Abstract
Survivin is a member of the inhibitor of apoptosis family of proteins and a biomarker of poor prognosis in aggressive B cell non-Hodgkin’s lymphoma (B-NHL). In addition to its role in inhibition of apoptosis, survivin also regulates mitosis. Here, we show that deletion of survivin during early B cell development results in a complete block at the cycling pre-B stage. In the periphery, B cell homeostasis is not affected, but survivin-deficient B cells are unable to mount humoral responses. Correspondingly, we show that survivin is required for cell division in response to mitogenic stimulation. Thus, survivin is essential for proliferation of B cell progenitors and activated mature B cells, but is dispensable for B cell survival. Moreover, a small molecule inhibitor of survivin strongly impaired the growth of representative B lymphoma lines in vitro, support the validity of survivin as an attractive therapeutic target for high-grade B-NHL.
INTRODUCTION
Survivin/TIAP (encoded by Birc5) is a ubiquitously-expressed member of the inhibitor of apoptosis (IAP) family of proteins that regulates apoptosis via association with effector caspases such as caspases 3 and 7, preventing their cleavage and subsequent activation by caspase 9 (1–3). In addition to the regulation of apoptosis, survivin is a component of the chromosomal passenger complex (CPC) that facilitates chromosome segregation during mitosis (4–6). Correspondingly, survivin is highly expressed in cells actively undergoing cell division, including fetal tissues, activated lymphocytes, and many types of cancer (7, 8).
Gene targeting experiments demonstrated a role for survivin in pre-TCR-driven expansion of early T cells, as well as in homeostatic and mitogen-induced proliferation of mature T cells (9). Further studies showed that T cell co-stimulation induces the expression of survivin, which regulates the activity of aurora B kinase and, subsequently, the activity of the CPC (10, 11). Despite the high level of interest in survivin as a therapeutic target in malignant B cells, the importance of survivin in normal B cells is unknown. To this end, we generated two mouse lines with B cell stage-specific inactivation of survivin. Loss of survivin during early B cell development resulted in a block at the cycling pre-B cell stage with a consequent loss of mature B cells. In contrast, B cell homeostasis was not altered following deletion of survivin at late stages of maturation in the spleen. We further show that survivin-deficient B cells exhibit impaired cell division in vitro and severely impaired humoral responses in vivo. Taken together, we establish that in B cells, survivin functions as an essential regulator of cell division, but does not directly regulate apoptosis.
MATERIALS AND METHODS
Mice
SurvivinL/L mice (9) were crossed with mb1Cre (12) or cd21Cre (13) mice. All animals were maintained in the animal facility of the Sanford Burnham Prebys Medical Discovery Institute. All protocols were approved by the Institutional Animal Care and Use Committee and were carried out in accordance with institutional guidelines and regulations.
Flow Cytometry and Antibodies
Single cell suspensions were prepared, counted, and stained with antibodies according to standard procedures. The following antibodies from eBioscience (San Diego, CA) were used: CD3 (145-2C11), IgM (II/41), IgD (11-26), CD19 (ID3), B220 (RA3-6B2), BP-1 (6C3), CD11b (M1/70), CD43 (S7), CD21/35 (4E3), CD23 (B3B4), CD4 (GK1.5), CD8 (53-6.7). The following antibodies from BD Pharmingen (San Diego, CA) were used: IgG1 (A85-1), Fas (Jo2). The antibody directed against pH2AX (20E3) was purchased from Cell Signaling Technology (Danvers, MA). Biotinylated reagents were detected with streptavidin (SA) conjugated to PerCP-Cy5.5 (BD Biosciences, San Jose, CA). To stain for pH2AX, cells were fixed with 2% paraformaldehyde in PBS for 10 mins at room temperature, washed, permeabilized with 70% methanol for 30 mins on ice, washed twice and incubated with the anti-pH2AX antibody for 1 hour on ice. To stain DNA content, cells were fixed with paraformaldehyde, permeabilized with 70% methanol overnight and stained with 500 µL DAPI solution (10 µg/mL DAPI + 0.1% TritonX in PBS). Data were collected using a FACSCanto or a BD LSR Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR), or using the Amnis ImageStreamX MkII Imaging Flow Cytometer (EMD Millipore, Billerica, MA).
Cell Culture, Survival, and Proliferation Assays
For 3H-thymidine incorporation assays, purified splenic B cells were cultured at a concentration of 1×106 cells/mL in 96-well round-bottom tissue culture plates at 37°C with different stimuli as indicated. After 48 hrs, cells were pulsed with 1 µCi 3H-thymidine for 16 hrs, and then collected and scintillation counted. To analyze proliferation, cells were loaded with the Cell Proliferation Dye eFluor670 (eBioscience) and cultured for 3 days in complete RPMI medium (RPMI (Corning Cellgro) + 10% FBS (Sigma) + 1× penicillin/streptomycin (Corning) + 1 mM sodium pyruvate (Cellgro) + 2 mM GlutaGro (Cellgro) + 1× MEM non essential amino acids (Cellgro) + 50 µM β-mercaptoethanol (Gibco)). The following stimuli were used: anti-IgM (Jackson Laboratories, West Grove, PA), LPS (Sigma, St.Louis, MO), anti-CD40 (eBioscience), rmBAFF (R&D Systems, Minneapolis, MN), IL-4 (eBioscience). To measure in vivo B cell turnover, mice were continuously provided 0.5 mg/mL BrdU (Sigma) + 2% sucrose in the drinking water for 7 weeks. Bone marrow and splenic cells were isolated and stained with antibodies as indicated. Cells were fixed with BD Cytofix/Cytoperm (BD Biosciences) and permeabilized with permeabilization buffer (eBioscience), followed by a second permeabilization step with 0.1% Triton X-100 (Sigma), fixed again and treated with DNase (Sigma). The cells were then stained with an anti-BrdU antibody and analyzed by flow cytometry. To analyze cell growth of different lymphoma lines 2×104 cells were plated in 100µl medium and incubated for 1,2 or 3 days. The survivin inhibitor S12 (Calbiochem, EMD Millipore, Billerica, MA) was dissolved in DMSO to a concentration of 100mM. Cells were treated with a 1: 20000 (5 µM), 1: 4000 (25 µM), 1: 3000 (33 µM), 1: 2000 (50 µM) dilution of the S12 stock solution. Untreated cells were cultured with 0.03% DMSO. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Rockville, MD) according to the manufacturers instructions. The optical density (OD) value obtained from a blank sample was subtracted from all values measured.
