Abstract
PTPN22 gene variation associates with multiple autoimmune diseases including type 1 diabetes and rheumatoid arthritis. Loss of function studies have demonstrated that PTPN22 impinges on the homeostatic behavior of regulatory T (Treg) cells, a lineage critical for immune tolerance. The frequency and absolute number of Treg cells is increased in Ptpn22 deficient mice, but the mechanism driving this increase is unknown. We show here that Ptpn22 knockdown (KD) promoted the expansion of the Treg cell compartment by up-regulating the glucocorticoid-induced TNF receptor family-related protein (GITR) and increasing GITR signaling. Ptpn22 KD did not accelerate cell division but instead prolonged Treg cell survival, as measured by a decrease in the frequency of apoptotic Treg cells. Loss of Ptpn22 caused a concomitant increase in the proportion of CD44hiCD62Llo ‘effector’ Treg cells, at the expense of CD44loCD62Lhi ‘central’ Treg cells. The increase in Treg cell numbers, but not their differentiation towards an effector phenotype, was dependent on GITR signaling, because blockade of GITR-L prevented Treg cell expansion caused by Ptpn22 KD. These findings indicate that GITR plays a key role in regulating the overall size of the Treg cell pool. Our results suggest that the size and composition of the Treg cell compartment are independently controlled, and have implications for the design of immunotherapies that seek to improve Treg cell function.
Introduction
PTPN22 is one of the non-HLA genes most highly associated with autoimmunity (1). Although the phosphatase encoded by PTPN22, termed Lyp in human and Pep in mouse, is involved in the function of multiple cell lineages (2), the most striking phenotype observed in Ptpn22 deficient mice is the expansion of the regulatory T (Treg) cell compartment. The loss of Ptpn22 was shown to increase both the absolute number and the frequency of Treg cells in two independent Ptpn22 knockout (KO) lines as well as in Ptpn22 knockdown (KD) mice (3–5). Published data suggest that Treg cell expansion caused by Ptpn22 deficiency does not derive from increased thymic output, but rather stems from altered homeostasis of peripheral Treg cells (3, 5). However, the mechanism by which Ptpn22 variation affects Treg cell homeostasis is unclear.
Insight into the requirements for Treg cell homeostasis was provided by a recent study of factors critical to the recovery of the Treg cell population following partial depletion (6). This study showed that Treg cell proliferation induced by acute depletion required both IL-2 and costimulation. Work by Campbell and colleagues further demonstrated that subpopulations of Treg cells, characterized by their relative expression of CD44, CD62L and CCR7, have distinct homeostatic requirements (7). ‘Central’ Treg (cTreg) cells that express low levels of CD44 and high levels of CD62L, depend largely on IL-2 for their maintenance and have a slower turnover rate than CD44loCD62Lhi ‘effector’ Treg (effTreg) cells that depend for their maintenance on costimulatory signals (7). effTreg cells were shown to have a higher proliferation rate under steady-state conditions, but also to be more prone to apoptosis, leading to a stable ratio of central to effector Treg cells. Current strategies to boost Treg cell numbers in patients with autoimmunity have not yet taken into account the heterogeneity of the Treg cell compartment (8,9).
In addition to their expression of high levels of CD25, Treg cells are characterized by increased GITR expression. CD25 sensitizes Treg cells to IL-2, in line with the critical role of this cytokine for Treg cell maintenance. In contrast, the role of GITR in Treg cell function has been controversial. Studies with tumor models suggested that GITR antibody-ligation is detrimental to Treg cell stability (10). However, the effect of agonist GITR antibody in this context required activating Fcγ receptors (11). The involvement of Fcγ receptors indicates that anti-GITR may lead to Treg cell depletion by antibody-dependent cell-mediated cytotoxicity or phagocytosis. Therefore, GITR ligation may not directly impair Treg cell function. Instead, it was shown that GITR stimulation can induce Treg cell proliferation (12) and that GITR ligation is in fact necessary for Treg cell function (13).
In seeking to determine how Ptpn22 silencing effected a change in Treg cell homeostasis, we found that Ptpn22 KD caused GITR upregulation and increased GITR signaling. Blocking GITR ligation prevented expansion of the Treg cell compartment following Ptpn22 KD, indicating that GITR plays a key role in the control of Treg cell homeostasis. Further, we found that loss of Ptpn22 did not increase Treg cell proliferation, but rather that it prolonged Treg cell survival. Concomitantly, Ptpn22 silencing increased the effTreg to cTreg cell ratio, but did so in a GITR-independent manner. Together, our data suggest a critical role for GITR in Treg cell homeostasis and indicate that Ptpn22 independently affects the differentiation status of Treg cells and their homeostatic behavior.
Materials and Methods
Animals
All mice were bred and maintained under specific pathogen-free conditions at Joslin Diabetes Centre in accordance with institutional guidelines. Lentiviral transgenic P2 and P4 mice were generated as described previously (5). Briefly, lentivirus carrying an inducible short hairpin RNA (shRNA) expression cassette that targets the sequence GATGAGGATTCCAGTTATA (P2) or GCATCTGTACACATCTTTA (P4) in the Ptpn22 mRNA was microinjected into NOD zygotes. For induction of gene silencing, mice were treated with 50 μg / mL doxycycline (Bio Basic Canada Inc.) in the drinking water. Age- and sex-matched WT NOD mice bred in the same colony were used as controls. Raspberry mice congenicaly marked with mRaspberry (14) were used as recipients in cell transfer experiments. All experiments were approved by the Joslin Diabetes Center IACUC.
Flow cytometry
Single cell suspensions of splenocytes and lymph node cells were stained with the following antibodies (all from Biolegend unless otherwise stated): anti-CD4-BV605/PB/PE/PE-Cy7, anti-CD25-APC/PE (Miltenyi Biotec)/PerCP-Cy5.5, anti-CD44-APC-Cy7, anti-CD62L-APC, anti-GITR-PE-Cy7, anti- Foxp3-PE/eFluor450 (eBioscience) and Ki-67-PerCP-eFluor710 (eBioscience), anti-caspase-3-PE (BD Pharmingen), anti-BrdU-PE (BD Pharmingen). Intranuclear staining for Foxp3 and Ki-67 was performed using Foxp3 Fixation/Permeabilization buffers (eBioscience), and caspase-3 staining was performed using Cytofix/Cytoperm kit as per manufacturer's instructions (BD Pharmingen). Prior to surface staining cells were stained with fixable viability dye eFluor780 (eBioscience) if indicated. The samples were acquired on the LSR II flow cytometer (BD) and analyzed using FlowJo software (Tree Star).
