Abstract
Purpose
The 34-gene classifier, ClearCode34, identifies prognostically distinct molecular subtypes of clear cell renal cell carcinoma (ccRCC) termed ccA and ccB. The primary objective of this study was to describe clinical characteristics and comorbidities of relevance in patients stratified by ClearCode34.
Patients and Methods
In this retrospective analysis, 282 patients from Moffitt Cancer Center with ccRCC with gene expression analyses of the primary tumor were identified and ClearCode34 was applied to identify tumors as ccA or ccB. The medical record and institutional databases were queried to define patient characteristics, comorbidities, and outcomes.
Results
We validated in this external cohort the superior overall survival (OS), cancer specific survival (CSS), and recurrence-free survival of ccA patients relative to ccB patients (p<0.001). Addressing other clinical characteristics, the ccA patients were more likely to be obese (48% versus 34%, p=0.021) and diabetic (26% versus 13%, p=0.035). The ccA patients also trended towards having been more frequent users of angiotensin system inhibitors (ASIs) (71% versus 52%, p=0.055). In multivariate analyses, ccB status is independently associated with inferior CSS (HR 3.26, 95% CI 1.84–5.79) and OS (HR 2.50, 95% CI 1.53–4.08).
Conclusions
ClearCode34, after considering distinct patterns of comorbidities in each molecular subtype, remains a strong prognostic tool in ccRCC patients. Obesity and DM emerged as factors that may influence ccRCC phenotypes and further studies investigating the impact of these metabolic conditions functionally onto tumor biology are warranted. Additionally, use of ASI could be studied in the context of ccRCC molecular classification in future studies to better understand its impact on ccRCC outcomes.
Keywords: ClearCode34, clear cell renal cell carcinoma, obesity, diabetes mellitus
1. Introduction
Cancers of the kidney and renal pelvis are among the more common cancers diagnosed in the United States with an estimated 61,560 new cases and 14,080 deaths for 2015 [1]. In adults, the overwhelming majority of these cases were renal cell carcinoma (RCC). Significant effort has been expended in developing ways to stratify RCC patients into clinically meaningful categories in order to inform clinical decisions. Classically, anatomical features (e.g. TNM classification or Fuhrman nuclear grade) were used to predict more aggressive disease [2]. Clinical prognostic systems are commonly used and include both clinical factors and laboratory data [3, 4]. Models including molecular testing have also received much attention in the prognostication of RCC, including multi-gene expression signatures [5–7]. The most comprehensive molecular analysis to date of clear cell RCC (ccRCC), the most common RCC histologic subtype, was The Cancer Genome Atlas (TCGA) study. The TCGA group analyzed over 400 tumors on multiple platforms and described numerous molecular changes that correlated significantly with patient outcomes [8]. However, despite these intense efforts, no models using molecular analyses are routinely utilized in the clinic to direct patient care.
Brannon et al. and others used gene expression microarrays to describe two dominant molecular subtypes of ccRCC tumors termed clear cell A (ccA) and clear cell B (ccB) [6, 8, 9]. The ccA patients demonstrated superior overall survival (OS), cancer-specific survival (CSS), and recurrence free survival (RFS) [6, 7]. However, these studies, in utilizing frozen tissue and large numbers of gene probes, have significant barriers to translational and clinical applications. Brooks et al. published a method that attempts to circumvent these barriers [7]. In their work, a model using 34 genes (coined ClearCode34) was developed that reliably classified individual tumors as ccA and ccB from formalin-fixed, paraffin embedded tumor specimens (Table 1). Prognostic models containing this signature outperformed traditional clinical nomograms [7]. Thus, ClearCode34 is poised for further study in readily available specimens.
Table 1.
