Abstract
Cyclophosphamide administered on an intermittent metronomic schedule induces strong immune-dependent regression in several glioma models. Here we investigate whether this immunogenic chemotherapy can be potentiated by combination with the immune stimulatory TLR9 agonist CpG-1826. CpG-1826 treatment of GL261 gliomas implanted in immune competent mice induced tumor growth delay associated with increased tumor recruitment of macrophages and B cells. Anti-tumor responses varied between individuals, with CpG-1826 inducing robust tumor growth delay in ~50% of treated mice. Both high and low CpG-1826-responsive mice showed striking improvements when CpG-1826 was combined with cyclophosphamide treatment. Tumor-associated macrophages, B cells, dendritic cells, and cytotoxic T cells were increased, T regulatory cells were not induced, and long-term GL261 glioma regression with immune memory was achieved when CpG-1826 was combined with either single cyclophosphamide dosing (90 mg/kg) or metronomic cyclophosphamide treatment (two cycles at 45 mg/ kg, spaced 12-days apart). B16F10 melanoma, a low immunogenic tumor model, also showed enhanced immune and anti-tumor responses to cyclophosphamide/CpG-1826 chemoimmunotherapy, but unlike GL261 tumors, did not regress. TLR9-based immunotherapy can thus be effectively combined with immunogenic cyclophosphamide treatment to enhance immune-based anti-tumor responses, even in poorly immunogenic cancer models.
Keywords: Metronomic cyclophosphamide, Chemoimmunotherapy, CpG oligodeoxynucleotide, Glioma
Introduction
Toll-like receptors (TLRs) are cell surface and endosomal receptors prominently expressed by many immune cells. TLRs recognize pathogen-associated molecular pattern molecules, such as viral double-stranded RNA (TLR3 agonists), bacterial lipopolysaccharide (TLR4 agonist) and unmethylated CpG oligonucleotides (TLR9 agonists), which are characteristic of bacterial and viral DNA, to initiate immune responses to foreign pathogens [1]. Three classes of synthetic CpG oligodeoxynucleotides (CpG-ODN) differing in DNA sequence have been developed as TLR9 agonists [2]. Type A CpG-ODN activate natural killer (NK) cells and stimulate interferon-α production by plasmacytoid dendritic cells, type B CpG-ODN trigger the activation and maturation of dendritic cells, proliferation of B cells and production of Th1 cytokines and T cells, and type C CpG-ODN are intermediate in their actions. Type B CpG-ODN can induce tumor regression in various preclinical cancer models, with long-term cures and immune memory sometimes seen in a subset of animals [3–7]. Some studies report a T cell dependence of the anti-tumor actions of type B CpG-ODN [5–8], while others show a requirement for NK cells, or both NK and T cells [3,9]. These findings highlight differences in mechanism between cancer models and underscore the need for improved understanding of immune responses to CpG-ODN in different cancer types.
In clinical trials, CpG-ODN treatment has yielded mixed results. CpG-ODN alone can stimulate immune responses in cancer patients [10], and improve immune responses to cancer vaccines [11,12] and radiotherapy in Non-Hodgkin lymphoma [13]. However, the immune stimulating effects of CpG-ODN do not necessarily lead to improved patient outcomes. Thus, CpG-ODN treatment did not enhance the effects of dacarbazine in late stage melanoma patients [14], despite its demonstrated ability to stimulate tumor antigen-specific immune responses [15]. Further, initial studies of CpG-ODN in non-small cell lung cancer were encouraging [16], but later studies showed that CpG-ODN did not improve patient responses to paclitaxel/carboplatin combination chemotherapy [17] or targeted therapy with erlotinib [18]. However, a phase II study in recurrent glioblastoma showed that CpG-ODN treatment increased long term survival compared to historical data, suggesting that a subset of patients respond well to CpG-ODN [19,20]. Given these disparate findings, further investigation is needed to identify tumor types and chemotherapeutic drug regimens and schedules likely to benefit most from CpG-ODN-based combination therapies.
Conventional chemotherapy is widely known to cause lymphodepletion and immunosuppression, a factor that could undermine immunotherapy in combined treatment regimens [21]. However, chemotherapy can also activate multiple immunostimulatory pathways and anti-tumor immune mechanisms. Several chemotherapeutic drugs, including doxorubicin, oxaliplatin and cyclophosphamide (CPA), can kill cancer cells by an immunogenic cell death mechanism, which involves ATP release, exposure of calreticulin at the tumor cell surface, and release of the alarmin molecule HMGB1 into the extracellular matrix [22–25]. Cancer chemotherapeutic drugs can also directly modulate immune cell populations. CPA depletes immune suppressive myeloid-derived suppressor cells and T regulatory cells, restores NK cell and T cell proliferation and cytotoxic activity, and promotes Th1 cytokine production [26,27]. Further, chemotherapy administered on a metronomic schedule [28,29] is increasingly recognized for its potential to induce unique immune-stimulatory responses [30–34]. Non-toxic doses of chemotherapeutic drugs may render tumor cells more immunogenic [35], highlighting the potential advantages of combining low dose chemotherapy with immunotherapy.
Few studies have tested metronomic dosing strategies in combination with immunotherapies, despite the potential for synergism based on the immune stimulatory effects of metronomic drug scheduling. While metronomic CPA treatment benefits IL-12 gene therapy and adoptive transfer of dendritic cells in mouse models [36,37], CPA is typically administered as a single bolus dose when combined with immunotherapy [38–41]. In some cases CPA as a bolus dose is more effective at stimulating an immune response than when given on a metronomic schedule [42], yet in other cases, immune responses are strongly impaired by bolus dosing [43]. These findings suggest that anti-tumor responses can be improved by consideration of drug scheduling and by combinations with immunotherapy that optimize the balance between the immune suppressive and immune stimulatory actions of chemotherapy. Additional studies are needed to understand how metronomic chemotherapy and immunotherapy can best be combined to optimize immune stimulation while avoiding the immune suppression common to many cancer chemotherapeutic drugs [44,45].
We previously reported that an intermittent metronomic schedule of CPA elicits strong anti-tumor immune responses leading to regression of large established gliomas [31,46–48]. Tumor regression was associated with strong activation of interferon signaling pathways [49], tumor infiltration by macrophages, dendritic cells, NK cells [31,46,48] and cytotoxic T cells, and acquisition of long-term immune memory [32]. Here, we examine whether this immunogenic response to CPA can be potentiated by combination with CpG-ODN therapy in an immune-responsive GL261 glioma model, as well as in a low-immunogenicity B16F10 melanoma model. Our findings show how CpG-ODN immunotherapy can complement metronomic chemotherapy, and establish that this CPA–CpG-ODN combination chemoimmunotherapy can elicit lasting anti-tumor immune responses while reducing both the length and the dose of chemotherapeutic drug administration.