BrdU pulse labeling
Mice were injected intraperitoneally with 2 mg bromodeoxyuridine (BrdU, Sigma) twice a day for 3 days. Tissues were harvested on day 1 and day 28 after the last injection. BrdU was detected by flow cytometry using BrdU Flow kit (BD Bioscience) following the manufacturer's instructions.
Cell transfer
CD4+ T cells were MACS-purified by negative selection as per the manufacturer's instructions (Miltenyi Biotec). Cells were then stained with fluorescent antibodies and sorted by FACS for CD4+CD25+CD62Lhi CD44lo cTreg cells or CD4+CD25+CD62LloCD44hi effTreg cells. Purity following FACS sort was routinely 95% ± 5%. WT Raspberry mice pre-treated with doxycycline or left untreated received 3×105 cells intravenously. Tissues were harvested 5 days later for analysis.
GITR-L blockade
Mice were injected intraperitoneally with one dose of 0.25 mg anti-GITR antibody (clone DTA-1, BioXCell) and sacrificed 7 days after, or with one dose of 0.25 mg anti-GITR-L antibody (clone 5F1, Biolegend) weekly for 4 weeks and sacrificed 7 days after the last injection.
In vitro proliferation assay
Single cell suspensions were prepared from mice pre-treated with doxycycline for 4 weeks or left untreated. CD4+ CD25+ T cells were MACS-purified as per the manufacturer's instructions (Miltenyi Biotec). Purity following the sort was routinely 95% ± 5%. Cells were then labeled with fixable proliferation dye eFluor450 according to manufacturer's instructions (eBioscience). Following this, cells were resuspended at 1×106 cells / ml in RPMI 1640 medium (Gibco), supplemented with 2mM Glutamine (PAA), 100 U / ml Penicillin (Gibco), 100 μg / ml Streptomycin (Gibco), 50 μM 2- mercaptoethanol (Gibco) and 10 % heat-inactivated fetal calf serum (FCS, HyClone). CD4+ CD25+ T cells were then stimulated for 5 days with 10 μg / ml DTA-1 in the presence or absence of 10 U / ml IL-2 (Preprotech) and 1 μg / ml doxycycline followed by flow cytometry analysis. Cell cultures were kept at 37°C in a humidified atmosphere at 5% CO2.
In vitro suppression assay
MACS-purified CD4+CD25+ T cells were purified from mice pre-treated with doxcycycline for 4 weeks followed by intraperitoneal injection with one dose of 0.25 mg anti-GITR antibody (DTA-1), or PBS as a control, and sacrificed 1 week later. MACS-sorted WT CD4+CD62L+ naïve T cells were mixed with CD4+CD25+ T cells at different ratios, and stimulated in the presence of irradiated splenocytes and 1 μg/ml anti-CD3 for 4 days. CD4+CD62L+ T cells were pre-labeled with fixable proliferation dye eFluor450 according to manufacturer's instructions (eBioscience). Unstimulated naïve CD4+ T cells and cells stimulated in the absence of Treg cells were used as controls. Suppressive capacity of CD4+CD25+ T cells was assessed by the dilution of proliferation dye in stimulated naïve T cells using flow cytometry.
Apoptosis assay
Single cell suspensions were prepared from spleens and lymph nodes of mice pre-treated with doxycycline for 4 weeks or left untreated. Cells were either immediately stained with anti-active caspase-3 antibody using Cytofix/Cytoperm kit as per manufacturer's instructions (BD Pharmingen), or resuspended at 4×106 cells/ml in supplemented RPMI-1640 medium in 48-well plates and incubated for 5h followed by caspase-3 staining. The frequency of cells with activated caspase-3 was measured by flow cytometry.
Western blotting
MACS-purified CD4+ CD25+ T cells isolated from mice pre-treated with doxycycline for 1 to 4 weeks were rested or stimulated with 10 μg / ml DTA-1 for indicated amount of time in serum-free RPMI 1640 medium. Cells were then centrifuged and resuspended in RIPA lysis buffer (Mitosciences) supplemented with protease inhibitor cocktail (Roche) on ice for 2 minutes. The protein concentration was measured with a Pierce 660 nm protein assay (Thermo Scientific). Target proteins were probed with antibodies against pERK1/2 (1:1000) (Cell Signaling Technology) and actin (1:1000) (Santa Cruz Biotechnology), and detected by secondary antibody incubation with ECL anti-mouse (actin) or anti-rabbit (pERK1/2) IgG horseradish peroxidase-linked whole antibody. Actin was reprobed on the same membrane used to visualize pERK1/2 following incubation in Restore Western Blot Stripping Buffer according to manufacturer's instructions (Thermo Scientific). The assay was developed using Pierce ECL Western Blotting substrate (Thermo Scientific).
Statistical analysis
All statistical analyses were performed using GraphPad Prism software. Unpaired t-test was used when comparing two groups. To compare three and more groups a one-way analysis of variance (ANOVA) was performed with Tukey's multiple comparison post-test. Data were considered statistically different with p < 0.05.
