Abstract
Dectin-1 and TLR9 play distinct roles in the recognition and induction of innate immune responses to Aspergillus fumigatus and Candida albicans. Dectin-1 is a receptor for the major fungal cell wall carbohydrate β-1,3 glucan that induces inflammatory cytokines and controls phagosomal maturation through Syk activation. TLR9 is an endosomal Toll-like receptor that also modulates the inflammatory cytokine response to fungal pathogens. In this study, we demonstrate that β-1,3 glucan beads are sufficient to induce dynamic redistribution and accumulation of cleaved TLR9 to phagosomes. Trafficking of TLR9 to A. fumigatus and C. albicans phagosomes requires Dectin-1 recognition. Inhibition of phagosomal acidification blocks TLR9 accumulation on phagosomes containing β-1,3 glucan beads. Dectin-1 mediated Syk activation is required for TLR9 trafficking to β-1,3 glucan, A. fumigatus, and C. albicans containing phagosomes. In addition, Dectin-1 regulates TLR9 dependent gene expression. Collectively, our study demonstrates that recognition of β-1,3 glucan by Dectin-1 triggers TLR9 trafficking to β-1,3 glucan-containing phagosomes, which may be critical in coordinating innate anti-fungal defense.
INTRODUCTION
Invasive fungal infections by Candida albicans and Aspergillus fumigatus cause significant morbidity and mortality in immunocompromised patients despite the widespread use of potent anti-fungal medications, suggesting that the immune system plays a critical role in determining the outcome of the infection (1, 2). Advances in the treatment of invasive fungal infections require elucidation of the molecular mechanisms that govern the host immune response.
Pathogen recognition by the host requires binding of highly conserved microbial pathogen-associated molecular patterns (PAMPs) epitopes by germline-encoded pattern-recognition receptors (PRRs) including Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) (3). Subcellular localization of TLRs is critical in recognition of microbial ligands, downstream signaling, and self vs. non self-discrimination (4). TLR-1, −2, −4, −5, and −6 are expressed at the plasma membrane and recognize bacterial and fungal cell wall components, whereas TLR-3, −7, −8, and −9 are localized to the intracellular compartments and recognize bacterial and viral nucleic acids (5). A role for TLR9 has been implicated in host defense against A. fumigatus and C. albicans (6, 7). Activation of TLR9 homodimers in response to unmethylated CpG DNA requires proteolytic cleavage of its N-terminal ectodomain to generate a functional receptor, as well as a conformational change in the cytoplasmic signaling domains that is required for the recruitment of adaptor molecules (8–11). This N-terminal proteolytic cleavage of TLR9 is also required for recruitment to fungal containing phagosomes (9, 12). Unlike other TLRs, macrophages lacking TLR9 show enhanced anti-fungal immunity (13). The molecular mechanisms governing regulation of TLR9 trafficking to fungal phagosomes remains to be determined.
Dectin-1, a C-type lectin receptor, recognizes the carbohydrate β-1,3 glucan, which constitutes the major fungal cell wall component of multiple pathogenic fungi including A. fumigatus, C. albicans, and Pneumocystis jirovecii (14–16). Dectin-1 plays an essential role in pulmonary defense against A. fumigatus and chronic mucocutaneous Candida infections (15, 17). Upon ligand recognition, the cytoplasmic ITAM motif of Dectin-1 is phosphorylated by Src family kinases resulting in the recruitment of spleen tyrosine kinase (Syk). Syk triggers an intracellular signaling cascade resulting in pro-inflammatory cytokine production and induction of Th17 cells, central to the control of mucosal fungal infections (18–20). Dectin-1 interacts with TLR2 and TLR4, leading to a specific innate immune response against C. albicans (19, 21). Dectin-1 controls phagosome maturation (22), but its ability to regulate trafficking of intracellular TLRs has not been established.
We sought to define the role of Dectin-1 and Syk signaling on TLR9 subcellular redistribution in antigen presenting cells to phagosomes containing pathogenic fungi. Using “fungal-like particles” that display monodispersed β-1,3 glucan on a polystyrene platform (23), as well as C. albicans and A. fumigatus, our data show that the presence of β-1,3 glucan in phagosomes is sufficient for TLR9-GFP recruitment. We further demonstrate that Dectin-1 controls TLR9 trafficking to C. albicans and multiple A. fumigatus spore stages. Endolysosomal acidification is required for TLR9 recruitment to β-1,3 glucan beads-containing phagosomes. Using chemical inhibitors of Syk and the signaling incompetent Dectin-1, which are incapable of activating Syk, we demonstrate that Syk activation is required for TLR9 recruitment to β-1,3 glucan beads and fungal-containing phagosomes.
MATERIALS & METHODS
Reagents
Bafilomycin A1 (BafA1) was purchased from A.G. Scientific, Inc (Billerica, MA). CpG phosphorothioate oligodeoxynucleotide 1826 (5’-TCCATGACGTTCCTGACGTT-3’) was synthesized by Integrated DNA Technologies (Coralville, IA). Mouse anti-double stranded DNA monoclonal antibody (IgG) was purchased from Millipore (Temecula, CA). Mouse monoclonal antibody to β-1,3 glucan (IgG) was purchased from Biosupplies Australia Pty Ltd (Victoria, Australia). Mouse monoclonal GFP-HRP antibody and R406, a Syk inhibitor, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines and Cell culture
The mouse macrophage-like cell line, RAW 264.7 and human embryonic kidney cell line, HEK293T were purchased from ATCC (American Type Cell Culture Collection, Manassas, VA). RAW cells were cultured in Dulbecco’s-modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% L-glutamine (ThermoScientific, Logan, UT) (DMEM complete media). Dectin-1 deficient immortalized macrophages were a gift from Gordon Brown (University of Aberdeen) and Stuart Levitz (University of Massachusetts Medical School). Dectin-1 deficient macrophages were cultured in RPMI-GlutaMax (Life Technologies) containing 10% heat-inactivated FBS, 1% penicillin/streptomycin, 1% HEPES buffer, and 2 µM of β-mercaptoethanol (RPMI complete media). Puromycin (5 µg/mL) was used for selection of transduced cells.
Candida albicans, SC5314, a wild-type strain and Aspergillus fumigatus strain 293 were gifts from Eleftherios Mylonakis (Brown Medical School). A. fumigatus strain 293 expressing cytosolic RFP was a gift from Michelle Momany (University of Georgia) (24). C. albicans was grown in YPD media (Sigma-Aldrich) with ampicillin overnight at 30°C in a shaker incubator at 250 rpm. The next morning, yeast was washed three times in PBS, heat-killed at 95°C for 15 min in 500 µl of PBS, and counted by hemacytometer. A. fumigatus was grown on Sabouraud dextrose agar plates supplemented with 100 µg/mL ampicillin at 30°C for three days. Conidia were harvested by scraping and washing three times in ice-cold PBS. Conidia were stored at 4°C for use or immediately heat-killed at 100°C for 20 min. A. fumigatus swollen conidia were prepared by incubation in complete RPMI media at 37°C in water bath for 6 h. Spore germination stages were distinguished by visual inspection using light microscopy. Resting conidia are 2–5 microns and swollen conidia are 6–8 microns. After 6 h, the majority (>95%) of conidia were determined to be swollen.
Viral transduction and plasmids
The retroviral pMSCV vector containing murine TLR9 fused at the C-terminus to GFP (pMSCV-TLR9-GFP) and plasmids encoding VSV-G and gag-pol were gifts from Hidde Ploegh (Whitehead Institute for Biomedical Research, Cambridge, MA). Murine TLR9 fused at the C terminus to mCherry encoded in the retroviral pMSCV vector was a gift from Melanie Brinkmann (Helmholtz Center for Infection Research, Germany). Retroviral transduction was performed as described (12). GFP-Dectin-1 or GFP-Dectin-1ΔY15 was subcloned into pHAGE II (22). HEK293T cells were used to generate lentivirus as described (25).
Generation of CpG+IgG beads
CpG+IgG beads were generated similar to (26) with slight modifications. Briefly, 3 µM amine-terminated polystyrene beads (Polysciences, Warrington, PA) were washed three times in PBS and incubated with 1 µM of CpG in acetonitrile overnight at 4°C. The next morning, CpG beads were washed in PBS and incubated with 0.25 µg-1.25 µg of anti-double stranded DNA antibody in PBS/BSA for 1 h. After incubation, CpG+IgG beads were washed and stored at 4°C in PBS for later use. β-1,3 glucan conjugated beads were generated as described (23).
Surface fluorescence labeling of β-1,3 glucan beads and CpG+IgG beads
β-1,3 glucan beads and CpG+IgG beads were labeled for live cell imaging by mixing approximately 5x106 beads with 30 µg of N-hydroxysuccinimidyl ester Alexa fluor dye (Invitrogen AF658 and AF647) in DMF for 1 h at room temperature followed by three washes in PBS. Then, labeled beads were resuspended in PBS.
Confocal Microscopy
Macrophages were plated onto 8-chambered coverglass (LabTek, Thermoscientific, Rochester, NY) (13, 22, 23). Cells were incubated with stimuli at 37°C for specified times. Coverglass were then mounted on Nikon Ti-E inverted microscope equipped with CSU-X1 confocal spinning-disk head (Yokogawa, Sugarland, TX). A coherent, 4 Watt laser (Coherent, Santa Clara, CA) was used as an excitation light source to produce excitation wavelengths 488, 568, and 647 nm using an acoustic optical tunable tuner. To acquire high-quality fluorescence images, a high-magnification, high-numerical aperture objective was used (Nikon, 1003, 1.49 numerical aperture, oil immersion) was used. A polarizer (Nikon, MEN 51941) and Wollaston prisms (Nikon, MBH76190) were used to acquire differential interference contrast (DIC) images. Emission light from the sample was collected after passage through the appropriate emission filters (Semrock, Rochester, NY). Images were acquired using an EM-CCD camera (Hamamatsu, C9100-13, Bridgewater, NJ). Image acquisition was performed using MetaMorph software (Molecular Devices, Dowington, PA). Raw image data files were processed using Adobe Photoshop CS4 and assembled in Adobe Illustrator, version CS4 (Adobe Systems, San Jose, CA).