Materials and methods
Cell culture and tumor models
GL261 mouse glioma cells were authenticated by and obtained from the Developmental Therapeutics Program Tumor Repository (National Cancer Institute, Frederick, MD) and cultured in RPMI-1640 media. B16F10 mouse melanoma cells were authenticated by and obtained from ATCC (Manassas, VA) and cultured in DMEM media. Cells were grown at 37 °C in a humidified 5% CO2 environment in media supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Six-week old male C57BL/6NTac mice (Taconic, Germantown, NY) were housed and treated under approved protocols and federal guidelines. GL261 cells (4 × 106) or B16F10 cells (1 × 106) were suspended in 0.2 ml of serum-free media and injected into the mice subcutaneously at each posterior flank using a U-100 insulin syringe with a 28.5 gauge needle. Tumor dimensions were measured twice weekly by using a Vernier caliper and volumes calculated from the formula: Volume = (π/6)*(L*W)3/2. Treatment with CPA and/or CpG-1826 (detailed below) was initiated when tumor volumes reached an average of 500 mm3. Tumor volume data are normalized to the first day of drug treatment, based on n mice per group, as specified. Mice were considered cured when tumors regressed to < 200 mm3 without subsequent detectable regrowth for at least 4 weeks. The acquisition of persistent tumor immunity was assayed by injection of cured mice with 4 × 106 drug-naïve GL261 cells at a subcutaneous site in the posterior flank separate from the site of initial tumor implantation (“tumor rechallenge”).
Drug treatment
CPA was administered as a monohydrate (Sigma Aldrich, St. Louis, MO; Cat. # C0768), with doses reported here based on the non-hydrated molecular weight of 261. CPA was dissolved in 1X phosphate-buffered saline (PBS), filter sterilized and administered at a dose of 45, 90 or 140 mg/kg by intraperitoneal injection using a 1 ml syringe and 27.5 gauge needle. Fully phosphorothioated CpG-ODN were synthesized and purified by Eurofins MWG Operon (Huntsville, AL); the CpG-1826 sequence is 5′-tccatgaCGttcctgaCGtt-3′ (CpG bases shown in upper case) and control GpC-1826 sequence is 5′-tccatgaGCttcctgaGCtt-3′. CpG-ODNs were characterized by gel electrophoresis, mass spectrometry, and reversed phase HPLC analysis by Eurofins (purity up to 98% for material used in most of the studies reported here). CpG-ODN were dissolved in sterile 1X PBS at 2 mg/ml and stored at −20 °C in aliquots. CpG-ODN were administered intratumorally at 100 μg per tumor per treatment. For each treatment, CpG-ODN were injected in a total volume of 50 μl distributed between two separate injection sites per tumor and injected at a rate of 1 μl/second using a syringe pump (Cat # 702212, Harvard Apparatus, Holliston, MA) outfitted with a 1 ml syringe and a 30 gauge needle.
qPCR analysis of marker genes
Changes in tumor-infiltrating immune cells were monitored by changes in the expression of immune cell marker genes, as determined by qPCR analysis of total tumor RNA. Changes in the marker genes reported here are indicative of changes in the corresponding marker protein levels and immune cell numbers, as we established previously for metronomic CPA-treated GL261 and other gliomas by immunohistochemistry and/or flow cytometry [31,32,46,47]. RNA isolation, cDNA synthesis, and qPCR were performed as described [46]. Briefly, total RNA was isolated from each tumor using Trizol (Life Technologies, Grand Island, NY) followed by DNase I treatment (Promega, Madison, WI) and cDNA synthesis using the Applied Biosystems High-Capacity cDNA Reverse Transcription kit (Life Technologies). qPCR was performed using Power SYBR Green (Life Technologies) and primers previously described [46], and processed on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Grand Island, NY). Results were analyzed using the comparative Ct method normalized to the 18S RNA content of each RNA sample.
Flow cytometry
Tumor tissue was excised and single-cell suspensions were generated using a GentleMACS Tissue Dissociator (Miltenyi Biotec, San Diego, CA) using the manufacturer’s instructions for mouse implanted tumor tissue, on ice. Briefly, tumor tissue was dissected into 1 mm pieces and placed in a Miltentyi Biotec C tube with 5 ml of dissociation buffer (1X PBS containing 0.5% BSA and 2 mM EDTA). Tissue was mechanically dissociated by running the GentleMACS program m_implanted_tumor_1 program twice, and the solution was passed through a 70 μm filter and washed once in dissociation buffer. The cells were then incubated in red blood cell lysis buffer (eBioscience) according to the manufacturer’s instructions, resuspended in 100 μl buffer and incubated for 30 minutes on ice with fluorescent-conjugated antibodies to the following proteins: CD11b (2.5 μg/ml final staining concentration, clone M1/70), CD11c (1 μg/ml, clone N418), F4/80 (1 μg/ml, clone BM8), CD3e (1.5 μg/ml, clone 145-2C11) and CD8a (1 μg/ml, clone 53–6.7) (all from TONBO Biosciences); CD45 (2 μg/ml, clone 30-F11, BD Biosciences, Franklin Lakes, NJ), and NK1.1 (1 μg/ml, clone PK136, eBioscience). Cells were washed once in buffer and resuspended for analysis on a BD FACSCalibur instrument (BD Biosciences) and data analyzed using FlowJo software version 7.6.5. Cells were gated by selecting a main population on forward scatter versus side scatter to exclude cell debris, then positive events selected by comparison to unstained samples; representative gating plots are presented in Appendix S1.
Immunohistochemical staining
Tumor sections (6 μm) were prepared using a Leica CM1950 cryostat. Sections were fixed in 1% paraformaldehyde in 1X PBS for 30 min at room temperature, washed twice in 1X PBS, incubated with 1% Triton-X100 in 1% sodium citrate at 4°C for 5 min, washed twice and incubated in 3% hydrogen peroxide for 10 min at 4°C. After two washes, slides were incubated with 2% rabbit serum with avidin (Vector Laboratories, Burlingame, CA) in 1X PBS for 20 min at room temperature, followed by incubation with 4 μg/ml rat anti-mouse CD68 antibody (Cat. # MCA1957, AbD Serotec, Raleigh, NC) in 2% rabbit serum with biotin for 90 min at room temperature. Slides were then washed twice and incubated with 2.5 μg/ml biotinylated rabbit anti-rat secondary antibody (Cat. # BA-4001, Vector Laboratories) in 2% rabbit serum for 90 min at room temperature, washed twice, incubated in Vectastain Elite ABC reagent (Cat. # PK-6100, Vector Laboratories) for 30 min at room temperature, washed twice, stained with DAB (3, 3′-diaminobenzidine) solution (Vector Laboratories) for 0.5 min, and washed in tap water for 5 min. Slides were dehydrated by sequential immersion in 95% ethanol (2 min, twice), 100% ethanol (2 min, twice) and 100% xylene (3 min, twice) and sealed with permanent mounting solution. Images were captured at 10× magnification on an Olympus FSX100 instrument. Staining intensity was quantified using NIH ImageJ software and expressed as percent stained area, mean ± SE for each tumor, based on all images (typically 7–15 per tumor) for n = 3–4 tumors per treatment group.