Immunization and enzyme-linked immunosorbent assay (ELISA)
For TI-II immunization, mice were immunized (i.p.) with 10 µg TNP(24)-AECM-Ficoll (Biosearch Technologies, Novato, CA) in PBS and serum was collected prior to and five days post-injection. To detect antigen specific antibodies or the total IgM and IgG serum levels, polystyrene plates were coated with TNP(26)-BSA (Biosearch Technologies) or polyclonal anti-mouse IgM or IgG and blocked with BSA. Serial dilutions of serum collected at the indicated time points were added followed by detection using anti-IgM or anti-IgG coupled to AP (Bethyl Laboratories, Montgomery, TX). Mouse reference serum was used for quantitation of innate Ig (Bethyl Laboratories). For antigen specific antibodies, a sample of pooled sera served as standard defining arbitrary units. PNPP (Sigma-Aldrich) was added and absorbance was measured at 405nm. For TD immunization, mice were i.p. injected with 100 µL of packed sheep red blood cells (SRBC) and euthanized 7 days later. The spleen was collected for the analysis of the germinal center reaction by flow cytometry and histology. Serum was collected on day 0 and day 7. To measure the levels of SRBC-specific antibodies in the serum, 20 µL of packed SRBC were incubated with serial serum dilutions, washed and incubated with anti-mouse IgG1 and IgM. Mean fluorescence intensities of the bound IgM and IgG1 were determined by flow cytometry.
Histology
Whole spleens were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek U.S.A., Torrance, CA) and frozen at −80°C. Six µm cryo-sections were mounted on microscope slides, fixed for 10 mins in cold acetone, blocked for 1 hour with 1% BSA + 5% FBS in PBS, and stained with the indicated antibodies. Sections were mounted with Gel/Mount (Biomeda Corp, Foster City, CA) and sealed with glass coverslips. Images were acquired using a Zeiss Axio ImagerM1 microscope (Zeiss, Thornwood, NY) and Slidebook software (Intelligent Imaging Innovations, Denver, CO).
Results
Survivin is required for B cell development in the bone marrow
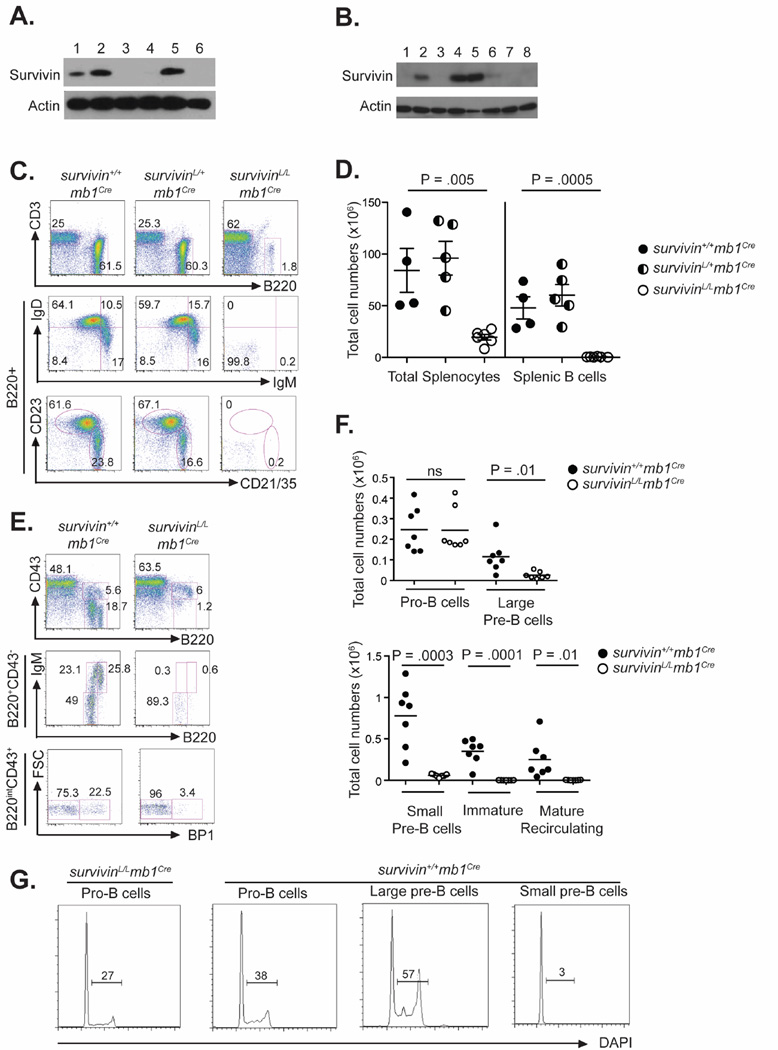
Survivin is known to associate with the mitotic spindle and thus can be found in proliferating cells such as fetal tissue and most tumor cell types (8). In the B cell lineage, we found survivin to be expressed in CD43+B220+IgM−IgD− cells in the bone marrow (Fig. 1A). This population includes large pre-B cells, a population of highly proliferating cells. Survivin expression was maintained through the small pre-B cell stage and lost in immature B cells (Fig. 1A). Neither mature recirculating B cells in the bone marrow, nor naïve B cells in the spleen expressed detectable levels of survivin (Fig. 1A). Survivin expression was induced in mature B cells after stimulation with all mitogens tested (Anti-IgM (Fab2), LPS, CpG) (Fig. 1B). Survivin levels were low in cell lysates from anti-CD40 stimulated cells; in agreement with low mitogenic properties of this stimulant (Fig. 1B). Unstimulated cells, cells stimulated with the pro-survival stimulants BAFF and APRIL and cells stimulated with an intact anti-IgM antibody, which is able to simultaneously engage the B cell receptor and inhibitory Fc-receptors, did not lead to survivin expression (Fig. 1B). Ex vivo germinal center B cells (GC), which are known to be highly proliferative, expressed survivin (Fig. 1A). Taken together, these data suggest that survivin expression is induced by a broad array of mitogenic stimulants and is present in proliferating B cell subpopulations.
Figure 1. Survivin expression is required for B cell development in the bone marrow.
(A) Survivin expression in: (1) pro- and large pre-B cells (CD43+, B220+, IgM−, IgD−), (2) small pre-B cells (CD43−, B220+, IgM−, IgD−), (3) immature B cells (CD43−, B220+, IgM+, IgD−), (4) mature recirculating cells (CD43−, B220+, IgM+, IgD+), (5) GC B cells (CD11c−, CD43−, IgD−) and (6) non GC B cells (CD11c−, CD43−, GL7−) determined by western blot. Actin was used as a loading control. One of two independent experiments is shown. (B) Survivin expression in (1) unstimulated B cells or treated with (2, 3) 10µg/mL anti-IgM (intact or F(ab’)2 fragment, (4) 10 µg/mL LPS, (5) 5 µg/mL CpG (6) 5 µg/mL anti-CD40, (7) 25 ng/mL BAFF or (8) 100ng/mL APRIL (C) Flow cytometric analysis of splenocytes from survivinL/Lmb1Cre, survivinL/+mb1Cre, and survivin+/+mb1Cre mice with indicated antibodies. (D) Number of total splenocytes and splenic B cells from survivinL/Lmb1Cre, survivinL/+mb1Cre, and survivin+/+mb1Cre mice. (E) Flow cytometric analysis of early B cell compartment in the BM of survivinL/Lmb1Cre and survivin+/+mb1Cre mice. (F) Absolute numbers of B lineage cells at each stage of maturation in the BM of survivinL/Lmb1Cre and survivin+/+mb1Cre mice. (G) Cell cycle analysis of pro- and pre- B cells from survivinL/Lmb1Cre and survivin+/+mb1Cre mice. Results are representative of 2 independent experiments.
