Abstract
Purpose
Low maternal socioeconomic position (SEP) has been associated with adverse neonatal outcomes including preterm birth, low birth weight, intrauterine growth restriction, and infant mortality. A key biological mechanism that has been proposed to explain this association is hypothalamic-pituitary-adrenal (HPA) activity, yet the association between SEP and HPA activity in pregnancy has received little attention. In this study we aimed to examine the association between SEP and two forms of maternal cortisol regulation: diurnal slope and wakening response across pregnancy. Furthermore, we aimed to assess if this association differed by the sex of the fetus.
Methods
217 pregnant women aged 18–40 with singleton pregnancies participated. Women were excluded from participating if they were < 18 or > 40 years old, and if they were at risk for maternal or obstetric complications. Women provided information on socioeconomic characteristics of adults contributing to the participants’ household to compute a Hollingshead score of SEP. Women provided salivary cortisol samples upon awakening, 30 minutes after wake-up, and at bedtime at three times over pregnancy and once 30 days postpartum to calculate the diurnal slope and cortisol awakening response (CAR). Using linear regression analyses, we examined the relations between maternal SEP and maternal diurnal slope and CAR. We explored the relations between maternal SEP and cortisol by fetal sex using linear regression analyses. We also explored links between maternal SEP, maternal cortisol, and infant birth outcomes.
Findings
Women of lower SEP displayed smaller awakening responses and less change over the day compared to women of higher SEP. SEP was significantly associated with attenuated diurnal slope only among women carrying female fetuses, while for CAR, the association between SEP and attenuated CAR was significant only for women carrying male fetuses. Lower SEP was associated with decreased birth weight, and this association was partially explained by maternal HPA activity in pregnancy.
Implications
Women of low SEP displayed attenuated HPA activity across the perinatal period, and patterns varied by fetal sex and cortisol metric. Findings are in need of replication. More research is needed to understand links between SEP, HPA activity, and neonatal health.
Keywords: cortisol, pregnancy, socioeconomic position, sex
Introduction
Socioeconomic position (SEP) reflects the social standing of an individual or group, and is often measured as a combination of education, income, and occupation1. Socioeconomic deprivation has been consistently associated with morbidity and mortality in non-pregnant adults2–5. In pregnancy, low socioeconomic position is robustly associated with adverse pregnancy outcomes including infant mortality, prematurity, low birth weight, and intrauterine growth restriction6–9. Past studies have cited poor nutrition and access the health care as some factors explaining links between low SEP and adverse neonatal outcomes10–12. A key physiological process that has been proposed to explain the link between low SEP and poor health is the hypothalamic-pituitary-adrenal (HPA) axis13. Low SEP may represent a chronic stressor that results in wear and tear on the HPA axis, resulting in HPA dysregulation and aberrant patterns of cortisol production. Low SEP has been associated with altered cortisol patterns in non-pregnant adults, with evidence for both elevated and attenuated concentrations among individuals of low SEP13. In addition, some studies of non-pregnant adults suggest that the association between SEP and cortisol may differ by gender; Steptoe et al. (2003)14 found that low SEP was associated with lower levels of cortisol among women but higher levels among men, and Kunz-Ebrecht (2004) also reported lower cortisol levels among low SEP women (who also reported low job demands)15.
The association between SEP and HPA activity in pregnancy has not been extensively studied despite evidence from the literature demonstrating that glucocorticoid overexposure in utero is one key pathway linking prenatal adversity to adverse infant outcomes16. Emerging evidence suggests that SEP is associated with altered diurnal patterns of HPA activity in pregnancy. Diurnal slope and CAR are under different regulatory control and may respond differently to environmental stress17 ; thus diurnal cortisol and CAR have been examined separately in past studies. Valladares et al. (2009) reported that low social resources in pregnancy were associated with higher evening cortisol levels in the second half of pregnancy18. Consistent findings were reported by Thayer et al. (2014), who found that greater economic deprivation was associated with elevated evening, but not morning, cortisol levels in late pregnancy19. Corwin et al. (2013) also reported higher third trimester diurnal cortisol levels and glucocorticoid resistance (i.e., an inability of cortisol to inhibit pro-inflammatory signaling pathways) among low income minority women20, and Raikkenon et al. (2014) reported increased placental glucocorticoid receptor expression among pregnant women with less education and lower occupational status21.