Phagosome Isolation
Phagosome isolation was adopted from previous protocols (22, 27). For cell lysates and phagosome isolations, RAW cells were plated into 6-well dishes (Corning, Tewksbury, MA). 2x106 RAW-TLR9-GFP cells were incubated with 4x106 β-1,3 glucan beads or CpG+IgG beads for indicated times. Cells were washed in ice-cold PBS three times. Cells were then mechanically sheared in a hypotonic lysis buffer (2 mM MgCl2, 6 mM β-mercaptoethanol, 10 mM HEPES with protease inhibitors (Roche, Indianapolis, IN) by passing through a 1cc syringe fitted with a ½-inch 26G needle (Fisher, Agawam, MA) for 15 cycles. 62% sucrose solution was added to adjust the final solution to 40% sucrose. A discontinuous gradient was then constructed in high-speed ultracentrifugation tubes (Beckman Polyallomer, Brea, CA) overlaying 2 mL of 62%, 40% of lysed cell suspension, 30%, 25%, and 10%. Sucrose gradient was then centrifuged at 80,000 x g for 1 h at 4°C (Beckman, SW-28 rotor, L8- M ultracentrifuge, Brea, CA). Phagosomes were isolated at the interface of the 25%/10% sucrose layers, washed in PBS, and counted using a hemacytometer in preparation for flow cytometry and immunoblot analysis.
Immunoblot analysis
RAW macrophages were incubated with R406 at the concentration of 10 µM for 30 min or 100 nM of BafA1 for 15 min in complete DMEM media. Cells were lysed in 1% nonidet P40 (NP-40; American Bioanalytical, Natick, MA) with protease inhibitors (Roche, Indianapolis, IN) for 1 h at 4°C. 1x106 phagosomes or cell lysate in 1X loading buffer/reducing agent (Invitrogen) were heated to 95°C for 5 min and proteins resolved by SDS-PAGE using 4–12% gels (Invitrogen). Following electrophoresis, gels were removed and methanol-activated PVDF membrane (PerkinElmer, Waltham, MA) were applied to the gel in transfer buffer (0.025 M Tris, 0.192 M glycine, 20% methanol). All buffer components were from National Diagnostics (Atlanta, GA) or Sigma-Aldrich. The gel and PVDF membrane were sandwiched between transfer sponge and blotting paper and subjected to electrophoretic transfer at 100V for 1 h.
For detection of GFP proteins, PVDF-immobilized gel transfers were blocked with 5% milk in PBS-0.01% Tween-20 (PBS-T, Sigma-Aldrich), overnight at 4°C. Blots were incubated with anti-GFP-HRP in 1% milk in PBS-T for 1 h at room temperature. Following three 5 min washes in PBS-T and PBS, the blot was visualized using the Western Lighting Plus ECL chemiluminescent substrate (PerkinElmer, Waltham, MA) on Kodak BioMax XAR Film (Sigma). Films were then scanned, cropped, and contrast was adjusted evenly to the entire image using Adobe Photoshop CS4.
Microarray analysis
4x106 wild-type B6 and Toll-like receptor 9 knockout (TLR9KO) immortalized murine macrophages were plated onto 10 cm cell culture dishes (Corning, Tewksbury, MA) and were stimulated either with β-1,3 glucan beads at MOI of 20:1 for 4 hr or unstimulated. Total RNA was extracted using RNA easy mini kit (Qiagen, Valencia, CA). Biotinylated cDNA were prepared according to the standard Affymetrix protocol from 100 ng total RNA using amplification kit WT Plus, followed by labeling of 5.5 µg cDNA. Following fragmentation, 90 µl of cDNA were hybridized for 16 hr at 45°C on GeneChip Mouse 1.0 ST Array in an Affymetrix GeneChip Hybridization oven 645 (Santa Clara, California). GeneChips were washed and stained in the Affymetrix Fluidics station 450 (Santa Clara, California). GeneChips were scanned using the Affymetrix GeneChip Scanner 3000 7G running Affymetrix Gene Command Console ver 3.2 (Santa Clara, California). The data were normalized using the Robust Multichip Average summary program RMAexpress (Pubmed ID: 12538238). Differentially expressed genes were identified using the mercury linear fit model. Transcripts with fold change > 2.0 between TLR9KO and wild-type B6 macrophages with a p-value < 0.001 were identified. Among these genes, transcripts with fold change > 1.8 between macrophages stimulated with β-1,3 glucan beads and without stimulation were considered differentially regulated between TLR9KO and wild-type B6 macrophages. GEO accession number GSE73474. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE73474
Phagocytosis Assay
1.8x105 RAW-TLR9-GFP macrophages plated onto 8-chambered coverglass were stimulated with 9x105 CpG beads, CpG+IgG beads, β-1,3 glucan beads, β-1,3 glucan+ IgG beads for 2 h at 37°C supplemented with 5% CO2. Green fluorescence around the beads was assessed by confocal microscopy. The percentage of TLR9-GFP positive phagosomes was calculated by dividing the number of engulfed beads that were GFP positive by the total number of engulfed beads in approximately 25 macrophages per condition. Three independent experiments were performed per condition.
RESULTS
Phagosomes containing β-1,3 glucan beads specifically recruit TLR9
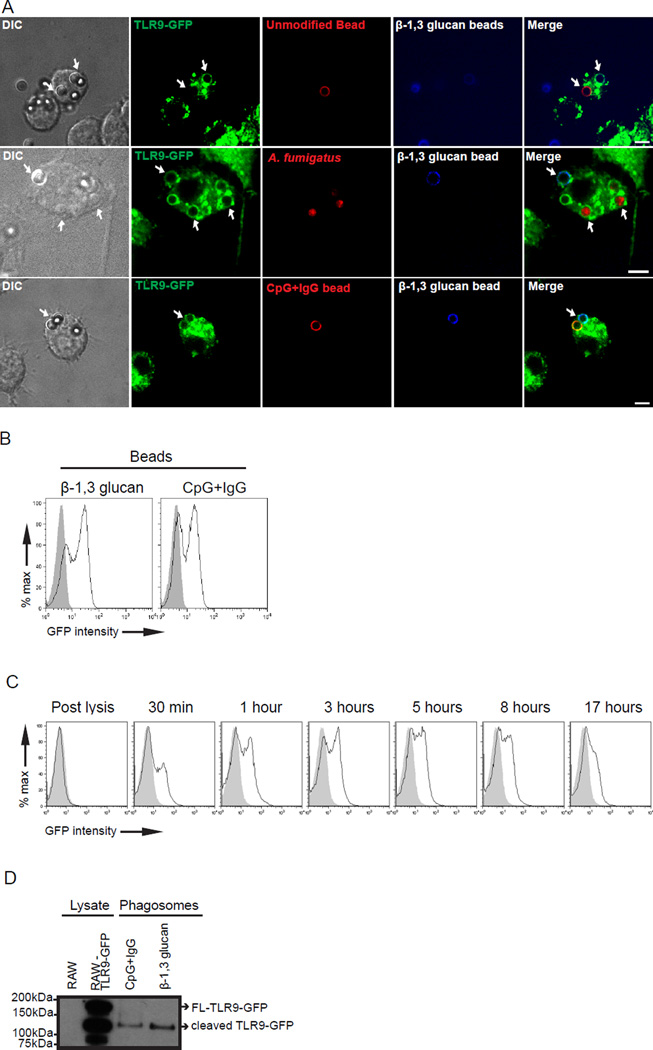
Our previous findings indicate that TLR9 is recruited to phagosomes containing either Aspergillus fumigatus or Candida albicans (12, 13). We hypothesized that a constituent of the fungal cell wall triggered redistribution and accumulation of TLR9 to the fungal-containing phagosomes. The fungal cell wall is composed of a complex mixture of carbohydrates including β-1,3 glucan polymers and proteins (3, 18). We took advantage of the development of polystyrene beads conjugated with pure, fungal-derived β-1,3 glucan to probe the response of TLR9 (23). Phagocytosis of β-1,3 glucan beads is Dectin-1 dependent. While CR3, a serum complement protein, can also mediate uptake of glucan particles into cells (28), our system used serum lacking functional CR3 (i.e. heat-inactivated serum). Within 30 min of bead internalization, TLR9 redistributes from the ER to endolysosomal compartments and is enriched on phagosomes containing β-1,3 glucan beads, whereas an uncoated, size-matched polystyrene bead-containing phagosome in the same cell failed to recruit TLR9 (Fig. 1A, top panel). Pre-treatment of β-1,3 glucan beads with DNase confirmed TLR9-GFP recruitment is likely not due to any contaminating DNA adsorbed to the beads (data not shown). A. fumigatus conidia triggers recruitment of TLR9 to the phagosome (12). To demonstrate that the recruitment of TLR9 to β-1,3 glucan beads is comparable to Aspergillus conidia, we stimulated TLR9-GFP macrophages with both β-1,3 glucan beads and A. fumigatus. Recruitment of TLR9-GFP to phagosomes containing β-1,3 glucan beads was comparable to that of A. fumigatus within the same cell (Fig. 1A, middle panel).