To determine the role of survivin in B lymphocyte development, we intercrossed mice bearing a loxP-flanked survivin allele (survivinL/+) with mb1Cre/+ mice in which Cre expression is induced in early pro-B cells (12). Spleens from survivinL/Lmb1Cre mice were devoid of mature B cells, in stark contrast with control survivin+/+mb1Cre (WT) mice (Fig. 1C,D). Notably, survivinL/+mb1Cre mice were essentially identical to WT controls indicating that one copy of survivin is sufficient to support normal B cell development (Fig. 1C,D). Similar results were found upon analysis of lymph nodes (LNs) from WT and survivinL/Lmb1Cre mice (data not shown). T cell subsets and numbers in spleens and LNs were not different between WT, heterozygous, and survivinL/Lmb1Cre mice (data not shown).
To analyze the role of survivin in early B cell development, we examined the bone marrow (BM) of control and survivinL/Lmb1Cre mice. SurvivinL/Lmb1Cre mice showed normal numbers of pro-B cells (B220+, CD43+, BP1−), but significantly decreased numbers of large (B220+, CD43+, BP1+) and small (B220+, IgM−, CD43−) pre-B cells (Fig. 1E,F). Immature (B220+, IgM+, CD43−) and mature recirculating (B220hi, IgM+, CD43−) B cells were absent from the bone marrow of survivinL/Lmb1Cre mice (Fig. 1E,F). The developmental stage of large pre-B cells is characterized by a proliferative burst, and we show that survivinL/Lmb1Cre B cells do not develop beyond this stage (Fig. 1G).
Survivin is dispensable for the maintenance of mature recirculating B cells
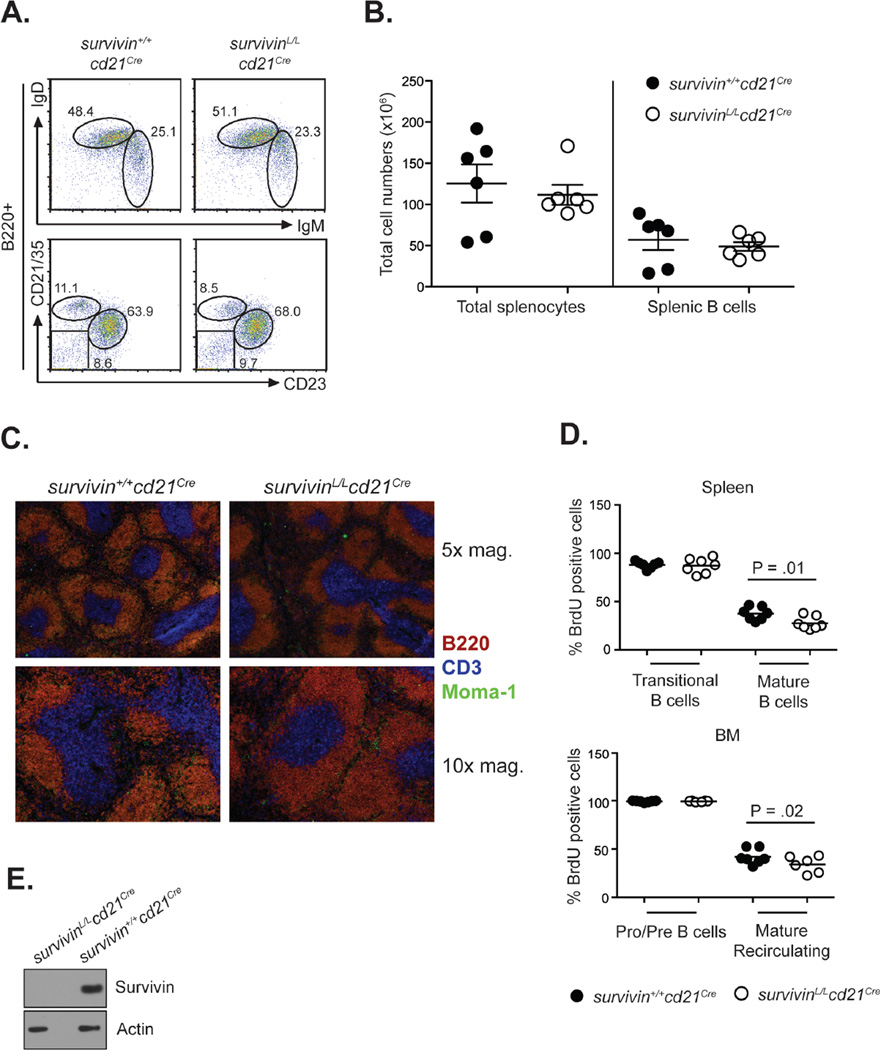
While B cell development in the BM requires proliferation, mature recirculating B cells are largely quiescent, but depend on tonic signaling via the BCR and BAFF-R for continued survival. We used cd21Cre mice to generate a mouse in which survivin is deleted during the transitional phase of B cell maturation in the spleen (13). We found that the relative frequency and absolute numbers of peripheral B cell populations were not significantly different between survivin+/+cd21Cre and survivinL/Lcd21Cre mice (Fig. 2A, B). Consistent with these data, no differences were observed in the splenic architecture between WT and survivinL/Lcd21Cre mice (Fig. 2C).
Figure 2. Survival of mature B cells is survivin-independent.
(A) Flow cytometric analysis of splenocytes from survivinL/Lcd21Cre and survivin+/+cd21Cre mice with indicated antibodies. (B) Enumeration of splenocytes and splenic B cells in survivinL/Lcd21Cre and survivin+/+cd21Cre mice. (C) Immunohistology of spleens from survivinL/Lcd21Cre and survivinL/+cd21Cre mice. Red = B220+ B cells; Blue = CD3+ T cells; Green = moma-1+ macrophages. (D) SurvivinL/Lcd21Cre and survivin+/+cd21Cre mice were continuously provided BrdU in the drinking water for a 7 week period. The graphs show the percentage of BrdU-positive cells in the indicated B cell subpopulations in the spleen and BM. (E) Deletion efficiency of survivin in LPS (10 µg/mL) stimulated B cells from survivinL/Lcd21Cre and survivin+/+cd21Cre mice after 2 days of culture.
To analyze the turnover rate of the mature B cell compartment in the absence of survivin, survivinL/Lcd21Cre mice were provided BrdU continuously in their drinking water and sacrificed after 7 weeks. As expected, all pro/pre B cells in the bone marrow from both survivinL/Lcd21Cre and survivin+/+cd21Cre mice had incorporated BrdU at this time point (Fig. 2D). Similarly, transitional splenic B cells from both survivinL/Lcd21Cre and survivin+/+cd21Cre mice were efficiently labeled with BrdU at this time point. The average percentage of BrdU+ mature B cells in the spleen and in the bone marrow in survivin+/+cd21Cre mice was 38% and 42%, respectively. This observation is consistent with the reported half-life of 6 weeks for normal B cells (Fig. 2D) (14). The percentage of BrdU+ B cells in the population of mature B cells in the spleen and bone marrow was slightly, but significantly lower in survivinL/Lcd21Cre mice than in survivin+/+cd21Cre mice (28% and 34%, respectively, Fig. 2D). The slower turnover of mature B cells in survivinL/Lcd21Cre mice indicates that propagation of mature B cells does not require survivin.