The literature on the relations between SEP and maternal cortisol is sparse, but suggest an association between SEP and cortisol regulation in pregnancy. Past findings are limited to an examination of cortisol concentrations in late pregnancy only. It is therefore unclear if the association between SEP and maternal cortisol differs during different gestational time periods. This is an important gap in the literature given the dramatic changes in HPA activity over the course of gestation22, and the importance of trajectories of HPA activity over pregnancy in promoting positive maternal and neonatal health23–26. It is also unclear from the literature if the association between SEP and cortisol in pregnancy endures into the postpartum period. Thus, the primary aim of the current study was to examine the association between SEP and maternal cortisol over pregnancy and in the early postpartum period in a socioeconomically diverse sample.
Another aim of this study was to understand whether the association between SEP and maternal cortisol differs according to the sex of the fetus. Past studies have reported that maternal cortisol levels vary over gestation depending on the sex of the fetus; DiPietro and colleagues (2010) found that women carrying male fetuses had higher salivary cortisol levels relative to women carrying female fetuses prior to 30 weeks gestation, at which point there was a cross-over, and women carrying female fetuses displayed elevated cortisol levels27. The influence of SEP in the association between maternal cortisol and fetal sex was not examined in the DiPietro study. In addition, a recent study reported that associations among SEP and methylation of the placental 11-β hydroxysteroid dehydrogenase gene (an enzyme responsible for the inactivation of maternal cortisol, protecting the fetus from overexposure to maternal glucocorticoids) differed by fetal sex; Appleton et al. (2013) found that women reporting the greatest economic adversity in pregnancy had the highest placental 11β methylation, particularly if they were carrying a male fetus28. Given evidence of differences in HPA activity over pregnancy by fetal sex, and evidence of gender differences in the SEP-cortisol association in non-pregnant adults, we also explored whether associations between SEP and maternal cortisol differed by fetal sex. Finally, given past evidence of links between maternal SEP and adverse neonatal outcomes6,7, we explored associations between maternal SEP and birth outcomes, and whether associations were mediated by maternal cortisol levels.
Methods
Participants
Participants were 217 pregnant women with singleton pregnancies who were part of a larger study of the effects of maternal mood on fetal and infant development (Behavior and Mood in Mothers, Behavior in Infants (BAMBI)). Maternal and infant characteristics, categorized according to low and high SEP and infant sex, are presented in Table 1. Thirty-nine percent of the sample was considered “low” SEP (score of 4 or 5 on the Hollingshead29). Approximately 12% of the sample had a score of 1 on the Hollingshead, indicating “high” SEP. Women in the current study were on average 27 years old (SD=6), from racially and ethnically diverse backgrounds (45% non-Hispanic White, 17% non-Hispanic Black, 26% Hispanic, 6% > 1 race, 4% Asian, 1% ‘other’, 1% American Indian), 45% were married, 46% of pregnancies were planned, 35% had a high school education or less, and 17% of the sample reported an annual income of less than $10,000. Approximately 25% of women in the study received public insurance.
Table 1.
Maternal and Infant Characteristics by SEP and Infant Sex
| Maternal Characteristics | ||||
|---|---|---|---|---|
|
| ||||
| Low SEP/ Male | Low SEP/ Female | High SEP/ Male | High SEP/ Female | |
|
| ||||
| Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | |
| Age (years) | 23 (4) | 25 (6) | 28 (5) | 28 (5) |
| Gravida | 3 (1) | 3 (2) | 2 (1) | 2 (1) |
| Education (% < High School) | 31% | 39% | 3% | 3% |
| Race (% White) | 8% | 42% | 52% | 57% |
| Annual Income (% < $10K) | 26% | 39% | 8% | 7% |
| Marital Status (% married) | 6% | 6% | 61% | 62% |
| Pre-pregnancy BMI* | 26 (7) | 28 (8) | 26 (7) | 25 (6) |
| Cigarettes in pregnancy | 113 (348) | 41 (166) | 23 (111) | 11 (49) |
| Units of alcohol in pregnancy | 6 (13) | 5 (9) | 7 (10) | 7 (12) |
| Hypertension (%yes) | 3% | 3% | 7% | 6% |
| Gestational Diabetes (%yes) | 11% | 0% | 7% | 10% |
| Infant Characteristics | ||||
|---|---|---|---|---|
|
| ||||
| Low SEP/ Male | Low SEP/ Female | High SEP/ Male | High SEP/ Female | |
|
| ||||
| Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | |
| Gestational Length (weeks) | 39 (4) | 39 (1) | 39 (1) | 40 (1) |
| Birth Weight (kilograms) | 2.14 (0.76) | 3.24 (0.47) | 3.45 (0.49) | 3.38 (0.45) |
| APGAR score at 5 minutes | 9 (1) | 9 (0.3) | 9 (0.5) | 9 (1) |
Note.