Statistical analysis
Data were analyzed for statistical significance using student’s t-test for comparison of 2 groups or one-way analysis of variance (ANOVA) with Dunnett’s post-test for comparisons of multiple groups to controls, implemented using GraphPad Prism 4.1. Significance is indicated in each Figure by *p < 0.05, **p < 0.01, ***p < 0.001, or ****p < 0.0001, or as specified.
Results
GL261 gliomas are sensitive to CpG-1826 immunotherapy
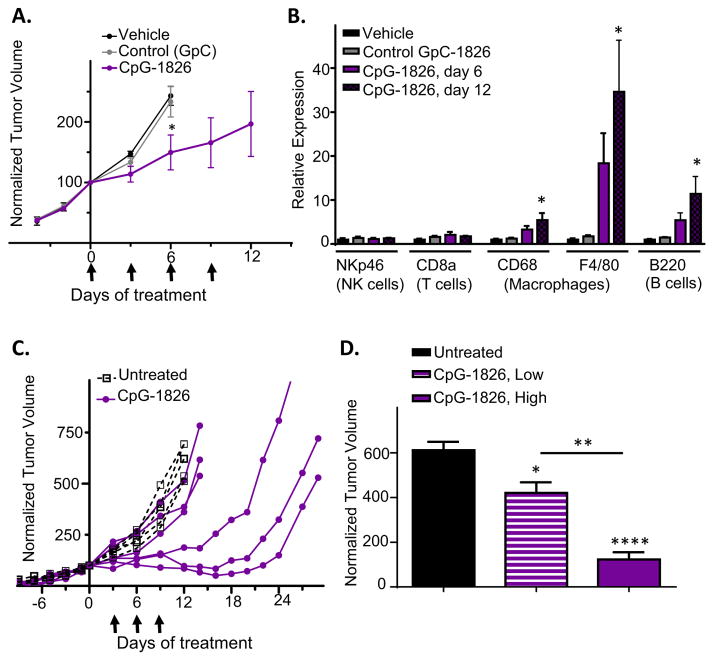
We first investigated the effects of CpG-1826 given as a monotherapy for large, established GL261 gliomas implanted in C57BL/6 mice. Mice were given up to 4 intratumoral injections of CpG-1826 every 3 days. Beginning with the first injection, CpG-1826 slowed tumor growth compared to tumors treated with an oligonucleotide containing GpC in place of CpG sequences, or vehicle controls (Fig. 1A). qPCR analysis revealed large and significant increases in tumor-infiltrating immune cell marker genes for macrophages (F4/80 and CD68) and B cells (B220, which is also expressed on some dendritic cell subsets) [50]. NK cell (NKp46) and T cell markers (CD8a) were not consistently increased (Fig. 1B).
Fig. 1.
CpG-1826 elicits tumor growth delay and immune activation in GL261 gliomas. (A) Shown are GL261 tumor volumes normalized to 100% on the first day of treatment with CpG-1826 or controls (t = 0 days), mean ± SE, for n mice per group: vehicle and GpC controls, n = 4; CpG-1826, n = 9 until day 6, n = 5 until day 9, n = 4 until day 12. *, p < 0.05 for CpG-1826 versus controls on day 6 (one-tailed t-test). Arrows: days of treatment with CpG-1826 or controls. (B) qPCR analysis of tumor RNA samples isolated from tumors in (A) and collected on treatment day 6 (vehicle, control GpC, and CpG-1826 treatment) or day 12 (CpG-1826 treatment). *p < 0.05, n = 4 mice per group. (C) Tumor growth curves in individual mice showing variable responses to CpG-1826 given on days 3, 6, and 9 (arrows). (D) Normalized tumor volumes on CpG-1826 treatment day 12 for CpG-1826 low-responsive mice (“CpG-1826, Low”) versus CpG-1826 high-responsive mice (“CpG-1826, High”), from panel C, with significance by one-way ANOVA with Tukey’s post-test comparing CpG-1826-treated to untreated mice, and low-responsive versus high-responsive mice, as indicated.
Examination of individual tumor growth curves revealed large differences in responses to CpG-1826 treatment, with half of the mice showing full tumor growth inhibition lasting up to 16–20 days, and half showing only minor growth delay following three CpG-1826 injections (Fig. 1C; also see Fig. 2B, below). The highly responsive mice had mean tumor volumes ~75% lower than the mice showing low responses three days after the third CpG-1826 injection (Fig. 1D). Tumor volumes were negatively correlated with levels of the macrophage marker F4/80 quantified by qPCR, or with the abundance of CD45 + tumor-infiltrating immune cells detected by flow cytometry (Appendix S1), suggesting that the degree of tumor regression is determined by the extent of immune cell infiltration.
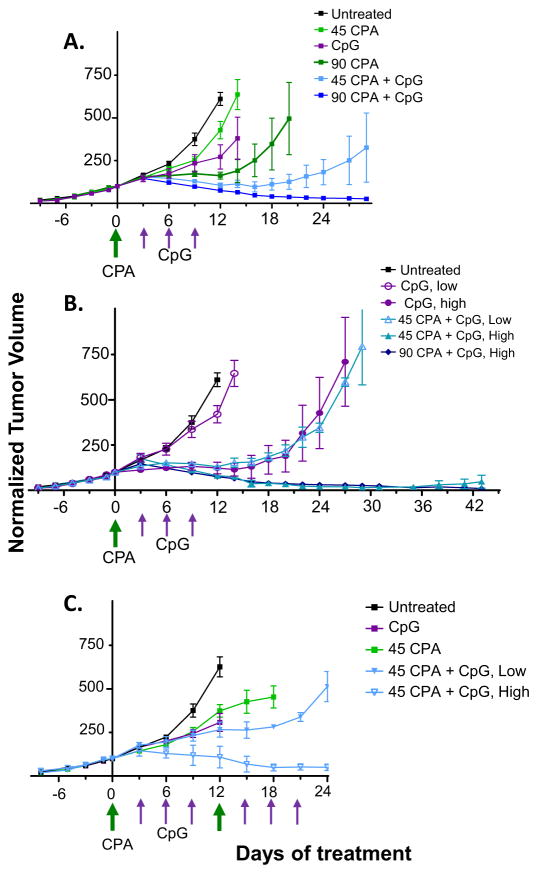
Fig. 2.
