Abstract
Plasmacytoid dendritic cells (pDCs) are vital to antiviral defense, directing immune responses via secretion of huge concentrations of IFN-α. These cells are critical in protecting the lung against clinically relevant respiratory viruses, particularly influenza (Flu), a virus responsible for substantial worldwide morbidity and mortality. How pDC responses to such viral pathogens are regulated, however, is poorly understood in humans. Using an unbiased approach of gene chip analysis, we discovered that Flu significantly impacts metabolism in primary human pDCs. We demonstrate that Flu and RV, another common respiratory virus, induce glycolysis in pDCs and that this metabolic pathway regulates pDC antiviral functions including IFN-α production and phenotypic maturation. Intranasal vaccination of human volunteers with live influenza virus also increases glycolysis in circulating pDCs, highlighting a previously unrecognized potential role for metabolism in regulating pDC immune responses to viral infections in humans.
Introduction
Influenza viruses cause significant morbidity and mortality worldwide, imposing an enormous public health burden (1). Plasmacytoid dendritic cells (pDCs) are critical in directing antiviral responses against many viruses (2) and are recruited to the sites of viral replication during respiratory Flu infections (3, 4). In mice, pDCs promote viral clearance and are essential for protecting the lung against Flu infection (5).
pDCs direct immune responses through their unique ability to produce massive concentrations of type I IFNs upon viral encounter (6). Type I IFNs promote an antiviral state, limiting viral replication while also activating multiple immune cells including macrophages, DCs, as well as B- and T-lymphocytes (7). pDCs are thus crucial for both innate and adaptive antiviral responses (8).
The regulation of pDC responses to Flu is incompletely understood in humans. In mice, TLR7 and MyD88 are essential for Flu-induced secretion of type I IFN (9). TLR7 is also required for pDC IFN-α responses to Flu in humans (10), and a role for PI3K in pDC IFN-α production has also been demonstrated (11). In addition, several signaling pathways including IL18R, FcεR1 and Ig-like transcript 7 have been shown to negatively regulate Flu-induced pDC IFN-α production in humans (12–14). Whether additional pathways regulate human pDC responses to Flu is unknown. We utilized an unbiased approach of gene chip analysis of Flu-exposed primary human pDCs to address this question. We discovered that metabolism, and specifically glycolysis, plays a critical, previously unrecognized role in human pDC antiviral responses. Flu and RV, both ssRNA viruses, and the TLR7-agonist gardiquimod all induce glycolysis in human pDCs and this metabolic pathway is essential for pDC functions including viral-induced IFN-α secretion and phenotypic maturation. The discovery that live attenuated influenza vaccine (LAIV) promotes increased glycolytic rates in circulating human pDCs highlights the potential role of glycolysis in human pDC responses to viral infections in vivo.
Materials and Methods
Human subjects
For ex vivo studies, enriched leucocyte packs were obtained from a blood bank. For in vivo studies, informed consent was obtained from 16 healthy adults (7 males and 9 females with no recent history of Flu infection or vaccination). Blood was drawn before, 1 and/or 3 days after administration of live attenuated influenza vaccine (LAIV; Flumist Quadrivalent, MedImmune). Nasal samples were collected by instilling saline (0.9%; 2 sprays) into each nare and collecting material expelled by forcible nasal exhalation (15). Samples were then mixed with PBS, centrifuged (1300 G-force/10 min), and stored for PCR analysis. All procedures were performed according to an IRB-approved protocol.
Purification of blood pDCs, culture conditions and reagents
pDCs were purified from PBMCs by negative selection using Ab-coated magnetic beads (Stem Cell Technologies); purity (Lineage−HLA-DR+CD11c−CD123+) was greater than 90%. pDCs were cultured in complete RPMI media (cRPMI) with 10 ng/ml IL-3 (R&D Systems) (14). The conditions evaluated were: control, Flu (A/PR/8/34 [H1N1], 0.1–0.25 PFU/cell; Charles River Laboratories), RV (RV-16, 106–107 PFU/ml; gift from J. Gern, University of Wisconsin), gardiquimod (Gard, a TLR7 agonist, 1 μg/ml; Invivogen). Cells were harvested by centrifugation and supernatants stored at −80°C for IFN-α ELISA and lactate assays. In some experiments, pDCs were harvested for RNA isolation or flow cytometry. For glycolysis inhibition studies, pDCs were cultured with 10 mM 2-DG (a glycolytic inhibitor; Sigma-Aldrich) and 10 mM D-glucose (Sigma-Aldrich). pDC viability was assessed using trypan blue (Sigma-Aldrich).
Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) measurements
To determine ECAR and OCR, stimulated pDCs were seeded onto Cell-Tak (BD Biosciences) coated 24-well plates in extracellular flux (XF) media (with 2 mM L-glutamine, pH 7.4) for 1–2 h at 37°C. ECAR and OCR were measured in real time using the XF-24 Analyzer (Seahorse Biosciences). The following treatments were evaluated over 135 min: 1) no glucose, 2) 10 mM D-glucose, 3) 1 mM oligomycin (to induce maximal glycolysis) and 4) 100 mM 2-DG. Basal ECAR and OCR were evaluated simultaneously in XF media with 25 mM glucose.
Lactate quantification by Gas Chromatography/Mass Spectrometry
To quantify glucose-derived-13C-lactate, pDCs were cultured in glucose-free cRPMI media, 10% dialyzed FBS (Invitrogen) and 10 mM D-[1,6-13C]-glucose (Cambridge Isotope Laboratories). Lactate was extracted from supernatants using methanol, chloroform and water. Samples were evaporated and derivatized by trimethylsilylation (Tri-Sil HTP reagent; Thermo Scientific) and injected into an Agilent 6970 gas chromatograph networked to an Agilent 5973 mass selective detector. Fragment ions m/z 117–119 were used to monitor lactate enrichment and 13C-lactate amount was calculated based on 13C-lactate enrichment and total lactate abundance.
IFN-α quantification
IFN-α was measured using the Human IFN-α (pan-specific) ELISA kit (MabTech) and analyzed on an ELISA reader DTX 880 Multimode detector (Beckman Coulter). For LAIV experiments, the IFN-α2a Ultra-Sensitive Kit was used and data analyzed by MESO SECTOR S 600 (Meso Scale Discovery).
Flow cytometry
pDCs were stained (14) with the following anti-human Abs: HLA-DR APC-Cy7, CD80-FITC and CD86-PeCy5 (BD Biosciences), acquired on a BD LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star). The Molecules of Equivalent Soluble fluorochrome (MESF) values of HLA-DR, CD80 and CD86 were measured using the Ultra Rainbow Calibration kit (BD Biosciences). For mitochondrial staining, MitoTracker Red CMXRos (200 nM; Invitrogen) was utilized.
Protein and RNA Analysis
Total RNA was extracted from pDC pellets using Arcturus® Picopure® RNA isolation kit (Applied Biosystems) and quantified by NanoDrop 2000 (Thermo Scientific). Total protein was measured using the BCA protein assay kit (Thermo Scientific). Quantitative real time PCR (qRT-PCR) for IFN-α, CD80, CD86, HLA-DR, beta glucuronidase (GUSB), interleukin-3 receptor alpha (IL3Rα), CD2-associated protein (CD2AP), CD317 (tetherin), beta actin (ACTB), hexokinase 2 (HK2), lactate dehydrogenase A (LDHA) and interferon regulatory factor 7 (IRF7) was performed using Taqman probe/primers (Applied Biosystems) and results normalized to peptidylprolyl isomerase A (PPIA).
ELISPOT assay
ELISPOT assay was used to measure the frequency of H1N1 Flu peptide-specific IFN-γ producing T cells. PBMCs (105) were placed in 96-well IFN-γ-coated-plates and stimulated with or without H1N1 Flu peptide (20 μg/ml) or anti-CD3 (5 μg/ml) for 24h at 37°C. After washing, plates were incubated with biotinylated anti-IFN-γ Ab (1 μg/ml) and developed using streptavidin-HRP and AEC (3-amino-9-ethyl carbazole). Plates were analyzed using a Bio-Sys-Bioreader 5000 ELISPOT reader. Data was normalized to the number of spots obtained +/− anti-CD3 stimulation.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism version 6 and included student t-test, one- and two-way ANOVA. Pearson Correlation analysis was performed in select experiments. Data are presented as mean ± SEM. p ≤ 0.05 indicated significant differences.