To confirm that deletion was efficient in mature B cells from the survivinL/Lcd21Cre line, we performed western blots on cultured splenic B cells. For comparison, we chose LPS stimulated cells, since survivin expression is strongly induced in proliferating cells (Fig. 1B). While survivin was strongly expressed in LPS-stimulated survivin+/+cd21Cre B cells, no survivin protein was detected in LPS-stimulated survivinL/Lcd21Cre B cells (Fig. 2E).
Survivin is required for the generation of natural antibodies
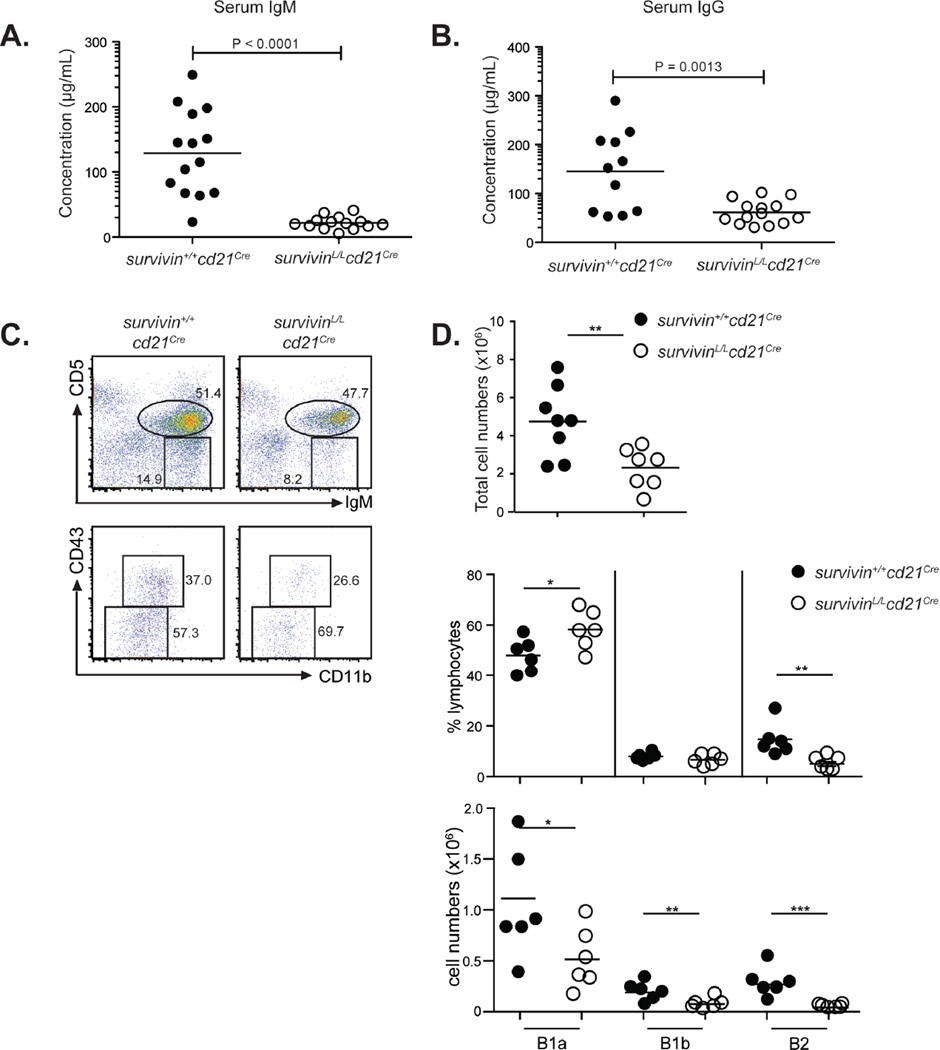
Peritoneal B cells represent a heterogeneous mixture of cells with unique signaling properties and function. B-1 cells are able to undergo homeostatic proliferation, often express BCRs directed against common bacterial pathogens and are believed to be the main producers of natural antibodies (15). We analyzed the levels of natural antibodies in the serum from survivinL/Lcd21Cre and survivin+/+cd21Cre mice and found that both IgM and IgG levels were significantly decreased in the serum from naïve survivinL/Lcd21Cre mice (Fig. 3A,B). Analysis of peritoneal B cell subpopulations revealed that the absolute cell numbers of B1a (CD5lo, IgM+), B1b (CD5−, IgM+, CD43+, CD11b+) and B2 (CD5−, IgM+, CD43−, CD11b−) cell populations were all significantly decreased in survivinL/Lcd21Cre mice (Fig. 3C,D). In summary, these data suggest that survivin is necessary for the generation of natural antibodies, possibly by supporting the homeostasis of peritoneal B cells.
Figure 3. Survivin is required for the production of natural antibodies.
(A) Total serum IgM and (B) total serum IgG levels from unimmunized survivinL/Lcd21Cre and survivin+/+cd21Cre mice. (C) Flow cytometric analysis of peritoneal cavity cells from survivinL/Lcd21Cre and survivin+/+cd21Cre mice with indicated antibodies. (D) Graphs show total cell numbers in the peritoneal cavity (top panel), the relative frequency of B1a, B1b and B 2 cells in the population of lymphocytes (middle) and the absolute cell numbers of B1a, B1b and B 2 cells (bottom) in the peritoneal cavities of survivinL/Lcd21Cre and survivin+/+cd21Cre mice.