Results
Loss of Ptpn22 increases GITR expression
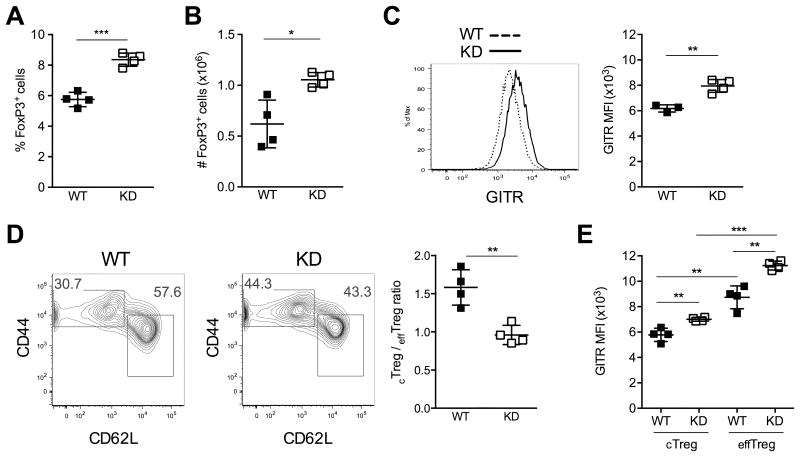
We previously described the generation of nonobese diabetic (NOD) mice in which Ptpn22 silencing is inducible by administration of doxycycline (5). Our most significant finding in these mice was the expansion of the Treg cell compartment following prolonged Ptpn22 KD. Treating transgenic mice with doxycycline for a duration of 4 weeks caused an increase in both the frequency and absolute number of FoxP3+CD4+ regulatory T cells (Figs. 1A and 1B). Continued Ptpn22 KD did not increase Treg cell numbers further (data not shown), and we speculate that loss of Ptpn22 enforces a new set-point onto the size of the Treg cell compartment. That is, Treg cells expand for a limited time and stop increasing in number once they reach their new maximal compartment size. In searching for an explanation for this expansion, we discovered that Ptpn22 KD caused the upregulation of the glucocorticoid-induced TNF receptor family protein (GITR) (Fig. 1C). None of the other cell surface markers measured, including CD25 and CTLA-4, were affected (data not shown). Of interest, the ratio of CD62LhiCD44lo cTreg to CD62LloCD44hi effTreg cells shifted in favor of the latter (Fig. 1D). We also noted that effTreg cells expressed markedly higher levels of GITR than cTreg cells, and that Ptpn22 KD increased GITR expression in both Treg subpopulations (Fig. 1E). Results from these experiments were concordant in both Ptpn22 KD lines (P2 and P4) generated in our laboratory. The phenotype of Ptpn22 KD animals not treated with doxycycline consistently resembled that of WT mice, and doxycycline treatment had no effect on Treg cells in non-transgenic animals (data not shown), as we also described previously (5). Thus, Ptpn22 KD caused both a change in GITR expression and in the differentiation status of Treg cells. We therefore sought to determine whether the increased GITR signaling in Ptpn22 KD mice underlies the elevated frequency of Treg cells, and of effTreg cells in particular.
Figure 1. Ptpn22 KD expands peripheral FoxP3+ Treg cells, and increases the expression of GITR and the frequency of effTreg cells.
Lymph node Treg cells from wild-type (WT) and Ptpn22 KD mice (KD) were analyzed after 4 weeks of doxycycline treatment. Ptpn22 KD increased the frequency (A) and absolute number (B) of FoxP3+ Treg cells. Ptpn22 silencing also increased the expression of GITR on FoxP3+ Treg cells (C) and the frequency of CD44hiCD62Llo effTreg cells within the FoxP3+ Treg cell population (D). GITR expression was further compared between effTreg cells and cTreg cells of both WT and Ptpn22 KD mice (E). Left panels in C and D show FACS analyses representative of the data used to calculate the mean fluorescence intensity (MFI) of GITR expression (C, right panel) and cTreg to effTreg ratio (D, right panel). Mean values +/- SEM from 3 to 5 mice per group are shown. Data are representative of 5 experiments. *p<0.05, **p<0.01, ***p<0.001.
Ptpn22 KD amplifies the effect of GITR stimulation in vitro and in vivo
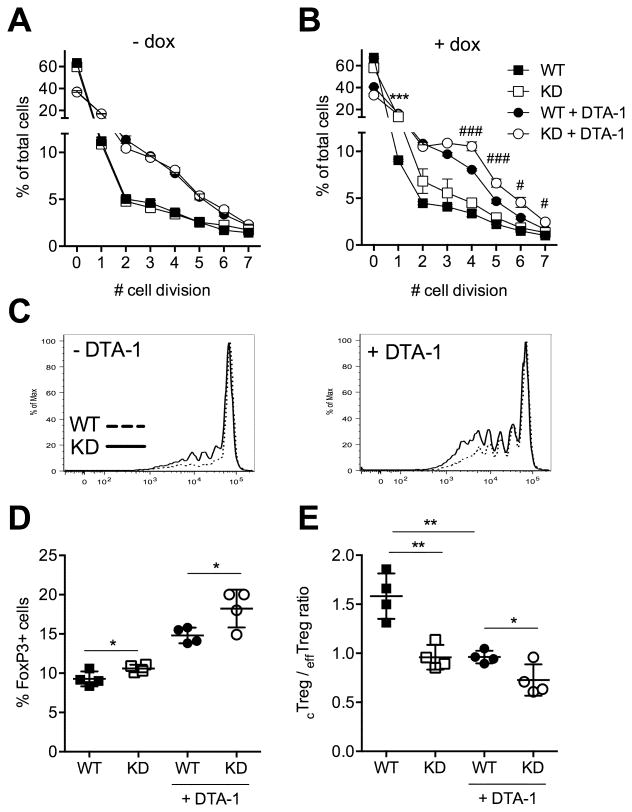
We first tested if GITR ligation could in principle promote Treg cell expansion in the NOD background. We stimulated CD4+CD25+ Treg cells from WT and Ptpn22 KD mice in vitro using the GITR agonist antibody DTA-1 (15). Proliferation dye dilution showed that DTA-1 stimulation increased Treg cell division in this setting (Fig. 2A). The proliferative effect of GITR ligation was enhanced by concomitant Ptpn22 silencing (Fig. 2B and 2C), and we speculate that GITR upregulation by Ptpn22 KD could explain the increased response to DTA-1.
Figure 2. Ptpn22 KD increases GITR-stimulated Treg cell division in vitro and Treg cell expansion in vivo. Treg cell division in vitro was measured after stimulation with the GITR agonist antibody DTA-1.