GL261 tumor regression induced by CPA/CpG-1826 chemoimmunotherapy. Shown are normalized tumor volumes for GL261 glioma-bearing mice treated with CpG-1826 and/or CPA (45 or 90 mg/kg/treatment), on days marked by arrows along the X-axis. Drug treatments were given for a single cycle (A, B) or two cycles (C). (A) Mean +/− SE tumor volumes for: untreated, n = 5 mice; 45 mg/kg CPA, n = 6; CpG-1826, n = 6; 90 mg/kg CPA, n = 14 until day 6, then n = 11 until day 14, then n = 4; 45 mg/kg CPA + CpG-1826, n = 6; 90 mg/kg CPA + CpG-1826, n = 12 until day 14, then n = 4. Tumor volume at day 12 was significantly lower in all treated groups compared to untreated control, p < 0.01 by two-tailed t-test. Tumor volume at day 12 was significantly lower in both combination treatment groups compared to their respective monotherapies, p < 0.05 by two-tailed t-test. (B) Data from panel A regraphed to show tumor volumes for high-responsive (“High”) and low-responsive (“Low”) mice in each treatment group. (C) Mean +/− SE tumor volumes for: untreated, n = 11 until day 6, then n = 8; CpG-1826, n = 8; CPA, n = 12 until day 6, then n = 10 until 12, then n = 4; CPA + CpG-1826 high-response group, n = 12 until day 12, then n = 5; and CPA + CpG-1826 low-response group, n = 3 until day 12, then n = 2. Some mice were sampled at days 6 and 12 to analyze tumor immune-infiltrates. Tumor volume at day 12 was significantly lower in the high-responsive combination group compared to CPA alone, p < 0.05.
Neoadjuvant CPA treatment sensitizes GL261 tumors to CpG-1826 immunotherapy
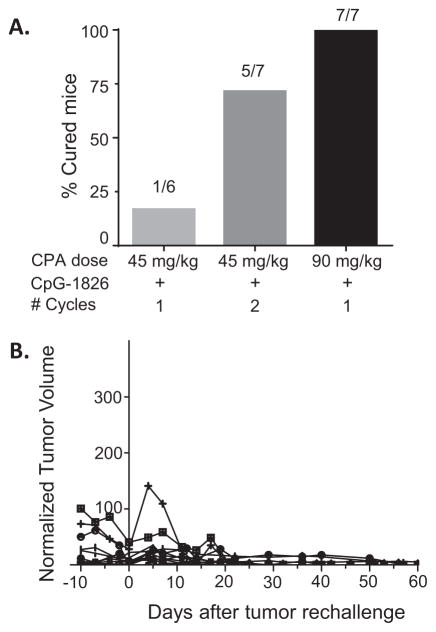
Next, we tested whether responses to CpG-1826 immunotherapy could be improved by neoadjuvant CPA treatment. GL261 tumor-bearing mice were given a single injection of CPA at 45 mg/kg (low dose) or 90 mg/kg (high dose), either alone or followed beginning 3 days later by three CpG-1826 injections spaced 3 days apart. Low dose CPA alone induced tumor growth delay for 2–3 days, while high dose CPA resulted in a 12 day growth delay followed by tumor regrowth (Fig. 2A). CPA at both doses significantly improved the anti-tumor response to CpG-1826 compared to either agent as a monotherapy. Notably, the individual heterogeneity of response seen with CpG-1826 alone (Fig. 1C) was also seen when low dose CPA was combined with CpG-1826. For CpG-1826 low-responsive tumors, growth stasis extended until day ~16, while for CpG-1826 high-responsive tumors, strong regression persisted long term (Fig. 2B). Tumors were completely eliminated in 1 of 6 mice treated with low dose CPA + CpG-1826 and in 7 of 7 mice treated with high dose CPA + CpG-1826 (Fig. 3A), with no regrowth seen as late as day 52. Thus, neoadjuvant CPA treatment sensitized GL261 gliomas to CpG-ODN immunotherapy.
Fig. 3.
CPA + CpG-1826 chemoimmunotherapy cures glioma-bearing mice and elicits long-term immunity. (A) Number of cured mice in each treatment group (see Methods), based on the individuals represented in Fig. 2. (B) Mice treated with 90 mg/ kg CPA + CpG-1826 (1 cycle, n = 7), 45 mg/kg CPA + CpG-1826 (1 cycle, n = 1), or 45 mg/ kg CPA + CpG-1826 (2 cycles, n = 5) that completely regressed (Fig. 3) were rechallenged (day 0) and then monitored for tumor regrowth up to day 60.
Therapeutic responses were improved when a second cycle of low dose CPA, given on a 12-day metronomic schedule, was combined with CpG-1826 treatment (Fig. 2C). The frequency of complete regression increased >4-fold compared to a single treatment cycle (Fig. 3A), with 5 out of 7 mice exhibiting strong and durable tumor regression and 2 out of 7 mice showing prolonged growth delay followed by tumor rebound (Fig. 2C). Mice cured by either high or low dose CPA + CpG-1826 were rechallenged with a second injection of GL261 cells. All mice rejected the GL261 rechallenge, with no tumor growth seen at least 60 days later (Fig. 3B), consistent with the induction of long-term anti-tumor immune memory.