Figure 1.
TLR9 is specifically recruited to β-1,3 glucan phagosomes. (A) Confocal microscopy of RAW macrophages expressing TLR9-GFP (green) incubated with Alexa fluor 647-labeled β-1,3 glucan beads (blue) and Alexa fluor 568-labeled polystyrene beads (red-top panel), or A. fumigatus-RFP (red-middle panel), or Alexa fluor 568-labeled CpG-IgG beads (red-bottom panel) for 30 min. Size bar indicates 5µm. Original magnification X60 or X100. (B) Purified phagosomes containing either β-1,3 glucan beads or CpG-IgG beads were assessed for TLR9-GFP recruitment (black line) by phagoFACS and compared with polystyrene beads control (gray shaded histogram). (C) Kinetics of TLR9-GFP recruitment (black line) to purified β-1,3 glucan-containing phagosomes were assessed by phagoFACS for the indicated times and compared to polystyrene bead-only control (gray shaded histogram). β-1,3 glucan beads were added to cell lysates assessed for TLR9-GFP recruitment (D) β-1,3 glucan beads and CpG-IgG beads were incubated with 2x106 RAW TLR9-GFP cells for 3 h. Lysate control and 1x106 purified phagosomes were analyzed by immunoblot and blotted for GFP. Arrows indicate full-length-TLR9GFP (FL-TLR9-GFP) and cleaved TLR9-GFP as labelled. Data are representative of five independent experiments.
A previous study shows that CpG beads with anti-double stranded DNA antibody (CpG+IgG) have enhanced TLR9 recruitment compared to CpG beads alone (26). We sought to determine whether the addition of anti-β-1,3 glucan antibody would enhance TLR9 recruitment to β-1,3 glucan phagosomes. As expected, the CpG beads adsorbed with anti-double stranded DNA antibody (CpG+IgG) demonstrated potent recruitment of TLR9 to the phagosomes. However, β-1,3 glucan beads conjugated with β-1,3 glucan antibody (β-1,3 glucan + IgG) failed to enhance TLR9 recruitment in RAW macrophages compared to β-1,3 glucan beads as shown by Western blot, flow cytometric, and live cell imaging analyses (Supplementary Fig. 1A–C). Henceforth, the CpG+IgG beads were used as a positive control for TLR9 recruitment. As individual cells may express varying levels of TLR9-GFP, we sought to compare directly phagosomes containing either CpG+IgG or β-1,3 glucan beads within the same cell. There was comparable recruitment of TLR9-GFP to phagosomes containing β-1,3 glucan beads or CpG+IgG beads within the same macrophage (Fig. 1A, bottom panel).
To quantify the amount of TLR9-GFP on the phagosomal membrane, we isolated phagosomes from RAW TLR9-GFP cells incubated with β-1,3 glucan beads or CpG+IgG beads for 3 h. This type of analysis permits interrogation of at least 20,000 phagosomes per population. Flow cytometry analysis of isolated phagosomes revealed a comparable amount of TLR9 recruited to β-1,3 glucan beads as compared to CpG+IgG beads as determined by emission from GFP (Fig. 1B). Thus, our results indicate that TLR9-GFP is specifically recruited to phagosomes containing β-1,3 glucan beads and is comparable to levels achieved by CpG+IgG beads. Our previous findings indicate loss of β-1,3 glucan phagosomal-associated GFP-Dectin-1 within 90 min of phagocytosis (22). We explored the kinetics of TLR9-GFP translocation to β-1,3 glucan beads following phagocytosis. We stimulated RAW TLR9-GFP macrophages with β-1,3 glucan beads for up to 17 h and subjected the isolated β-1,3 glucan containing-phagosomes to flow cytometry. Unlike Dectin-1, which is lost from the phagosome within 30 min (22), TLR9-GFP was recruited within 30 min and remained on β-1,3 glucan-containing phagosomes for up to 17 h (Fig. 1C). Recruitment of the cleaved fraction of TLR9 was confirmed by immunoblot (Supplementary Fig. 2).
Proteolytic cleavage of TLR9 is required for its activation and signaling in primary antigen presenting cells (8, 9). We sought to determine whether full length TLR9-GFP or cleaved TLR9-GFP accumulated on β-1,3 glucan containing phagosomes. We incubated RAW TLR9-GFP cells with β-1,3 glucan beads for 3 h followed by phagosome isolation, and phagosomal proteins were resolved by SDS-PAGE. We recovered only the cleaved fraction of TLR9-GFP from phagosomes-containing β-1,3 glucan beads as well as CpG+IgG beads (Fig. 1D). In resting conditions, about 50% of TLR9-GFP in unstimulated RAW macrophages localizes to endolysosomes (29), and the lysate control confirms this finding (Fig. 1D). Incubation of β-1,3 glucan beads with lysates from RAW cells expressing TLR9-GFP failed to permit sufficient TLR9-GFP to be visualized by immunoblot, and phagoFACS indicating TLR9-GFP on phagosomes is not due to post-lysis effects (Fig. 1C and Supplementary Fig. 2). Our findings indicate that the proteolytically cleaved form of TLR9 accumulates on phagosomes containing β-1,3 glucan beads.
Dectin-1 is required for TLR9 recruitment to fungal phagosomes
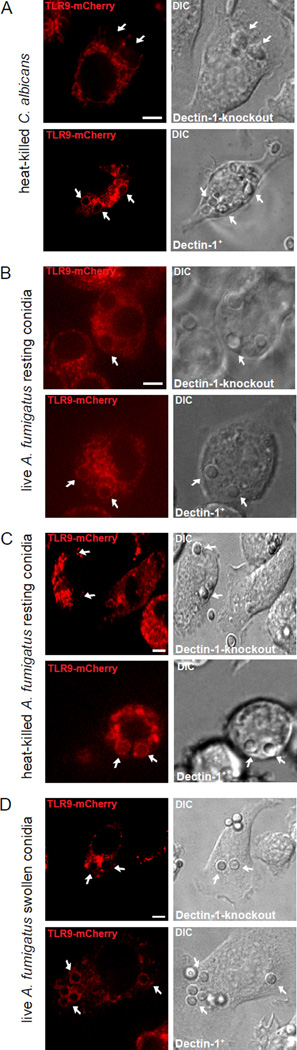
The β-1,3 glucan receptor, Dectin-1, synergizes with TLR2 and TLR4 in recognition of A. fumigatus or C. albicans in innate immune cells and cytokine production (30, 31). Dectin-1 activation controls maturation of phagosomes-containing β-1,3 glucan beads and C. albicans (22). Therefore, we predicted that Dectin-1 controls TLR9 recruitment to fungal phagosomes. To test this hypothesis, we expressed TLR9-mCherry in Dectin-1-knockout (D1KO) macrophages and D1KO macrophages reconstituted with GFP-Dectin-1. These macrophages were incubated with heat-killed (HK) C. albicans for 40 min as heat-killing of C. albicans exposes more β-1,3 glucan on the surface (32, 33). Confocal microscopy revealed that despite adequate expression of TLR9-mCherry and phagocytosis of HK C. albicans, TLR9-mCherry did not recruit to phagosomes in D1KO macrophages (Fig. 2A, top panel). Functional complementation of Dectin-1 deficient macrophages restored the ability of TLR9-mCherry to translocate successfully to HK C. albicans phagosomes (Fig. 2A, bottom panel). Our data support the notion that TLR9 recruitment to phagosomes containing β-1,3 glucan requires Dectin-1.
Figure 2.
TLR9 trafficking to fungal phagosomes requires Dectin-1. (A) Confocal microscopy of Dectin-1 knockout macrophages expressing TLR9-mcherry (red) in the absence (top panel) or presence (bottom panel) of Dectin-1. (A–D) Cells were incubated with heat-killed C. albicans (A), live A. fumigatus resting conidia (B), heat-killed A. fumigatus resting conidia (C), or live A. fumigatus swollen conidia (D) for 40 min. Scale bar indicates 5µm. Original magnification X100. (Top panels A–D). Data are representative of five independent experiments.
We wished to generalize our finding of Dectin-1 dependent TLR9 recruitment to other pathogenic fungi. A. fumigatus conidial spore germination starts with resting conidia and then swells and progresses to germtube formation with eventual hyphal branching (34, 35). During the A. fumigatus germination process, conidial swelling increases surface exposure of fungal β-1,3 glucan (35). We have previously shown that the signal required for TLR9 recruitment appears to be continuously present throughout the different spore stages of A. fumigatus (12). To test the dependence of Dectin-1 in TLR9 recruitment to different A. fumigatus spore stages, D1KO macrophages expressing TLR9-mCherry were incubated with live A. fumigatus resting conidia for 40 min. Despite phagocytosis, we observed no TLR9 recruitment to A. fumigatus conidia (Fig. 2B, top panel). In sharp contrast, reconstituted Dectin-1 deficient macrophages incubated with A. fumigatus resting conidia showed recruitment of TLR9-mCherry to fungal phagosomes (Fig. 2B, bottom panel). Furthermore, metabolically active conidia do not elicit a robust inflammatory response, as the surface hydrophobin and carbohydrate layers on the conidia mask PAMPs including β-1,3 glucan (35–37). Our previous study shows that TLR9 properly recruited and did not distinguish between different A. fumigatus spore stages (12). We examined the role of Dectin-1 in TLR9 recruitment to HK resting conidia. The process of heat-killing halts spore germination and disrupts the fungal cell wall, thereby increasing β-1,3 glucan exposure (38). After incubating D1KO macrophages expressing TLR9-mCherry with HK resting conidia for 40 min, we observed no recruitment of TLR9-mCherry around fungal phagosomes (Fig. 2C, top panel). In contrast, in reconstituted D1KO macrophages expressing TLR9-mCherry, HK A. fumigatus containing phagosomes recruited TLR9-mCherry (Fig. 2C, bottom panel).