BMI = Body mass index.
Infants were born at 39 weeks gestation (SD=2), average weight was 3.36 kilograms (SD=506), and APGAR (Appearance, Pulse, Grimace, Activity, and Respiration) score at 5 minutes after delivery was 9 (SD=1) demonstrating good health among babies born in this sample. 52% of babies were male. Women were excluded from participating if they were < 18 or > 40 years old and if they were at risk for maternal or obstetric complications. This study was approved by the Women & Infants Hospital and Lifespan Hospital Institutional Review Boards. All women provided written consent prior to their participation.
Procedure
Pregnant women completed 1–3 study sessions during pregnancy (Session 1: 24 (SD=3) weeks; Session 2: 30 (SD=1) weeks; Session 3: 36 (SD=1) weeks) and one session in the postpartum period (approximately 30 days after delivery). 100% of participants completed at least 1 study session, 81% completed at least 2 study sessions, 72% completed all three pregnancy sessions, and approximately 85% completed the postpartum session. At baseline, participants provided information on socioeconomic characteristics of adults contributing to the participants’ household, maternal characteristics, and pregnancy health information. For three days following each session, participants provided saliva samples (passive drool) upon awakening, 30 minutes after wake-up, and bedtime. After participants completed the 3 days of saliva collection, study staff retrieved samples from participants’ homes and provided payment.
Maternal medical history was assessed via chart review at enrollment and following delivery. Neonatal outcomes, including fetal sex, gestational age at birth, birth weight, and APGAR scores, were collected from medical chart review following delivery.
Measures
Socioeconomic Position (SEP)
Participants were asked to self-report the current occupation and highest level of education for themselves and another contributing adult at their first study session. To measure SEP we utilized the Hollingshead Four Factor Scale29. This measure is an index of socioeconomic position based on weighted scores of education and occupation of contributing adults. Occupations were coded according to the Hollingshead Four Factor occupational codes. Occupation scores range from 1–9, with scores of 1 indicating menial laborers and 9 indicating higher executives and major professionals. Educational attainment is scored on a scale of 1–7 with 1 indicating completion of less than 7th grade and 7 indicating graduate training. Hollingshead scores range from 1–5, with scores of 4 and 5 indicating low SEP. The Hollingshead measure is one of the most frequently used measures of socioeconomic position and has demonstrated good inter rater reliability in past research30.
Maternal cortisol
Up to 36 total saliva samples were collected from each participant throughout the study and returned to the lab by study staff, aliquoted, and stored at −80°C until analysis. Samples were then shipped to the laboratory of Clemens Kirschbaum, Ph.D. (Dresden University). Cortisol concentrations were analyzed with an immunoassay with time-resolved fluorescence detection. The intra and inter-assay coefficients of variation were < 8%. A subset of the sample (17%) were given Medication Event Monitoring System (MEMS) caps (AARDEX, Zurich, Switzerland) that time stamp every opening of bottles containing tubes used to collect maternal saliva. For participants with both self-reported and MEMS-recorded saliva sampling times, there was an average time discrepancy of 8 minutes (SD=5) suggesting that, in a subset of participants, women were adherent to the sampling protocol.
Statistical Approach
Cortisol data reduction
We calculated the cortisol awakening response (CAR) on each day of saliva collection by taking the difference between cortisol values upon awakening and 30 minutes after wake-up and then averaging CAR across the three days of collection. The three CARs over pregnancy were also averaged to calculate an average CAR in pregnancy. Morning saliva samples that were < 20 or > 40 minutes apart were omitted from analyses in order to accurately capture the morning awakening response. We calculated a measure of diurnal cortisol slope by taking the difference between samples collected upon awakening and bedtime on each day of collection. Slopes across the three days of collection were then averaged. The three slopes over pregnancy were also averaged to calculate an average pregnancy diurnal slope measure. CAR and diurnal cortisol slope are regulated by different biological processes17 and thus were analyzed separately. CAR and diurnal slope values were log transformed due to skewed distributions.