(A, B) Frequency of Treg cells from untreated (-dox, A) or doxycycline treated (+dox, B) mice that have undergone the indicated number of cell divisions. Treg cells were cultured in the presence of IL-2 with or without 10 μg / ml DTA-1 antibody for 5 days. Cell proliferation was measured by dye-dilution of Treg cells pre-stained after ex vivo purification. Mean +/- SEM of triplicates are shown. (C) Representative histograms showing the dilution of proliferation dye as analyzed by flow cytometry. (D, E) Frequency of Treg cells (D) and cTreg to effTreg cells ratio (E) in lymph nodes of DTA-1 treated mice with or without concomitant Ptpn22 KD. Mice were treated with doxycycline for 4 weeks, injected once with 0.25 mg anti-GITR antibody (DTA-1), and lymph node cells were analyzed 7 days later. Mean values +/- SEM from 4 mice per group are shown. Data are representative of two (A-C) and three (D, E) experiments. In B, * shows significance between untreated, and # between DTA-1 treated, WT and KD animals. */#p<0.05, **p<0.01, ***/###p<0.001.
We proceeded to test the effect of GITR agonist antibody in vivo. Ptpn22 KD alone increased the frequency of Treg cells, as shown previously. A single injection of DTA-1 antibody caused an even more pronounced expansion of the Treg cell population (Fig. 2D). Notably, the response to DTA-1 administration was higher in Ptpn22 KD mice (71% increase in Treg cells vs. 59% increase in WT mice), suggesting that GITR upregulation on Ptpn22 KD Treg cells amplifies the response to GITR ligation, and could thus be the cause for Treg cell expansion following Ptpn22 silencing. Additionally, we observed that DTA-1 treatment affected the cTreg to effTreg cell ratio in a manner similar to Ptpn22 KD (Fig. 2E). Collectively, these results indicate that GITR stimulation using an agonist antibody resembles the effects of Ptpn22 KD, and that increased GITR signaling could be the cause for Treg cell expansion in Ptpn22 KD mice. We and others have shown that the suppressive capacity of Ptpn22 deficient Treg cells in vitro was similar to that of WT Treg cells (4,5). To test the effect of GITR stimulation on Treg cell function in the context of Ptpn22 KD, we compared Treg cells from WT and Ptpn22 KD mice either stimulated or not with GITR agonist antibody in vivo. The suppression of naïve T cell proliferation by GITR-stimulated Treg cells was comparable to suppression by non-stimulated cells (supplemental Fig. S1). These data indicate that GITR signaling does not alter Treg cell function and are consistent with results from earlier studies (16).
Increased GITR signaling precedes the expansion of Ptpn22 KD Treg cells
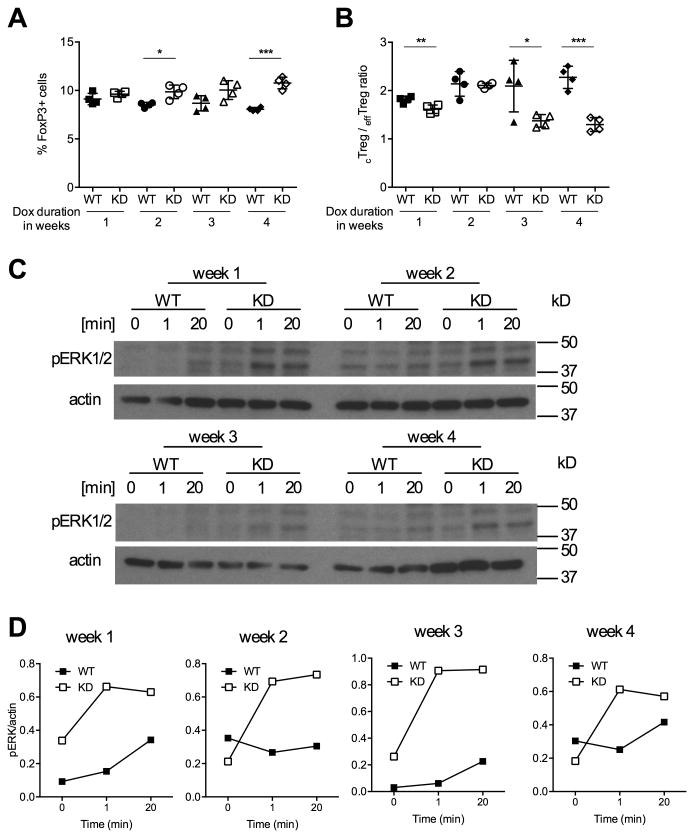
We reasoned that if GITR signaling was causal for Treg cell expansion, increased GITR signaling should be measurable before the increase in Treg cells. We therefore treated WT and Ptpn22 transgenic mice with doxycycline for 1, 2, 3 or 4 weeks before measuring both GITR signaling and Treg cell frequency. We found that Foxp3+ T cell frequency started increasing 2 weeks after beginning doxycycline treatment and that the difference between WT and Ptpn22 KD mice continued to increase until 4 weeks of Ptpn22 silencing (Fig. 3A). We observed a similar pattern, albeit in the opposite direction, for the cTreg to effTreg ratio that decreased with increasing duration of Ptpn22 KD (Fig. 3B). We then evaluated GITR signaling by measuring ERK phosphorylation after ex vivo Treg cell stimulation with DTA-1. These measurements showed that one week of doxycycline treatment was sufficient to increase GITR signaling (Fig. 3C, D), suggesting that the effect of Ptpn22 KD on GITR signaling preceded, and could be the cause for the expansion of the Treg cell compartment.
Figure 3. Ptpn22 silencing leads to increased GITR signaling prior to Treg cell expansion.
The percentage of Foxp3+ Treg cells (A) and the ratio between cTreg to effTreg cells (B) were measured in WT and Ptpn22 KD mice after 1, 2, 3 and 4 weeks of doxycycline (dox) treatment. Mean values +/- SEM from 4 mice per group are shown. C and D: GITR signaling was measured in Treg cells sorted from lymph node and spleen cells of WT and Ptpn22 KD mice treated with doxycycline for a duration of 1 to 4 weeks and stimulated ex vivo with DTA-1 (10 μg/ml). Data are representative of four (A, B) and two (C, D) experiments. *p<0.05, **p<0.01, ***p<0.001.