ClearCode34 Gene Expression Profile
| ccA | ccB | |
|---|---|---|
| ARNT | NRP1 | CCNO |
| BNIP3L | PDGFD | FLJ23867 |
| C11orf1 | PHYH | FOXM1 |
| CDH5 | PRKAA2 | GALNT10 |
| EHBP1 | RGS5 | GALNT4 |
| EPAS1 | SLC4A4 | KCNN4 |
| ESD | SPRYD7 | MOXD1 |
| FZD1 | ST13 | ROR2 |
| GIPC2 | STK32B | SERPINA3 |
| LEPROTL1 | TCEA3 | SLC4A3 |
| MAOB | TLR3 | |
| MAPT | VCAM1 | |
While new technology will facilitate translational investigations, much remains to be learned regarding the patients who constitute the ccA and ccB cohorts. Some clinical annotation has previously been described, such as lower stage and Fuhrman nuclear grade among ccA patients [6]. However, further clinical description, for example the anatomic metastatic pattern, of these patient cohorts is lacking. Furthermore, while evidence emerges that comorbidities and their treatment may influence kidney cancer outcomes [10, 11], the distribution of these among ccA and ccB patients remain to be addressed. These are analyses which may prove important in understanding the biology and clinical relevance of these ccRCC molecular subtypes.
2. Patients and methods
2.1. Patients and clinical samples
Gene expression data was obtained from primary ccRCC tumors of patients treated with nephrectomy at Moffitt Cancer Center (MCC) from 1/1/1998 to 10/25/2011 and organized through the Total Cancer Care (TCC) initiative [12]. Priority was given to samples with sufficient quantity to satisfy both clinical and research requirements. Tissue was collected and snap frozen within 15 minutes of surgical extirpation with cold ischemia times typically <5 minutes. Subsequently, frozen sections were prepared and stained. In order to be eligible for molecular analysis, tissue was evaluated by TCC pathologists for quality control and central pathology review. Macro-dissection was performed in liquid nitrogen to maintain the frozen tissue and enrich tumor. RNA extraction was performed and specimens arrayed on Affymetrix HuRSTA-2a520709 GeneChips (Affymetrix, Santa Clara, CA) chips with over 60,000 probe sets representing over 22,000 unique genes. Further details on the MCC TCC initiative and protocol were previously published [12]. Inclusion criteria included adult patients (>18 years old) with ccRCC. A total of 282 ccRCC primary tumors with gene expression data in TCC were identified; follow-up data including survival and vital status were obtained as part of the TCC initiative. Median follow-up was 74 months for all patients (74 for ccA and 76 for ccB). Patients’ medical records were reviewed to further define study variables. Clinical characteristics such as age, BMI [weight (kg)/height2 (m2)], and comorbidity status and treatment were gathered at time of diagnosis. A patient was classified as obese if BMI was 30 kg/m2 or greater. Patients were considered to have hypertension, hyperlipidemia, or diabetes mellitus (DM) if they reported use of a medication typically used to treat the condition. All samples and data were obtained with appropriate institutional review board approvals.
2.2. Application of gene expression classifier of ccA or ccB
The ClearCode34 consists of a 34 gene panel (Table 1) that was previously published [7] and assigns tumors as either ccA or ccB according to gene expression level. Tumor classification was performed in R program (www.r-project.org) using Prediction Analysis of Microarrays for R (PAMR), a centroid-based classification algorithm [7].
2.3. Statistical analysis
All continuous variables were described with the median and range values. OS was defined as the time from diagnosis to death of any cause. CSS was defined as the time from diagnosis to death from ccRCC; patients who remained alive, died of other causes, or had unknown cause of death were censored at the time of last follow-up or death. RFS was defined as the date from nephrectomy to the earliest date that recurrence or metastasis was described by imaging or pathology report. The probability of death or recurrence was determined by using the Kaplan-Meier method, and comparisons were performed using a log-rank test. Significance of categorical analyses was determined with two-tail Fisher’s exact test (two variables) or chi-squared (three variables). Significance of medians was evaluated with Mann-Whitney U test. Cox regression multivariate analyses were performed with SPSS version 21.
