Abstract
Limited oral fluid (OF) pharmacokinetic data collected with commercially available collection devices after controlled cocaine administration hinder OF result interpretations. Ten cocaine-using adults provided OF, collected with Oral-Eze® (OE) and StatSure Saliva Sampler™ (SS) devices, an hour prior to and up to 69 h after 25 mg intravenous (IV) cocaine administration. Cocaine and benzoylecgonine (BE) were quantified by a validated 2D-GC-MS method. Large inter-subject variability was observed. Cocaine was detected in OF in the first 0.17 h sample after IV administration, with much more rapid elimination than BE. OE median observed Cmax (range) was 932 (394–1,574) μg/L for cocaine and 248 (96.9–953) μg/L for BE. SS median (range) observed cocaine and BE Cmax trended lower at 732 (83.3–1,892) μg/L and 360 (77.2–836) μg/L, respectively. OE and SS cocaine OF detection times were 12.5 and 6.5 h and for BE 30.5 and 28.0 h, respectively at 1 μg/L. There were no significant pharmacokinetic differences between OE and SS OF collection devices, except cocaine half-life was significantly shorter in SS OF specimens. This difference could be attributed to differences in stabilizing buffers present in OF collection devices, which may affect cocaine stability in OF specimens, or decreased recovery from collection pads. Both OE and SS OF collection devices were effective in monitoring cocaine and metabolite concentrations with similar detection windows. Furthermore, we demonstrated that different confirmatory OF cutoffs can be selected to produce shorter or longer cocaine and metabolite detection windows to address specific needs of clinical and forensic drug testing programs.
Keywords: Cocaine, Benzoylecgonine, Oral Fluid, Oral-Eze®, Pharmacokinetics, StatSure Saliva Sampler™
Introduction
Despite declining cocaine use prevalence in the general population, cocaine remains a widely used illicit drug in Europe (3.4 million users) [1] and the United States (1.5) million users [2], behind only cannabis and methamphetamine [3]. Cocaine was the second most prevalent illicit drug (after cannabis) in the general driving population (0.42%), in Europe, [4] and the second most prevalent drug in nighttime drivers (1.4%) in the United States [5].
Alternative matrices, such as oral fluid (OF), are increasingly employed for detecting recent drug consumption due to the non-invasive and observed collection, no need for same-sex collectors, and little potential for adulteration, dilution and substitution compared to urine [6–9]. OF cocaine concentrations were significantly correlated to those in plasma [10–13] and blood [14], making OF an attractive matrix for estimating cocaine-associated impairment and windows of detection. However, studies have demonstrated large inter- and intra-subject variability in OF to plasma ratios precluding calculating one concentration from the other [10, 15]. Detection windows vary based on dose, analyte, administration route, drug intake frequency, cutoffs, and OF collection methods. Ideal drug detection windows differ depending upon the monitoring purpose. For driving under the influence of drugs (DUID) programs detection windows should mirror the period of impairing effects; however, for workplace and pain management drug testing, longer detection windows are ideal due to widely separated specimen collections [16].
Cocaine pharmacokinetics are well studied in blood, plasma, and urine after various administration routes, but less is known about cocaine disposition in OF following controlled drug administration. Previous studies investigated OF cocaine and metabolite concentrations following controlled intravenous (IV) [12, 13, 17–21], smoking (SM) [18–21], intranasal (IN) [18, 20, 21], oral [21, 22], and subcutaneous (SC) administration [10]; however, only three studies examined cocaine and metabolite OF pharmacokinetics [10, 20, 22], and none evaluated commercially available OF collection devices containing stabilizing and elution buffers. Furthermore, cocaine and metabolite detection windows are needed for recently published US Substance Abuse and Mental Health Services Administration (SAMHSA) National Laboratory Certification Program (NLCP) Mandatory Guidelines for federally-mandated oral fluid testing [23] and established DRUID OF guidelines [24].
Cocaine was previously quantified in OF collected via citric-acid stimulated expectoration between 4.1–17.7 h following 25 mg IV, 32 mg IN, 42 mg SM, and 75 mg/70 kg and 150 mg/70 kg SC cocaine administration [10, 21]. For benzoylecgonine (BE), the primary inactive cocaine metabolite, OF detection times ranged from 5.0–47 h. Following repeated oral cocaine administration (375–2000 mg), OF cocaine and BE were identified up to 21.2 and 50.0 h, respectively [21], indicating extended windows of detection with repeated dosing. It is unclear if OF collected with various commercially available devices, containing different elution and stabilization buffers, will exhibit similar cocaine concentration time profiles and kinetics over time. SAMHSA and DRUID proposed confirmatory OF cocaine cutoff concentrations of 8 and 10 μg/L, respectively [23, 24], and 8 and 10 μg/L for BE. Eight μg/L also was recommended for suspected drug-impaired driving cases [25], and a 10 μg/L OF cocaine concentration was suggested by the Talloires recommendations [26].
The aims of this study were to characterize for the first time cocaine and BE OF pharmacokinetics collected with the Oral-Eze® (OE) and StatSure Saliva Sampler™ (SS) devices (two of the most commonly used commercial devices) following controlled IV cocaine administration. Additionally, we investigated detection windows with different OF collection devices at the recommended SAMHSA and DRUID OF testing guidelines.