Survivin-deficient mice fail to mount a humoral immune response
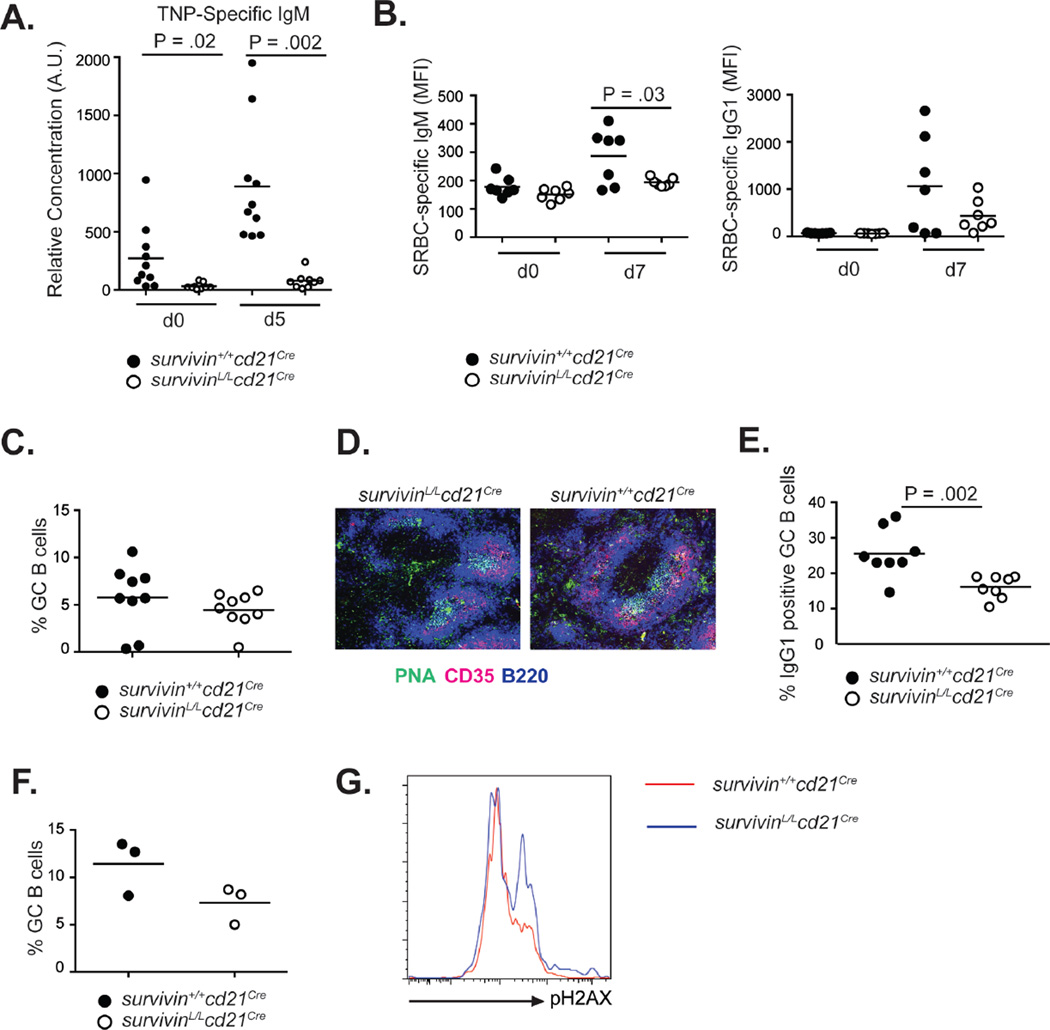
In response to antigen, B cells undergo several rounds of cell division before initiating immunoglobulin isotype switching or committing to terminal differentiation into plasma cells. To study the role of survivin in the humoral immune response, we immunized survivinL/Lcd21Cre and survivin+/+cd21Cre mice with the thymus-independent (TI) antigen trinitrophenyl (TNP)-conjugated Ficoll (TNP-Ficoll). SurvivinL/Lcd21Cre mice exhibited a profound defect in the production of TNP–specific IgM as compared to WT controls (Fig. 4A). Next, we immunized survivinL/Lcd21Cre and survivin+/+cd21Cre mice with sheep red blood cells (SRBC), a thymus-dependent (TD) antigen and analyzed the levels of SRBC-specific antibodies at the peak of the immune response. In comparison to control animals, survivinL/Lcd21Cre mice produced slightly lower levels of SRBC-specific IgG1 and significantly reduced levels of SRBC-specific IgM (Fig. 4B). For both IgM and IgG, there was little increase in antigen-specific titers relative to pre-immune levels in survivinL/Lcd21Cre mice (Fig. 4B). Since an important aspect of a TD immune response is the generation of germinal center (GC) B cells, which are characterized by a high rate of proliferation, we analyzed the frequency of GC B cells in the spleens from SRBC immunized survivinL/Lcd21Cre and survivin+/+cd21Cre mice. As determined by flow cytometry, we found that the frequency of GC B cells (B220+, Fas+, GL7+) in survivinL/Lcd21Cre mice was slightly lower in comparison to survivin+/+cd21Cre mice, however this difference did not reach statistical significance (Fig. 4C). GCs were also readily detectable by histology in the spleens from both, survivinL/Lcd21Cre and survivin+/+cd21Cre mice (Fig. 4D). On the other hand, the frequency of GC B cells which have undergone isotype switching to IgG1 were significantly reduced in survivinL/Lcd21Cre mice (Fig. 4E). While GCs are only present in the spleens of immunized mice, Peyer‘s patches in naïve mice harbor large GCs due to constant exposure to microbial antigens from the gut lumen. Similarly to the spleen, B cells in the Peyer‘s patches from survivinL/Lcd21Cre mice were able to generate GCs (Fig. 4F), however a higher percentage of GC B cells were positive for the DNA damage marker pH2AX, suggesting increased occurrence of DNA strand breaks in survivin-deficient GC B cells.
Figure 4. Survivin is required for TI-2 and TD antibody responses.
(A) Relative concentration of TNP-specific IgM in sera from survivinL/Lcd21Cre and survivin+/+cd21Cre mice immunized with TNP-Ficoll. (B) Relative concentration of SRBC specific IgM and IgG1 in the sera from survivinL/Lcd21Cre and survivin+/+cd21Cre mice immunized with SRBC. (C) Relative frequency of GC B cells (B220+, GL7+, Fas+) within the splenic B cell compartment of survivinL/Lcd21Cre and survivin+/+cd21Cre mice 7 days post-SRBC immunization. (D) Immunohistology of spleens from survivinL/Lcd21Cre and survivin+/+cd21Cre mice 7 days post-SRBC immunization. B220 was used to detect B cells. PNA was used to detect GC B cells and CD35 was used to detect follicular dendritic cells. (E) Percentage of IgG1+ GC B cells on day 7 post-SRBC immunization. (F) Frequency of GC B cells within the B cell compartment in Peyer‘s Patches isolated from unimmunized survivinL/Lcd21Cre and survivin+/+cd21Cre mice. (G) Expression levels of phospho-H2AX in GC B cells from Peyer‘s patches isolated from unimmunized survivinL/Lcd21Cre and survivin+/+cd21Cre mice. Histograms are representative of 3 independent experiments.