Next, we induced conidial swelling which also unmasks β-1,3 glucan (35). Cells were incubated with live A. fumigatus swollen conidia for 40 min and TLR9 recruitment was observed. TLR9-mCherry recruitment was impaired in the absence of Dectin-1 whereas, reconstituted D1KO macrophages recruited TLR9-mCherry to swollen conidial phagosomes (Fig. 2D). Taken together, our results indicate Dectin-1 is required for TLR9 recruitment to phagosomes containing C. albicans and A. fumigatus, independent of spore stages.
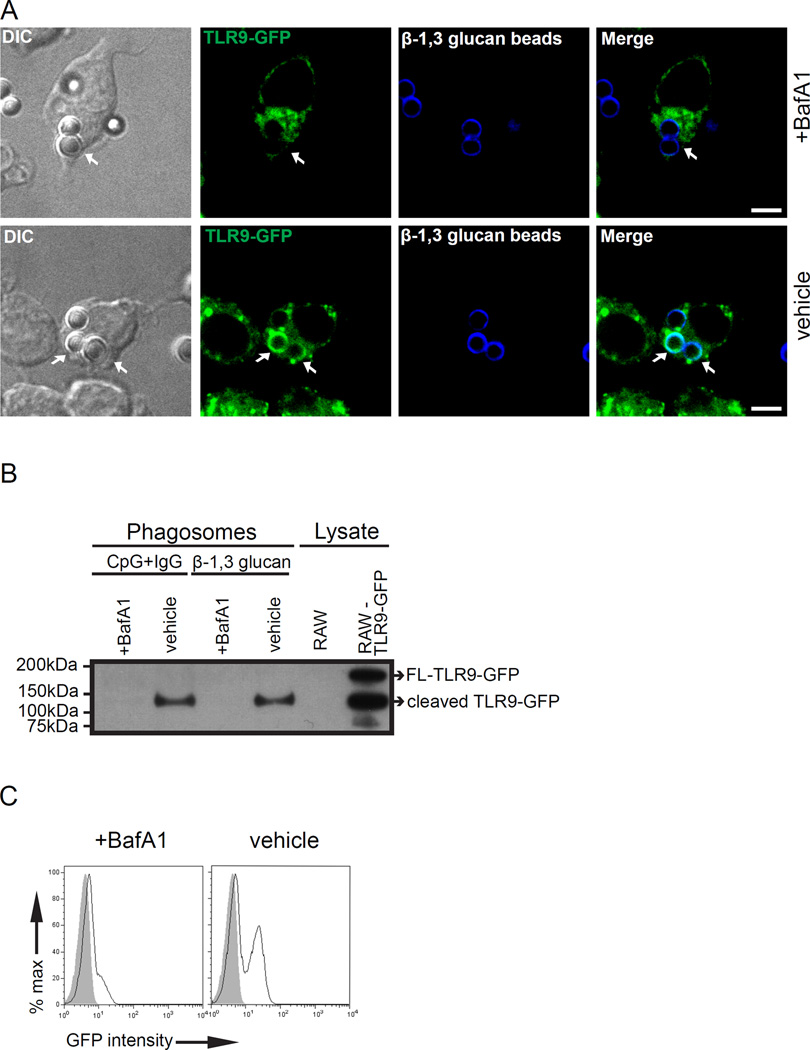
Phagosomal acidification is required for TLR9 recruitment to β-1,3 glucan phagosomes
Compartmentalized proteolysis of TLR9 is an essential step for nucleic acid recognition (8). The full-length form of TLR9 must undergo proteolysis in the endolysosomal compartments for successful intracellular trafficking, subcellular compartmental retention, and generation of a functionally-competent receptor to signal in response to CpG (8, 9, 29). Our recent findings indicated a direct correlation between Dectin-1 retention to phagosomes containing β-1,3 glucan and phagosomal pH (22). We hypothesized that selective accumulation of the cleaved TLR9 to β-1,3 glucan phagosomes (Fig. 1B) is dependent on phagosomal acidification. To evaluate this hypothesis, we pre-treated cells with the vacuolar-type H+ -ATPase inhibitor, bafilomycinA1 (BafA1). BafA1 blocks endolysosomal acidification as well as signaling by TLR9, TLR7, and TLR3 (39). BafA1 treatment had no effect on phagocytosis of β-1,3 glucan beads (22). TLR9 recruitment to β-1,3 glucan bead phagosomes was impaired in RAW cells expressing TLR9-GFP when pre-treated with BafA1 (Fig. 3A, top panel). In sharp contrast, TLR9 retained to β-1,3 glucan phagosomes when treated with a vehicle control (DMSO) (Fig. 3A, bottom panel). To confirm further this finding biochemically, we isolated β-1,3 glucan beads-containing phagosomes from RAW cells expressing TLR9-GFP that were treated with either BafA1 or vehicle control. Phagosomes from vehicle-treated cells retained cleaved TLR9-GFP at 3 h, whereas TLR9-GFP cells pre-treated with BafA1 showed no TLR9-GFP (Fig. 3B). As a control, phagosomes-containing CpG+IgG beads from TLR9-GFP macrophages pre-treated with BafA1 showed no TLR9, as expected (Fig. 3B). To assess a larger number of phagosomes simultaneously, we pre-treated TLR9-GFP expressing macrophages with BafA1 and DMSO for 15 min followed by incubation with β-1,3 glucan beads for 3 h. When isolated phagosomes were analyzed by flow cytometry, we observed trace green fluorescence on β-1,3 glucan-containing phagosomes from cells pre-treated with BafA1 (Fig. 3C). In contrast, phagosomes from macrophages exposed to DMSO demonstrated robust green fluorescence, indicating potent recruitment of TLR9-GFP. Our results demonstrate that phagosomal acidification is required for retention of TLR9 to β-1,3 glucan phagosomes.
Figure 3.
TLR9 recruitment to β-1,3 glucan phagosomes requires acidification. (A) RAW TLR9-GFP cells pre-treated with BafA1 or vehicle control for 15 min and incubated with either β-1,3 glucan beads and CpG-IgG beads for 3 h. TLR9-GFP recruitment to β-1,3 glucan phagosomes was assessed by confocal microscopy. Original magnification X60. Scale bar indicates 5µm. (B) Purified phagosomes and lysate control were analyzed by immunoblot and blotted for TLR9-GFP. Lysate controls indicate FL-TLR9-GFP and cleaved TLR9-GFP. (C) TLR9-GFP recruitment (black line) to purified β-1,3 glucan phagosomes in the presence of BafA1 or vehicle control was assessed by phagoFACS and compared to bead-only control (gray shaded histogram). Data are representative of three independent experiments.