Impact of combination chemoimmunotherapy on immune cell infiltration
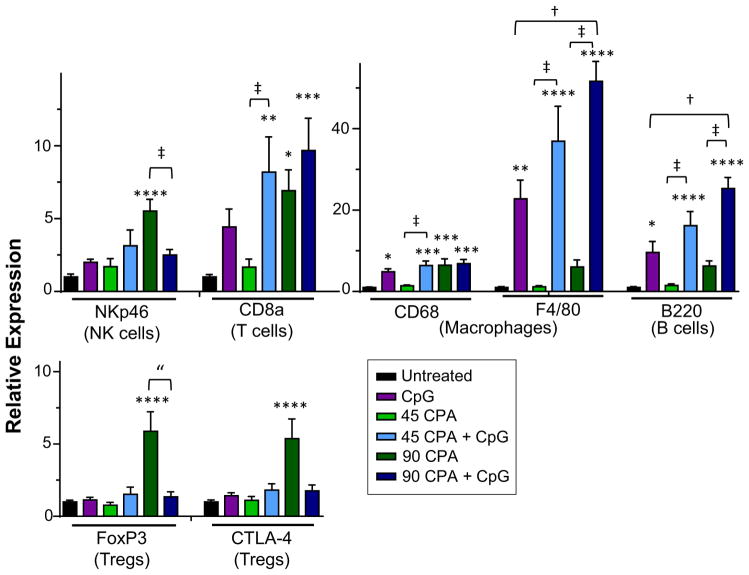
Markers for tumor-infiltrating NK cells, T cells, B cells and macrophages were significantly increased by 90 mg/kg CPA treatment, as shown by qPCR analysis of total tumor RNA (Fig. 4). Stronger responses were seen after 12 days compared to after 6 days CPA treatment (Appendix S1), consistent with the day 12 peak in immune response seen in our earlier study of CPA-treated GL261 tumors in SCID adaptive immune-deficient mice [48]. Immune responses to 45 mg/kg CPA were lower than the peak responses elicited by 90 mg/kg CPA. Moreover, at the 45 mg/kg CPA dose, several of the immune cell markers induced after 6 days declined back to baseline levels by day 12 (Appendix S1). Markers for T regulatory cells (FoxP3 and CTLA-4) were unchanged at day 6 and increased significantly at day 12 by 90 mg/kg CPA treatment (Appendix S1). Combination of CPA with CpG-1826 significantly increased T cell (CD8a), B cell (B220), and macrophage markers (F4/80, CD68), but not NK cell (NKp46) or T regulatory cell (FoxP3, CTLA-4) markers, at both CPA doses (Fig. 4). The combination treatments were generally more effective at eliciting immune infiltration compared to CPA treatment alone. CpG-1826 thus enhances the transient immune activation elicited by low dose CPA, resulting in increased tumor immune cell infiltration.
Fig. 4.
CPA + CpG-1826 treatment increases immune cell markers identified by qPCR. GL261 tumors from treated and untreated mice were excised on treatment day 12 (Fig. 2) and analyzed for the indicated tumor-infiltrating immune cell markers by qPCR. Data are shown for: untreated, n = 11; CpG-1826, n = 7; 45 mg/kg CPA, n = 7; 45 mg/kg CPA + CpG-1826, n = 8; 90 mg/kg CPA, n = 8; and 90 mg/kg CPA + CpG-1826, n = 5 mice per group. Significance is indicated by: *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001; combination treatment groups were compared to CpG-1826 alone (dagger symbol) or CPA at the appropriate dose (double-dagger) by two-tailed t-test, p < 0.05 or one-tailed t-test (double quotation marks), p < 0.05.
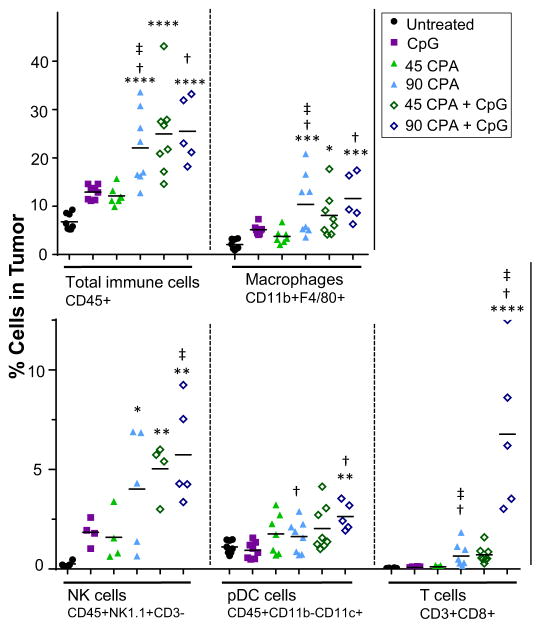
The increased immune cell response to the CPA + CpG-1826 combination therapy was verified by flow cytometry. We analyzed macrophages and dendritic cells, which both express TLR9 [51–53], and cytotoxic T cells, which may be critical for the observed long-term immune memory response. CPA increased tumor infiltration of total immune cells (CD45+), macrophages (CD11b + F4/80+) and cytotoxic T cells (CD3 + CD8+), with responses peaking at 6 days for low dose CPA but generally sustained through day 12 for high dose CPA (Appendix S1). On treatment day 12, neither low dose CPA nor CpG-1826 significantly increased total tumor-infiltrating immune cells or tumor-associated macrophages, although these immune cell levels were about 2-fold higher than in untreated tumors (Fig. 5, Appendix S1). Similarly, plasmacytoid dendritic cells (CD45 + CD11b-CD11c+) and cytotoxic T cells were not significantly increased by either monotherapy alone. In contrast, total tumor-infiltrating immune cells were increased 3–4-fold by high dose CPA, low dose CPA + CpG-1826, or high dose CPA + CpG-1826, reflecting significant increases in macrophages and NK cells (Fig. 5). The drug-induced increase in macrophage levels (CD68-marked) was also confirmed by immunohistochemistry (Appendix S1). Plasmacytoid dendritic cells and cytotoxic T cells were significantly increased by high dose CPA + CpG-1826. Further, a 20-fold increase in cytotoxic T cells that did not reach statistical significance was seen for the low dose CPA + CpG-1826 combination. Thus, combination of low dose CPA with CpG-1826 induces two antigen-presenting cell responses, macrophages and plasmacytoid dendritic cells, as well as a cytotoxic T cell response, although to a lesser extent than high dose CPA + CpG-1826 treatment.
Fig. 5.
Flow cytometry analysis of tumor-infiltrating immune cells. Flow cytometry of tumors excised on treatment day 12 (Fig. 2) were analyzed for increases in the indicated tumor-infiltrating immune cells by flow cytometry using the indicated immune cell markers. Data comparing untreated and CPA-treated tumors are shown in Appendix S1, and representative flow cytometry gating images are shown in Appendix S1. Significance for comparisons to untreated tumors is indicated by: *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. Combination treatment groups were compared to CpG-1826 alone (dagger symbol) or CPA (double dagger) by two-tailed t-test, p < 0.05.