Hypothesis testing
To assess the influence of potential covariates, we performed Pearson correlation analyses (for continuous variables) and one-way analysis of variance (for categorical variables) to examine associations among maternal and infant characteristics and maternal cortisol concentrations. Characteristics that were significantly associated with maternal cortisol were included as covariates in subsequent analyses. We performed linear regression analyses to examine the association between maternal SEP and 1) average maternal CAR and diurnal slope in pregnancy, and 2) average maternal CAR and diurnal slope by all study sessions (three times in pregnancy and one time postpartum). Gestational age at sample collection was included as a covariate in regression analyses examining SEP and cortisol at separate gestational windows in pregnancy. To explore whether associations between SEP and maternal cortisol differed by fetal sex, we performed linear regression analyses examining the association between SEP and cortisol separately for women carrying male and female fetuses. Finally, we performed linear regression analyses to examine associations between maternal SEP, cortisol, and infant outcomes (gestational age at birth, birth weight, and APGAR score at 5 minutes after delivery).
Results
Assessment of Potential Covariates
We first examined associations between maternal cortisol and maternal characteristics of age, gravida, parity, race/ethnicity, pre-pregnancy BMI, number of cigarettes smoked and number of alcoholic drinks consumed in pregnancy, gestational diabetes, hypertension, hyper/hypothyroidism, maternal infection in pregnancy, and maternal steroid use in pregnancy. Only maternal hypertension was significantly associated with maternal CAR (F=18.08, p<.001) and was included as a covariate in subsequent analyses. Maternal age (r=.21, p=.004), the number of alcoholic drinks consumed in pregnancy (r=.13, p=.05), and race/ethnicity (F=5.63, p<.001) were significantly associated with diurnal cortisol slope and were included as covariates in subsequent analyses.
Socioeconomic Position and Cortisol
Maternal SEP was significantly associated with average diurnal slope (β=−.26, p=.002) and average CAR (β=−.18, p=.02) in pregnancy, such that women of lower SEP displayed smaller awakening responses and smaller slopes in pregnancy compared with women of higher SEP. When we examined the association within each study session separately, we found that for slope, the association between SEP was significant at 24 weeks GA (β=−.21, p=.02), 30 weeks GA (β=−.23, p=.01), and 36 weeks GA (β=−.23, p=.01) such that lower SEP was associated with smaller slopes at each time point. SEP was associated with CAR at 36 weeks gestation (β=−.20, p=.02), but not at 24 (β=−.11, p=.28) or 30 weeks gestation (β=−.05, p=.53); lower SEP was associated with smaller CAR only at 36 weeks gestation. The association between postpartum CAR and SEP was not significant (β=.09, p=.42). Postpartum slope was significantly associated with SEP (β=−.32, p=.001); women who reported lower SEP had greater cortisol slopes in the postpartum period. See Table 2.
Table 2.
Associations among maternal SEP and maternal cortisol by fetal sex
| Average Cortisol in Pregnancy | 24 weeks’ Gestationc | 30 weeks’ Gestationc | 36 weeks’ Gestationc | 30 Days Postpartum | |
|---|---|---|---|---|---|
|
| |||||
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Total Sample | |||||
| CARa | −.18 (.02) * | −.11 (.38) | −.05 (.42) | −.20 (.36) * | 0.9 (.49) |
| Slopeb | −.26 (.02) ** | −.21 (.02) * | −.23 (.02) * | −.23 (.02) * | −.32 (.74) ** |
| Males Only | |||||
| CARa | −.24 (.03) * | −.12 (.52) + | .01 (.59) | −.26 (.52) * | .33 (.61) * |
| Slopeb | −.07 (.02) | −.24 (.03) | −.09 (.03) | −.09 (.03) | −.36 (1.05) ** |
| Females Only | |||||
| CARa | −.12 (.03) | −.12 (.57) | −.12 (.61) | −.12 (.51) | −.12 (.75) |
| Slopeb | −.36 (.02) ** | −.19 (.02) | −.33 (.03) * | −.28 (.03) * | −.29 (1.04) + |
Note:
Average CAR analyses controlled for gestational hypertension.
Average diurnal slope analyses controlled for maternal age, number of alcoholic drinks in pregnancy, and race/ethnicity.
Analyses within separate gestational ages also included gestational age at cortisol sampling as a covariate.
p<.05;
p<.01;
p<.10
Socioeconomic Position and Cortisol by Fetal Sex
To explore whether the association between maternal SEP and cortisol differed by fetal sex, we ran regression analyses stratified by sex of the fetus. Results of these analyses revealed that SEP was significantly associated with average maternal slope in pregnancy only among women carrying female fetuses (β=−.36, p=.002), but was not significant for women carrying male fetuses (β=−.07, p=.58). This pattern reversed in the postpartum period; SEP was associated with cortisol slope among women who delivered male babies (β=−.36, p=.004), but was not significantly associated among women who delivered female babies (β=−.29, p=.06). Women of lower SEP in pregnancy and who had male babies displayed attenuated cortisol slope in the postpartum period.