Materials and Methods
Participants
Participants provided written informed consent for this NIDA Institutional Review Board and Food and Drug Administration-approved study. Eligibility criteria included healthy adults ages 18–50 years who smoked or used IV cocaine for at least six months and at least three times per month during the three months prior to screening. Exclusion criteria included pregnant or nursing women; current physical dependence on any drug other than cocaine, caffeine, or nicotine; current clinically significant medical or psychiatric disorder; hemoglobin less than 12.5 g/dL or blood donation within eight weeks of study entry; current hypertension or blood pressure readings consistently above 140 mm Hg systolic or 90 mm Hg diastolic while at rest; heart rate consistently above 90 or below 50 bpm while at rest; abnormal 12-lead ECG; history of clinically significant adverse reaction to cocaine, acetazolamide, or quinine; or interest in drug abuse treatment within three months of study screening.
Drug Administration
Participants resided on a secure research unit for 13 days and 12 nights, and received a single dose of 25 mg IV cocaine through a peripheral venous catheter on three separate days (Days 1, 5, and 10). Cocaine was administered alone on Day 1, in combination with oral acetazolamide on Day 5, and with oral quinine on Day 10. The study’s primary aims were to evaluate potential pharmacodynamic and pharmacokinetic interactions between cocaine and acetazolamide and quinine, as they are being considered as medication compliance markers for cocaine use disorder treatment pharmacotherapies, similar to a study performed with oxycodone and quinine by Babalonis et al [27].
Oral Fluid Specimen Collection
OF was collected with either the Oral-Eze® (Quest Diagnostics) or StatSure Saliva Sampler™ (StatSure Diagnostic Systems) devices by placing the absorptive pad under the tongue until the volume-adequacy indicator turned blue, indicating 1 mL OF was collected, or 5 min had elapsed, whichever occurred first. OF was collected with a single device type prior to and following each IV cocaine dose in each participant. All participants had OF collected with each device type. OE collection tubes contained 2 mL stabilizing buffer, yielding a 3-fold OF dilution, whereas SS tubes contained 1 mL buffer for a 2-fold dilution. Following manufacturer’s recommendations, the pad was removed and placed in the stabilizing buffer and left at room temperature (OE) or 4°C (SS) for ≥12 h for drug elution. Serum separators depressed into the collection tube were utilized to facilitate decanting into a 3.6 mL Nunc® CryoTube®, with samples stored refrigerated at 4° C prior to analysis. The majority of OF specimens were analyzed within 4 months, although all specimen analysis was complete within 8 months. No oral intake or smoking was permitted 10 min before OF collection at −1.0, 0.17, 0.5, 1, 1.5, 2, 3, 4, 6.5, 9.5, 12.5 and 21 h from dosing. Additional collections were obtained at 28 (Day 10 only), 33 (Day 1 only), 45 and 69 h (Days 1 and 5) post-administration.
Quantification of Cocaine and Benzoylecgonine in Oral Fluid
Cocaine and BE OF were quantified with modifications of a previously validated venous blood analytical method [28]. Calibrators were prepared at drug concentrations from 1–100 μg/L in 0.75 mL (OE: 0.25 mL blank OF + 0.5 mL OE stabilizing buffer) or 0.5 mL (SS: 0.25 mL blank OF + 0.25 mL SS buffer) solutions at the same dilutions as authentic specimens, therefore, accounting for the dilution factor. Quality controls (QCs) were prepared at 3, 25, and 75 μg/L in the same manner as calibrators, although from different standard ampoules. Briefly, 25 μL internal standard (IS) (250 μg/L D3-cocaine and D8-BE) were added to either 0.75 or 0.5 mL OF with 2 mL phosphate buffer (pH 6). After vortexing and centrifugation, the filtrate was decanted onto preconditioned UCT Clean Screen DAU 200 mg 10 mL SPE cartridges. Columns were washed (water, 0.1 M hydrochloric acid, methanol), dried for 20 min, and eluted into conical glass centrifuge with dichloromethane:isopropanol (80:20, v/v prepared for 100 mL) mixed with 2 mL ammonium hydroxide. After evaporating to dryness, samples were derivatized with 20 μL ethyl acetate:MTBSTFA + 1% t-BDMS (50:50 v/v) for 40 min at 70°C. The derivatized extracts (2 μL) were analyzed by an electron impact two-dimensional (2D) GC-MS method [28] with oven temperature program modifications for a total run time of 18.05 min (Supplemental Table 1). Instrument parameters were described in detail previously [28] and are outlined in Supplemental Table 1. The upper limit of linearity was 100 μg/L; specimens exceeding this limit were diluted with oral fluid and buffer mixture (2:1, v/v; 1:1 v/v), re-extracted and analyzed.
Method Validation
OF methods were validated in accordance with the Scientific Working Group for Forensic Toxicology recommendations [29]. Linearity from 1–100 μg/L with 1/x2 weighting was achieved for cocaine and BE in OF (OE and SS) with calibration curve (n=5) coefficients (R2) above 0.9916 ± 0.0040 and residuals <15%. The limits of detection (LOD) for cocaine and BE were 1 and 0.5 μg/L, respectively, with a 1 μg/L limit of quantification (LOQ) for each analyte for OE and SS assays, respectively. Bias and imprecision were evaluated in four replicates over five days (n = 20) at each QC concentration with a One-way Analysis of Variation approach to calculate combined within- and between-run imprecision. Bias was ±9.2% and 14% for cocaine and BE, respectively. OE within- and between-run imprecision was <3.9% and <4.3%, whereas SS exhibited <3.4% (within-run) and <5.7% (between-run) imprecision for cocaine and BE. Cocaine OE and SS extraction efficiencies were 93.1–95.5% and 96.4–97.2%, respectively, with BE results 70.9–73.5% and 79.7–82.7%, respectively. Analytes were stable in the autosampler for 72 h at low and high QC concentrations with percent differences between −3.9% and 1.7% (n=4). Short-term stability was evaluated at room temperature for 24 h and at 4°C for 72 h. Analytes were considered stable under evaluated conditions (% difference < ±16.7%).