Combination treatment in a low immunogenicity model
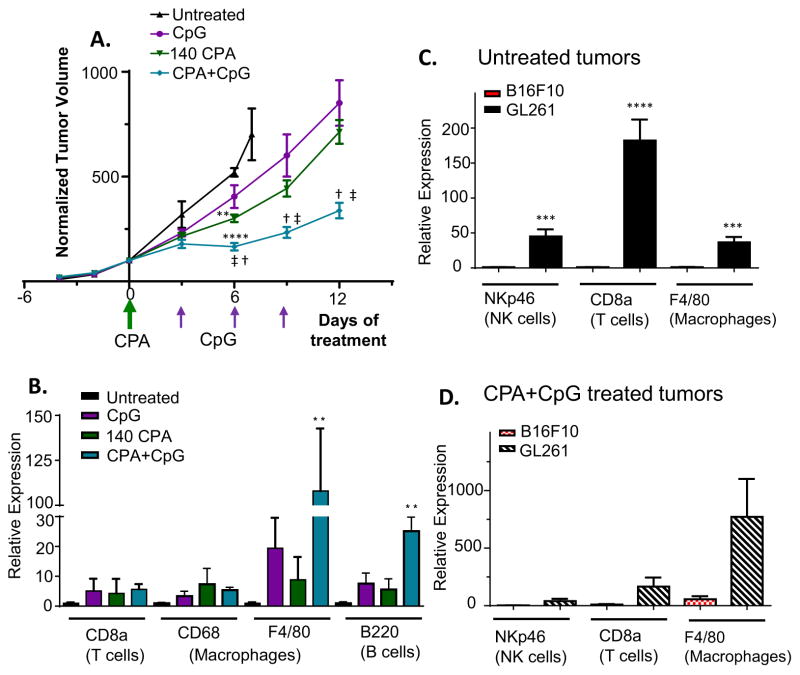
The studies above demonstrate that the combination of CPA with CpG-1826 can elicit strong anti-tumor and anti-tumor immune responses leading to complete regression and an apparent acquisition of anti-cancer immune memory in a metronomic CPA immune-responsive glioma model. We next tested whether this combination treatment is effective in B16F10 melanoma, which is widely studied as a low immunogenic tumor model [54,55]. We found that B16F10 tumor cells are intrinsically sensitive to the direct cytotoxic effects of CPA when assayed in cell culture but, in contrast to GL261 tumors, do not mount a strong immune response or undergo tumor regression when implanted in C57BL/6 mice and treated with CPA on a 6-day repeating metronomic schedule (unpublished experiments). B16F10 tumors respond to either CPA (140 mg/kg) or CpG-1826 monotherapy with modest growth delay. In contrast, the CPA + CpG-1826 combination induced tumor growth stasis for 6 days followed by growth at a significantly lower rate compared to either monotherapy (Fig. 6A). Further, the combination treatment, but neither single agent, significantly increased tumor levels of macrophage (F4/80) and B cell (B220) markers (Fig. 6B). An increase in CD8 T cells was seen, but did not reach statistical significance. However, in contrast to GL261 tumors, B16F10 tumors did not regress in response to the CPA + CpG-1826 combination. This lower responsiveness of B16F10 tumors may reflect the substantially lower level of infiltrating immune cells compared to GL261 tumors, which is indicated by comparing marker gene levels in each model, for both untreated tumors and for CPA + CpG-1826-treated tumors (Fig. 6C, Fig. 6D).
Fig. 6.
CPA + CpG treatment delays tumor growth in the non-immunogenic B16F10 melanoma model. (A) Normalized B16F10 tumor volumes, mean ± SE, for: untreated n = 3, CpG-1826, n = 5 until day 6, then n = 4; CPA n = 5, CPA + CpG-1826 n = 5 until day 6 then n = 4. Tumor growth was significantly slowed by CPA (**p < 0.01) and by CPA + CpG-1826 treatment (****p < 0.0001) compared to untreated controls at day 6, as indicated. Tumor volume on days 6, 9 and 12 was significantly lower at p < 0.05 in the CPA + CpG-1826 combination group compared to treatment with CpG alone (dagger symbol) or CPA alone (double-dagger), as indicated. CPA treatment was at 140 mg/kg/injection. (B) qPCR analysis of immune cell marker genes in untreated B16F10 tumors and in treated tumors excised on day 12 (mean ± SE, n = 4–5 mice per group). (C) Basal levels of immune cell marker gene expression in untreated B16F10 tumors compared to untreated GL261 tumors, determined by qPCR, mean ± SE, n = 9–10 mice per group. (D) Comparison of levels of immune cell marker genes induced by CPA + CpG-1826 treatment in B16F10 tumors (day 12, as in panel A), versus GL261 (day 12, as in Fig. 2C), determined by qPCR, mean ± SE, n = 4–5 mice per group.
Discussion
We investigated the combination of immunotherapy using the TLR9 agonist CpG-1826, a class B CpG-ODN, with an inherently immune-stimulatory chemotherapeutic regimen based on CPA treatment of syngeneic mouse tumors. In GL261 gliomas, CpG-1826 induced strong anti-tumor immune responses, complementing and enhancing the immune stimulatory actions of low dose, metronomic CPA treatment. Individual GL261 glioma-bearing mice responded variably to CpG-1826 given as a monotherapy, with CpG-1826 high-responsive mice showing extended tumor stasis as compared to only a short growth delay for CpG-1826 low-responsive mice. However, even CpG-1826 low-responsive GL261-bearing mice benefited significantly from CPA + CpG-1826 combination chemoimmunotherapy. Improved anti-tumor responses were also seen with the CPA + CpG-1826 combination in mice bearing B16F10 tumors, which have low intrinsic immunogenicity [54,55] and do not exhibit the strong immune response to intermittent metronomic CPA treatment that characterizes GL261 and several other tumor models [32,46]. While CpG-1826 treatment alone increased tumor-infiltrating macrophages markedly, an even more important response may be the apparently synergistic increase in CD8 + cytotoxic T cells, which was achieved using CPA + CpG-1826 combinations based on either high dose CPA or low dose metronomic CPA treatment, and led to long-term tumor ablation with resistance to tumor rechallenge, indicating acquisition of anti-tumor immunity. These findings demonstrate the strong potential of chemoimmunotherapy, in particular, using low dose, metronomic CPA scheduling, and identify GL261 as a tumor model that may be useful for studying predictors and biomarkers of individual responsiveness in vivo.