For CAR, the opposite pattern was observed; the association between SEP and average CAR in pregnancy was significant for women carrying male fetuses (β=−.24, p=.03) but not among women carrying female fetuses (β=−.12, p=.30). A consistent pattern was observed in the postpartum period; SEP was associated with CAR among women who had male infants (β=.33, p=.02) but not female infants (β=−.12, p=.45). Women of lower SEP in pregnancy and who had male babies displayed attenuated CAR in pregnancy and the postpartum period. See Table 2.
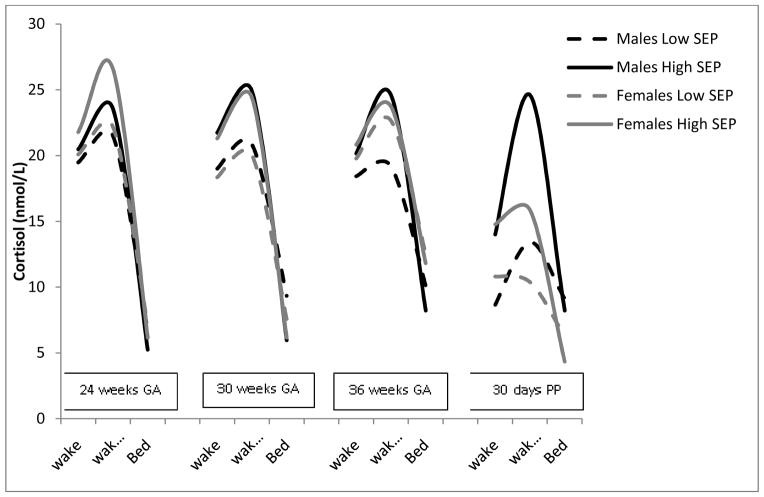
Examination of associations between SEP and maternal cortisol within each gestational window stratified by fetal sex revealed that, for women carrying female fetuses, SEP was significantly associated with maternal diurnal slope at 30 weeks GA (β=−.33, p=.01) and 36 weeks GA (β=−.28, p=.02) but was not statistically significant at 24 weeks GA (β=−.19, p=.18). Among women carrying male fetuses, the association between SEP and maternal diurnal slope was not significant (β values >−.10, p values >.09). For CAR, results revealed that associations among SEP and CAR were significant for women carrying male fetuses at 36 weeks gestation (β=−.26, p=.02) but not at 24 (β=−.12, p=.39) or 30 weeks gestation (β=.01, p=.87). There were no significant associations between maternal SEP and CAR at any gestational time point among women carrying female fetuses (β values > −.13, p values > .27). See Table 2 and Figure 1. In Figure 1 we present maternal cortisol patterns stratified by SEP category for ease of interpretation; however SEP was examined as a continuous variable in analyses.
Figure 1.
Socioeconomic positiona and maternal diurnal cortisol differs by fetal sex
Note: aSEP = Socioeconomic Position (measured using the Hollingshead Four Factor Index of social position). PP= postpartum. Cortisol values were log transformed for analyses. Raw values are presented in the figure.
Socioeconomic Position and Neonatal Outcomes
Associations among SEP and neonatal outcomes revealed a significant association between infant birth weight and SEP (β=−.19, p=.007); women with lower SEP in pregnancy had babies that weighed less at birth than women of higher SEP in pregnancy. This association was significant for male (β=−.21, p=.03) but not female infants (β=−.16, p=.12). The association between SEP and infant birth weight remained significant when average maternal cortisol slope over pregnancy was added to the model (β=−.20, p=.01) but became non-significant when adjusting for average maternal CAR in pregnancy (β=−.12, p=.16). These results suggest that the association between lower SEP and reduced birth weight is partially explained by maternal HPA functioning in pregnancy. There were no significant associations between SEP and gestational length or APGAR score at 5 minutes after delivery (p values > .35).
Maternal Cortisol and Neonatal Outcomes
Associations among maternal cortisol and neonatal outcomes were not statistically significant (p values >.06).