Statistical and Pharmacokinetic Analyses
Visual inspection of data and evaluation by D’Agostino-Pearson normality test (omnibus K2) indicated non-normal data distribution. Therefore, statistical comparisons were conducted with non-parametric tests (Mann-Whitney or Wilcoxon signed-rank) using GraphPad Prism Version 5.02 (GraphPad Software Inc, La Jolla, CA). Comparisons were considered significant if P < 0.05. Pharmacokinetic analyses were performed utilizing noncompartmental analysis with Phoenix WinNonlin® 6.4 for Windows (Pharsight, Mountain View, CA). Maximum concentration (Cmax), time to maximum concentration (Tmax), areas under the curve (AUC) from 0 to 21 h post-dose (AUC0-69), half-life (T1/2), time of last observed concentration (Tlast), and last observed concentration (Clast) were calculated. At least five data points on the descending linear limb of the time-concentration curve were employed to calculate T1/2. For statistical purposes, concentrations less than the LOQ were set to 0.
Results
Participants
Participant demographics and self-reported cocaine histories are detailed in Table 1. Ten subjects (9 men, 1 woman, 5 black, 4 white, 1 > one race) aged 35–50 participated in the study, with nine completing all dosing sessions. One participant was medically discharged prior to the third cocaine dose (Day 10) due to elevated heart rate (Participant I). Frequency of cocaine use (smoked or intravenous) among participants ranged from daily to once per week, with a median duration of cocaine use of 16.6 years (range: 4.2–31.5 years).
Table 1.
Demographics and self-reported cocaine histories for 10 cocaine users. Participant I was discharged prior to the third cocaine dose, however, all Oral-Eze and StatSure oral fluid specimens were collected prior to discharge.
| Participant | Race | Sex | Age (years) | Weight (lbs) | Days used in past 14 (SM or IV) | Mean use (SM or IV) | Age first use (SM or IV) (years) | Lifetime cocaine use (years) |
|---|---|---|---|---|---|---|---|---|
| B | AA | M | 38.1 | 202 | 11 SM | 5/week | 32 | 6.1 |
| C | 1+ | M | 42.4 | 162 | 5 IV | 3/week | 32 | 10.4 |
| 0 SM | 3–4/month | 21 | 21.4 | |||||
| D | W | M | 35.2 | 165 | 3 SM | 1/week | 25 | 10.2 |
| F | W | M | 44.0 | 197 | 5 SM | Daily | 18 | 26 |
| G | W | M | 42.5 | 180 | 6 IV | 3/week | 28 | 14.5 |
| 7 SM | 2/week | 31 | 11.5 | |||||
| H | AA | M | 43.7 | 150 | 14 SM | Daily | 21 | 22.7 |
| I | AA | M | 49.9 | 175 | 10 SM | 4–5/week | 25 | 24.9 |
| J | AA | M | 46.5 | 176 | 1 SM | 3/week | 15 | 31.5 |
| K | W | M | 39.2 | 175 | 0 IV | N/A | 35 | 4.2 |
| 0 SM | Daily | 11 | 28.2 | |||||
| M | AA | F | 36.6 | 140 | 0 SM | 2/week | 20 | 16.6 |
SM smoked, IV intravenous, AA African-American, 1+ more than one race, W white, M male
Oral Fluid Results
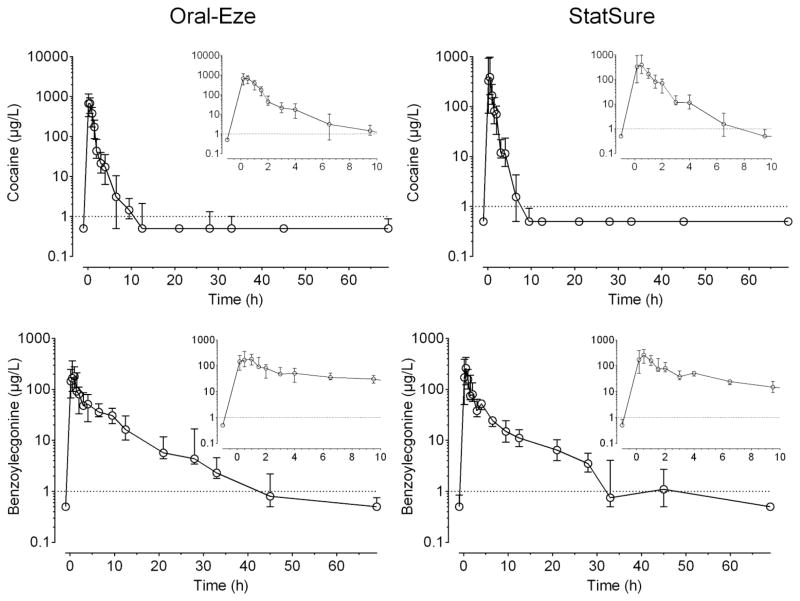
In total, 139 and 141 OF specimens were collected with the OE and SS collection devices from 1 h prior to 69 h post-administration and analyzed for cocaine and BE. Median (interquartile range) OF cocaine and BE concentration time courses are illustrated in Figure 1 for OE and SS collection devices. Cocaine exhibited higher concentrations than BE for approximately 2 h for both OE and SS. OE cocaine and BE median OF concentrations (range) immediately following administration (0.17 h) were 673 (76.5–1,574) μg/L and 145 (0–953) μg/L, respectively. Concentrations of cocaine and BE 2 h post-dose were 43.8 (19.3–194) μg/L and 80.6 (30–150) μg/L, respectively. Most participants’ cocaine concentrations were below the method LOQ at 9.5 h, while BE was detectable up to 33 h. Median (range) SS cocaine and BE concentrations immediately after dosing (329 (26.4–1,892) μg/L and 172 (10.9–836) μg/L) trended lower than OE OF specimens, and fell to 70.4 (15.7–115) μg/L and 79.9 (42–271) μg/L at 2 h. In contrast to OE OF specimens, most participants’ cocaine concentrations were below the LOQ at 6.5 h, whereas BE concentrations remained detectable for 28 h with the exception of a few specimens at 45 h (4/7 OF specimens). There was high inter-subject variability in cocaine and metabolite concentrations in OF from both collection devices.
