosphor-mTOR when stimulated by IL-12/IL-18, but when the NK cells have sufficient arginine, more mTOR molecules become activated. We propose two reasons why there is no difference in MFI of phosphor-4EBP1. On the other hand, activated mTOR can complexed into mTORC1 and mTORC2, and mTORC2 does not activate 4EBP1. Another possibility would be due to the effects seen at various time points. At this 48h time point, we observed no difference in MFI, however, it doesn’t exclude the possibility that the MFI would be different over a longer suppression of the mTOR pathway. The importance of the mTOR pathway to NK cell activation was also recently demonstrated in mice, where the absence of mTOR signaling impaired nutrient uptake and acquisition of effector function, particularly IFN-γ, in NK cells (26). Given that the liver is essential for amino acid metabolism, it is intriguing to contemplate how the effect of MDSCs on NK cells would be further compounded by the metabolic status of the liver during HCV infection. In fact, postprandial increases in viral titers are well documented in HCV patients (52). However, the specific contribution of amino acids to virus production and metabolic regulation of hepatic immune responses is largely unknown and may prove useful in preventing liver damage and promoting recovery following tissue injury.
Importantly, arginase-1 mediated inhibition of NK cell effector function was limited to IFN-γ as granzyme B production was unaffected in the presence of HCV-induced MDSCs (Fig. 1E-F). Differences in processing and storage of granzyme B and IFN-γ may explain the IFNγ-specific defect in NK cells cultured with HCV-conditioned CD33+ cells. Granzyme B, a serine protease that induces apoptosis in target cells, is stored in preformed granules in human peripheral blood lymphocytes (53). In contrast, NK cells store IFN-γ as transcript, which is then translated upon stimulation (54). Our results demonstrate that IFN-γ production in NK cells is inhibited at the post-transcriptional stage, as IFN-γ mRNA was present in equal amounts in NK cells cultured with mock- or HCV-conditioned CD33+ cells (Fig. 2C). Because granzyme B release by NK cells is not dependent on translation, it could explain why the effect of HCV-induced MDSCs on NK cells was limited to IFN-γ production.
Surprisingly, the specific defect in IFN-γ production in NK cells was not due to changes in differentiation status. As shown in Fig. 2D-E, expression of CD56 was not significantly altered in NK cells cultured with HCV-conditioned CD33+ cells. CD56 is a marker of differentiation in human NK cells: CD56bright cells readily produce cytokines, have minimal cytolytic activity, and are thought to give rise to the more mature, CD56dim population that is both cytolytic and capable of producing cytokines (55). Although both hepatic and peripheral blood NK cells express CD56, liver-resident NK cells are defined as CD56+ while 90% of NK cells found in blood are CD56dim (16). Because our analysis was performed on NK cells derived from PBMCs of healthy individuals, they most likely represent the responses of mature CD56dim NK cells that would infiltrate the liver during infection (56). It would be interesting to compare our results to those of hepatic NK cells in humans, as liver-resident NK cells have been distinguished from conventional NK cells in mice both developmentally and in effector function (57).
Indeed, the importance of NK cells to hepatic inflammation cannot be understated as they are a major lymphocytic subset in the liver (10). Hepatic NK cells are essential not only for conventional immune responses against pathogens, but also for maintenance of tissue homeostasis. For example, NK cells regulate the development of liver fibrosis by killing hepatic stellate cells, which are the major source of matrix deposition during liver injury (58). NK cells also control hepatic inflammation by stimulating the production of IL-6 by Kupffer cells (59) and inducing apoptosis in activated NKT and T cells (60). These findings are particularly informative when considering that dysregulated activation of macrophages and lymphocytes drives immunopathology in chronic inflammatory diseases. Given that chronic inflammation is a hallmark of numerous liver diseases, such as viral hepatitis, alcoholic and non-alcoholic steatohepatitis, autoimmune hepatitis, and hepatocellular carcinoma, the extensive crosstalk between NK cells and other hepatic cells may play a critical role in both removing the inflammatory insult and in restoring tissue homeostasis. Consequently, our findings describing a role for suppressive myeloid populations in controlling NK cell responses may be equally beneficial in shifting chronic inflammation into an anti-inflammatory response.
In conclusion, we show that HCV-induced MDSCs suppress NK cell IFN-γ production via an arginase-1-dependent loss of L-arginine, resulting in defective mTOR signaling. Given that NK cells play a crucial role in anti-viral immunity via cytolysis of infected cells and cytokine production, understanding how MDSCs affect NK cells provides novel insight into mechanisms that regulate NK cell function. Moreover, these results challenge us to consider the effect of MDSCs on other cells in the liver including Kupffer cells, hepatocytes, and stellate cells, all of which are key players in the progression of chronic liver diseases. Further exploration of the interplay between myeloid cells and other hepatic immune cells may thus help identify key molecular regulators that can resolve chronic inflammation and restore immune homeostasis.