Results and Discussion
Viral exposure enhances glycolysis and activates HIF-1α in human pDCs
We employed gene chip analysis to identify novel pathways involved in pDC antiviral responses, using Flu exposure as our model. Functional annotation analysis of approximately 2600 differentially regulated genes revealed 75 biological pathways impacted by Flu. In addition to expected pathways related to antiviral defense and cytokine production (16), Flu significantly influenced metabolism in pDCs; 17 pathways related to metabolism were impacted (Supplemental Fig. 1A). Glycolysis and OXPHOS, the major energy producing pathways, were differentially affected by Flu. While most genes related to glycolysis were upregulated, OXPHOS pathway genes were not. Expression of key glycolytic genes HK2 and LDHA as well as hypoxia-inducible factor 1α (HIF-1α), a transcription factor known to regulate cellular metabolism, was significantly increased in Flu-exposed pDCs (Supplemental Fig. 1B). These findings were confirmed by qRT-PCR and similar effects were observed with RV, another ssRNA virus and the TLR7 agonist Gard (Supplemental Fig. 1C). Flu and RV also induced significant increases in ATP in pDCs (Supplemental Fig. 1D). Collectively, these data indicate that these respiratory viruses significantly impact pDC metabolism.
HIF-1α is a principal regulator of metabolism, controlling most genes encoding glycolytic enzymes in mammalian cells (17). A central role for HIF-1α has also been observed during inflammation and viral infections (18, 19). Flu infection in macaques promotes HIF-1α expression in blood leukocytes and tissue macrophages, highlighting a potential role for HIF-1α in respiratory viral infections (20). Our data reveal that Flu induces significant increases in both HIF-1α protein expression and nuclear translocation in pDCs (Supplemental Fig. 1E, 1F, 1G), suggesting that viral-induced metabolic changes are regulated in part by HIF-1α in these cells.
Flu, RV and Gard induce glycolysis in human pDCs
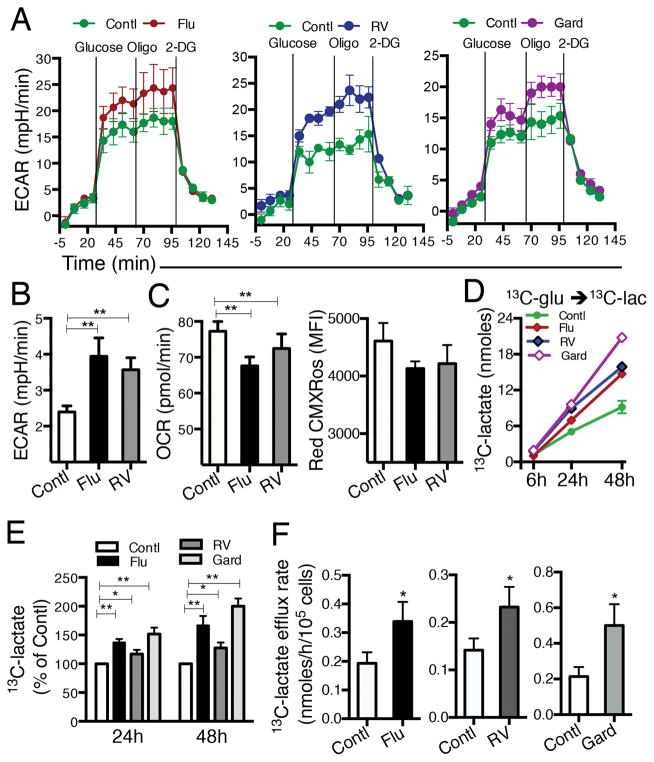
To demonstrate the impact of viral stimulation on the glycolytic pathway in pDCs, we measured ECAR, an indicator of glycolysis in real time. pDCs were first exposed to Flu, RV or Gard followed by the various treatments depicted in Figure 1A. Flu, RV and Gard-exposed pDCs generated elevated ECAR upon addition of glucose and this persisted after treatment with oligomycin, an ATP synthase inhibitor added to induce maximal glycolysis (Fig. 1A). In contrast to the viral-mediated increase in ECAR (Fig. 1B), OCR, a reflection of mitochondrial OXPHOS, was reduced in pDCs exposed to Flu and RV (Fig. 1C, left). Mitochondrial membrane potential was also unaffected by Flu or RV exposure (Fig. 1C, right). A longer-term assessment of glycolysis was accomplished by quantifying extracellular lactate, a product of glycolysis, in supernatants of pDCs exposed to viruses over 48 h. Time-dependent production of 13C-glucose-derived-13C-lactate was significantly increased by Flu, RV, and Gard exposure compared to control conditions (Fig. 1D, 1E). Flu-, RV- and Gard-activated pDCs also exhibited elevated rates of lactate production (Fig. 1F). These results demonstrate that viral exposure enhances glycolytic flux but not mitochondrial OXPHOS in pDCs and provide evidence that pDCs preferentially induce glycolysis to potentially meet biosynthetic demands during viral infections.