Figure 1.
Median (interquartile range) cocaine and benzoylecgonine oral fluid concentration-time curves up to 69 h from 10 participants after 25 mg intravenous cocaine collected with Oral-Eze and StatSure oral fluid collection devices. Inserts represent time course from 0.17 to 10 h post-administration. Dotted lines represent the 1 μg/L analytical limit of quantification (LOQ). Concentrations <LOQ are represented as ½ LOQ.
Detection Rates of Oral Fluid Cocaine and Benzoylecgonine
Cocaine and BE detection rates 0.17–69 h after IV cocaine are in Table 2. Cocaine and BE initially appeared in OF as early as 0.17 h, the first specimen collected after administration, in both collection devices, with the exception of one participant where BE was not detected until 0.5 h. In total there were 2, 32 and 88 OE and 0, 41, and 81 SS OF cocaine only, BE only, and cocaine and BE positive specimens, respectively.
Table 2.
Detection rates for cocaine (COC), benzoylecgonine (BE), and COC and/or BE in oral fluid collected with Oral-Eze (OE) and StatSure (SS) collection devices up to 69 h from 10 participants after 25 mg intravenous cocaine. Detection rates (%) were calculated at the assay limit of quantification (1 μg/L). Note that not all oral fluid collection time points were n= 10 as different cocaine dosing days had different oral fluid collection times beyond 21 h.
| Time (h) | Oral-Eze Detection Rates (%) | StatSure Detection Rates (%) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | COC | BE | COC and/or BE | n | COC | BE | COC and/or BE | |
| −1 | 10 | 0 | 10 | 10 | 10 | 10 | 20 | 20 |
| 0.17 | 10 | 100 | 90 | 100 | 10 | 100 | 100 | 100 |
| 0.5 | 10 | 100 | 100 | 100 | 10 | 100 | 100 | 100 |
| 1 | 10 | 100 | 100 | 100 | 10 | 100 | 100 | 100 |
| 1.5 | 10 | 100 | 100 | 100 | 10 | 100 | 100 | 100 |
| 2 | 10 | 100 | 100 | 100 | 10 | 100 | 100 | 100 |
| 3 | 10 | 100 | 100 | 100 | 10 | 100 | 100 | 100 |
| 4 | 10 | 90 | 90 | 90 | 10 | 100 | 100 | 100 |
| 6.5 | 10 | 60 | 100 | 100 | 10 | 60 | 100 | 100 |
| 9.5 | 10 | 80 | 100 | 100 | 10 | 20 | 100 | 100 |
| 12.5 | 10 | 30 | 100 | 100 | 10 | 10 | 100 | 100 |
| 21 | 10 | 10 | 100 | 100 | 10 | 0 | 100 | 100 |
| 28 | 4 | 25 | 100 | 100 | 3 | 0 | 100 | 100 |
| 33 | 3 | 33.3 | 100 | 100 | 4 | 0 | 50 | 50.0 |
| 45 | 6 | 0.0 | 50 | 50 | 7 | 14.3 | 57.1 | 57.1 |
| 69 | 6 | 16.7 | 16.7 | 33.3 | 7 | 0 | 14.3 | 14.3 |
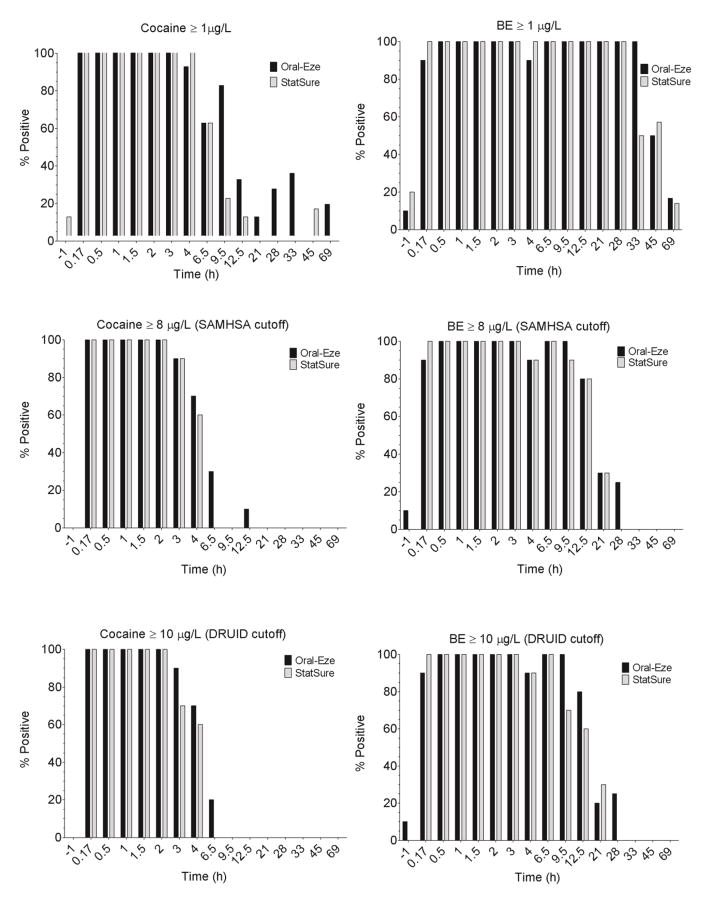
Figure 2 depicts detection rates for up to 69 h after IV cocaine based on the LOQ, SAMHSA (8 μg/L) and DRUID (10 μg/L) OF cutoffs. Typically, OE OF detection times for cocaine and BE were up to 6.5 and 28 h (SAMHSA and DRUID cutoffs). At the LOQ, increased detection rates for cocaine and BE occurred with detection times up to 21 and 69 h, respectively. SS last positive times were typically shorter than for OE with cocaine detected up to 4 h and BE 21 h (SAMHSA and DRUID cutoffs) and 12.5 and 69 h post-administration (LOQ cutoffs), respectively. Detection times were extended (21–69 h) when either cocaine and/or BE was tested as compared to cocaine testing only (4–21 h). Cocaine was always present in combination with BE, with the exception two OE specimens at 0.17 and 69 h where only cocaine was quantified at the LOQ, thus increasing % positives from 90% to 100% at 0.17 h and 16.7% to 33.3% at 69 h, and overall from 86.3% to 87.8% (Table 2). Although there was a small increase in OE % positives at 0.17 and 69 h compared to BE testing only, detection windows were unchanged. SS detection rates with either cocaine and/or BE were identical to BE only testing.