Supplementary Material
Acknowledgements
We thank the members of the Hahn lab for providing critical advice on this work. In particular, we specially thank Ms. Sowmya Narayanan for thoughtful discussion on our studies and critical comments on the manuscript.
This work was supported by NIH Grants AI057591 and U19 AI066328 to Y.S.H., and T32 GM08715-14 and T32 AI07496 to C.G. This work was supported in part by a NIGMS MARC-U-STAR grant # 1T34GM105550.
Abbreviations
- HCV
hepatitis C virus
- NK
natural killer
- IFN-γ
interferon-γ
- PBMC
peripheral blood mononuclear cell
- MDSC
myeloid derived suppressor cell
- Arg-1
arginase-1
- ROS
reactive oxygen species
- iNOS
inducible nitric oxide synthase
- L-NMMA
L-NG-monomethyl L-arginineacetate
- Nor-NOHA
N(ω)-hydroxy-nor-L-arginine
- mTOR
mammalian target of rapamycin
- 4EBP1
elongation initiation factor 4E
References
- 1.Chinnadurai R, Velazquez V, Grakoui A. Hepatic Transplant and HCV: A New Playground for an Old Virus. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 2.Roohvand F, Kossari N. Advances in hepatitis C virus vaccines, part one: advances in basic knowledge for hepatitis C virus vaccine design. Expert Opin Ther Pat. 2011 doi: 10.1517/13543776.2011.630662. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH. Hepatitis C: The Clinical Spectrum of Disease. Hepatology. 1997:173–195. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 4.Tacke RS, Tosello-Trampont A, Nguyen V, Mullins DW, Hahn YS. Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J. Biol. Chem. 2011;286:10847–10855. doi: 10.1074/jbc.M110.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn YS. Subversion of immune responses by hepatitis C virus: immunomodulatory strategies beyond evasion? Curr. Opin. Immunol. 2003;15:443–449. doi: 10.1016/s0952-7915(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 6.Lee H-C, Sung S-SJ, Krueger PD, Jo Y-A, Rosen HR, Ziegler SF, Hahn YS. Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology. 2013;57:1314–1324. doi: 10.1002/hep.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 8.Hartling HJ, Gaardbo JC, Ronit A, Knudsen LS, Ullum H, Vainer B, Clausen MR, Skogstrand K, Gerstoft J, Nielsen SD. CD4? and CD8? regulatory T cells (Tregs) are elevated and display an active phenotype in patients with chronic HCV mono-infection and HIV/HCV co-infection. Scand J Immunol. 2012;76:294–305. doi: 10.1111/j.1365-3083.2012.02725.x. [DOI] [PubMed] [Google Scholar]
- 9.Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell. Mol. Immunol. 2013;10:202–212. doi: 10.1038/cmi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walzer T, Dalod M, Vivier E, Zitvogel L. Natural killer cell-dendritic cell crosstalk in the initiation of immune responses. Expert Opin Biol Ther. 2005;5(Suppl 1):S49–59. doi: 10.1517/14712598.5.1.s49. [DOI] [PubMed] [Google Scholar]
- 14.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J. Exp. Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, Titerence RH, Liang TJ, Rehermann B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–9. doi: 10.1053/j.gastro.2011.06.069. 1239.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeromski J, Mozer-Lisewska I, Kaczmarek M, Kowala-Piaskowska A, Sikora J. NK cells prevalence, subsets and function in viral hepatitis C. Arch. Immunol. Ther. Exp. (Warsz.) 2011;59:449–455. doi: 10.1007/s00005-011-0145-y. [DOI] [PubMed] [Google Scholar]
- 17.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56 brightnatural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y-Y, Yang B-Y, Wu C-Y. Phenotypically and functionally distinct subsets of natural killer cells in human PBMCs. Cell Biol. Int. 2008;32:188–197. doi: 10.1016/j.cellbi.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez VD, Falconer K, Björkström NK, Blom KG, Weiland O, Ljunggren H-G, Alaeus A, Sandberg JK. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J. Immunol. 2009;183:6612–6618. doi: 10.4049/jimmunol.0901437. [DOI] [PubMed] [Google Scholar]
- 20.Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 2013;255:210–221. doi: 10.1111/imr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacke R, Lee H-C, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T cell responses through the production of reactive oxygen species. Hepatology. 2011 doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce EL, Poffenberger MC, Chang C-H, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454–1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert V, Hall MN. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2014;33C:55–66. doi: 10.1016/j.ceb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Marçais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, Rabilloud J, Mayol K, Tavares A, Bienvenu J, Gangloff Y-G, Gilson E, Vivier E, Walzer T. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014;15:749–757. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandagopal N, Ali AK, Komal AK, Lee S-H. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions. Front Immunol. 2014;5:187. doi: 10.3389/fimmu.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altomare DA, Khaled AR. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr. Med. Chem. 2012;19:3748–3762. doi: 10.2174/092986712801661130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonenberg N, Gingras AC. The mRNA 5' cap-binding protein eIF4E and control of cell growth. Curr. Opin. Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 31.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am. J. Physiol. Endocrinol. Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, Zou Y, Mao D, Sun D, Gao G, Shi J, Liu X, Zhu C, Yang M, Ye W, Hao Q, Li R, Yu L. The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J. Cell Biol. 2014;206:173–182. doi: 10.1083/jcb.201403009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Showkat M, Beigh MA, Andrabi KI. mTOR Signaling in Protein Translation Regulation: Implications in Cancer Genesis and Therapeutic Interventions. Mol Biol Int. 2014;2014:686984–14. doi: 10.1155/2014/686984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boultwood J, Yip BH, Vuppusetty C, Pellagatti A, Wainscoat JS. Activation of the mTOR pathway by the amino acid (L)-leucine in the 5q- syndrome and other ribosomopathies. Adv Biol Regul. 2013;53:8–17. doi: 10.1016/j.jbior.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golden-Mason L, Rosen HR. Natural killer cells: multifaceted players with key roles in hepatitis C immunity. Immunol. Rev. 2013;255:68–81. doi: 10.1111/imr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 40.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaraj S, Schrum AG, Cho H-I, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 43.Morris SM. Recent advances in arginine metabolism: roles and regulation of the arginases. Br. J. Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong X, Tan B, Yin Y, Gao H, Li X, Jaeger LA, Bazer FW, Wu G. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J. Nutr. Biochem. 2012;23:1178–1183. doi: 10.1016/j.jnutbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao W, Sun B, Feitelson MA, Wu T, Tur-Kaspa R, Fan Q. Hepatitis C virus targets over-expression of arginase I in hepatocarcinogenesis. Int. J. Cancer. 2009;124:2886–2892. doi: 10.1002/ijc.24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, Xu M, Fu Y, Zhou J, Tang X. Clinical Significance and Functional Studies of Myeloid-Derived Suppressor Cells in Chronic Hepatitis C Patients. J. Clin. Immunol. 2013 doi: 10.1007/s10875-012-9861-2. [DOI] [PubMed] [Google Scholar]
- 48.Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, Singh KP, Thomas N, Das A, Chen A, Fusai G, Bertoletti A, Cantrell DA, Kennedy PT, Davies NA, Haniffa M, Maini MK. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat. Med. 2015 doi: 10.1038/nm.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Q-L, Yang B, Sun H-Q, Feng G-H, Jin L, Zou Z-S, Zhang Z, Zhang J-Y, Wang F-S. Myeloid-derived suppressor cells are associated with viral persistence and downregulation of TCR ζ chain expression on CD8(+) T cells in chronic hepatitis C patients. Mol. Cells. 2014;37:66–73. doi: 10.14348/molcells.2014.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamas B, Vergnaud-Gauduchon J, Goncalves-Mendes N, Perche O, Rossary A, Vasson M-P, Farges M-C. Altered functions of natural killer cells in response to L-Arginine availability. Cell. Immunol. 2012;280:182–190. doi: 10.1016/j.cellimm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Oberlies J, Watzl C, Giese T, Luckner C, Kropf P, Müller I, Ho AD, Munder M. Regulation of NK cell function by human granulocyte arginase. J. Immunol. 2009;182:5259–5267. doi: 10.4049/jimmunol.0803523. [DOI] [PubMed] [Google Scholar]
- 52.Felmlee DJ, Sheridan DA, Bridge SH, Nielsen SU, Milne RW, Packard CJ, Caslake MJ, McLauchlan J, Toms GL, Neely RDG, Bassendine MF. Intravascular transfer contributes to postprandial increase in numbers of very-low-density hepatitis C virus particles. Gastroenterology. 2010;139:1774–83. doi: 10.1053/j.gastro.2010.07.047. 1783.e1–6. [DOI] [PubMed] [Google Scholar]
- 53.Bratke K, Kuepper M, Bade B, Virchow JC, Luttmann W. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur. J. Immunol. 2005;35:2608–2616. doi: 10.1002/eji.200526122. [DOI] [PubMed] [Google Scholar]
- 54.Hodge DL, Martinez A, Julias JG, Taylor LS, Young HA. Regulation of nuclear gamma interferon gene expression by interleukin 12 (IL-12) and IL-2 represents a novel form of posttranscriptional control. Mol. Cell. Biol. 2002;22:1742–1753. doi: 10.1128/MCB.22.6.1742-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proceedings of the National Academy of Sciences. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moretta L. Dissecting CD56dim human NK cells. Blood. 2010;116:3689–3691. doi: 10.1182/blood-2010-09-303057. [DOI] [PubMed] [Google Scholar]
- 57.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 59.Cheng C-W, Duwaerts CC, Rooijen NV, Wintermeyer P, Mott S, Gregory SH. NK cells suppress experimental cholestatic liver injury by an interleukin-6-mediated, Kupffer cell-dependent mechanism. J. Hepatol. 2011;54:746–752. doi: 10.1016/j.jhep.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Sun R, Wei H, Dong Z, Gao B, Tian Z. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J. Hepatol. 2006;44:446–454. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.