Discussion
Findings from this study revealed an association between maternal SEP and diurnal cortisol over pregnancy and in the postpartum period, such that women of lower SEP displayed smaller diurnal cortisol slope and CAR. Attenuated cortisol production may be indicative of dysregulation of the HPA axis and have been associated with poor health in past research31. Low SEP may represent a chronic stressor that results in wear and tear on the HPA axis, resulting in HPA attenuation and reductions in cortisol production over pregnancy. Decreases in cortisol production toward the end of gestation may impair production of fetal lung surfactant (a lipoprotein that increases the ability of the lungs and thorax to expand) and slow maturation of fetal lung development32.
The association between SEP and cortisol diurnal slope was significant across pregnancy and postpartum time points, while the association between SEP and CAR was only significant in late third trimester. Over typically developing pregnancies, CAR and cortisol reactivity to stress become blunted as pregnancy progresses and circadian rhythms are maintained22. In light of the typical changes in HPA activity over pregnancy, these results suggest that CAR attenuation and disruption in circadian patterns may be particularly pronounced among women of lower SEP. Findings are consistent with the small number of previous studies showing that socioeconomic deprivation is associated with altered diurnal cortisol patterns in late pregnancy18,19. However, future studies are needed to further understand the SEP-cortisol association in the perinatal period.
We also examined whether the association between SEP and cortisol differed by the sex of the fetus. Results from this study revealed that women of low SEP who were carrying female fetuses displayed smaller diurnal cortisol slopes at 30 and 36 weeks gestation, whereas we observed no significant associations between SEP and diurnal cortisol slope in pregnancy among women carrying male fetuses. The pattern changed in the postpartum period; women of lower SEP who delivered male infants displayed attenuated diurnal slope. In contrast, lower SEP women carrying male fetuses displayed smaller CAR, but only at 36 weeks gestation and postpartum. These results complement, in part, the findings reported by DiPietro and colleagues27 who found differences in maternal cortisol regulation according to the sex of the fetus and the gestational window of cortisol sampling. Results also support the hypothesis that fetal sex hormones may interact with environmental cues to dictate maternal HPA activity and cortisol production in pregnancy33.
The diurnal cortisol slope represents the average rate of decline in cortisol from wake-up to bedtime, while the CAR represents the increase in cortisol from wake-up to 30–45 minutes after wake-up. Diurnal slope and CAR are under different regulatory control and may respond differently to environmental stress17. While in the current study the associations among SEP and these indices of diurnal cortisol rhythm varied by fetal sex, together, findings suggest that, among women of lower SEP, there is an overall reduction in cortisol production in pregnancy. Cortisol production modulates the inflammatory response34,35. Chronic stress, such as experiencing low SEP, may alter the effectiveness of cortisol in regulating the inflammatory response, resulting in increased inflammation36. Diminished cortisol production among low SEP women, particularly at the end of gestation and if they are carrying male fetuses, may in turn increase risk for inflammation. Elevated inflammation is a risk factor for adverse neonatal outcomes including preterm birth37, and male fetuses are more likely to be born preterm38. Taken together, results from the current study suggest that male fetuses of lower SEP women may be particularly vulnerable to preterm birth, and one mechanism explaining this vulnerability may be diminished maternal cortisol production (and concomitant risk for inflammation/infection) in late pregnancy. However, women in this study were selected to be at low risk for obstetric and neonatal complications, and we did not observe an association between SEP and gestational length, so our data do not support the previously observed link between SEP and preterm birth.
We explored associations between maternal SEP and infant birth outcomes. Results revealed an inverse association between maternal SEP and infant birth weight, particularly among male infants, such that women of lower SEP delivered infants that weighed less, reflecting perhaps a sub-optimal intrauterine environment. These results suggest that, even in this sample of predominantly healthy pregnancies, low SEP may have had a significant effect on fetal growth. This association became non-significant after adjusting for maternal CARsuggesting that maternal HPA activity explained some of the variance in the relation between SEP and infant birth weight.
Results from this study are the first, to our knowledge, to examine the association between SEP and cortisol by fetal sex, and findings extend the current literature by demonstrating an association between SEP and maternal cortisol at multiple gestational time periods. Results from this study, however, should be interpreted in light of several limitations. The aim of this study was to examine associations between maternal SEP and one biological system that may serve as a pathway to adverse neonatal outcomes. Other important behavioral factors that have been linked to birth outcomes, including health care utilization and nutrition10–12, were not examined. These factors are important to consider in future studies. The Hollingshead measure of SEP incorporates data on the education and occupation of adults contributing to the household. This may not be an accurate representation of social status because it does not take into account other indices of social status including income, neighborhood factors, and perceived social status. We also did not ask participants to report on socioeconomic characteristics of their childhood, despite research suggesting that childhood socioeconomic characteristics may be more predictive of adult stress physiology than adult SEP39. In addition, we collected only two cortisol samples each morning over three days to capture the CAR. Ideally, we would have collected three or more samples per day for up to six days to accurately capture the CAR40,41. Another limitation of this study was our lack of assessment of the sex of previous pregnancies. Some literature suggests that having a male fetus in a prior pregnancy increases preterm birth risk in subsequent pregnancies42, leading to the speculation that the sex of a prior pregnancy may prime maternal hormonal profiles in subsequent pregnancies33. This is an important consideration for future research.