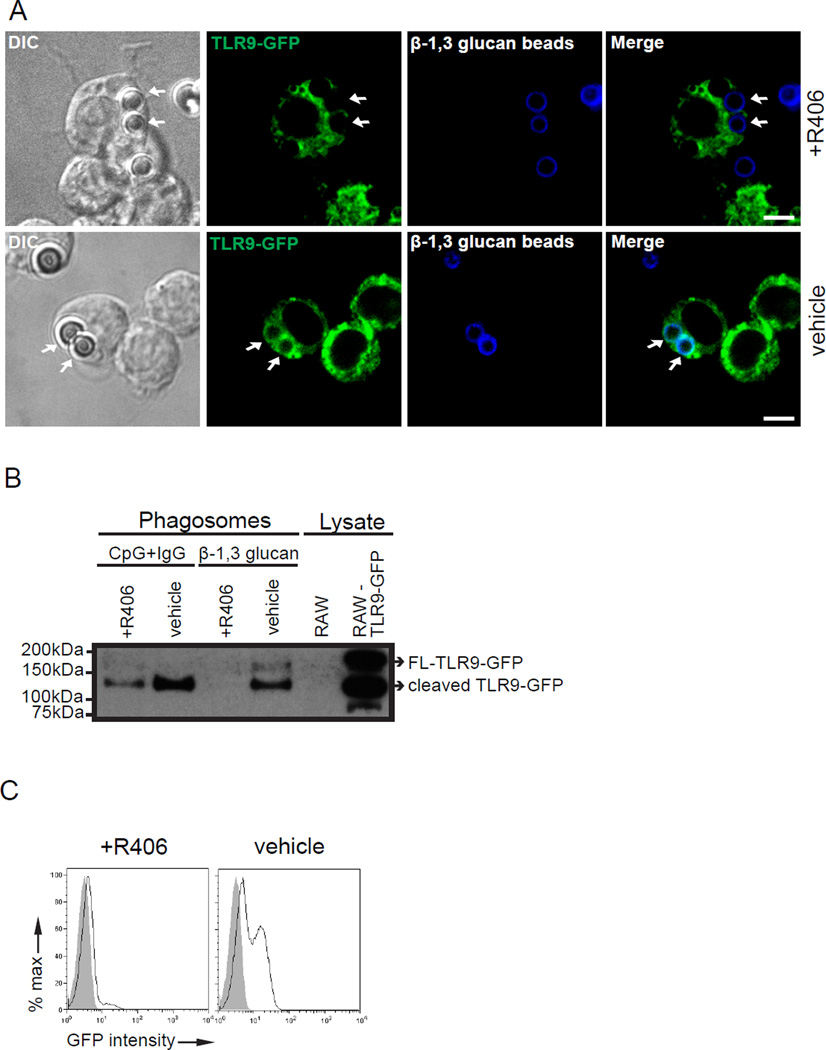
TLR9 recruitment to β-1,3 glucan phagosomes requires Syk signaling
Syk-coupled PRRs collaborate with Myd88-coupled TLRs to induce enhanced cytokine production (40). Blockade of Syk activation arrests β-1,3 glucan-containing phagosomes at an early endosomal stage (22). We hypothesized that Syk activation is required for successful TLR9 rearrangement to β-1,3 glucan phagosomes. To investigate the role of Syk activation in TLR9 trafficking, we pre-treated RAW TLR9-GFP macrophages with a Syk inhibitor (R406) or vehicle control for 30 min and incubated these cells with β-1,3 glucan beads for 3 h. R406 treatment had no effect on phagocytosis of beads as determined by microscopy (data not shown). Despite adequate expression of the GFP, TLR9-GFP recruitment to phagosomes containing β-1,3 glucan beads was impaired in R406-pre-treated cells. In contrast, TLR9-GFP successfully translocated to β-1,3 glucan beads in macrophages exposed to the vehicle control (Fig. 4A). To confirm our findings by microscopy, we exposed RAW-TLR9-GFP cells to either β-1,3 glucan beads or CpG+IgG beads for 3 h and performed biochemical analysis on isolated phagosomes to assess thousands of phagosomes simultaneously. Given that Fc receptors use Syk to initiate downstream signaling events (20), we used CpG-IgG beads as a control for Fc-dependent Syk activation. Although Syk inhibition blocks IgG/Fc receptor phagocytosis, granulin facilitates the uptake and delivery of CpG to TLR9 positive compartments (41). Indeed, we observed uptake of CpG beads by macrophages treated with a Syk inhibitor. Phagosomal proteins resolved by SDS-PAGE and probed for GFP showed cleaved TLR9-GFP for CpG+IgG and β-1,3 glucan bead-containing phagosomes from vehicle treated cells, as expected (Fig. 4B). β-1,3 glucan beads-containing phagosomes from R406 pre-treated cells showed no TLR9-GFP whereas recruitment of TLR9-GFP to CpG+IgG phagosomes was present although impaired (Fig. 4B). We confirmed these findings by flow cytometry and observed no GFP signal (TLR9-GFP) to β-1,3 glucan beads in the presence of R406, whereas vehicle pre-treatment showed TLR9 recruitment as expected (Fig. 4C). Our results indicate that Syk signaling is required for TLR9 recruitment to β-1,3 glucan phagosomes.
Figure 4.
TLR9 recruitment to β-1,3 glucan phagosomes requires Syk activation. (A-C) RAW macrophages expressing TLR9-GFP were pre-treated with R406 or vehicle control for 30 min. Cells were stimulated with either β-1,3 glucan beads or CpG+IgG beads for 3 h. (A) Confocal microscopy of RAW TLR9-GFP recruitment to β-1,3 glucan phagosomes in the presence of R406 or vehicle control. Original magnification X100. Scale bar indicates 5µm. (B) TLR9-GFP recruitment to purified β-1,3 glucan or CpG-IgG phagosomes in the presence of R406 or vehicle were analyzed by immunoblot. Lysate control of indicated cell type were used to indicate FL-TLR9-GFP and cleaved TLR9-GFP. (C) TLR9-GFP recruitment (black line) to purified β-1,3 glucan phagosomes in the presence of R406 or vehicle control was assessed by phagoFACS and compared to bead-only control (gray shaded histogram). Data are representative of three independent experiments.
Dectin-1 dependent Syk activation is required for TLR9 recruitment to β-1,3 glucan and fungal containing phagosomes
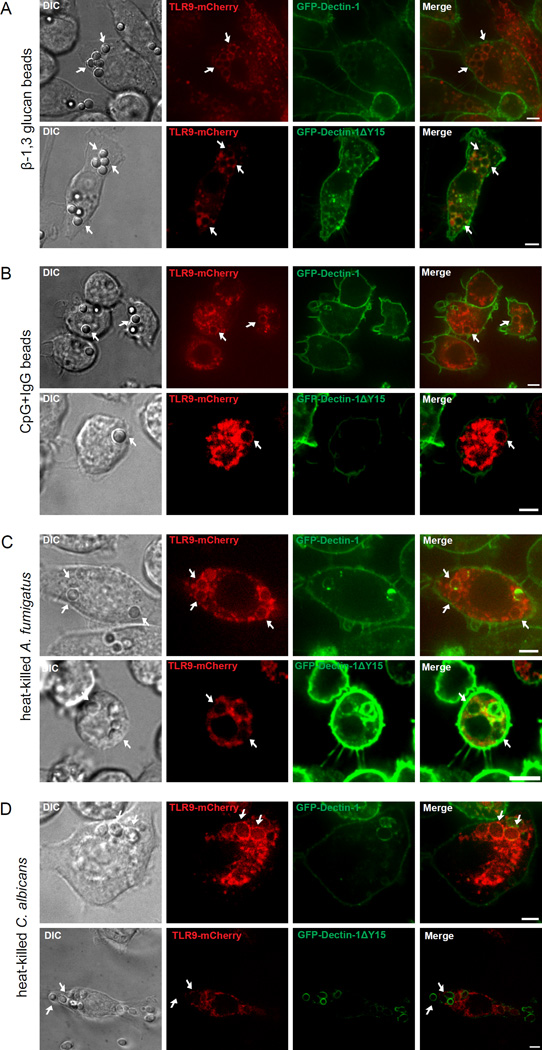
To eliminate any off-target effects of chemical inhibitors, we used a molecular approach to demonstrate a direct role of Dectin-1 dependent signaling through Syk phosphorylation for TLR9 recruitment to β-1,3 glucan containing phagosomes. To study TLR9 intracellular trafficking, we stably transduced Dectin-1-knockout macrophages with TLR9-mCherry and GFP-Dectin-1 or GFP-Dectin-1ΔY15, a signaling incompetent mutant of Dectin-1 that ablates Syk-mediated downstream signaling (19, 22). We then stimulated these cells with β-1,3 glucan beads for 2 h. As expected, we observed loss of phagosomal-associated GFP-Dectin-1 within 2 h of stimulation (22), but TLR9-mCherry was retained on β-1,3 glucan-containing phagosomes (Fig. 5A, top panel). In contrast, recruitment of TLR9-mCherry was impaired but not completely absent on β-1,3 glucan bead containing phagosomes in the presence of GFP-Dectin-1ΔY15 (Fig. 5A, bottom panel).
Figure 5.
Dectin-1 dependent Syk activation is required for TLR9 recruitment to β-1,3 glucan and fungal phagosomes. (A-D) Confocal microscopy of Dectin-1-knockout macrophages expressing TLR9-mCherry and GFP-Dectin-1 or GFP-Dectin-1ΔY15 incubated with β-1,3 glucan beads for 3 h (A and B), CpG+IgG beads for 2h (C and D), heat-killed A. fumigatus (E and F), or heat-killed C. albicans (G and H). Original magnification X60 and X100. Scale bar indicates 5µm. Data are representative of five independent experiments.
In the absence of Dectin-1 dependent Syk signaling, phagosomal maturation and acidification of β-1,3 glucan is blocked (22). Our data consistently show that the form of TLR9 recruited to β-1,3 glucan beads phagosomes is cleaved. To assess the size of the TLR9-mCherry on β-1,3 glucan beads in the presence of GFP-Dectin-1ΔY15, we performed immunoblot analysis on isolated phagosomes. Our data show that cleaved TLR9-mCherry was recruited as early as 1 h, suggesting TLR9 N-terminal proteolytic cleavage occurs either prior to or immediately after its trafficking to β-1,3 glucan bead containing phagosomes (data not shown). To demonstrate that the requirement of Dectin-1 dependent Syk activation for TLR9 recruitment is specific for β-1,3 glucan, we incubated macrophages with CpG+IgG beads for 2 h and showed TLR9 recruitment in both GFP-Dectin-1 and GFP-Dectin-1ΔY15 expressing macrophages (Fig. 5B). We then fed CpG+IgG and β-1,3 glucan beads to GFP-Dectin-1ΔY15 and TLR9-mCherry expressing macrophages and observed TLR9-mCherry recruitment to CpG+IgG beads. Specifically, TLR9-mCherry recruited to β-1,3 glucan bead in the same cell (Supplementary Fig. 3). We extended our observation of Dectin-1 dependent Syk signaling of TLR9 recruitment to fungal organisms. We incubated GFP-Dectin-1 and TLR9-mCherry-coexpressing D1KO macrophages with HK A. fumigatus for 40 min and observed robust TLR9-mCherry recruitment at the fungal phagosomal surface with weak GFP-Dectin-1 signal (Fig. 5C, top panel). In the TLR9-mCherry-expressing GFP-Dectin-1ΔY15 macrophages, HK conidia failed to recruit TLR9-mCherry with moderate levels of GFP-Dectin-1ΔY15 around the fungal phagosome (Fig. 5C, bottom panel). Next, to demonstrate this observation is not only specific to A. fumigatus, we exposed HK C. albicans to the D1KO macrophages expressing TLR9-mCherry and GFP-Dectin-1 and observed robust TLR9-mCherry recruitment to phagosomes (Fig. 5D, top panel). In agreement with previous findings, we observed normal decay of GFP-Dectin-1 signal from the phagosomal membrane of C. albicans within 20 min of phagocytosis (22). In contrast, GFP-Dectin-1ΔY15 retained to HK C. albicans phagosomes after 40 min of incubation whereas, TLR9-mCherry failed to traffic on fungal phagosomal compartments (Fig. 5D, bottom panel). Our results indicate Dectin-1 dependent Syk-activation is critical for triggering TLR9-mCherry accumulation on β-1,3 glucan bead and fungal-containing phagosomes.