3. Results
3.1. Patient characteristics of molecular subtypes
Of the 282 ccRCC tumors studied, 226 (80%) were classified as ccA and the remainder as ccB (Table 2). Univariate analyses (UVAs) demonstrated that ccA patients, relative to ccB patients, presented with lower Fuhrman nuclear grade (p<0.001) and smaller tumors (median 4.6 cm versus 7.2 cm, p=0.003) and were less likely to have nodal involvement (4% versus 11%, p=0.038) or metastatic disease (23% versus 60%, p=0.003). There were no statistically significant distinctions in anatomic pattern of metastastic spread between the ccA and ccB patients: lung-only (19% versus 31%, p=0.201), lung (79% versus 63%, p=0.130), bone (45% versus 45%, p=1.000), brain (21% versus 13%, p=0.393), and liver (9% versus 13%, p=0.723).
Table 2.
Clear Cell Renal Cell Carcinoma Molecular Subtypes and Patient Characteristics
| ccA | ccB | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 226 | 80 | 56 | 20 | |
| Age (years), Median (± IQR) | 62 (53–71) | 68 (56–73) | 0.090 | ||
| Gender | 0.539 | ||||
| Men | 144 | 64 | 33 | 59 | |
| Women | 82 | 36 | 23 | 41 | |
| Race | 0.071 | ||||
| White | 214 | 95 | 49 | 88 | |
| Non-White | 12 | 5 | 7 | 13 | |
| Fuhrman Nuclear Grade | <0.001 | ||||
| I | 5 | 2 | 2 | 4 | |
| II | 95 | 42 | 11 | 20 | |
| III | 106 | 47 | 22 | 39 | |
| IV | 19 | 8 | 21 | 38 | |
| Tumor Size (cm), Median (± IQR) | 4.6 (3.2–7.0) | 7.2 (3.4–10.4) | 0.003 | ||
| Node Positive | 8 | 4 | 6 | 11 | 0.038 |
| Metastatic Disease | 53 | 23 | 32 | 60 | 0.003 |
| Metastatic Pattern | |||||
| Lung Only | 10 | 19 | 10 | 31 | 0.201 |
| Lung | 42 | 79 | 20 | 63 | 0.130 |
| Bone | 24 | 45 | 14 | 44 | 1.000 |
| Brain | 11 | 21 | 4 | 13 | 0.393 |
| Liver | 5 | 9 | 4 | 13 | 0.723 |
| Length of Follow-Up (months) | 74 | 76 | |||
ccA = clear cell renal cell carcinoma subtype A; ccB = clear cell renal cell carcinoma subtype B cm = centimeters; IQR = interquartile range
3.2. Overall survival, cancer specific survival, and recurrence-free survival of molecular subtypes
Consistent with prior publications, the ccB patients demonstrated inferior OS relative to ccA patients (median OS 151 months versus 31 months, log-rank p<0.001) with a hazard ratio (HR) of 5.52 (95% CI, 3.17–9.61) (Fig. 1a) as well as inferior CSS (median CSS 253 versus 33 months, p<0.001) with a HR of 12.45 (95% CI, 6.45–24.03) (Fig. 1b). The ccB patients were also inferior for recurrence-free survival (RFS) in non-metastatic patients after nephrectomy (median not reached, p<0.001) with a HR of 12.20 (95% CI, 4.48–33.17) (Fig. 1c).
Figure 1. ClearCode34 defines ccRCC subtypes with significantly different patient outcomes.