Despite several limitations, we believe that results from this study make significant contributions to the extant literature. A consistent association between SEP and cortisol has been observed in non-pregnant samples13, but this association has not been adequately examined in pregnancy or the early postpartum period. Results from this study add to our understanding of the relations between SEP and cortisol during the perinatal period. This study is also the first, to our knowledge, to suggest that the relations between SEP and maternal cortisol may differ by fetal sex. This difference is particularly important to consider in light of robust evidence indicating that male fetuses are at greater risk for preterm delivery38, and suggest that sex hormones may interact with environmental inputs (i.e., SEP) to result in differences in maternal cortisol production. Findings need to be replicated in larger samples of pregnant women, including those at risk for adverse maternal and neonatal outcomes, in order to further understand the links between SEP, cortisol, and maternal and infant health.
Conclusions
The current study provides evidence supporting an association between maternal SEP and HPA activity in the perinatal period and adds to the current literature by assessing this link among a variety of diurnal cortisol measures, across several periods of gestation, and examining differences by fetal sex. Future research utilizing larger sample sizes is needed to explore the interactive effects of fetal sex, SEP, and maternal cortisol patterns, as well as pathways to adverse neonatal outcomes based on fetal sex and maternal SEP. These findings provide preliminary evidence for potential biomarkers that may serve as pathways to sex differences in adverse neonatal outcomes.
Acknowledgments
This research was supported in part by National Institutes of Health grant MH079153 to LRS. The sponsor was not involved in study design, data collection or analysis, the interpretation of the data, or in the decision to submit this article for publication. All authors have approved the final article. We thank the study staff for their efforts in data collection. We thank the women in the study for their participation.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Association AP. Socioeconomic Status. 2015. [Accessed August 7, 2015]. [Google Scholar]
- 2.Clark A, DesMeules M, Luo W, Duncan A, Wielgosz A. Socioeconomic status and cardiovascular disease: risks and imlications for care. Nature Reviews Cardiology. 2009;6(11):712–722. doi: 10.1038/nrcardio.2009.163. [DOI] [PubMed] [Google Scholar]
- 3.Adler N, Boyce W, Chesney M, Folkman S, Syme S. Socioeconomic inequalities and health: no easy solution. Journal of the American Medical Association. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 4.Schoeni R, Martin L, Andreski P, Freedman V. Persistent and growing economic disparities in disability among the elderly: 1982–2002. American Journal of Public Health. 2005;95:2065–2070. doi: 10.2105/AJPH.2004.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elo I, Martikainen P, Smith K. Socioeconomic differentials in mortality in Finland and the United States: the role of education and income. European Journal of Population. 2006;22(2):179–203. [Google Scholar]
- 6.Kramer M, Sequin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly. Paediatric and Perinatal Epidemiology. 2000;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 7.Kramer M. Determinants of low birth weight: methodological assessment and meta-analysis. Bulletin of the World Health Organization. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 8.Parker J, Schoendorf K, Kiely J. Associations between measures of socioeconomic status and low birthweight, small for gestational age, and premature delivery in the United States. Annals of Epidemiology. 1994;4:271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 9.Kogan M. Social causes of low birth weight. Journal of the Royal Society of Medicine. 1995;88:611–615. doi: 10.1177/014107689508801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiologic Reviews. 2010;(32):5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- 11.Batty G, Shipley M, Gunnell D, et al. Height, wealth, and health: an overview with new data from three longitudinal studies. Economics and human biology. 2009;7(2):137–152. doi: 10.1016/j.ehb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Feijen-de Jong E, Jansen D, Baarveld F, van der Schans C, Schellevis F, Reijneveld S. Determinants of late and/or inadequate use of prenatal healthcare in high-income countries: a systematic review. European Journal of Public Health. 2012;22(6):904–913. doi: 10.1093/eurpub/ckr164. [DOI] [PubMed] [Google Scholar]