FIGURE 1.
Flu, RV and gardiquimod (Gard) induce glycolysis in human pDCs. (A) pDCs were cultured in cRPMI with no stimulant (Contl), Flu, RV, or Gard for 24h, washed, and reincubated in non-buffered media. Real-time ECAR was measured in supernatants of pDC cultures using a XF-24 analyzer. Vertical lines indicate addition of glucose (glycolysis substrate), oligomycin (ATP synthase inhibitor) and 2-DG (glycolysis inhibitor). One of 3 independent experiments shown. (B) Summary of pDC basal ECAR and (C, left) basal OCR measured simultaneously in the same well. (C, right) Red CMXRos mean fluorescence intensity (MFI) was quantified by flow cytometry in pDCs cultured +/− Flu for 48 h. Data represent mean ± SEM, N=3 for B and C. (D) pDCs were exposed to Flu, RV, or Gard for 6, 24 and 48 h and glucose-derived-13C-lactate measured in supernatants by mass spectrometry. One of 3 experiments shown. (E) Mean ± SEM values of 13C-lactate normalized to control in Flu-, RV- and Gard-treated pDCs after 24 and 48 h; N=4–6. (F) Lactate efflux rate (nmoles/h/105 cells) in pDCs exposed to Flu, RV or Gard, N=4–6. *p ≤ 0.05; **p ≤ 0.01, paired t-test, control versus treatment.
Inhibition of glycolysis impairs pDC antiviral responses
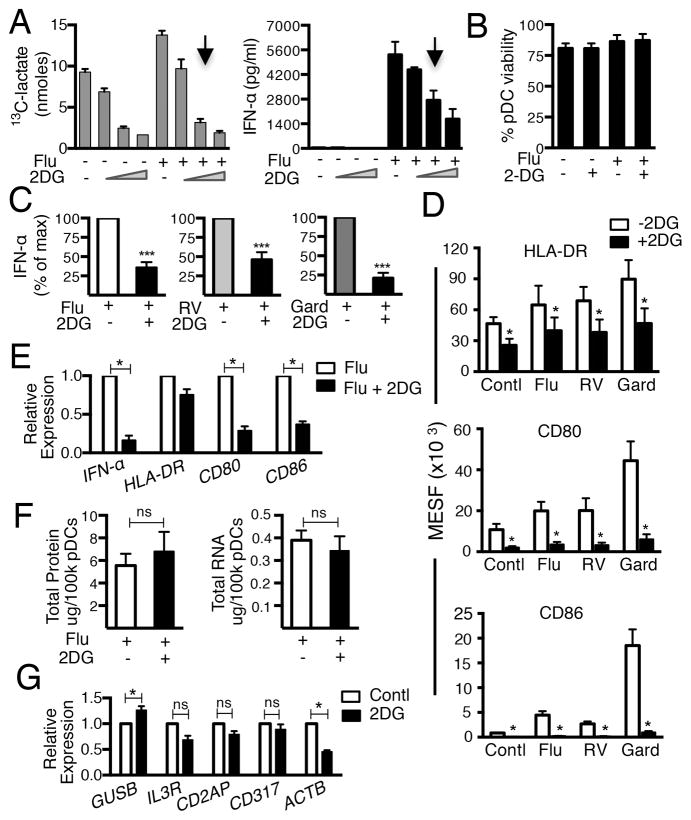
To investigate the role of glycolysis in regulating pDC antiviral responses we used 2-DG, a glycolysis inhibitor. Addition of 2-DG significantly reduced 13C-lactate efflux by pDCs and concomitantly impaired Flu-induced IFN-α secretion in a dose-dependent manner (Fig 2A) without decreasing pDC viability (Fig 2B). Inhibition of glycolysis also interrupted RV- and Gard-induced pDC IFN-α secretion (Fig. 2C). Viral and Gard-induced pDC maturation, assessed as up-regulation of surface HLA-DR, CD80 and CD86, was similarly impaired by 2-DG (Fig. 2D). Additionally, Flu-induced expression of IFN-α, CD-80 and CD-86 mRNA was also significantly inhibited by 2-DG (Fig. 2E) suggesting that glycolysis may regulate these antiviral genes at the transcriptional level. Taken together, these data highlight a critical role for glycolysis in pDC antiviral functional responses including IFN-α production and up-regulation of co-stimulatory molecules.