GITR ligand blockade prevents Treg cell expansion upon Ptpn22 KD
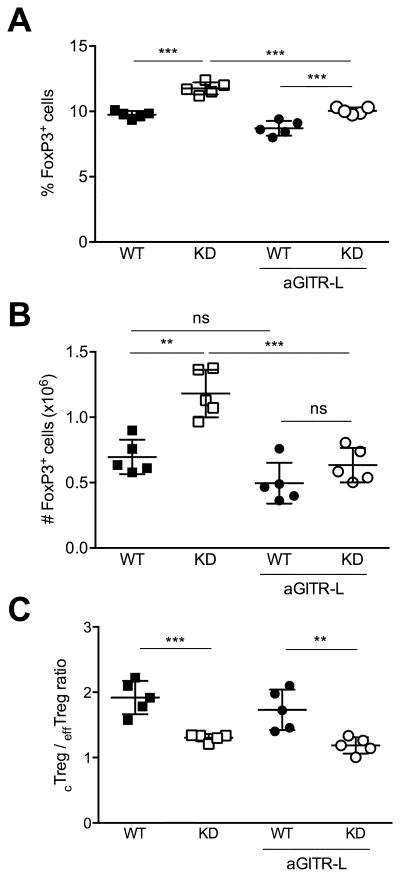
To directly test the hypothesis that the Treg cell expansion caused by Ptpn22 KD was driven by GITR, we used a blocking antibody against GITR ligand (GITR-L). We treated transgenic and WT mice with doxycycline for a duration of 4 weeks to induce Treg cell expansion. At the same time, we injected anti-GITR-L once a week to inhibit GITR ligation. GITR-L blockade prevented the increase in both the frequency and absolute number of Treg cells elicited by Ptpn22 KD (Fig. 4A and 4B). These results indicate that the expansion of the Treg cell compartment caused by Ptpn22 KD is dependent on GITR signals. In contrast, we found that anti-GITR-L injection did not prevent the phenotypic shift from CD62LhiCD44lo cTreg cells towards CD62LloCD44hi effTreg cells (Fig. 4C). The ratio of cTreg to effTreg cells was similarly diminished in both Ptpn22 KD mice that did and did not receive anti-GITR-L. We conclude that Ptpn22 silencing indirectly modulates Treg cell homeostasis by way of GITR upregulation. However, the increase in effTreg cell frequency caused by loss of Ptpn22 appears to be independent of GITR.
Figure 4. GITR-L blockade prevents Treg cell expansion caused by Ptpn22 KD.
Frequency (A) and absolute numbers (B) of Treg cells in lymph nodes from WT and Ptpn22 KD mice treated or not with anti-GITR-L and doxycycline. (C) cTreg to effTreg ratio in WT and Ptpn22 KD mice treated with doxycycline for 4 weeks, and injected or not with 0.25 mg anti-GITR-L once a week for the duration of doxycycline treatment. Mean values +/- SEM from 5 mice per group are shown. Data are representative of two similar experiments. **p<0.01, ***p<0.001, ns, not significant.
Ptpn22 KD prolongs Treg cell survival and promotes cTreg to effTreg cell transition
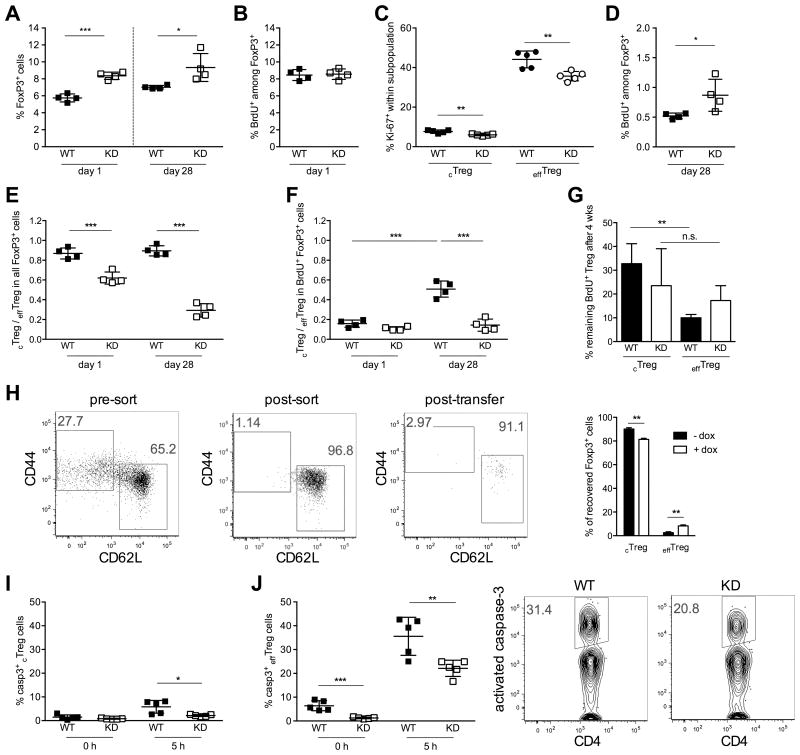
We next wanted to determine whether Treg cell expansion was caused by a change in the proliferative rate of Treg cells. We performed BrdU pulse labeling in mice treated with doxycycline for 4 weeks and measured the frequency of BrdU-labeled FoxP3+ Treg cells 1 and 28 days after BrdU pulse. The overall frequency of Treg cells was higher in Ptpn22 KD mice than in WT animals at both time points, as expected (Fig. 5A). Significantly, the frequency of BrdU+ Treg cells immediately after labeling was identical in WT and Ptpn22 KD mice, indicating that diminished Ptpn22 expression did not increase Treg cell cycling (Fig. 5B). These results were confirmed by Ki-67 staining, which showed that Ptpn22 KD did not increase the frequency of Ki-67+ cells in either the cTreg or effTreg cell subpopulation after 4 weeks of doxycycline treatment (Fig. 5C). Ptpn22 KD in fact decreased the proportion of Ki-67+ cells, although not their absolute number (data not shown). We also measured the frequency of Ki-67+ Treg cells at earlier time points following doxycycline treatment, and observed no increase in Treg cell proliferation at any time after Ptpn22 KD, with the exception of a small and transient increase in the frequency of Ki-67+ cTreg cells in the first week of treatment (supplemental Fig. S2). Thus, we conclude that the expansion of Treg cells by Ptpn22 silencing is not caused by accelerated cell cycling. The increased proliferation of Ptpn22 KD Treg cells observed in vitro in response to GITR ligation (see Fig. 2) may not be representative of changes affecting Treg cells in vivo, perhaps due to the supra-physiological stimulus provided by the DTA-1 antibody. Of note, Ki-67 staining also confirmed that effTreg cells cycle more frequently than cTreg cells (Fig. 5C and supplemental Fig. S2), as reported previously (7). Analysis of BrdU-pulsed animals 4 weeks after BrdU injection revealed instead that Ptpn22 KD increased the longevity of BrdU-labeled Treg cells (Fig. 5D). The frequency of BrdU+ Treg cells recovered 28 days after labeling was higher in Ptpn22 KD mice, and this finding suggests that the loss of Ptpn22 increases Treg cell survival. Analysis of Treg cell subpopulations showed that sustained Ptpn22 KD had a marked effect on the phenotype of Treg cells. Indeed, while 4 weeks of doxycycline treatment decreased the cTreg to effTreg cell ratio, 8 weeks of Ptpn22 silencing caused an even greater shift from in cTreg cells towards effTreg cells (Fig. 5E).