Survivin is required for B cell proliferation, but not for inhibition of apoptosis
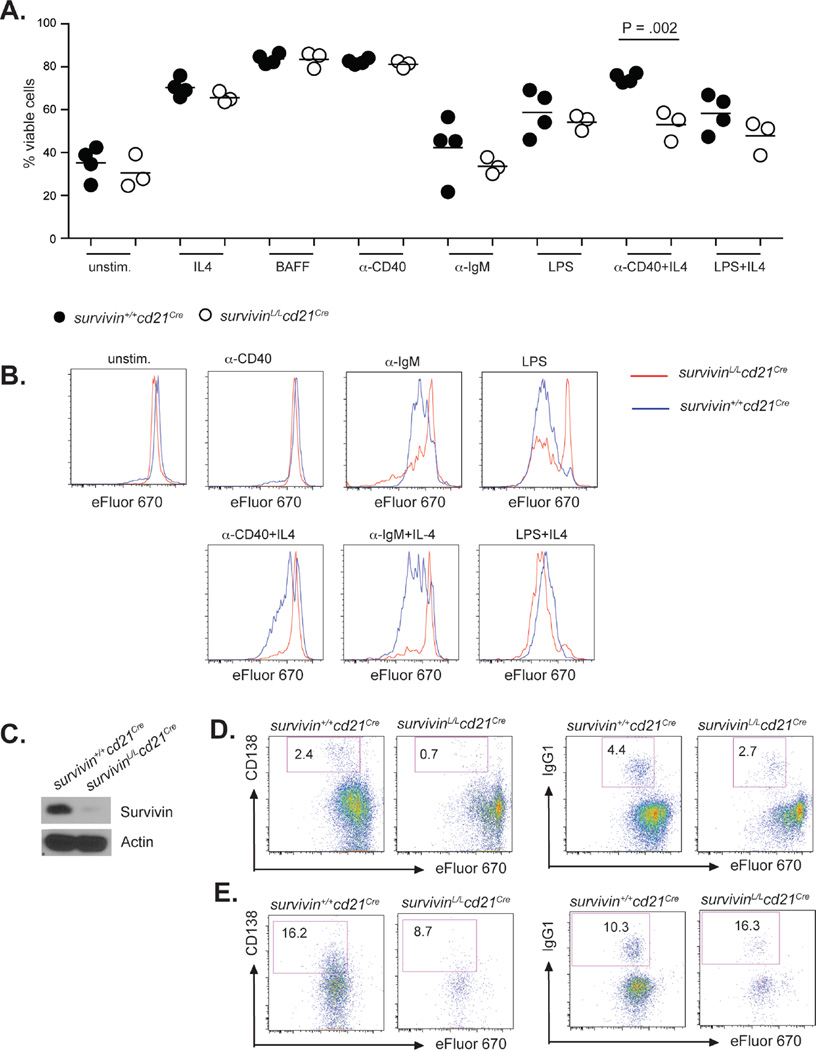
In other cell types, survivin has been shown to play a role in preventing apoptosis and supporting proliferation (3). To elucidate the role of survivin in B cell survival and proliferation, we cultured survivinL/Lcd21Cre B cells in the presence of the pro-survival stimuli IL-4, BAFF and anti-CD40, and mitogenic stimuli anti-IgM, LPS, anti-CD40+IL-4 and LPS+IL-4. Unstimulated survivinL/Lcd21Cre and survivin+/+cd21Cre B cells showed comparable viability after 3 days of cell culture (Fig. 5A). IL-4, BAFF and anti-CD40 enhanced viability equally well in survivinL/Lcd21Cre and survivin+/+cd21Cre B cells (Fig. 5A), suggesting that survivin does not play a major role in B cell survival in response to non-mitogenic stimulation. However, the frequency of viable SurvivinL/Lcd21Cre B cells was significantly decreased in the presence of anti-CD40+IL-4 as compared to survivin+/+cd21Cre B cells (Fig. 5A). The frequency of viable SurvivinL/Lcd21Cre B cells was also slightly decreased following treatment with anti-IgM-, LPS- or LPS+IL-4, but this difference did not reach statistical significance (Fig. 5A).
Figure 5. Survivin is required for B cell proliferation, but not survival of activated B cells.
(A) Splenic B cells from survivinL/Lcd21Cre and survivin+/+cd21Cre mice were cultured in the presence of the indicated stimuli for 3 days. Viability was determined by flow cytometry. The following concentrations were used: 10 ng/mL IL4; 10 ng/mL BAFF, 5 µg/mL anti-CD40, 13 µg/mL anti-IgM F(ab‘)2 fragments, 10 µg/mL LPS. Statistics were performed using the student’s t-test. (B) Splenic B cells from survivinL/Lcd21Cre and survivin+/+cd21Cre mice were cultured with the indicated stimuli for 3 days. Graphs show the dilution of the dye eFluro670 as a measure of proliferation. Histograms are representative for 3 independent experiments. (C) Survivin expression in B cells stimulated with LPS (10 µg/mL) plus IL-4 (10 ng/mL) for 3d. (D, E) Splenic B cells from survivinL/Lcd21Cre and survivin+/+cd21Cre mice were stimulated with anti-CD40 (5 µg/mL) plus IL-4 (10 ng/mL) (D) or LPS (10µg/mL) plus IL-4 (10ng/mL) (E). Plasma cell generation (left) and isotype switching (right) were measured by flow cytometry on day 3 of culture. Results are representative of 3 independent experiments.
Anti-IgM and LPS stimulation efficiently induced proliferation of survivin+/+cd21Cre B cells, however proliferation was strongly decreased in stimulated survivinL/Lcd21Cre B cells (Fig. 5B). Since IL-4-mediated B cell survival seems to be intact in survivinL/Lcd21Cre B cells, we included IL-4 in our cultures to test whether this pro-survival signal would rescue proliferation of survivinL/Lcd21Cre B cells. Interestingly, while proliferation of survivinL/Lcd21Cre B cells was not detected after anti-CD40+IL-4 and anti-IgM+IL-4 stimulation, LPS+IL-4-stimulated survivinL/Lcd21Cre B cells were able to proliferate (Fig. 5B). Deletion of survivin in these cells was confirmed by western blot (Fig. 5C). Differentiation into antibody-secreting cells and isotype switching are linked to proliferation. Consistently, survivinL/Lcd21Cre B cells failed to efficiently switch to IgG1 and to differentiate into plasma cells/plasmablasts after stimulations that failed to induce proliferation in survivinL/Lcd21Cre B cells such as anti-CD40+IL-4 (Fig. 5D). Notably, LPS+IL-4-stimulated survivinL/Lcd21Cre B cells that were able to undergo cell division, did not show defects in plasma cell generation or isotype switching (Fig. 5E).