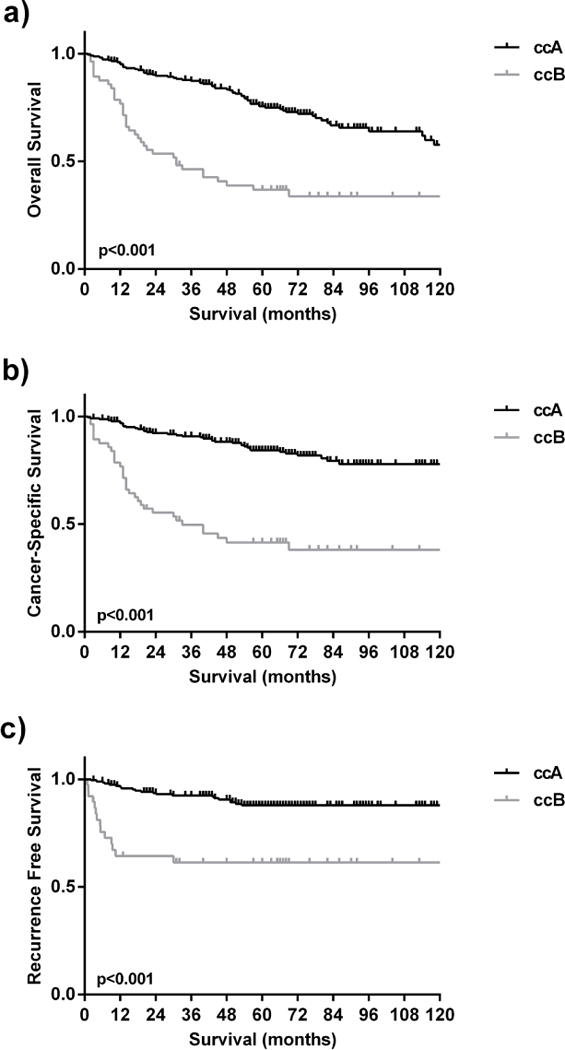
The ccA patients had superior a) overall survival, b) cancer-specific survival and c) recurrence free survival relative to ccB patients.
3.3. Comorbidities in relationship to ClearCode34 subtypes
There is a growing appreciation for the impact of comorbidities, such as hypertension and hyperlipidemia, and their treatment on RCC outcomes. Therefore, we sought to evaluate whether comorbidities and their treatments at time of diagnosis contribute to the divergent outcomes observed for the ClearCode34 subtypes. Intriguingly, the ccA patients have a higher median BMI at diagnosis relative to the ccB patients (29.7 versus 27.7, p=0.019) and a higher frequency of obesity (48% versus 34%, p=0.021; Table 3). In addition, there was a higher rate of DM among ccA patients relative to ccB patients (26% versus 13%, p=0.035). The risk of RCC in patients with hypertension has been observed to be related to the degree of hypertension [13–16]. However, similar rate of hypertension was observed in each subtype (63% versus 56%, p=0.355) though there was a trend towards increased utilization of ASI among the ccA patients with hypertension (71% versus 52%, p=0.055). Similar rates of hyperlipidemia and statin utilization were observed. Despite being associated with advanced RCC [17], smoking rates were similar in each subtype (Table 3).
Table 3.
Comorbidities in Relationship to Clear Cell Renal Cell Carcinoma Molecular Subtypes
| ccA | ccB | p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| BMI, Median (± IQR) | 29.7 (25.8–35.1) | 27.7 (23.8–32.3) | 0.019 | ||
| Rate of Obesity | 0.021 | ||||
| BMI < 30 | 112 | 52 | 31 | 66 | |
| BMI ≥ 30 | 103 | 48 | 16 | 34 | |
| Diabetes Mellitus | 58 | 26 | 7 | 13 | 0.035 |
| Metformin | 26 | 45 | 1 | 14 | 0.224 |
| No Metformin | 32 | 55 | 6 | 86 | |
| Hyperlipidemia | 83 | 37 | 17 | 31 | 0.436 |
| Statin | 77 | 93 | 16 | 94 | 0.464 |
| No Statin | 6 | 7 | 1 | 6 | |
| Hypertension | 142 | 63 | 31 | 56 | 0.355 |
| ASI | 101 | 71 | 16 | 52 | 0.055 |
| Thiazide | 40 | 28 | 7 | 23 | 0.658 |
| Other Treatment | 78 | 55 | 24 | 77 | <0.001 |
| No. Drugs = 1 | 65 | 46 | 14 | 45 | 1.000 |
| No. Drugs ≥ 2 | 77 | 54 | 17 | 55 | |
| Smoking | 0.680 | ||||
| Ever Smoker | 130 | 58 | 32 | 57 | 0.881 |
| Current Smoker | 34 | 15 | 6 | 11 | 0.523 |
| No. of Pack-Years, Median (± IQR) | 30 (26–76) | 30 (15–60) | 0.523 | ||
ccA = clear cell renal cell carcinoma subtype A; ccB = clear cell renal cell carcinoma subtype B IQR = interquartile range; BMI = body mass index; ASI = angiotensin system inhibitor
3.4. Univariate and multivariate Cox proportional hazards regression for cancer-specific survival and overall survival