FIGURE 2.
Inhibiting glycolysis impairs viral-induced pDC antiviral responses without disrupting viability. (A) 13C-lactate and IFN-α in supernatants of pDCs exposed to Flu in media containing 10 mM D-glucose with or without 2-DG (1 mM, 10 mM and 20 mM). One of 3 experiments shown. Arrows indicate 2-DG concentration (10 mM) utilized in all subsequent experiments. (B) Effects of 2-DG on pDC viability. (C) IFN-α (pg/ml) in supernatants of pDCs exposed to Flu, RV or Gard for 24 h +/− 2-DG. (D) Surface expression of HLA-DR, CD80 and CD86 on pDCs cultured for 48 h in conditions shown in (C). (E) Relative expression of IFN-α, HLA-DR, CD80 and CD86 to PPIA in pDCs exposed to Flu +/− 2-DG for 24 h. (F) Total protein and total RNA in pDCs exposed to Flu +/− 2-DG for 24 h. (G) Impact of 2-DG on expression of GUSB, IL3R, CD2AP, CD317 and ACTB. Data represent mean ± SEM, N= 3–8 in B-G, *p ≤ 0.05; ***p ≤ 0.001, paired t-test.
We next evaluated the impact of glycolysis inhibition on total protein and total RNA concentrations in Flu-exposed pDCs. No differences were induced by 2-DG treatment (Fig. 2F), suggesting that glycolysis is not a global regulator of all transcription and protein synthesis in these cells. To assess whether glycolysis impacted transcription of pDC genes not induced by viral exposure, we measured the effect of 2-DG on transcription of the following genes for which expression was unaltered by Flu in our DNA microarray experiments (Genebank accession # GSE68849; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=qfarmocyrvcdvsb&acc=GSE68849): GUSB (encodes an essential lysosomal enzyme), ACTB (maintains cell motility), IL-3R (a receptor that maintains pDC viability), CD2AP (regulates pDC migration), CD317 (a receptor that negatively regulates proinflammatory cytokine secretion) and PPIA (encodes cyclophilin A, a regulator of cellular protein folding and trafficking) (21–25). Results confirmed that glycolysis is not required for global pDC gene expression (Fig. 2G); GUSB expression was actually increased while IL-3R, CD2AP, and CD317 were unaltered by inhibition of glycolysis. PPIA expression was similarly unaffected by glycolysis inhibition and was used to normalize this data. Conversely, 2-DG treatment resulted in decreased actin mRNA expression in pDCs suggesting that some cellular processes, in addition to viral-induced pDC IFN-α and maturation responses are likely also regulated by glycolysis in these cells.
As IFN-β has been shown to directly regulate glucose metabolism (26), it seemed possible that viral-induced glycolysis in pDCs could be mediated by IFN-α/β. To test this, we exposed pDCs to IFN-α and measured 13C-lactate production. pDC lactate efflux was not induced by IFN-α treatment (Supplemental Fig. 2A). Furthermore, IFN-α/β receptor blockade did not inhibit Flu-induced increases in lactate, suggesting that type I IFN does not regulate Flu-induced glycolysis in pDCs. To evaluate the role of TLR7 activation we utilized chloroquine to disrupt endosomal acidification, a required component of TLR7 signaling (27). Chloroquine inhibited Flu- and Gard-induced increases in both 13C-lactate and IFN-α production, providing evidence that TLR7 signaling is involved in viral-induced up-regulation of glycolytic pathways in pDCs (Supplemental Fig. 2B, 2C).