Dectin-1 regulates TLR9 dependent gene expression upon stimulation by β-1,3 glucan beads
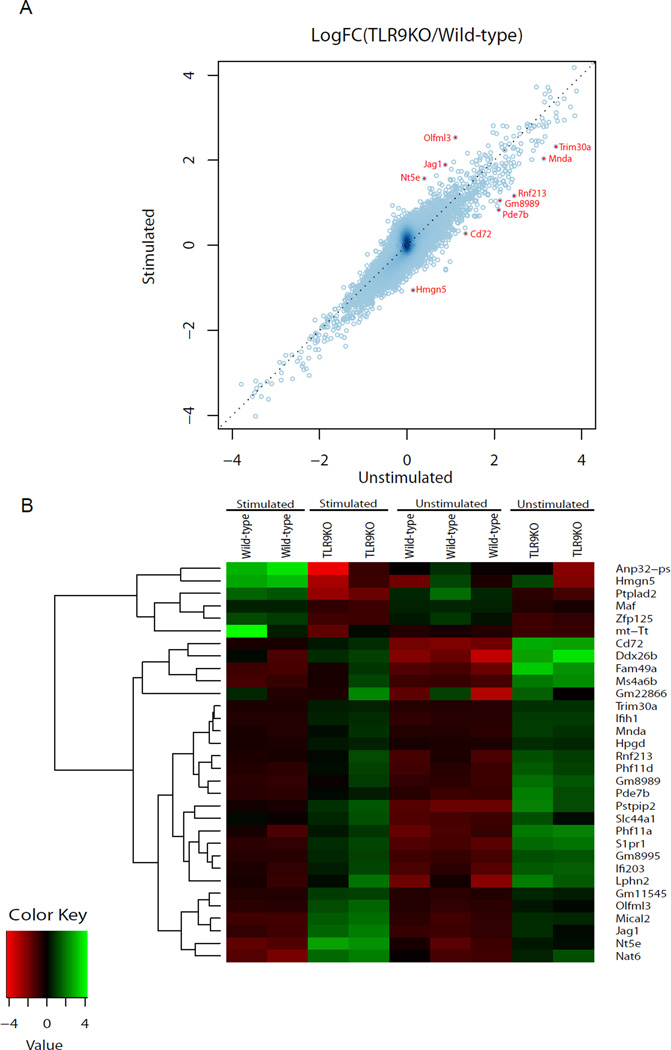
Our data indicate that Dectin-1 controls the recruitment of TLR9 to phagosomes containing β-1,3 glucan. To establish if this recruitment is functionally relevant, we sought to determine whether the absence of TLR9 affects the transcriptome in β-1,3 glucan stimulated macrophages. We stimulated wild-type and TLR9KO immortalized macrophages with β-1,3 glucan beads for 4 h, harvested RNA, and conducted a microarray analysis to identify differentially expressed genes using the cut off 1.8-fold change in expression levels. Gene expression values from unstimulated wild-type and TLR9KO macrophages were used to determine the baseline differences in expression levels. Analysis of microarray data revealed 32 differentially expressed genes that are dependent on TLR9 in β-1,3 glucan stimulated macrophages (Table 1 and Fig. 6). Of the 32 differentially expressed genes, the Interferon-inducible (IFI) family genes: IFI203, Mnda, and Ifih1 (MDA5), and Trim30a are directly implicated in the regulation of immune signaling (42–45). Trim30a is also a negative regulator of TLR9 signaling (45). These data indicate Dectin-1 modulates TLR9-dependent gene expression in response to β-1,3 glucan beads in macrophages.
Table 1.
List of differentially expressed genes in TLR9KO and wild-type immortalized macrophages.1
| Gene Symbol |
Gene Description | Delta_LogFC | Gene Function |
|---|---|---|---|
| Olfml3 | Olfactomedin-like 3 | 1.42 | Multicellular organismal development |
| Rnf213 | Ring finger protein 213 | 1.30 | Ubiquitin-transferase activity |
| Pde7b | Phosphodiesterase 7B | 1.27 | Signal transduction |
| Hmgn5 | High-mobility group nucleosome binding domain 5 |
1.20 | Regulation of transcription |
| Nt5e | 5’ nucleotidase, ecto | 1.17 | Negative regulator of inflammatory response |
| Gm8989 | Predicted gene 8989 | 1.14 | Unknown function |
| Trim30a | Tripartite motif-containing 30A | 1.09 | Negative regulator of immune signaling |
| Mnda | Myeloid cell nuclear differentiation antigen | 1.09 | Type I IFN induced genes |
| Cd72 | CD72 antigen | 1.07 | Carbohydrate binding |
| Jag1 | Jagged 1 | 1.01 | Cell differentiation |
| Ifi203 | Interferon induced gene 203 | 0.99 | Type I IFN induced genes |
| Phf11d | PHD finger protein 11D | 0.99 | Regulation of transcription |
| Zfp125 | Zinc finger protein 125 | 0.98 | Unknown function |
| Phf11a | PHD finger protein 11A | 0.97 | Regulation of immune response |
| Nat6 | N-acetyltransferase 6 | 0.96 | Transferase activity |
| mt-Tt | Mitochondrially encoded tRNA threonine | 0.95 | Translation |
| Fam49a | Family with sequence similarity 49, member A | 0.93 | Unknown function |
| Ms4a6b | Membrane-spanning 4-domains, subfamily A, member 6B |
0.92 | Unknown function |
| Mical2 | Microtubule associated monooxygenase, calponin and LIM domain containing 2 |
0.89 | Actin filament depolarization |
| Slc44a1 | Solute carrier family 44, member 1 | 0.88 | Choline transporter |
| Lphn2 | Latrophilin-2 | 0.88 | Unknown function |
| Gm8995 | Predicted gene 8995 | 0.87 | Unknown function |
| S1pr1 | Sphingosine-1-phosphate receptor 1 | 0.85 | Cell adhesion |
| Pstpip2 | Proline-serine-threonine phosphatase- interacting protein 2 |
0.84 | Actin binding |
| Gm22866 | Gm22866 | 0.84 | Unknown function |
| Ifih1* | Interferon induced with helicase C domain 1 | 0.84 | Type I IFN induced genes |
| Ptplad2 | Protein tyrosine phosphatase-like A domain containing 2 |
0.84 | Fatty acid elongation |
| Anp32-ps | Acidic nuclear phosphoprotein 32 family, member A |
0.84 | Regulation of transcription |
| Ddx26b | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 26B |
0.84 | Unknown function |
| Gm11545 | Predicted gene 11545 | 0.83 | Unknown function |
| Maf | Avian musculoponeurotic fibrosarcoma (v-maf) AS42 oncogne homolog |
0.82 | Regulation of transcription |
| Hpgd | Hydroxyprostaglandin dehydrogenase 15 (NAD) | 0.80 | Oxidoreductase activity |
List of TLR9 dependent genes in response to β-1,3 glucan beads are determined by microarray gene analysis. Transcripts with fold change > 2.0 between TLR9KO and wild-type B6 macrophages with a p-value < 0.001 were identified. Among these genes, 32 transcripts with fold change > 1.8 between macrophages stimulated with β-1,3 glucan beads and without stimulation were considered differentially regulated between TLR9KO and wild-type B6 macrophages
Ifih1 encodes for MDA5 previously described as host defense against Candida infections.
Figure 6.
Dectin-1 regulates TLR9 dependent gene expression. (A–B) Microarray data analysis of TLR9KO and wild-type B6 immortalized macrophages in response to β-1,3 glucan beads. (A) A scatter plot indicating a ratio of fold changes in gene expression between wild-type and TLR9KO macrophages. Off-diagonal dots (red) indicate differentially regulated TLR9 dependent genes that are fold change > 2.0 between macrophages stimulated with β-1,3 glucan beads and unstimulated. (B) A heat map of the 32 genes differentially expressed (fold change > 1.8) between TLR9KO and wild-type macrophages in response to β-1, 3 glucan beads.
DISCUSSION
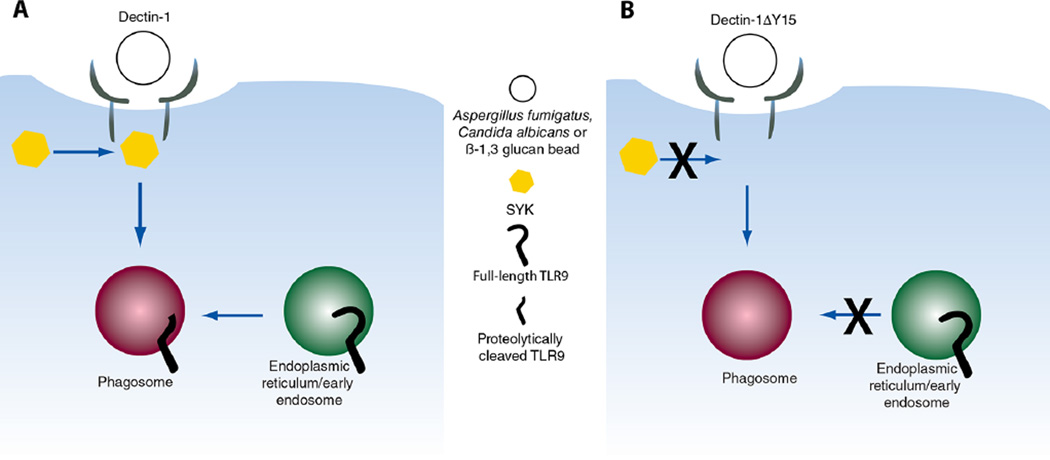
Our data support a model in which phagosomes containing β-1,3 glucan are recognized by Dectin-1 and trigger Syk activation, which leads to TLR9 recruitment and retention of the proteolytically-cleaved TLR9 to the phagosome (Fig. 7). In addition, we identified two requirements for redistribution of TLR9 to these phagosomes: phagosomal acidification and Dectin-1 dependent Syk activation. Finally, we show that TLR9 modulates gene expression in a Dectin-1 dependent manner, suggesting a functional role for TLR9 in regulating the immune response to β-1,3 glucan.