While prior work showed ccA patients have a lower stage and grade of disease, the current study also identified a higher BMI and rate of obesity, higher rate of DM, and of a trend towards increased utilization of ASIs in the ccA patients. As BMI and ASI use are reported to be associated with improved outcomes in kidney cancer, we sought to determine whether comorbidities and their treatment were confounding the survival analyses for ClearCode34 subtypes and could thus explain the improved outcomes seen in ccA patients. UVA and multivariate analysis (MVA) using the Cox regression model for OS and CSS included these novel variables (BMI, DM status, ASI utilization) as well as established variables (age, grade, tumor size, nodal status, metastatic disease; Tables 4 and 5). In the CSS analysis, ccB status was associated with inferior survival relative to ccA patients in both UVA and MVA (UVA: p<0.001; HR 5.01, 95% CI 3.14–8.00 and MVA: p<0.001; HR 3.26, 95% CI 1.84–5.79). Similarly, in the OS analysis, ccB status was also associated with inferior survival in both UVA and MVA (UVA: p<0.001; HR 3.23, 95% CI 2.16–4.86 and MVA: p<0.001; HR 2.50, 95% CI 1.53–4.08). Among the novel variables, the UVA showed DM was not associated with survival, neither for CSS (p=0.125, HR 0.84, 95% CI 0.47–1.51) nor for OS (p=0.484; HR 1.17, 95% CI 0.75–1.83). However, in the MVA, DM was associated with inferior CSS (p=0.011; HR 2.56, 95% CI 1.24–5.30) and inferior OS (p=0.001; HR 2.43, 95% CI 1.41–4.18). The use of ASI was not associated with survival in any of these analyses.
Table 4.
Univariate and Multivariate Cox Proportional Hazards Regression for Cancer Specific Survival
| Univariate
|
Multivariate
|
|||||
|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | |
| Age | 1.03 | 1.01–1.05 | 0.010 | 1.06 | 1.03–1.08 | <0.001 |
| Grade | ||||||
| I–II | Referent | Referent | ||||
| III–IV | 5.98 | 2.97–12.02 | <0.001 | 3.92 | 1.50–10.23 | 0.005 |
| Tumor Size | 1.21 | 1.16–1.27 | <0.001 | 1.11 | 1.04–1.19 | 0.002 |
| Node Positive | 5.80 | 2.93–11.47 | <0.001 | 1.74 | 0.81–3.76 | 0.158 |
| Metastatic Disease | 7.10 | 4.45–11.31 | <0.001 | 17.65 | 7.71–40.40 | <0.001 |
| ClearCode34 | ||||||
| ccA | Referent | Referent | ||||
| ccB | 5.01 | 3.14–8.00 | <0.001 | 3.26 | 1.84–5.79 | <0.001 |
| BMI | 0.97 | 0.93–1.01 | 0.125 | 0.99 | 0.94–1.04 | 0.686 |
| Diabetes Mellitus | 0.84 | 0.47–1.51 | 0.556 | 2.56 | 1.24–5.30 | 0.011 |
| ASI | 0.64 | 0.39–1.04 | 0.074 | 1.13 | 0.61–2.09 | 0.700 |
ccA = clear cell renal cell carcinoma subtype A; ccB = clear cell renal cell carcinoma subtype B BMI = body mass index at diagnosis; ASI = angiotensin system inhibitor; CI = 95% confidence interval
Table 5.