Figure 5. Ptpn22 KD increases Treg cell longevity and promotes the differentiation of cTreg into effTreg cells.
Frequency of total (A) and BrdU-labeled (B and D) FoxP3+ Treg cells in lymph node cells from WT and KD mice 1 day (A and B) and 28 days (A and D) after BrdU pulse. Doxycycline treatment was started 4 weeks prior to BrdU pulse and continued until the end of the experiment. (C) Percentage of Ki-67+ cTreg and effTreg cells in WT and KD after 4 weeks of doxycycline treatment. (E, F) Ratio of cTreg to effTreg cells within the total (E) or BrdU-labeled (F) Treg cell population. (G) Frequency of BrdU-labeled cTreg and effTreg cells 28 days after BrdU-pulse relative to BrdU+ cell numbers measured 1 day after in vivo labeling. (H) Phenotype of FACS-sorted Ptpn22 KD cTreg cells 5 days after transfer into WT NOD Raspberry mice treated or not with doxycycline. (I, J) Percentage of Treg cells with activated caspase-3+ within the cTreg (I) and effTreg (J) cell population, measured in splenocytes ex vivo (0 hours) and 5 hours after in vitro culture. Representative flow cytometry data for activated caspase-3 measurements are shown in J. Mean values +/- SEM from 3 (H), 4 (A, B, D-G) or 5 (C, I, J) mice per group are shown. Data are representative of two (A-G, I, J) or three (H) similar experiments. *p<0.05, **p<0.01, ***p<0.001.
The ratio of cTreg to effTreg cells within the BrdU-labeled population was markedly lower than the ratio measured in the total Treg cell population (Fig. 5F), and this can be attributed to the fact that cTreg cells proliferate less than effTreg cells. Consequently, the majority of cells that incorporated BrdU belonged to the effTreg cell subpopulation. This was reflected in a very low BrdU+ cTreg cell to BrdU+ effTreg cell ratio in both WT and Ptpn22 KD mice immediately after the BrdU pulse. Because effTreg cells have a higher apoptotic rate and are thus shorter-lived (7), this low ratio changed over the course of the following 4 weeks in favor of cTreg cells (Fig. 5F). Strikingly, the proportion of cTreg cells within the BrdU+ population remained low in Ptpn22 KD mice, suggesting that the loss of BrdU+ effTreg cells observed in WT mice did not occur in transgenic animals. We further quantified the proportion of BrdU+ cTreg and effTreg cells that remained in circulation after 4 weeks, relative to the number of labeled cells measured 1 day after the BrdU pulse. In WT mice, the fraction of BrdU-labeled cTreg cells detected after 4 weeks was significantly higher than the fraction of labeled effTreg cells still found in circulation (Fig. 5G). This observation is consistent with the notion that cTreg cells are longer lived than effTreg cells. In Ptpn22 KD mice, the fraction of BrdU+ effTreg cells recovered after 4 weeks was higher than in WT mice and not significantly different from the fraction of persisting BrdU+ cTreg cells. Together, these results suggest that Ptpn22 KD caused more BrdU+ effTreg cells to remain in circulation over a 4 week period.
Maintenance of a larger BrdU-labeled effTreg cell population could be due to either a higher transition rate from the cTreg to effTreg compartment, prolonged survival of effTreg cells, or both. To investigate these possibilities, we first sorted Ptpn22 KD cTreg cells by flow cytometry and transplanted them into Raspberry transgenic NOD mice (14). Donor and recipient mice were either treated or not with doxycycline to induce Ptpn22 gene silencing. Transferred Treg cells were identified as GFP+Raspberry- FoxP3+ T cells, and subdivided on the basis of CD44 and CD62L staining. Ptpn22 silencing in transplanted cTreg cells increased their propensity to differentiate into effTreg cells (Fig. 5H). These results indicate that Ptpn22 KD promotes the differentiation of cTreg cells towards an activated effector phenotype. We then examined the effect of Ptpn22 KD on Treg cell apoptosis. We measured the frequency of Treg cells with activated caspase-3 in WT and Ptpn22 KD mice. We observed a marked reduction in apoptotic Treg cells in Ptpn22 KD animals, both immediately after cell isolation and after 5 hours of culture in vitro, conditions under which Treg cell apoptosis increased sharply (Fig. 5I and 5J). The survival advantage of Ptpn22 KD Treg cells was particularly apparent within the effTreg cell population (Fig. 5J). Decreased apoptosis was also apparent in cTreg cells, albeit more modestly (Fig. 5I). These findings were confirmed by Annexin V and propidium iodide staining (data not shown). Collectively, these data suggest that the increase in Treg cell frequency following Ptpn22 KD is caused by prolonged Treg cell survival. Further, the enlargement of the effTreg cell compartment is a combined result of facilitated cTreg cell differentiation and decreased effTreg cell apoptosis.