Earlier studies found that single dose chemotherapy can enhance the anti-tumor activity of CpG-based immunotherapy [38,56,57], but with some significant limitations. In a rhabdomyosarcoma model, a single, very high dose of CPA (200 mg/kg) followed by multiple systemic CpG-ODN injections over several weeks induced tumor remission and long-term survival. However, while 70% of mice with minimal disease burden at the time of initial drug treatment were apparently cured, only 15% of mice with large disease burden showed this response [38]. The CPA treatment employed did not induce anti-tumor immunity, and while the CpG-ODN responses appeared to be dependent on cytotoxic T cells, the cured mice were not significantly resistant to tumor rechallenge [38]. Similarly, while other studies reported benefits of CpG-ODN when combined with chemotherapy, long-term cures were generally achieved in fewer than ~50% of mice, even in immunogenic tumor models [57] or with continued drug treatment [56]. In contrast, our study describes a uniquely efficacious combination chemoimmunotherapy that requires only limited exposure to chemotherapy.
In earlier studies from this laboratory, CPA administered to GL261-bearing C57BL/6 mice at 140 mg/kg on a 6 day repeating metronomic schedule induced extensive immune-based GL261 glioma ablation in 100% of the mice, however, when the CPA dose was reduced to 90 mg/kg, the initial anti-tumor response was not sustained in a subset of mice (36%), and tumors regrew [32]. Here, we show that CpG-1826 immunotherapy is highly effective against GL261 gliomas when combined with either one cycle (90 mg/kg) or two cycles of fractionated (45 mg/kg × 2) CPA treatment spaced 12 days apart, under conditions that augment immune-based anti-tumor responses. These treatments activated long-term immune stimulation and achieved sustained remission with a substantial decrease in overall exposure to cytotoxic chemotherapy when compared to metronomic CPA given repeatedly on an every 6-day schedule [32]. Importantly, the second treatment cycle did not compromise the anti-tumor response, for example, by ablating the immune cell response [44,45]. The 12 day cycle of CPA + CpG-1826 treatment employed here is based on our earlier finding in GL261 tumors implanted in SCID mice that immune responses to CPA are initially repressed, then increase and reach maximal levels after 12 days [48]. However, in those studies, the 12-day schedule, while initially effective, ultimately led to decline of the initial immune response and tumor escape in almost all of the treated mice [48]. The present findings suggest that introduction of CpG-1826 immunotherapy during the 12-day gap between CPA treatment cycles may yield an optimal anti-tumor response by enhancing and sustaining immune responses induced by CPA treatment. Decreasing the interval between metronomic CPA treatments to every 6 days, while highly effective when CPA is given as a single agent over many treatment cycles [32], may be suboptimal in the context of the present combination chemoimmunotherapy, where we administered CpG-1826 after CPA treatment to minimize ablation by CPA of the responding immune cell populations [32,48].
Given the strong immune modulatory capacity of CPA, the schedule of CPA administration may be a critical determinant of whether, and to what extent, combination with CpG-1826 is beneficial. Optimization of chemoimmunotherapy schedules [25] needs to take into account the finding that CPA-induced lymphodepletion is transient, and is followed by a recovery period during which dendritic cells and CD4 + and CD8 + T cells return to normal levels or even expand further [58–62]. CPA-mediated T regulatory cell depletion [63] is also reversible, and effective combination treatments may require that immunotherapy be administered before the decline in chemotherapy-induced immune stimulatory responses. Further, while CpG-1826 administration prior to CPA, or concurrently with low dose daily metronomic CPA dosing, could boost pre-chemotherapy levels of tumor-infiltrating immune cells, it might lead to a reduction in overall efficacy by way of ablation of the CpG-induced immune responses upon CPA treatment.
We previously found in the same syngeneic mouse GL261 model that intratumoral T regulatory cells are depleted 1–3 days after CPA treatment, return to basal levels at day 6, and then strongly increase by days 9–12 in the absence of a second CPA treatment [32]. Consistent with those findings, here we observed a strong increase in T regulatory cells (Foxp3 marker) 12 days after CPA treatment at the 90 mg/kg dose, and further, we found that CpG, when combined with CPA, blocks that increase in T regulatory cells. The same pattern of response was seen for a second immunosuppressive marker associated with T regulatory cells, CTLA-4: strong increase on day 12 following CPA treatment at 90 mg/kg, but no increase when CPA is combined with CpG treatment (Fig. 4). Together, these findings suggest that the increased anti-tumor immunity of the CPA + CpG-1826 combination is at least in part due to the blocking of T regulatory cells-dependent immunosuppression.
Metronomic chemotherapy schedules investigated in preclinical studies range from daily administration through the drinking water [31,64] to injections given every 3–12 days [32,47,48,65,66]. Clinical metronomic regimens are also diverse, with common schedules of CPA treatment ranging from twice daily to once every 2 weeks, with low dose daily regimens most commonly used [33,67]. Further preclinical studies are needed to determine whether other metronomic chemotherapy schedules, including daily low dose schedules most commonly used in the clinic, are amenable for combination chemoimmunotherapy using CpG-ODN.
The CPA + CpG-1826 combination stimulated large increases in tumor-infiltrating immune cells in B16F10 melanomas, where a significant increase in anti-tumor activity was achieved as compared to either CPA or CpG-1826 treatment alone. However, in contrast to GL261 tumors, B16F10 tumors did not show strong regression, and were not cured by CPA + CpG-1826 treatment. B16F10 tumors are less sensitive to intermittent metronomic CPA treatment than GL261 tumors, showing only modest growth delay and little immune response to CPA treatment alone, despite the intrinsic chemosensitivity of B16F10 tumor cells to activated CPA. Further, we found that basal and also CPA + CpG-1826-induced levels of in-filtrating immune cells were substantially lower in B16F10 tumors than in GL261 tumors (Fig. 6), which may explain the much weaker anti-tumor responses achieved in B16F10 tumors. Given these findings, an important question relevant to the evaluation of CpG-ODN and other TLR agonists under clinical development [68] is how to identify individual patients most likely to respond well to these immunotherapies. One possibility, that pre-treatment levels of immune infiltration are predictive of tumor responsiveness [69], is supported by our findings in GL261 compared to B16F10 tumors. Supporting this, tumor cell immunogenicity may be correlated with sensitivity to immunotherapies, in particular therapies involving T cell modulation [70,71]. Further, an “immunoscore” quantifying cytotoxic T cell infiltration into tumors may have utility in predicting patient responses to certain chemotherapy and immunotherapy regimens [72–74]. Immune cell profiles in lymph nodes and tumor cell immunogenicity, based on MHC class I expression, may also help predict responsiveness to therapy [75]. Future studies comparing high-responsive and low-responsive tumors may extend these findings and identify useful markers that predict responsiveness to therapy. Such markers may include immunosuppressive factors, several of which show significant increases following metronomic chemotherapy, as seen in highly immune responsive glioma models [49].
Supplementary Material
Acknowledgments
Supported in part by NIH grant CA049248 (to DJW).