Recent discoveries indicate that metabolic pathways regulate mDC and T cell responses to activation signals including TLR agonists, growth factors and antigens (28–30). Our results reveal that viral exposure promotes metabolic activation in pDCs, and that these metabolic changes regulate critical pDC antiviral functions. Why viruses such as Flu and RV specifically induce glycolysis in human pDCs is unclear. Since immune cells face substantial bioenergetic challenges to generate immune responses, it is possible that rapid generation of ATP, one advantage of glycolysis over OXPHOS (31), could provide energy required for immediate secretion of IFN-α by pDCs. Increased glycolysis could also support the anabolic demands of viral-exposed pDCs by providing intermediate precursors for biosynthesis of nucleotides, non-essential amino acids and lipids as demonstrated in TLR agonist-induced murine DCs (32). We were unable to detect such precursor macromolecule intermediates in our experimental system, however, due to the limited number of pDCs available from human donors.
Inflammation and infection depletes tissue oxygen and nutrient levels, providing optimal conditions for glycolysis and not OXPHOS. pDCs are known to be recruited to such sites (3). Preferential induction of glycolysis upon pathogen encounter could thus promote generation of pDC antiviral responses in these anaerobic microenvironments.
LAIV results in increased glycolysis in human pDCs
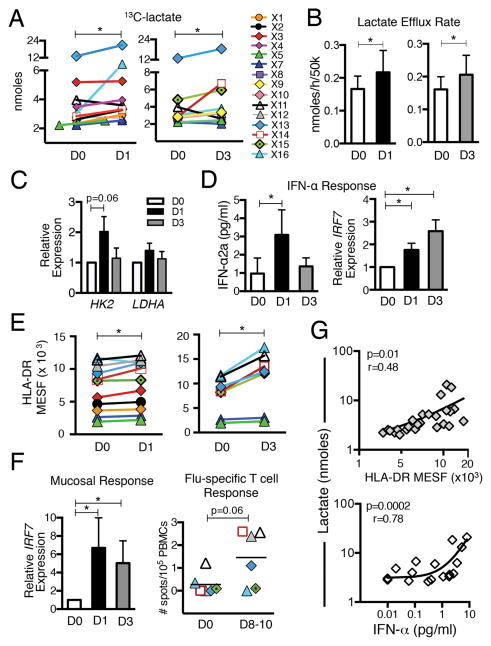
To evaluate the impact of in vivo viral infection on pDC metabolism we utilized LAIV. Blood pDCs were purified from healthy human donors (median 3×105; range 1–7×105 pDCs/donor) before and after intranasal LAIV administration and ex vivo pDC lactate efflux was measured. Despite substantial variation in baseline rates of pDC lactate production among donors, pDCs isolated after LAIV demonstrated significant increases in ex vivo lactate secretion and lactate efflux rates (elevated glycolysis) (Fig. 3A, 3B). Transcription of HK2 was also increased in pDCs isolated after LAIV, although the increase not significant (Fig. 3C). LAIV also significantly increased pDC IFN-α secretion, IRF7 mRNA expression (Fig. 3D) and surface HLA-DR expression (Fig. 3E), further demonstrating a systemic pDC response to the locally administered LAIV-induced Flu infection.
FIGURE 3.