- 13.Dowd J, Simanek A, Aiello A. Socio-economic status, cortisol, and addostatis load: a review of the literature. International journal of epidemiology. 2009;38(5):1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steptoe A, Kunz-Ebrecht S, Owen N, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosomatic Medicine. 2003;65(3):461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- 15.Kunz-Ebrecht S, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Social Science and Medicine. 2004;58(8):1532–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- 16.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007 May;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Valladares E, Pena R, Ellsberg M, Persson L, Hogberg U. Neuroendocrine response to violence during pregnancy - impact on duration of pregnancy and fetal growth. Acta Obstetrica et Gynecologica. 2009;88:818–823. doi: 10.1080/00016340903015321. [DOI] [PubMed] [Google Scholar]
- 19.Thayer Z, Kuzawa C. Early origins of health disparities: maternal deprivation predicts maternal evening cortisol in pregnancy and offspring cortisol reactivity in the first few weeks of life. American Journal of Human Biology. 2014;26:723–730. doi: 10.1002/ajhb.22532. [DOI] [PubMed] [Google Scholar]
- 20.Corwin E, Guo Y, Pajer K, et al. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 2013;38:1786–1796. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raikkonen K, O'Reilly J, Pesonen A, et al. Associations between maternal level of education and occupational status with placental glucocorticoid regeneration and sensitivity. Clinical Endocrinology. 2014;81:175–182. doi: 10.1111/cen.12412. [DOI] [PubMed] [Google Scholar]
- 22.Sandman CA, Davis EP, Buss C, Glynn L. Prenatal Programming of Human Neurological Function. International Journal of Peptides. 2011;2011:1–9. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buss C, Entringer S, Reyes JF, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009 Oct;201(4):398e391–398. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011;73:469–474. doi: 10.1097/PSY.0b013e31821fbf9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadhwa P, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. American Journal of Obstetrics and Gynecology. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 26.Giurgescu C. Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neonatal Nurs. 2009 Jul-Aug;38(4):377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 27.DiPietro J, Costigan K, Kivlighan K, Chen P, Laudenslager M. Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology. 2011;36(4):588–591. doi: 10.1016/j.psyneuen.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appleton A, Armstrong D, Lesseur C, et al. Patterning in Placental 11-B Hydroxysteroid Dehydrogenase Methylation According to Prenatal Socioeconomic Adversity. PLoS One. 2013;8(9):e74691. doi: 10.1371/journal.pone.0074691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollingshead A. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 30.Cirino P, Chin C, Sevcik R, Wolf M, Lovett M, Morris R. Measuring Socioeconomic Status: Reliability and Preliminary Validity for Different Approaches. Assessment. 2002;9(2):145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 31.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;113:24–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Mescher E, Platzker A, Ballard P, Kitterman J, Clements J, Tooley W. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma corticoids in the fetal lamb. Journal of Applied Physiology. 1975;39(6):1017–1021. doi: 10.1152/jappl.1975.39.6.1017. [DOI] [PubMed] [Google Scholar]
- 33.Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and preterm birth. Placenta. 2013;34:95–99. doi: 10.1016/j.placenta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Shelton M, Schminkey D, Groer M. Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biological research for nursing. 2015;17(3):295–302. doi: 10.1177/1099800414543821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilder R. Hormones, pregnancy, and autoimmune diseases. Annals of the New York Academy of Sciences. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 36.Tian R, Hou G, Yuan T. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. The Scientific World Journal. 2014;2014:780616. doi: 10.1155/2014/780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Espinoza J, Goncalves L, Kusanovic J, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in Reproductive Medicine. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeitlin J, Saurel-Cubizolles M-J, de Mouzon J, et al. Fetal sex and preterm birth: are males at greater risk? Human Reproduction. 2002;17(10):2762–2768. doi: 10.1093/humrep/17.10.2762. [DOI] [PubMed] [Google Scholar]
- 39.Miller G, Chen E, Walker H, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences in the United States of America. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clow A, Hucklebridge F, Thorn L. The cortisol awakening response in context. International Review of Neurobiology. 2010;93:153–175.22. doi: 10.1016/S0074-7742(10)93007-9. [DOI] [PubMed] [Google Scholar]
- 41.Hellhammer D, Wust S, Kudielka B. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Mortensen L, Nielsen H, Cnattingius S, Andersen A-M. Sex of the first-born and risk of preterm birth in the subsequent pregnancy. Epidemiology. 2011;22:328–332. doi: 10.1097/EDE.0b013e31820e8600. [DOI] [PubMed] [Google Scholar]