Figure 7.
Schematic representation of Dectin-1 dependent TLR9 recruitment to fungal- and β-1, 3 glucan-containing phagosomes. (A) Recognition and ligation of Dectin-1 with A. fumigatus, C. albicans, or β-1,3 glucan bead results in phagocytosis and Syk activation triggering the recruitment of TLR9 to the phagosomes and retention of the proteolytically cleaved version of TLR9. (B) Signaling incompetent Dectin-1ΔY15 can mediate phagocytosis of fungi or β-1,3 glucan beads but is incapable of activating Syk, resulting in failed TLR9 recruitment to the phagosome.
In this study, we demonstrate that TLR9 recruitment to phagosomes containing β-1,3 glucan is controlled by Dectin-1. Furthermore, our data indicate that β-1,3 glucan in phagosomes is sufficient for TLR9 recruitment. Our experiments rely on the exclusive use of TLR9-GFP to report on the activity of TLR9. It remains possible that TLR9-GFP may not faithfully replicate all features of endogenous TLR9. Unlike Dectin-1, retention of TLR9 on the phagosomes is long-lived and remains present for >16 h. To our knowledge, this observation is the first to demonstrate control of TLR trafficking by a CLR.
The redistribution of TLR9 to phagosomes requires phagosomal acidification and Dectin-1 dependent Syk activation. Lysosomal cysteine protease, cathepsin L and cathepsin S, are required for TLR9 cleavage (8). Inhibition of Dectin-1-dependent phagosomal acidification (22) may also block activation of lysosomal cathepsins, which, in turn, inhibit cleavage of TLR9 (8, 9). Thus, it is formally possible that TLR9 may still be recruited to the phagosome, but the failure to undergo cleavage may prevent its retention to β-1,3-glucan-containing phagosomes.
Receptor signaling by TLRs at the plasma membrane is regulated precisely by mechanisms that control receptor coupling to signal transduction machinery and subcellular distribution of signaling components (4, 5). Considerably less is known about mechanisms that regulate TLR signaling from endosomes. Defects in these mechanisms can have profound effects including increased susceptibility to infections or immunodeficiency (46). Unc93B1, an ER resident protein, controls the egress of TLR9 and other endosomal TLRs from the ER to the endosomal compartments (47, 48). To become fully competent in interferon signaling, TLR9 must be localized in AP-3+ compartments (49). Proteolytically cleaved TLR9 recruits MyD88 in response to CpG (8). It remains to be determined whether TLR9 that is recruited to β-1,3-glucan phagosomes assembles a functional myddosome for signaling. TLR9 signaling by A. fumigatus phagosomes can bypass MydD88 (50). Therefore, it is possible that Dectin-1 dependent Syk activation modulates TLR9 signaling independent of myddosome formation.
Dectin-1 signaling provides a powerful activating signal and triggers potent inflammatory cytokines in response to β-1,3 glucan (51). The concept that Dectin-1 induces an immunomodulatory pathway has been previously established. SOCS-1 is induced by Dectin-1 in mouse bone marrow-derived dendritic cells and macrophages, which leads to decreased and abbreviated NF-κB activation in DCs and macrophages triggered by TLR9 (52). It is noteworthy that IL-12 and IL-10 secretion were inhibited by SOCS-1. The precise role of SOCS-1 in this model of fungal infection remains to be determined.
In this study, we extend the role of Dectin-1 in triggering inflammatory responses to regulating recruitment of TLR9 to fungal phagosomes. We have previously shown that TLR9 serves to modulate inflammatory response (13). Thus, the Dectin-1-TLR9 axis may represent a feedback loop to control the activation of innate immune cells. Upon ligation with a fungal pathogen that contains β-1,3 glucan, Dectin-1 activates Src which, in turn, activates Syk (19). Card9 activation and ROS production are downstream events from Dectin-1 dependent Syk activation that direct the elaboration of inflammatory cytokines (53). Dectin-1 dependent Syk activation triggers acidification of the phagosome and permits trafficking and retention of TLR9 to these compartments. TLR9 signaling thus serves to modulate the inflammatory response by down regulating cytokine production (13). Topologically, β-1,3 glucan is closer to the surface of fungal cell walls when compared to chitin (54). After sufficient exposure within the phagolysosome, deeper structures of the fungi such as chitin may be exposed or fungal DNA may be released. Our data indicate that the N-terminal cleaved TLR9 is the predominant form found on these phagosomes, which is capable of signaling. While the precise identity of the TLR9 ligand is not known on fungal organisms, it is interesting to note that the recognition of fungal chitin appears to trigger IL-10 production in a TLR9 dependent manner (55), thereby providing the final step in this feedback loop.
Importantly, recruitment of TLR9 to phagosomes is not equivalent to TLR9 signaling. Microarray analysis revealed a subset of 32 genes that are differentially expressed in TLR9KO macrophages compared to wild-type macrophages when stimulated with β-1,3 glucan beads (Table 1 and Fig. 6). Since Dectin-1 is the receptor for β-1,3 glucan, these data demonstrate that TLR9 modulates gene expression in a Dectin-1 dependent manner and suggests a functional role for TLR9 in regulating the immune response to fungal pathogens. The effects of β-1,3 glucan on TLR9-dependent gene expression may be direct or indirect and does not suggest TLR9 is directly binding β-1,3 glucan. Comparison of our differentially expressed genes to previously published data probing macrophages with CpG (56) identified 4 common genes, suggesting that TLR9 signaling in response to β-1,3 glucan beads shares minor overlap with CpG induced changes in gene expression. Genes that are differentially regulated in response to CpG would not necessarily be influenced by β-1,3 glucan as CpG is a direct TLR9 ligand. Our study identified TLR9 dependent changes in gene expression that are specifically regulated by Dectin-1 (Table 1 and Fig. 6).
Along with CLRs, TLRs orchestrate anti-fungal innate immune responses and generate an effective host defenses against clinically-relevant fungal pathogens (30, 57). Bacterial and viral DNA containing unmethylated CpG motifs are well-known ligands of TLR9. The specificity of TLR9 is not only restricted to microbial DNA but can also directly bind malarial hemozoin resulting in TLR9 conformational changes (58). For fungal organisms, TLR9 can be triggered by fungal DNA (7, 59, 60). TLR9 can recognize fungal species of various groups including C. albicans, A. fumigatus, Cryptococcus neoformans, Saccharomyces cerevisiae, Paracocci brasiliensis, and Malassezia furfur (12, 13, 59). TLR9 plays an essential and protective role in P. brasiliensis infection as TLR9−/− mice succumb to death yet had higher TNFα and IL-6 expression (59). In contrast, TLR9−/− mice had a survival advantage compared to wild-type mice when challenged with C. albicans and A. fumigatus (6). Also, our previous study demonstrated TLR9 deficiency increases macrophage fungicidal activity and enhanced anti-fungal effector response against C. albicans and S. cerevisiae (13). Thus, the precise role of TLR9 in the host defense against invasive fungal infections is not completely understood, but it appears to be distinct and pathogen dependent.
Interestingly, we identified several immunity-related genes that were upregulated in TLR9KO macrophages when stimulated with β-1,3 glucan beads (Table 1 and Fig. 6). For example, the IFI genes (Ifih1, IFI203, and Mnda) play a role in inflammation (42, 43, 61). Ifih1 which encodes MDA5, a RIG-I-like receptor directly participates in anti-fungal host defense (42). Type I Interferon signaling confers a protective role in mice with C. albicans infection (62, 63). Therefore, upregulation of IFI genes in the absence of TLR9 could provide a possible explanation for why mice that lack TLR9 are more resistant to C. albicans and A. fumigatus infections (6). IFI family proteins also recognize cytoplasmic dsDNA and regulate gene expression (64). Therefore, we speculate that IFI genes could be upregulated in the absence of TLR9 to participate in nucleic acid sensing pathways.
In this study, we sought to determine the role of Dectin-1 in controlling TLR9 trafficking to β-1,3-glucan and fungal containing phagosomes. The model we propose suggests that Dectin-1-dependent Syk activation is required for TLR9 recruitment (Fig. 7). Our results also indicate that Dectin-1 functions as a regulator for TLR9 dependent gene expression in response to β-1,3 glucan beads. Overall, these findings highlight a role for TLR9 in the cellular and molecular mechanisms that govern the innate immune response to fungal pathogens.
Supplementary Material
Acknowledgments
We thank Dr. Gordon Brown, Dr. Stuart Levitz, Dr. Eleftherios Mylonakis, and Dr. Michelle Momany for reagents and Arch Macinnes for assistance with the artwork. We also thank Fei Ji and Ruslan Sadreyev from Department of Molecular Biology at Massachusetts General Hospital for performing the microarray analysis.