Figure 2.
Percentages of positive specimens in oral fluid (OF) at different confirmatory cutoffs for 10 participants after 25 mg controlled intravenous cocaine administration. Cutoffs evaluated were: cocaine ≥ 1 μg/L, benzoylecgonine (BE) ≥ 1 μg/L, cocaine ≥ 8 μg/L (SAMHSA), BE ≥ 8 μg/L (SAMHSA), cocaine ≥ 10 μg/L (DRUID), and BE ≥ 10 μg/L (DRUID). Note that not all oral fluid collection time points were n= 10 as different cocaine dosing days had different oral fluid collection times beyond 21 h: four Oral-Eze (OE) and three StatSure (SS) OF specimens were collected at 28 h; three OE and four SS OF specimens at 33 h; and six OE and seven SS OF specimens at 45 and 69 h.
Pharmacokinetics of Cocaine and Benzoylecgonine in Oral Fluid
Table 3 presents the median (range) cocaine and BE pharmacokinetic parameters in OF collected via OE and SS. After IV cocaine, OF cocaine concentrations increased rapidly for OE and SS devices with median Cmax concentrations of 932 (394–1,574) and 732 (83.3–1,892) μg/L with corresponding Tmax of 0.34 and 0.17 h, respectively. One participant had cocaine Tmax at 1 h corresponding to Cmax of 737 μg/L (OE). Median OE and SS BE Cmax were approximately 26.6% and 49.2% of median cocaine Cmax with Tmax observed at 0.5 h. Time to cocaine Cmax peaked significantly earlier than BE for OE collections only, with cocaine Tlast as late as 12.5 h post-dose, with BE up to 30.5 h. SS cocaine (6.5 h) and BE (28 h) tended to be shorter than OE Tlast. Although significant differences in Tlast were observed between the two analytes, Tlast were not significantly different between the two collection devices. No significant differences were observed in AUC between analytes and collection methods. Cocaine half-lives (1.3 and 0.89 h) were significantly shorter than BE (30.5 and 28 h) for both OE and SS, indicating faster elimination than its primary metabolite.
Table 3.
Median (range) pharmacokinetic parameters for cocaine and benzoylecgonine (BE) in oral fluid (N = 10 participants) following 25 mg intravenous (IV) cocaine administration collected with Oral-Eze (OE) and StatSure (SS) collection devices up to 69 h.
| Oral-Eze | StatSure | |||
|---|---|---|---|---|
|
| ||||
| Cocaine | BE | Cocaine | BE | |
| Cmax (μg/L) | 932 (394–1,574)* | 248 (96.9–953)* | 732 (83.3–1,892) | 360 (77.2–836) |
| Tmax (h) | 0.34 (0.17–1)* | 0.5 (0.17–1)* | 0.17 (0.17–0.5) | 0.5 (0.17–1) |
| T1/2 (h) | 1.3 (0.6–2.2)*† | 6.9 (4.4–12.1)* | 0.89 (0.57–1.4)§† | 6.6 (4.1–14.1)§ |
| AUC0-69h (h x μg/L) | 802 (314–1,447) | 814 (425–1,801) | 467 (174–1,378) | 670 (390–1,298) |
| Tlast (h) | 12.5 (4–69)* | 30.5 (21–69)* | 6.5 (4–45)§ | 28 (21–69)§ |
| Clast (μg/L) | 1.9 (1.0–35.1) | 2.8 (1.1–20.8) | 5.3 (1.0–26.6) | 3.5 (1–11.4) |
Cmax maximum concentration detected, Tmax time of maximum concentration, T1/2 terminal half-life, AUC area under the curve, Clast last detected concentration, Tlast time of last detected concentration
Significant difference P < 0.05:
OE cocaine versus BE;
SS cocaine versus BE;
OE versus SS cocaine;
OE versus SS BE
Comparison of Oral Fluid Collection Methods
No statistically significant differences were observed between OE and SS OF cocaine or BE pharmacokinetics, with the exception of cocaine T1/2 (Table 3). OF collected with OE exhibited significantly longer T1/2 (1.3 h) than SS (0.89 h) (Figure 1). Detection times were longer in OE than in SS-collected specimens (Table 2). This could be attributed to the OE elution buffer that may have stabilized cocaine and BE better or improved recovery from the collection device more efficiently than the SS buffer.