In vivo viral infection (LAIV) increases glycolysis in human pDCs and correlates with pDC antiviral responses. Blood pDCs were purified from healthy donors before (D0), 1 (D1) and/or 3 (D3) days after LAIV and pDCs (5×104) were cultured ex vivo for 24 h. (A) 13C-lactate quantification and (B) lactate efflux rate in pDC supernatants. (C) Relative pDC mRNA expression of HK2 and LDHA. (D) IFN-α2a secretion (left) and relative IRF7 expression (right) in pDCs cultured ex vivo for 24 h. (E) Effect of LAIV on pDC HLA-DR expression. (F) Nasal and T lymphocyte responses to LAIV. Nasal cell IRF7 expression normalized to PPIA (left); Flu-specific IFN-γ secreting T cells measured by ELISPOT before (D0) and after (day 8–10) LAIV (right). (G) Correlations between pDC glycolysis (lactate production) and HLA-DR expression (upper) and IFN-α secretion (lower). Data from 3 time points (D0, D1 and D3) displayed. Data in histograms represent mean ± SEM. In A and E, lines connect data from individual donors. N= 6–11 *p ≤ 0.05, paired t-test.
To confirm that LAIV induced a local innate response, we also measured IRF7 expression in nasal samples. Expression of IRF7 mRNA was significantly increased at both 1 and 3 days after vaccination (Fig 3F, left), providing evidence of LAIV-induced antiviral response in the nasal mucosa. Although beyond the scope of our study, we also measured adaptive responses to LAIV in a subset of participants. Increased Flu-specific T-cell IFN-γ responses, although not significant (p=0.06) were detected after LAIV (Fig. 3F, right), providing evidence that LAIV induced both innate and adaptive immune responses. More importantly, significant associations between glycolysis and two critical components of innate antiviral pDC responses were revealed by our study. Positive correlations between pDC HLA-DR expression (Fig. 3G, upper) and pDC IFN-α secretion (Fig. 3G, lower) versus ex vivo lactate production were observed, supporting the notion that activation state and metabolism may be linked in pDCs. Results from this in vivo viral infection model thus complement the ex vivo findings and highlight a potential regulatory role for glycolysis in human pDC early responses to viral infections.
These data provide the first indication that pDCs utilize glycolysis, a key energy producing metabolic pathway, to perform critical antiviral functions. Our findings offer new insight into the regulation of pDC function and provide a foundation for potential development of novel strategies to augment immune responses in humans. Production of IFN-α, a potent antiviral cytokine, represents a primary pDC function. Considering the extensive biologic effects of IFN-α, glycolysis in pDCs - now linked to IFN-α production- could thus impact multiple components of antiviral responses extending beyond the pDC itself. IFN-α promotes pathogen clearance and also augments antigen presentation and cytokine production of other innate immune cells (7). The concept of manipulating glycolysis, a model already under investigation in cancer (33) to potentially impact pDC IFN-α production represents one potential application of our findings. This could be applied toward potential development of treatment strategies for viral infections such as chronic hepatitis C (HCV) and HIV (34, 35), where deficient pDC IFN-α responses are associated with clinical disease. Augmenting pDC IFN-α secretion through manipulation of pDC glycolysis could theoretically be evaluated in any clinical scenario associated with aberrant pDC IFN-α responses. TLR7 agonists, known to activate pDCs, have already been evaluated to treat infections associated with human papilloma and HCV (36). In our study, the TLR7 agonist gardiquimod induced pDC glycolysis and IFN-α secretion and could thus also be examined as a potential method to enhance antiviral immune responses. This strategy, however, is not pDC-specific, and could also activate other TLR7-expressing cells. It is important to note that as new approaches are developed to specifically target genetic pathways in distinct cell types, manipulation of the pDC glycolysis-IFN-α axis could provide an avenue to enhance general antiviral responses.
Despite the challenges of working with primary human pDCs, our study demonstrates a critical role for glucose metabolism in the regulation of pDC responses to common respiratory viral pathogens responsible for considerable morbidity and mortality in humans. Development of strategies to enhance pDC metabolic pathways to potentially improve antiviral responses represents one possible application of this discovery.
Supplementary Material
Acknowledgments
Funded by NIH NIAID R01AI098077 (to MAG), grant awards from Children’s Medical Center CCRAC (to MAG) and the Crystal Charity Ball of Dallas, TX (to MAG and JDF). Support also provided by the NIH National Center for Advancing Translational Sciences, award Number UL1TR001105.
We thank J. Gern and Y. Bochkov for providing rhinovirus, A. Ali for help with metabolic flux experiments, R. Kong for assistance with imaging flow cytometry and J. Seidel for assistance with obtaining LAIV.