Footnotes
NIH Grant 5R01 A1 092084, 1R01 A1 097519, M.K.M is supported by NIH/NIAID 1K08AI110655, J.L.R is supported by T32 A1007061-35 and KL2 TR001100.
Abbreviations used in this paper: Syk, spleen tyrosine kinase; BafA1, bafilomycin; RAW, RAW 264.7 macrophage-like cell line; DIC, differential interference contrast; PAMPs, pathogen associated molecular patterns; PRR, pattern-recognition receptor; CLR, C-type lectin receptor; TLR, Toll-like receptor; YPD, yeast extract peptone dextrose agar; D1KO, Dectin-1-knockout; TLR9KO, Toll-like receptor 9-knockout; IFI, Interferon-inducible; HK, heat-killed.
REFERENCES
- 1.Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 2.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25:736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, Sarfati J, D’Angelo C, Perruccio K, Bonifazi P, Zagarella S, Moretti S, Bistoni F, Latge JP, Romani L. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183:2407–2414. doi: 10.4049/jimmunol.0900961. [DOI] [PubMed] [Google Scholar]
- 4.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 5.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez-Ortiz ZG, Specht CA, Wang JP, Lee CK, Bartholomeu DC, Gazzinelli RT, Levitz SM. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun. 2008;76:2123–2129. doi: 10.1128/IAI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 11.Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, Kagan JC. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell. 2014;156:705–716. doi: 10.1016/j.cell.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasperkovitz PV, Cardenas ML, Vyas JM. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol. 2010;185:7614–7622. doi: 10.4049/jimmunol.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasperkovitz PV, Khan NS, Tam JM, Mansour MK, Davids PJ, Vyas JM. Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect Immun. 2011;79:4858–4867. doi: 10.1128/IAI.05626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 15.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 17.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinsbroek SE, Taylor PR, Martinez FO, Martinez-Pomares L, Brown GD, Gordon S. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog. 2008;4:e1000218. doi: 10.1371/journal.ppat.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev. 2010;234:335–352. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 21.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour MK, Tam JM, Khan NS, Seward M, Davids PJ, Puranam S, Sokolovska A, Sykes DB, Dagher Z, Becker C, Tanne A, Reedy JL, Stuart LM, Vyas JM. Dectin-1 activation controls maturation of beta-1,3-glucan-containing phagosomes. J Biol Chem. 2013;288:16043–16054. doi: 10.1074/jbc.M113.473223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam JM, Mansour MK, Khan NS, Yoder NC, Vyas JM. Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr Biol (Camb) 2012;4:220–227. doi: 10.1039/c2ib00089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS pathogens. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyas JM, Kim YM, Artavanis-Tsakonas K, Love JC, Van der Veen AG, Ploegh HL. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–7210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR, Sanjuan MA. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour MK, Tam JM, Khan NS, Seward M, Davids PJ, Puranam S, Sokolovska A, Sykes DB, Dagher Z, Becker C, Tanne A, Reedy JL, Stuart LM, Vyas JM. Dectin-1 activation controls maturation of beta-1,3-glucan-containing phagosomes. J Biol Chem. 288:16043–16054. doi: 10.1074/jbc.M113.473223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, Specht CA, Levitz SM. Relative contributions of dectin-1 and complement to immune responses to particulate beta-glucans. J Immunol. 2012;189:312–317. doi: 10.4049/jimmunol.1200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avalos AM, Kirak O, Oelkers JM, Pils MC, Kim YM, Ottinger M, Jaenisch R, Ploegh HL, Brinkmann MM. Cell-specific TLR9 trafficking in primary APCs of transgenic TLR9-GFP mice. J Immunol. 2013;190:695–702. doi: 10.4049/jimmunol.1202342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 31.Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol. 2006;176:3717–3724. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- 32.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, Brown GD, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CM, Vanier G, Urb M, Campoli P, Al Abdallah Q, Lehoux M, Chabot JC, Ouimet MC, Baptista SD, Fritz JH, Nierman WC, Latge JP, Mitchell AP, Filler SG, Fontaine T, Sheppard DC. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS Pathog. 2013;9:e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrion Sde J, Leal SM, Jr, Ghannoum MA, Aimanianda V, Latge JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol. 2013;191:2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 38.Tam JM, Mansour MK, Khan NS, Seward M, Puranam S, Tanne A, Sokolovska A, Becker CE, Acharya M, Baird MA, Choi AM, Davidson MW, Segal BH, Lacy-Hulbert A, Stuart LM, Xavier RJ, Vyas JM. Dectin-1 dependent LC3 recruitment to phagosomes enhances fungicidal activity in macrophages. The Journal of infectious diseases. 2014 doi: 10.1093/infdis/jiu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hacker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. Embo J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park B, Buti L, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, Nishihara M, Ploegh HL. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity. 2011;34:505–513. doi: 10.1016/j.immuni.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Jaeger M, van der Lee R, Cheng SC, Johnson MD, Kumar V, Ng A, Plantinga TS, Smeekens SP, Oosting M, Wang X, Barchet W, Fitzgerald K, Joosten LA, Perfect JR, Wijmenga C, van de Veerdonk FL, Huynen MA, Xavier RJ, Kullberg BJ, Netea MG. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur J Clin Microbiol Infect Dis. 2015;34:963–974. doi: 10.1007/s10096-014-2309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choubey D, Duan X, Dickerson E, Ponomareva L, Panchanathan R, Shen H, Srivastava R. Interferon-inducible p200-family proteins as novel sensors of cytoplasmic DNA: role in inflammation and autoimmunity. J Interferon Cytokine Res. 2010;30:371–380. doi: 10.1089/jir.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wimmer N, Huber B, Barabas N, Rohrl J, Pfeffer K, Hehlgans T. Lymphotoxin beta receptor activation on macrophages induces cross-tolerance to TLR4 and TLR9 ligands. J Immunol. 2012;188:3426–3433. doi: 10.4049/jimmunol.1103324. [DOI] [PubMed] [Google Scholar]
- 45.Hu Y, Mao K, Zeng Y, Chen S, Tao Z, Yang C, Sun S, Wu X, Meng G, Sun B. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J Immunol. 2010;185:7699–7705. doi: 10.4049/jimmunol.1001099. [DOI] [PubMed] [Google Scholar]
- 46.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clinical microbiology reviews. 2011;24:490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. The Journal of cell biology. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. eLife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, Armstrong-James D. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med. 2015;7:240–258. doi: 10.15252/emmm.201404556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Eberle ME, Dalpke AH. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. Journal of immunology. 2012;188:5644–5654. doi: 10.4049/jimmunol.1103068. [DOI] [PubMed] [Google Scholar]
- 53.Goodridge HS, Shimada T, Wolf AJ, Hsu YM, Becker CA, Lin X, Underhill DM. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. Journal of immunology. 2009;182:1146–1154. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CM, Vanier G, Urb M, Campoli P, Al Abdallah Q, Lehoux M, Chabot JC, Ouimet MC, Baptista SD, Fritz JH, Nierman WC, Latge JP, Mitchell AP, Filler SG, Fontaine T, Sheppard DC. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS pathogens. 2013;9:e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS pathogens. 2014;10:e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao JJ, Diesl V, Wittmann T, Morrison DC, Ryan JL, Vogel SN, Follettie MT. Bacterial LPS and CpG DNA differentially induce gene expression profiles in mouse macrophages. J Endotoxin Res. 2003;9:237–243. doi: 10.1179/096805103225001431. [DOI] [PubMed] [Google Scholar]
- 57.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. Journal of immunology. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 58.Coban C, Igari Y, Yagi M, Reimer T, Koyama S, Aoshi T, Ohata K, Tsukui T, Takeshita F, Sakurai K, Ikegami T, Nakagawa A, Horii T, Nunez G, Ishii KJ, Akira S. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell host & microbe. 2010;7:50–61. doi: 10.1016/j.chom.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Menino JF, Saraiva M, Gomes-Alves AG, Lobo-Silva D, Sturme M, Gomes-Rezende J, Saraiva AL, Goldman GH, Cunha C, Carvalho A, Romani L, Pedrosa J, Castro AG, Rodrigues F. TLR9 activation dampens the early inflammatory response to Paracoccidioides brasiliensis, impacting host survival. PLoS neglected tropical diseases. 2013;7:e2317. doi: 10.1371/journal.pntd.0002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka M, Ishii K, Nakamura Y, Miyazato A, Maki A, Abe Y, Miyasaka T, Yamamoto H, Akahori Y, Fue M, Takahashi Y, Kanno E, Maruyama R, Kawakami K. Toll-like receptor 9-dependent activation of bone marrow-derived dendritic cells by URA5 DNA from Cryptococcus neoformans. Infection and immunity. 2012;80:778–786. doi: 10.1128/IAI.05570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choubey D, Moudgil KD. Interferons in autoimmune and inflammatory diseases: regulation and roles. J Interferon Cytokine Res. 2011;31:857–865. doi: 10.1089/jir.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biondo C, Signorino G, Costa A, Midiri A, Gerace E, Galbo R, Bellantoni A, Malara A, Beninati C, Teti G, Mancuso G. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol. 2011;41:1969–1979. doi: 10.1002/eji.201141490. [DOI] [PubMed] [Google Scholar]
- 63.Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Muller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8:e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connolly DJ, Bowie AG. The emerging role of human PYHIN proteins in innate immunity: implications for health and disease. Biochem Pharmacol. 2014;92:405–414. doi: 10.1016/j.bcp.2014.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.