Univariate and Multivariate Cox Proportional Hazards Regression for Overall Survival
| Univariate
|
Multivariate
|
|||||
|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | |
| Age | 1.05 | 1.03–1.06 | <0.001 | 1.07 | 1.04–1.09 | <0.001 |
| Grade | ||||||
| I–II | Referent | Referent | ||||
| III–IV | 2.75 | 1.77–4.29 | <0.001 | 1.99 | 1.15–3.44 | 0.014 |
| Tumor Size | 1.17 | 1.12–1.22 | <0.001 | 1.09 | 1.03–1.16 | 0.004 |
| Node Positive | 5.46 | 2.95–10.11 | <0.001 | 2.34 | 1.15–4.74 | 0.019 |
| Metastatic Disease | 3.92 | 2.62–5.87 | <0.001 | 5.11 | 3.04–8.58 | <0.001 |
| ClearCode34 | ||||||
| ccA | Referent | |||||
| ccB | 3.23 | 2.16–4.85 | <0.001 | 2.50 | 1.53–4.08 | <0.001 |
| BMI | 0.96 | 0.92–0.99 | 0.013 | 0.98 | 0.94–1.02 | 0.259 |
| Diabetes Mellitus | 1.17 | 0.75–1.83 | 0.484 | 2.43 | 1.41–4.18 | 0.001 |
| ASI | 0.86 | 0.58–1.26 | 0.433 | 0.95 | 0.58–1.54 | 0.832 |
ccA = clear cell renal cell carcinoma subtype A; ccB = clear cell renal cell carcinoma subtype B BMI = body mass index at diagnosis; ASI = angiotensin system inhibitor; CI = 95% confidence interval
4. Discussion
This large, single-institution, retrospective study validated the prognostic significance of the ClearCode34 subtypes ccA and ccB in an independent cohort of ccRCC patients. In addition, we provide a novel description of the clinical characteristics of the patients that constitute these ccRCC subtypes. The ccA patients are more likely to be obese and diabetic. The ccB patients have an inferior prognosis, larger tumors, higher Fuhrman nuclear grade, increased nodal involvement, and are more likely to have tumor that is or becomes metastatic. Importantly, multivariate analyses for both OS and CSS demonstrate that even after considering factors such as tumor size, grade, and comorbidities, ccA/ccB distinction makes a significant and robust contribution to survival models. This work reinforces the concept that ccA and ccB defines distinct clinical cohorts with unique tumor biology.
In the current study, the prognostically superior ccA group has a higher rate of obesity while the ccB patients have a rate of obesity similar to other Americans of a similar age (Table 3) [18]. This observation highlights a paradox involving BMI and ccRCC. Obesity is a risk factor for RCC in both men and women [19] yet obese RCC patients have lower stage disease, improved survival, and improved outcomes when treated with targeted therapies relative to non-obese patients [20, 21]. Thus, our observation that ccA patients, with a higher BMI and rate of obesity, have superior outcomes is consistent with those observations. However, this retrospective data is not sufficient to conclude that obesity directly causes development of ccA tumors. Future studies should further explore how obesity impacts kidney tumor biology and how best to exploit this unique biology for the benefit of patients.
Increased incidence of solid tumors has long been recognized in patients with DM [22]. However, DM as a risk factor for RCC, and its impact on RCC outcomes, is controversial [23–25]. In the current study, ccA patients had increased incidence of DM at time of diagnosis relative to ccB patients. Interestingly, in MVA, DM was associated in inferior CSS and OS despite being associated with the superior prognostic group, ccA. Despite this conundrum, the prognostic validity of ClearCode34 persists as ccA status continues to correlate with improved CSS and OS in MVA that included DM. Given the increased incidence of obesity and DM in ccA patients, the question of whether host features can impact molecular subtypes and tumor biology is raised. Future, ideally prospective studies, are needed to address this question further.