Survivin deficient B cells accumulate aberrant levels of DNA after mitogenic stimulation
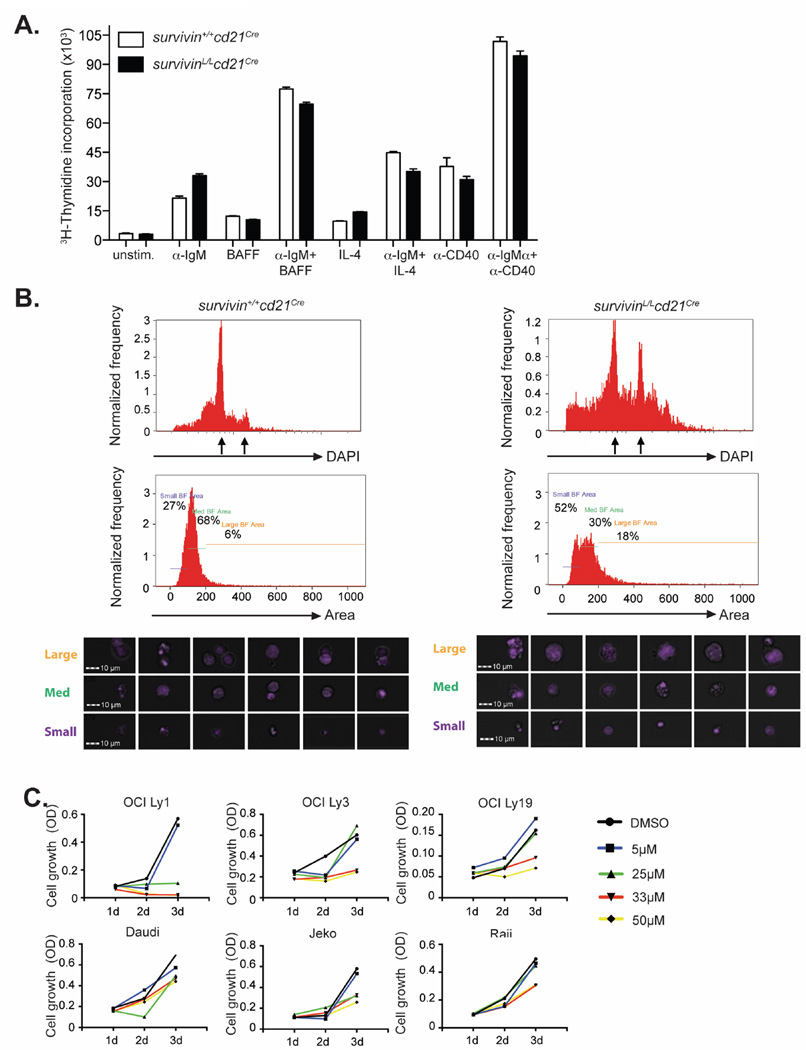
Since survivinL/Lcd21Cre B cells failed to proliferate after anti-IgM stimulation, we first tested whether these cells are able to undergo DNA replication. Survivin-deficient and control B cells showed similar levels of 3H-thymidine incorporation following stimulation with anti-IgM, indicating that in the absence of survivin, activated B cells can progress through S phase (Fig. 6A). Since survivin has been shown to associate with the mitotic spindle and to regulate cytokinesis (5, 6), we analyzed DNA content in LPS+IL4 stimulated cells. Survivin+/+cd21Cre samples included B cells in the G1/G0 -, S- and M- phases of the cell cycle (Fig. 6B). In addition, apoptotic cells with a subG1 DNA content could be detected. In stark contrast, survivinL/Lcd21Cre samples contained a prominent fraction of cells with >4N DNA content (Fig. 6B). Using the bright field area as a measure of cell size, we found abnormally large cells in the survivinL/Lcd21Cre samples that stained brightly for the DAPI DNA stain. Cells with these properties were absent from the survivin+/+cd21Cre samples. Events with a comparable bright field area value were less frequent in the survivin+/+cd21Cre samples and consisted mainly of cell aggregates. In summary, our data suggest that survivin-deficient B cells are able to initiate DNA synthesis, but cytokinesis is impaired.
Figure 6. Survivin-deficient B cells accumulate aberrant levels of DNA after mitogenic stimulation.
(A) B cells from survivinL/Lcd21Cre and survivin+/+cd21Cre mice were cultured unstimulated or in the presence of the indicated stimuli for 3 days. Graphs show 3H-thymidine incorporation. The following concentrations were used: 100ng/mL IL4; 25ng/mL BAFF, 5µg/mL anti-CD40, 10µg/mL anti-IgM (B) Morphology and DNA content of LPS (10 µg/mL) plus IL-4 (10 ng/mL) stimulated survivinL/Lcd21Cre and survivin+/+cd21Cre B cells on day 3 of culture was analyzed using an ImageStream Imaging Flow Cytometer. Arrows indicate 2n and 4n DNA content. Results are representative for 2 independent experiments. (C) Cell growth of OCI-Ly1 (GCB-DLBCL), OCI-Ly3 (ABC-DLBCL), OCI-Ly19 (GCB-DLBCL), Daudi (Burkitt‘s lymphoma), JeKo (Mantle cell lymphoma), Raji (Burkitt‘s lymphoma) cells 1, 2 and 3 days after the beginning of cell culture in the presence of the indicated concentration of the survivin inhibitor S12 was determined using a colorimetric cell counting assay. Displayed are the obtained OD values minus the value of a blank sample. The results are representative of three independent experiments.
Inhibition of survivin with a small molecule inhibitor reduces B lymphoma expansion
Survivin is expressed in the vast majority of cancer cell types and survivin over-expression is often associated with poor prognosis (8). To analyze whether survivin inhibition affects cell growth in lymphomas of B cell origin, we treated OCI-Ly1 (germinal center (GCB) diffuse large B cell lymphoma (DLBCL)), OCI-Ly3 (activated B cell (ABC) DLBCL), OCI-Ly19 (GCB DLBCL), Daudi (Burkitt‘s lymphoma), JeKo (Mantle cell lymphoma) and Raji (Burkitt‘s lymphoma) cells with increasing concentrations of the survivin inhibitor S12. Although the different lymphoma lines displayed variability in their sensitivity to survivin inhibition, 50 µM S12 showed an inhibitory effect on cell growth in all lines investigated (Fig. 6C). These results suggest that similar to normal proliferating B cells, transformed B cells require survivin for cell division.
Discussion
Survivin is highly expressed in many types of cancer and has been shown to regulate apoptosis and different aspects of the cell cycle (8). In cancer cells, elevated levels of survivin are often associated with an increased proliferative index (16–18) and inhibition of apoptosis (19, 20). Since survivin shows low expression in adult differentiated tissues, it has become an attractive target for cancer therapy. Understanding the role of survivin in normal tissue is therefore critical in order to assess side effects that survivin inhibition could have in cancer patients. In the present study, we analyzed the role of survivin in B cell development and differentiation. We found it to be required for B cell proliferation and therefore affecting B cell development and the humoral immune response. During B cell development, B cell precursors rearrange V(D)J segments in the immunoglobulin heavy chain locus. Productive rearrangement and successful expression of the pre-BCR induces clonal proliferation of large pre-B cells. We demonstrated that survivin is required to progress beyond this developmental stage.
Although survivin has been shown to promote survival in other cell types, deletion of survivin at the late transitional B cell stage in the spleen did not lead to a reduction in mature B cells. While this was not a surprising result given that survivin is not detectable by immunoblot in mature recirculating B cells, it remained possible that low or transient expression of survivin could be consequential. However, analysis of B cell turnover using BrdU labeling did not show any impairment in B cell survival in survivinL/Lcd21Cre mice. In agreement with this finding, survivin-deficient B cells showed comparable viability to control B cells when cultured in media alone or stimulated with the pro-survival factors BAFF, IL-4 or anti-CD40. Taken together, these findings establish that survivin is not essential for the survival of mature recirculating B cells.
In contrast to splenic follicular B cells, B cells in the peritoneal cavity were significantly reduced. This reduction in peritoneal B cells in survivinL/Lcd21Cre mice was accompanied by low titers of natural antibodies. B1 cells are the main producers of natural antibodies and homeostatic proliferation is believed to contribute to the maintenance of the B1 cell pool (15). Survivin deficiency may prevent B1 cell proliferation and thereby result in decreased B1 cell numbers in the peritoneal cavity. Apart from B1 cells, B2 cells are also found in the peritoneal cavity of mice and display a similar phenotype to follicular B cells of the spleen (21). Although surface marker expression on peritoneal B2 cells resembles that of follicular B cells, their gene expression and signaling properties are similar to those seen in B1 cells (21). Little is known about the origin and the maintenance of this B cell subpopulation; however peritoneal B2 cells have been shown to undergo homeostatic proliferation upon transfer into lymphopenic hosts (21). Similar to B1 cells, survivin expression may be necessary for B2 cell maintenance in the peritoneal cavity by facilitating cell division.