Discussion
OF drug testing for DUID, workplace, drug treatment and pain management monitoring is increasing due to its inherent advantages over other biological matrices. We present the first cocaine and BE OF data collected with commercially available devices after controlled IV cocaine administration. OE and SS collection devices contain stabilizing and elution buffers that preserve labile analytes like cocaine and improve drug recovery from the collection pad. Knowing detection rates and windows and pharmacokinetic parameters for cocaine markers in these OF collection devices is important for valid interpretation of results.
Similar cocaine and BE concentration-time courses were observed for OF collected with OE and SS collection devices (Figure 1). Peak cocaine concentrations occurred 0.17–1 h after dosing, with BE Cmax slightly later (0.17 and 1.5 h). There were significant differences in cocaine and BE Cmax and Tmax in OF specimens collected with the OE device, potentially due to buffer pH and composition differences. Although cocaine Tmax in this IV study was consistent with previous findings after SC administration [10], median BE Tmax occurred much earlier: 0.5 h versus 2–2.1 h, respectively. However, the previous SC cocaine study collected OF via citric-acid stimulated expectoration. This could contribute to the observed Tmax difference, as stimulation increases bicarbonate excretion, increasing OF pH and thus affecting cocaine and BE ion trapping into OF, thereby potentially increasing the time required to reach Cmax. Additionally, IV administration delivers cocaine more rapidly to the bloodstream than does SC administration, presumably contributing to an earlier Tmax.
Cocaine was quickly eliminated from OF, with median half-lives of 1.3 (0.6–2.2) and 0.89 (0.57–1.4) h in OE and SS OF specimens, respectively (Table 3). Our results are comparable to mean cocaine T1/2 after IV 0.58–2.45 [12, 17, 19, 20], SM (0.86–2.63 h) [19, 20], IN (1.61) [20], and oral (1.2 h) [22] dosing, but shorter than the median T1/2 after SC cocaine administration (2.6–3 h) [10]. This could be due to differences in administration routes, as IV cocaine should peak earlier and be eliminated more rapidly than SC administration. Additionally, differences in T1/2 are potentially due to the OF collection method used in previous studies, as citric-acid stimulated expectoration alters cocaine and BE ion trapping into OF (as explained above), and does not benefit from stabilization of analytes in the elution buffer. After self-reported cocaine use in the community, mean ± SEM cocaine T1/2 7.9 ±3.1 h, was much longer than observed in our study, potentially due to higher doses and bioaccumulation following repeated cocaine intake [30]. BE remained in the OF for a significantly longer time than cocaine with T1/2 of 6.9 (4.4–12.1) and 6.6 (4.1–14.1) h for OE and SS, respectively (Table 3). This is consistent with reports after SC administration (7.4–9.1 h) [10] and self-reported use (9.2 h) [30], and slightly longer than previous IV (2.3–6.5 h) [17, 20], SM 3.5 h, and IN 4.7 h T1/2 [20]. However, previous studies demonstrated large variability in half-lives based on a limited number of participants (n ≤ 7) that makes direct comparisons difficult between studies.
This is the first study of which we are aware that compared cocaine and BE pharmacokinetics in two OF collection devices containing different stabilizing and elution buffers. Although both devices were effective in monitoring cocaine and BE disposition in OF, SS OF concentrations trended lower and detection times were slightly shorter than those for OE. Significant differences in pharmacokinetics parameters were only observed for cocaine T1/2; SS exhibited significantly shorter cocaine T1/2 (Table 3), with cocaine eliminated more quickly (Figure 1) than in OE OF specimens. These observed differences could be due to differences in analyte recovery from the absorbent pad, and/or buffer pH and composition in the OF collection devices. There were no differences in time of analysis between the two collection devices for each participant; time of analysis should not be a contributing factor for the small differences observed between collection devices.
OE and SS devices performed comparably during method validation with acceptable extraction efficiencies and short term stability results, making these factors an unlikely source of variation. OE and SS extraction efficiencies ranged from 79.7–97.2% and 70.9–95.5% with short term stability results within ± 9.6% and ± 16.7% of target, respectively. In a prior study examining nine different OF collection devices, including SS, cocaine recoveries of fortified specimens were 85.4% and stability was within ± 17.4% of target up to 28 days stored at −18°C [31]. Although we did not observe issues in cocaine recovery and short term analyte stability in fortified OF samples during method development, it is possible that recovery from the absorbent pads and stability in authentic specimens might account for significant differences observed in cocaine T1/2 between the two devices and lower SS concentrations observed. OE devices may provide improved cocaine stabilization for specimens. Recovery and stability in fortified authentic or synthetic OF may not be the same as for authentic OF [32, 33]; however, this has not yet been evaluated for cocaine and its metabolites. Therefore, differences observed between OE and SS OF collection devices, although minor, could be attributed to variations in stabilizing buffers present in the devices or decreased recovery from the collection pad.