Abbreviations
- pDC
plasmacytoid dendritic cells
- Flu
influenza virus
- RV
rhinovirus
- Gard
gardiquimod
- IRF7
IFN regulatory factor 7
- HIF
hypoxia inducible factor
- OXPHOS
oxidative phosphorylation
- ECAR
extracellular acidification rate
- OCR
oxygen consumption rate
- XF
extracellular flux
- 2-DG
2-deoxyglucose
- LAIV
live attenuated influenza vaccine
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunological reviews. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swiecki M, Colonna M. Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur J Immunol. 2010;40:2094–2098. doi: 10.1002/eji.201040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill MA, Long K, Kwon T, Muniz L, Mejias A, Connolly J, Roy L, Banchereau J, Ramilo O. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. The Journal of infectious diseases. 2008;198:1667–1676. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminski MM, Ohnemus A, Cornitescu M, Staeheli P. Plasmacytoid dendritic cells and Toll-like receptor 7-dependent signalling promote efficient protection of mice against highly virulent influenza A virus. The Journal of general virology. 2012;93:555–559. doi: 10.1099/vir.0.039065-0. [DOI] [PubMed] [Google Scholar]
- 6.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 7.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum Immunol. 2002;63:1126–1132. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- 9.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 10.Di Domizio J, Blum A, Gallagher-Gambarelli M, Molens JP, Chaperot L, Plumas J. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114:1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao Y, Kaliaperumal N, Chretien AS, Tang S, Lee B, Poidinger M, Fairhurst AM, Connolly JE. Human plasmacytoid dendritic cells regulate IFN-alpha production through activation-induced splicing of IL-18Ralpha. Journal of leukocyte biology. 2014;96:1037–1046. doi: 10.1189/jlb.2A0813-465RR. [DOI] [PubMed] [Google Scholar]
- 13.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill MA, Bajwa G, George TA, Dong CC, Dougherty, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Jr, Calatroni A, Wildfire JJ, Gergen PJ, Cohen RT, Pongracic JA, Kercsmar CM, Khurana Hershey GK, Gruchalla RS, Liu AH, Zoratti EM, Kattan M, Grindle KA, Gern JE, Busse WW, Szefler SJ. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 18.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werth N, Beerlage C, Rosenberger C, Yazdi AS, Edelmann M, Amr A, Bernhardt W, von Eiff C, Becker K, Schafer A, Peschel A, Kempf VA. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS One. 2010;5:e11576. doi: 10.1371/journal.pone.0011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolnay AE, Baskin CR, Tumpey TM, Sabourin PJ, Sabourin CL, Long JP, Pyles JA, Albrecht RA, Garcia-Sastre A, Katze MG, Bielefeldt-Ohmann H. Extrapulmonary tissue responses in cynomolgus macaques (Macaca fascicularis) infected with highly pathogenic avian influenza A (H5N1) virus. Archives of virology. 2010;155:905–914. doi: 10.1007/s00705-010-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM. beta-Actin specifically controls cell growth, migration, and the G-actin pool. Molecular biology of the cell. 2011;22:4047–4058. doi: 10.1091/mbc.E11-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 23.Srivatsan S, Swiecki M, Otero K, Cella M, Shaw AS. CD2-associated protein regulates plasmacytoid dendritic cell migration, but is dispensable for their development and cytokine production. J Immunol. 2013;191:5933–5940. doi: 10.4049/jimmunol.1300454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: a key player for human disease. Cell death & disease. 2013;4:e888. doi: 10.1038/cddis.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke JD, Platanias LC, Fish EN. Beta interferon regulation of glucose metabolism is PI3K/Akt dependent and important for antiviral activity against coxsackievirus B3. Journal of virology. 2014;88:3485–3495. doi: 10.1128/JVI.02649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 28.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malinarich F, Duan K, Hamid RA, Bijin A, Lin WX, Poidinger M, Fairhurst AM, Connolly JE. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J Immunol. 2015;194:5174–5186. doi: 10.4049/jimmunol.1303316. [DOI] [PubMed] [Google Scholar]
- 30.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 32.Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, Artyomov MN, Jones RG, Pearce EL, Pearce EJ. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nature reviews Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 34.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 35.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 36.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nature medicine. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.