During an immune response, antigen-specific B cells undergo initial clonal expansion, which precedes immunoglobulin isotype switching and plasma cell differentiation (22). We showed that survivinL/Lcd21Cre mice produce low levels of antigen-specific antibody after immunization with TD or TI antigens. Interestingly, we were able to detect GC B cells in the spleens of SRBC-immunized survivinL/Lcd21Cre mice and in the Peyer‘s patches of unimmunized survivinL/Lcd21Cre mice. Although survivin was efficiently deleted in in vitro stimulated survivinL/Lcd21Cre B cells, it remains possible, that GCs in survivinL/Lcd21Cre mice originated from a few B cells that had escaped deletion. Alternatively, some survivin-deficient B cells may upregulate GC markers and initiate clonal expansion before undergoing proliferative collapse. In support of the latter, a higher frequency of pH2AX positive GC B cells was detected in the Peyer‘s patches from survivinL/Lcd21Cre mice. Histone H2AX has been shown to play a role in the recombination between immunoglobulin switch regions in normal GCs (23), but is also phosphorylated in response to DNA damage (24). Division abnormalities in survivin-deficient GC B cells could lead to genotoxic stress marked by H2AX phosphorylation and programmed cell death.
To better understand the role of survivin in B cell division, we analyzed proliferation after different mitogenic stimuli. As expected, most stimuli did not efficiently induce proliferation of survivinL/Lcd21Cre B cells. Addition of the pro-survival factor IL-4 also did not rescue the defect in proliferation. Since proliferation is necessary for class switch recombination and plasma cell differentiation, survivin-deficient cells showed impaired production of plasma cells and IgG1 positive cells. DNA synthesis in survivinL/Lcd21Cre B cells was normal, indicating that survivin-deficient B cells are able to enter the S-phase of the cell cycle. Interestingly, unlike anti-IgM or anti-CD40+IL-4 stimulated cells, a small percentage of LPS-stimulated survivinL/Lcd21Cre B cells was able to proliferate. Addition of IL-4 to the LPS culture resulted in strong proliferation of survivinL/Lcd21Cre B cells, comparable to the control B cells. Although these cells were able to proliferate, they displayed abnormalities in their cell size and DNA content. In comparison to control B cells, an increased percentage of survivinL/Lcd21Cre B cells showed sub-G1 DNA content, suggesting that these cells are undergoing apoptosis. Furthermore, abnormally large B cells with high DNA content could be found in survivinL/Lcd21Cre samples, consistent with the role of survivin in supporting cell division.
In summary, our results demonstrate that survivin is essential for B cell division, but does not affect survival of naïve B cells. Survival of proliferating B cells may be impacted indirectly by survivin deficiency due to increased genotoxic stress caused by failed chromosomal segregation. Furthermore, we show that chemical inhibition of survivin by the small molecule inhibitor impairs the growth of B-NHL cells. YM155 has also been shown to inhibit proliferation and induce apoptosis of stimulated chronic lymphocytic leukemia cells (CLL) (25), and has demonstrated anti-tumor activity in a human DLBCL xenograft mouse model (26, 27). Thus, survivin is becoming an attractive potential therapeutic target in various B cell malignancies.
ACKNOWLEDGEMENTS
We thank Drs. K. Rajewsky (Harvard University) and M. Reth (Max-Planck Institute) for providing the cd21Cre and mb1Cre mice, respectively; members of the Rickert laboratory for discussions and critical evaluation of the manuscript; and the SBP animal care staff for animal husbandry. We thank Yoav Altman and the SBP Flow Cytometry core for help with performing and analyzing experiments on the Image Stream Imaging Flow Cytometer.
Abbreviations used in this paper
- BM
bone marrow
- FO
follicular
- GC
Germinal center
- Ig
Immunoglobulin
- IAP
inhibitor of apoptosis
- LN
lymph node
- MZ
marginal zone
- SRBC
sheep red blood cells
- TD
Thymus-dependent
- TI-II
Thymus-independent type II
Footnotes
This work was supported by National Institutes of Health Grants HL088686 and AI41649 (R.C.R.) and F32CA132350 (A.V.M).
DISCLOSURES
The authors have no financial conflict of interest.
References
- 1.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer research. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 2.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 3.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 4.Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012;13:789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, Lai F, Ogawa H, Gudmundsdottir E, Gassmann R, Morrison CG, Ruchaud S, Earnshaw WC. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J Cell Biol. 2008;183:279–296. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skoufias DA, Mollinari C, Lacroix FB, Margolis RL. Human survivin is a kinetochore-associated passenger protein. The Journal of cell biology. 2000;151:1575–1582. doi: 10.1083/jcb.151.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Brattain MG. Role of the Survivin gene in pathophysiology. The American journal of pathology. 2006;169:1–11. doi: 10.2353/ajpath.2006.060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Molecular cancer therapeutics. 2006;5:1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 9.Xing Z, Conway EM, Kang C, Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med. 2004;199:69–80. doi: 10.1084/jem.20031588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 11.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher DA, Basten A. Influences on the lifespan of B cell subpopulations defined by different phenotypes. European journal of immunology. 1997;27:1188–1199. doi: 10.1002/eji.1830270521. [DOI] [PubMed] [Google Scholar]
- 15.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews. Immunology. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 16.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. International journal of oncology. 2002;21:315–320. [PubMed] [Google Scholar]
- 17.Mellai M, Caldera V, Patrucco A, Annovazzi L, Schiffer D. Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer research. 2008;28:109–118. [PubMed] [Google Scholar]
- 18.Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, Nakano T, Suzuki A. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer research. 1998;58:5071–5074. [PubMed] [Google Scholar]
- 20.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:127–134. [PubMed] [Google Scholar]
- 21.Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. European journal of immunology. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]
- 22.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nature reviews. Immunology. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 23.Reina-San-Martin B, Difilippantonio S, Hanitsch L, Masilamani RF, Nussenzweig A, Nussenzweig MC. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J Exp Med. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA repair. 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Purroy N, Abrisqueta P, Carabia J, Carpio C, Calpe E, Palacio C, Castellvi J, Crespo M, Bosch F. Targeting the proliferative and chemoresistant compartment in chronic lymphocytic leukemia by inhibiting survivin protein. Leukemia. 2014;28:1993–2004. doi: 10.1038/leu.2014.96. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, Keating AT, Shamsili S, Papadopoulos KP. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2012;118:3128–3134. doi: 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko N, Mitsuoka K, Amino N, Yamanaka K, Kita A, Mori M, Miyoshi S, Kuromitsu S. Combination of YM155, a survivin suppressant, with bendamustine and rituximab: a new combination therapy to treat relapsed/refractory diffuse large B-cell lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1814–1822. doi: 10.1158/1078-0432.CCR-13-2707. [DOI] [PubMed] [Google Scholar]