As ccA patients are more likely to have DM, it is not surprising that a trend was observed towards increased ASI use in ccA patients (71% of ccA patients versus 52% of ccB patients, p=0.055). Due to their proven benefit, guidelines for therapy of DM list multiple indications for ASIs in DM patients, including hypertension [26]. The current study did not support a protective effect for ASI in ccRCC (Tables 4 and 5). However, other studies have found that ASIs may offer survival benefits in kidney cancer in the targeted treatment era [11, 27]. Possibilities for this discrepancy include insufficient power in the current study to detect a benefit from ASIs. Another possibility is that ClearCode34 status was confounding the results of the other studies since ccA patients, a group with superior survival, may have increased rates of DM and ASI use. Thus, the benefit from ASI seen in other studies may have been partly due to an enrichment of ccA patients among ASI users. Further, ideally prospective, studies are needed to resolve this uncertainty regarding the benefits of ASIs in kidney cancer patients.
Limitations of the current study include the sample size as well as the retrospective nature of the study. In addition, the study period was long in order to maximize sample size. A prospective cohort study would better capture details of comorbidities in patients during the time of tumorigenesis (i.e. years prior to diagnosis of kidney cancer) but would require a very large sample size. Given practice patterns, the MCC electronic medical record lacks some data that would further illuminate the questions raised in the current study regarding comorbidities (e.g. hemoglobin A1C values). In addition, we identified more ccA tumors than ccB. While other studies too have observed a greater incidence of ccA relative to ccB [6, 8, 9], we cannot exclude unintended bias impacting selection of which patients go to surgery and which tumors get selected for study. Finally, heterogeneity within tumors has been documented for gene expression profiles (GEPs) [28]. Whether a scoring system integrating ClearCode34 classification from multiple sites of the primary tumor, or other strategies to capture the heterogeneity of an individual tumor, would impact the prognostic ability of this GEP is an important question going forward.
This work validates, in an external dataset, the ability of ClearCode34 to provide prognostic information in ccRCC [7]. The increased prevalence of obesity and DM in the ccA patients raises the question of whether comorbidities influence tumor biology and predispose to the development of a particular molecular subtype, or could, conversely, provide a protective effect that hinders disease progression. In addition, it is interesting that in the current MVAs, DM status is associated with inferior survival. While this observation requires validation, if true, the mechanism of this effect becomes an important research question with possibilities including tumor-promoting changes in the tumor microenvironment (i.e. diabetic nephropathy) versus a potential DM-associated metabolic effect. Furthermore, while other studies have demonstrated improved survival in RCC with ASI use, the current study fails to observe such effect. Since ASI users may enrich for prognostically superior ccA patients, this is a potential source of bias and thus ClearCode34 status may enhance future studies evaluating ASI benefit in ccRCC.
5. Conclusion
ClearCode34 identifies biologically-divergent molecular subtypes of kidney cancer that originates in patients with distinct comorbidities.
Highlights.
ClearCode34 subtypes clear cell RCC tumors in molecular subtypes, ccA and ccB
ccA patients have higher rate of obesity and diabetes mellitus
After considering host comorbidities, ccA patients remain prognostically superior
Acknowledgments
We would like to thank the patients and their families for their participation in the Moffitt Cancer Center Total Cancer Care (TCC) initiative.