Cocaine OF detection times (4–6.5 h at SAMHSA and DRUID cutoffs) from two commercially available collection devices following controlled IV cocaine administration are presented for the first time. Detection windows were similar to those reported in OF following 25 mg IV, 32 mg IN, and 42 mg SM cocaine (4.1–6.3 h) [21] and after 75 mg/70 kg and 150 mg/70 kg SC (8–9.8 h) [10] when OF was collected up to 12 and 48 h after dosing. BE detection times reported in this study were consistent with those after SC administration (28–32 h) with up to 48 h collection [10], but longer than after IV, IN, and SM cocaine administration (5.0–8.7 h) [21] with up to 12 h collection. Longer detection windows were achieved in our study with a 1 μg/L LOQ (cocaine 12.5–21 h; BE 69 h) compared to SC administration at a higher assay LOQ (2.5 μg/L) [10], especially for BE (cocaine 11.5–17.7 h; BE 32–47 h), with longer OF collection up to 69 h. After SC cocaine administration detection rates and windows were increased with the application of cocaine and/or BE confirmation cutoffs compared to BE alone [10]; however, this was not observed in our IV cocaine administration study. Although detection windows were not widened in our study, small increases in OE % positives were observed at 0.17 and 69 h when evaluating cocaine and/or BE compared to BE testing alone. These findings coincide with the earlier observed BE Tmax reported in our study compared to SC cocaine administration. It also is important to note that these differences could be due to stabilization and elution buffers present in the OF collection devices utilized in our study that may have stabilized cocaine, compared to OF specimens obtained via citric-acid stimulated expectoration following SC administration that had no stabilizers or elution buffer.
Our results highlight that cocaine and BE confirmatory cutoffs can be selected to meet different drug testing needs. SAMHSA (8 μg/L) and DRUID (10 μg/L) OF cocaine cutoffs yield detection times similar to the time course of cocaine’s impairing effects, making them useful for roadside testing. In contrast, the application of a low cutoff (LOQ) extended OF detection times, which could be beneficial for pain management drug testing programs. Additionally, including cocaine in the confirmation did not widen detection windows compared to BE only testing, as reported previously, due to BE being present in the OF immediately after controlled IV cocaine administration and perhaps to the presence of stabilizing and elution buffers in the collection devices.
Highlights.
Oral fluid (OF) pharmacokinetic data after intravenous cocaine are presented
Oral-Eze and StatSure OF collection device performance was evaluated
Oral-Eze had significantly longer cocaine OF T1/2 (1.3 h) than StatSure (0.89 h)
Oral-Eze and StatSure devices were effective in monitoring cocaine and BE in OF
Cocaine and BE confirmatory cutoffs can be selected to meet drug testing needs
Acknowledgments
This work was supported by the Intramural Research Program (IRP), National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH). Oral-Eze and StatSure oral fluid collection devices were provided to NIH through a Materials Transfer Agreement. The authors acknowledge the contributions of the clinical staff of the NIDA-IRP and the Clinical Research Unit, Johns Hopkins Bayview Medical Center, as well as the University of Maryland, Baltimore, a member of the Graduate Partnership Program, NIH, Drs. Sebastien Anizan and Jose Luiz da Costa, and Allan Barnes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.European Monitoring Centre Drugs and Drug Addiction (EMCDDA) European Drug Report – Trends and Developments. Lisbon, Portugal: 2015. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration (SAMHSA) Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015. HHS Publication No. SMA 15–4927, NSDUH Series H-50. [PubMed] [Google Scholar]
- 3.US Drug Enforcement Administration. National Forensic Laboratory Information System (NFLIS) - 2014 Midyear Report. Springfield, VA: 2015. [Google Scholar]
- 4.Schulze HSM, Urmeew R, Auerbach K, Alvarez J, Bernhoft Im, De Gier H, Hagenzieker M, Houwing S, Knoche A, Pilgerstorfer M, Zlender B. Driving Under the Influence of Drugs, Alcohol and Medicines in Europe – finding from the DRUID project. Lisbon, Portugal: 2012. [Google Scholar]
- 5.Kelley-Baker T, Moore C, Lacey JH, Yao J. Comparing drug detection in oral fluid and blood: data from a national sample of nighttime drivers. Traffic Inj Prev. 2014;15(2):111–118. doi: 10.1080/15389588.2013.796042. [DOI] [PubMed] [Google Scholar]
- 6.Bosker WM, Huestis MA. Oral Fluid Testing for Drugs of Abuse. Clin Chem. 2009;55(11):1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anizan S, Huestis MA. The Potential Role of Oral Fluid in Antidoping Testing. Clin Chem. 2013;60(2):307–322. doi: 10.1373/clinchem.2013.209676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verstraete AG. Oral fluid testing for driving under the influence of drugs: history, recent progress and remaining challenges. Forensic Sci Inter. 2005;150(2–3):143–150. doi: 10.1016/j.forsciint.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Drummer OH. Introduction and review of collection techniques and applications of drug testing of oral fluid. Ther Drug Monit. 2008;30(2):203–206. doi: 10.1097/FTD.0b013e3181679015. [DOI] [PubMed] [Google Scholar]