Transient Ptpn22 silencing is sufficient for prolonged expansion of the Treg cell population
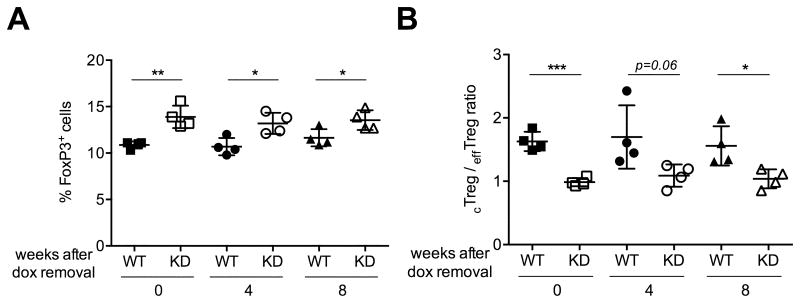
Gene silencing in Ptpn22 KD mice is reversible upon removal of doxycycline because RNAi is post-transcriptional and does not affect the integrity of the Ptpn22 gene. We made use of this feature to cause transient Ptpn22 silencing and to ask if Treg cell expansion required sustained loss of Ptpn22. We treated groups of WT and Ptpn22 KD mice with doxycycline for 4 weeks and then divided them into three cohorts: one cohort was given doxycycline for an additional 8 weeks, the second cohort was given doxycycline for another 4 weeks followed by 4 weeks without treatment , and the third cohort was taken off doxycycline treatment entirely for 8 weeks. The frequency of Treg cells was then analyzed in all mice 12 weeks after the start of treatment. We found that even 4 or 8 weeks after ceasing doxycycline treatment, the frequency of Treg cells remained elevated in Ptpn22 KD animals (Fig. 6A). Similarly, the cTreg to effTreg cell ratio was still markedly lower in transgenic mice 8 weeks after treatment was stopped (Fig. 6B). These results suggest that transient Ptpn22 silencing is sufficient to impart a survival advantage to Treg cells that is sustained for at least 2 months. This observation may have therapeutic implications, and should warrant further research into the mechanism underlying the effects of Ptpn22 silencing.
Figure 6. Transient Ptpn22 silencing is sufficient for prolonged expansion of the Treg cell population.
The frequency of Treg cells (A) and of effTreg cells (shown as cTreg/effTreg ratio) (B) remain increased up to 8 weeks after cessation of doxycycline (dox) treatment. WT and Ptpn22 transgenic mice were treated with doxycycline for 4 weeks and the treatment was either stopped after 4 weeks or continued for an additional 4 or 8 weeks. Lymph nodes cells from WT and Ptpn22 KD mice were analyzed after a total of 12 weeks. Mean values +/- SEM from 4 mice per group are shown. *p<0.05, **p<0.01, ***p<0.001.
Discussion
PTPN22 is broadly associated with autoimmune disease (2). PTPN22's effect on the risk of autoimmunity was initially ascribed to this gene's role in antigen receptor signaling (17–20). However, subsequent studies have shown that PTPN22 participates in a diverse array of signaling pathways, including Toll-like receptor and IFN-α receptor signaling (21, 22). The exact cause for PTPN22's association with type 1 diabetes and other autoimmune diseases thus remains elusive. Notwithstanding the pleiotropic nature of PTPN22, we focused on this gene's effect on Treg cell homeostasis. Treg cells are indispensable to immune tolerance and are considered among the most promising targets for the immunotherapy of autoimmune diabetes. Based on successful interventions in the NOD mouse model (23,24), trials are now ongoing that seek to increase the frequency of Treg cells in patients with type 1 diabetes (8,9). In this context, the finding by multiple groups that Ptpn22 deficiency markedly increases Treg cell numbers in mice (3–5) suggests that a better understanding of how Ptpn22 controls Treg cell homeostasis could help in improving current therapeutic strategies.
In the present study, we uncovered a role for GITR in regulating the longevity of Treg cells. By virtue of increasing GITR expression and signaling, Ptpn22 deficiency promoted the survival of circulating Treg cells, leading to the expansion of the Treg cell compartment. A role for GITR in Treg cell survival is consistent with the first described function of GITR, namely its ability to protect T cells from apoptosis (25, 26). Notably, GITR-deficiency was reported to reduce the frequency of circulating Treg cells by approximately 30% (16). The absence of GITR had been reported to have no effect on the frequency of Treg cells in the thymus, suggesting that GITR deficiency primarily affects the homeostasis of peripheral Treg cells. Recent work by Farrar and colleagues showed redundancy among GITR, OX40 and TNFR2 during thymic Treg cell development (27). This group further confirmed that GITR-deficiency has a pronounced effect the frequency of peripheral Treg cells. These data are in line with our finding that Ptpn22 KD, by upregulating GITR, causes the expansion of the circulating Treg cell population. Interestingly, the absence of GITR-L was found to have no impact on Treg cell numbers in the steady-state (13). However, GITR-L-deficiency had a marked negative effect on the expansion of Treg cells by Flt3L administration (13), supporting the notion that GITR signaling may be a limiting factor in sustaining an increase in Treg cell numbers. This may be relevant to the requirement for higher numbers of Treg cells in the resolution of immune responses and tissue inflammation. In this regard, Dittel and colleagues reported that the provision of GITR ligand by B cells was pivotal in the homeostasis Treg cells and their ability to drive the recovery from experimental autoimmune encephalomyelitis (28). This earlier report is in line with data presented here that implicate GITR in Treg cell homeostasis. Of interest, one study described that Treg cells from patients with type 1 diabetes had lower GITR expression compared to cells from healthy individuals, and decreased GITR expression correlated with poor Treg cell survival (29). Further investigation is needed to establish the molecular details of how PTPN22 affects GITR signaling, and how these together modify Treg cell longevity.