Research support: This work has been supported in part by Moffitt Total Cancer Care, the Cancer Informatics Core Facility, and the Department of Genitourinary Oncology at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). This research also received support from the National Research Service Award T32 ES007126 (SAB) from the National Institute of Environmental Health Sciences, the HHMI Translational Medicine Fellowship (SAB), and the National Cancer Institute K24 CA172355 (WKR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ACS. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Mian BM, Bhadkamkar N, Slaton JW, Pisters PW, Daliani D, Swanson DA, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. The Journal of urology. 2002;167:65–70. [PubMed] [Google Scholar]
- 3.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 5.Rini B, Goddard A, Knezevic D, Maddala T, Zhou M, Aydin H, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. The Lancet Oncology. 2015 doi: 10.1016/S1470-2045(15)70167-1. [DOI] [PubMed] [Google Scholar]
- 6.Brannon AR, Reddy A, Seiler M, Arreola A, Moore DT, Pruthi RS, et al. Molecular Stratification of Clear Cell Renal Cell Carcinoma by Consensus Clustering Reveals Distinct Subtypes and Survival Patterns. Genes Cancer. 2010;1:152–63. doi: 10.1177/1947601909359929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks SA, Brannon AR, Parker JS, Fisher JC, Sen O, Kattan MW, et al. ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. European urology. 2014;66:77–84. doi: 10.1016/j.eururo.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brannon AR, Haake SM, Hacker KE, Pruthi RS, Wallen EM, Nielsen ME, et al. Meta-analysis of clear cell renal cell carcinoma gene expression defines a variant subgroup and identifies gender influences on tumor biology. European urology. 2012;61:258–68. doi: 10.1016/j.eururo.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaffenberger SD, Lin-Tsai O, Stratton KL, Morgan TM, Barocas DA, Chang SS, et al. Statin use is associated with improved survival in patients undergoing surgery for renal cell carcinoma. Urologic oncology. 2015;33:21 e11–7. doi: 10.1016/j.urolonc.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS, et al. Angiotensin System Inhibitors and Survival Outcomes in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer journal (Sudbury, Mass) 2011;17:528–36. doi: 10.1097/PPO.0b013e318238216e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. European urology. 2011;60:615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. American journal of epidemiology. 2008;167:438–46. doi: 10.1093/aje/kwm321. [DOI] [PubMed] [Google Scholar]
- 15.Chow WH, Gridley G, Fraumeni JF, Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. The New England journal of medicine. 2000;343:1305–11. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 16.Sanfilippo KM, McTigue KM, Fidler CJ, Neaton JD, Chang Y, Fried LF, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension. 2014;63:934–41. doi: 10.1161/HYPERTENSIONAHA.113.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsivian M, Moreira DM, Caso JR, Mouraviev V, Polascik TJ. Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol. 2011;29:2027–31. doi: 10.1200/JCO.2010.30.9484. [DOI] [PubMed] [Google Scholar]
- 18.Fakhouri TH, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 19.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 20.Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. Journal of the National Cancer Institute. 2013;105:1862–70. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albiges L, Xie W, Lee J, Rini BI. The impact of body mass index (BMI) on treatment outcome of targeted therapy in metastatic renal cell carcinoma (mRCC): Results from the International Metastatic Renal Cell Cancer Database Consortium. J Clin Oncol. 2014;32 abstr 4576. [Google Scholar]
- 22.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocrine-related cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 23.Zucchetto A, Dal Maso L, Tavani A, Montella M, Ramazzotti V, Talamini R, et al. History of treated hypertension and diabetes mellitus and risk of renal cell cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2007;18:596–600. doi: 10.1093/annonc/mdl438. [DOI] [PubMed] [Google Scholar]
- 24.Psutka SP, Stewart SB, Boorjian SA, Lohse CM, Tollefson MK, Cheville JC, et al. Diabetes Mellitus is Independently Associated with an Increased Risk of Mortality Among Clear Cell Renal Cell Carcinoma Patients. The Journal of urology. 2014 doi: 10.1016/j.juro.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Antonelli A, Arrighi N, Corti S, Zanotelli T, Cozzoli A, Cosciani Cunico S, et al. Preexisting type-2 diabetes is not an adverse prognostic factor in patients with renal cell carcinoma: a single-center retrospective study. Urologic oncology. 2013;31:1310–5. doi: 10.1016/j.urolonc.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Executive summary: Standards of medical care in diabetes–2014. Diabetes care. 2014;37(Suppl 1):S5–13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 27.Izzedine H, Derosa L, Le Teuff G, Albiges L, Escudier B. Hypertension and angiotensin system inhibitors: impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2015 doi: 10.1093/annonc/mdv147. [DOI] [PubMed] [Google Scholar]
- 28.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]