- 10.Scheidweiler KB, Kolbrich Spargo EA, Kelly TL, Cone EJ, Barnes AJ, Huestis MA. Pharmacokinetics of Cocaine and Metabolites in Human Oral Fluid and Correlation With Plasma Concentrations After Controlled Administration. Ther Drug Monit. 2010;32(5):628–637. doi: 10.1097/FTD.0b013e3181f2b729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toennes SW, Kauert GF, Steinmeyer S, Moeller MR. Driving under the influence of drugs -- evaluation of analytical data of drugs in oral fluid, serum and urine, and correlation with impairment symptoms. Forensic Sci Int. 2005;152(2–3):149–155. doi: 10.1016/j.forsciint.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Cone EJ, Kumor K, Thompson LK, Sherer M. Correlation of saliva cocaine levels with plasma levels and with pharmacologic effects after intravenous cocaine administration in human subjects. J Anal Toxicol. 1988;12:200–206. doi: 10.1093/jat/12.4.200. [DOI] [PubMed] [Google Scholar]
- 13.Thompson LK, Yousefnejad D, Kumor K, Sherer M, Cone EJ. Confirmation of cocaine in human saliva after intravenous use. J Anal Toxicol. 1987;11:36–38. doi: 10.1093/jat/11.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Langel K, Gjerde H, Favretto D, et al. Comparison of drug concentrations between whole blood and oral fluid. Drug Test Anal. 2014;6(5):461–471. doi: 10.1002/dta.1532. [DOI] [PubMed] [Google Scholar]
- 15.Wille SMR, Raes E, Lillsunde P, et al. Relationship between oral fluid and blood concentrations of drugs of abuse in drivers suspected of driving under the influence of drugs. Ther Drug Monit. 2009;31(4):511–519. doi: 10.1097/FTD.0b013e3181ae46ea. [DOI] [PubMed] [Google Scholar]
- 16.Huestis MA, Verstraete A, Kwong TC, Morland J, Vincent MJ, De La Torre R. Oral-Fluid Testing: Promises and Pitfalls. Clin Chem. 2011;57(6):805–810. doi: 10.1373/clinchem.2010.152124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato K, Hillsgrove M, Weinhold L, Gorelick DA, Darwin WD, Cone EJ. Cocaine and metabolite excretion in saliva under stimulated and nonstimulated conditions. J Anal Toxicol. 1993;17:338–341. doi: 10.1093/jat/17.6.338. [DOI] [PubMed] [Google Scholar]
- 18.Cone EJ, Hillsgrove M, Darwin WD. Simultaneous measurement of cocaine, cocaethylene, their metabolites, and “Crack” pyrolysis products by gas chromatography-mass spectrometry. Clin Chem. 1994;40:1299–1305. [PubMed] [Google Scholar]
- 19.Jenkins AJ, Oyler JM, Cone EJ. Comparison of heroin and cocaine concentrations in saliva with concentrations in blood and plasma. J Anal Toxicol. 1995;19:359–374. doi: 10.1093/jat/19.6.359. [DOI] [PubMed] [Google Scholar]
- 20.Cone EJ, Oyler J, Darwin WD. Cocaine disposition in saliva following intravenous, intranasal, and smoked administration. J Anal Toxicol. 1997;21:465–475. doi: 10.1093/jat/21.6.465. [DOI] [PubMed] [Google Scholar]
- 21.Jufer R, Walsh SL, Cone EJ, Sampson-Cone A. Effect of repeated cocaine administration on detection times in oral fluid and urine. J Anal Toxicol. 2006;30(7):458–462. doi: 10.1093/jat/30.7.458. [DOI] [PubMed] [Google Scholar]
- 22.Jufer RA, Wstadik A, Walsh SL, Levine BS, Cone EJ. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24:467–477. doi: 10.1093/jat/24.7.467. [DOI] [PubMed] [Google Scholar]
- 23.SAMHSA. Mandatory Guidelines for Federal Workplace Drug Testing Programs. Federal Register. 2015;80(94):28054–28101. [Google Scholar]
- 24.Verstraete A, Knoche A, Jantos RR, Skopp G, Gjerde H, Vindenes V, et al. DRUID: Per se limits - Methods of defining cut-off values for zero tolerance. 2011 TREN-05-FP6TR-S07.61320-518404-DRUID. [Google Scholar]
- 25.Logan BK, Lowrie KJ, Turri JL, et al. Recommendations for toxicological investigation of drug-impaired driving and motor vehicle fatalities. J Anal Toxicol. 2013;37(8):552–558. doi: 10.1093/jat/bkt059. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JM, Verstraete AG, Huestis MA, Morland J. Guidelines for research on drugged driving. Addiction. 2008;103:1258–1268. doi: 10.1111/j.1360-0443.2008.02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babalonis S, Hampson AJ, Lofwall MR, Nuzzo PA, Walsh SL. Quinine as a potential tracer for medication adherence: A pharmacokinetic and pharmacodynamic assessment of quinine alone and in combination with oxycodone in humans. J Clin Pharmacol. 2015 doi: 10.1002/jcph.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellefsen KN, Da Costa JL, Concheiro M, et al. Cocaine and metabolite concentrations in DBS and venous blood after controlled intravenous cocaine administration. Bioanalysis. 2015;7(16):2041–2056. doi: 10.4155/bio.15.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J Anal Toxicol. 2013;37(7):452–474. doi: 10.1093/jat/bkt054. [DOI] [PubMed] [Google Scholar]
- 30.Moolchan ET, Cone EJ, Wstadik A, Huestis MA, Preston KL. Cocaine and metabolite elimination patterns in chronic cocaine users during cessation: plasma and saliva analysis. J Anal Toxicol. 2000;24:458–466. doi: 10.1093/jat/24.7.458. [DOI] [PubMed] [Google Scholar]
- 31.Langel K, Engblom C, Pehrsson A, Gunnar T, Ariniemi K, Lillsunde P. Drug testing in oral fluid-evaluation of sample collection devices. J Anal Toxicol. 2008;32(6):393–401. doi: 10.1093/jat/32.6.393. [DOI] [PubMed] [Google Scholar]
- 32.Anizan S, Bergamaschi MM, Barnes AJ, et al. Impact of oral fluid collection device on cannabinoid stability following smoked cannabis. Drug Test Anal. 2015;7(2):114–120. doi: 10.1002/dta.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D, Milman G, Schwope DM, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid Stability in Authentic Oral Fluid after Controlled Cannabis Smoking. Clin Chem. 2012;58(7):1101–1109. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]