In addition to modifying Treg cell survival, Ptpn22 silencing shifted Treg cells towards an activated ‘effector’ Treg cell phenotype. How an increase in the frequency of effTreg cells affects the overall functionality of the Treg cell compartment is as yet unclear. But it will be of interest to examine in more detail how current experimental strategies, and low-dose IL-2 in particular, impinge on the phenotype of Treg cells. This may be particularly relevant to the duration of any effect achieved by Treg cell expansion in patients, because effTreg cells are shorter lived than naïve Treg cells (7), suggesting that an expansion dominated by effTreg cell would inevitably be transient. Therefore, a therapeutic strategy that not only expands Treg cells but also promotes their survival would be most efficacious. The results presented here suggest that modifying the longevity of Treg cells is an achievable goal that warrants further research.
In sum, our data show that the loss of Ptpn22 has two distinct effects on Treg cell behavior. First, decreased Ptpn22 expression causes increased GITR signaling, promoting cell survival and expansion of the peripheral Treg cell pool. Second, Ptpn22 KD facilitates the differentiation of cTreg into effTreg cells, likely as a direct result of increased TCR signaling. These findings indicate that the size and composition of the Treg cell population are regulated independently. Identifying therapeutic agents capable of controlling the quantity and quality of Treg cells is a critical goal in seeking to restore immune tolerance. In this regard, it will be of interest to further explore how Treg cells can be manipulated more specifically to change either their frequency or differentiation status independently of each other, as these factors likely have distinct effects on the overall regulatory capacity of the Treg cell compartment.
Supplementary Material
Acknowledgments
The authors thank Dr. Cox Terhorst for critical reading of the manuscript. The authors also wish to acknowledge the Joslin DRC Flow Cytometry Core facility for help with cell sorting.
This work was supported in part by a Career Development Award from JDRF to S.K. (2-2010-383), by a Mary K. Iacocca fellowship from the Iacocca Foundation to D.J.N., and by a DRC award (NIH Award Number P30DK036836) to the Joslin Diabetes Center. The authors declare no conflict of interest.
Footnotes
Author Contributions: D.J.N. researched data. D.J.N. and S.K. analyzed the data and wrote the manuscript.
References
- 1.Stanford SM, Bottini N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat Rev Rheumatol. 2014;10:602–610. doi: 10.1038/nrrheum.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottini N, Peterson EJ. Tyrosine Phosphatase PTPN22: Multifunctional Regulator of Immune Signaling, Development, and Disease. Annu Rev Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlie RJ, Miosge La, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5:ra87. doi: 10.1126/scisignal.2003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maine CJ, Hamilton-Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS, Sherman La. PTPN22 alters the development of regulatory T cells in the thymus. J Immunol. 2012;188:5267–5275. doi: 10.4049/jimmunol.1200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng P, Kissler S. PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant. Diabetes. 2013;62:896–904. doi: 10.2337/db12-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schönefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas Aa, Luther RJ, Weaver CT, Dooley J, Gray DHD, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 9.Rosenzwajg M, Churlaud G, Mallone R, Six A, Dérian N, Chaara W, Lorenzon R, Long SA, Buckner JH, Afonso G, Pham HP, Hartemann A, Yu A, Pugliese A, Malek TR, Klatzmann D. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48–58. doi: 10.1016/j.jaut.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, Zhong H, Yang X, Houghton AN, Merghoub T, Wolchok JD. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res. 2013;1:320–331. doi: 10.1158/2326-6066.CIR-13-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee Da, Wilson NS, Dranoff G, Brogdon JL. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao G, Nayak S, Regueiro JR, Berger SB, Detre C, Romero X, de Waal Malefyt R, Chatila TA, Herzog RW, Terhorst C. GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int Immunol. 2010;22:259–270. doi: 10.1093/intimm/dxq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao G, O'Keeffe MS, Wang G, van Driel B, de Waal Malefyt R, Reinecker HC, Herzog RW, Terhorst C. Glucocorticoid-Induced TNF Receptor Family-Related Protein Ligand is Requisite for Optimal Functioning of Regulatory CD4(+) T Cells. Front Immunol. 2014;5:35. doi: 10.3389/fimmu.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirice E, Kahraman S, Jiang W, El Ouaamari A, De Jesus DF, Teo AKK, Hu J, Kawamori D, Gaglia JL, Mathis D, Kulkarni RN. Soluble factors secreted by T cells promote β-cell proliferation. Diabetes. 2014;63:188–202. doi: 10.2337/db13-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 16.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 17.Vang T, Congia M, Macis MD, Musumeci L, Orrú V, Zavattari P, Nika K, Tautz L, Taskén K, Cucca F, Mustelin T, Bottini N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 18.Menard L, Saadoun D, Isnardi I, Ng Y, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, Gregersen PK, Meffre E. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 123:4283–4293. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arechiga AF, Habib T, He Y, Zhang X, Zhang ZY, Funk A, Buckner JH. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, Luning Prak ET, Meyer-Bahlburg A, Sanda S, Greenbaum C, Rawlings DJ, Buckner JH. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188:487–496. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Shaked I, Stanford S, Zhou W, Curtsinger J, Mikulski Z, Shaheen Z, Cheng G, Sawatzke K, Campbell A, Auger J, Bilgic H, Shoyama F, Schmeling D, Balfour H, Hasegawa K, Chan A, Corbett J, Binstadt B, Mescher M, Ley K, Bottini N, Peterson E. The Autoimmunity-Associated Gene PTPN22 Potentiates Toll-like Receptor-Driven, Type 1 Interferon-Dependent Immunity. Immunity. 2013;39:111–122. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes DA, Suto E, Lee WP, Ou Q, Gong Q, Smith HRC, Caplazi P, Chan AC. Autoimmunity-associated protein tyrosine phosphatase PEP negatively regulates IFN-α receptor signaling. J Exp Med. 2015;212:1081–1093. doi: 10.1084/jem.20142130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207:1871–8. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–352. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- 27.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, Farrar MA. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–81. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–98. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xufré C, Costa M, Roura-Mir C, Codina-Busqueta E, Usero L, Pizarro E, Obiols G, Jaraquemada D, Martí M. Low frequency of GITR+ T cells in ex vivo and in vitro expanded Treg cells from type 1 diabetic patients. Int Immunol. 2013;25:563–74. doi: 10.1093/intimm/dxt020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.