Abbreviations
- CPA
cyclophosphamide
- CpG-ODN
CpG oligodeoxynucleotide
- NK
natural killer
- TLR
toll-like receptor
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2015.11.029.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic Acid Ther. 2012;22:77–89. doi: 10.1089/nat.2012.0340. [DOI] [PubMed] [Google Scholar]
- 3.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- 5.Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdorfer B, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 6.Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res. 2003;9:2693–2700. [PubMed] [Google Scholar]
- 7.Lonsdorf AS, Kuekrek H, Stern BV, Boehm BO, Lehmann PV, Tary-Lehmann M. Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J Immunol. 2003;171:3941–3946. doi: 10.4049/jimmunol.171.8.3941. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179:2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 9.Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, et al. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167:4878–4886. doi: 10.4049/jimmunol.167.9.4878. [DOI] [PubMed] [Google Scholar]
- 10.Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 11.Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32:6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karbach J, Neumann A, Atmaca A, Wahle C, Brand K, von Boehmer L, et al. Efficient in vivo priming by vaccination with recombinant NY-ESO-1 protein and CpG in antigen naive prostate cancer patients. Clin Cancer Res. 2010;17:861–870. doi: 10.1158/1078-0432.CCR-10-1811. [DOI] [PubMed] [Google Scholar]
- 13.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber JS, Zarour H, Redman B, Trefzer U, O’Day S, van den Eertwegh AJ, et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer. 2009;115:3944–3954. doi: 10.1002/cncr.24473. [DOI] [PubMed] [Google Scholar]
- 15.Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 16.Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G, et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3979–3986. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 17.Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2667–2674. doi: 10.1200/JCO.2010.32.8971. [DOI] [PubMed] [Google Scholar]
- 18.Belani CP, Nemunaitis JJ, Chachoua A, Eisenberg PD, Raez LE, Cuevas JD, et al. Phase 2 trial of erlotinib with or without PF-3512676 (CPG 7909, a Toll-like receptor 9 agonist) in patients with advanced recurrent EGFR-positive non-small cell lung cancer. Cancer Biol Ther. 2013;14:557–563. doi: 10.4161/cbt.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrie M, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 2010;12:401–408. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ursu R, Carpentier AF. Immunotherapeutic approach with oligodeoxynucleotides containing CpG motifs (CpG-ODN) in malignant glioma. Adv Exp Med Biol. 2012;746:95–108. doi: 10.1007/978-1-4614-3146-6_8. [DOI] [PubMed] [Google Scholar]
- 21.Litterman AJ, Dudek AZ, Largaespada DA. Alkylating chemotherapy may exert a uniquely deleterious effect upon neo-antigen-targeting anticancer vaccination. Oncoimmunology. 2013;2:e26294. doi: 10.4161/onci.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol. 2015;6:187. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2012;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 24.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 25.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matar P, Rozados VR, Gervasoni SI, Scharovsky GO. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50:588–596. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358:100–106. doi: 10.1016/j.canlet.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andre N, Carre M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11:413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 30.Hao YB, Yi SY, Ruan J, Zhao L, Nan KJ. New insights into metronomic chemotherapy-induced immunoregulation. Cancer Lett. 2014;354:220–226. doi: 10.1016/j.canlet.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Chen CS, Doloff JC, Waxman DJ. Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia. 2014;16:84–96. doi: 10.1593/neo.131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Waxman DJ. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8 T-cell responses and immune memory. Oncoimmunology. 2015;4:e1005521. doi: 10.1080/2162402X.2015.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer. 2013;49:3387–3395. doi: 10.1016/j.ejca.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Loven D, Hasnis E, Bertolini F, Shaked Y. Low-dose metronomic chemotherapy: from past experience to new paradigms in the treatment of cancer. Drug Discov Today. 2012;18:193–201. doi: 10.1016/j.drudis.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Luo J, Shurin MR. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr) 2011;34:97–106. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 36.Son CH, Shin DY, Kim SD, Park HS, Jung MH, Bae JH, et al. Improvement of antitumor effect of intratumoral injection of immature dendritic cells into irradiated tumor by cyclophosphamide in mouse colon cancer model. J Immunother. 2012;35:607–614. doi: 10.1097/CJI.0b013e31826f79a6. [DOI] [PubMed] [Google Scholar]
- 37.Denies S, Cicchelero L, Van Audenhove I, Sanders NN. Combination of interleukin-12 gene therapy, metronomic cyclophosphamide and DNA cancer vaccination directs all arms of the immune system towards tumor eradication. J Control Release. 2014;187:175–182. doi: 10.1016/j.jconrel.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 38.Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003;9:3105–3114. [PubMed] [Google Scholar]
- 39.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 40.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewan MZ, Vanpouille-Box C, Kawashima N, DiNapoli S, Babb JS, Formenti SC, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. 2012;18:6668–6678. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malvicini M, Alaniz L, Bayo J, Garcia M, Piccioni F, Fiore E, et al. Single low-dose cyclophosphamide combined with interleukin-12 gene therapy is superior to a metronomic schedule in inducing immunity against colorectal carcinoma in mice. Oncoimmunology. 2012;1:1038–1047. doi: 10.4161/onci.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litterman AJ, Zellmer DM, Grinnen KL, Hunt MA, Dudek AZ, Salazar AM, et al. Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol. 2013;190:6259–6268. doi: 10.4049/jimmunol.1203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20:648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Romiti A, Cox MC, Sarcina I, Di Rocco R, D’Antonio C, Barucca V, et al. Metronomic chemotherapy for cancer treatment: a decade of clinical studies. Cancer Chemother Pharmacol. 2013;72:13–33. doi: 10.1007/s00280-013-2125-x. [DOI] [PubMed] [Google Scholar]
- 46.Doloff JC, Waxman DJ. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012;72:1103–1115. doi: 10.1158/0008-5472.CAN-11-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doloff JC, Chen CS, Waxman DJ. Anti-tumor innate immunity activated by intermittent metronomic cyclophosphamide treatment of 9L brain tumor xenografts is preserved by anti-angiogenic drugs that spare VEGF receptor 2. Mol Cancer. 2014;13:158. doi: 10.1186/1476-4598-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J, Waxman DJ. Metronomic cyclophosphamide schedule-dependence of innate immune cell recruitment and tumor regression in an implanted glioma model. Cancer Lett. 2014;353:272–280. doi: 10.1016/j.canlet.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doloff JC, Waxman DJ. Transcriptional profiling provides insights into metronomic cyclophosphamide-activated, innate immune-dependent regression of brain tumor xenografts. BMC Cancer. 2015;15:375. doi: 10.1186/s12885-015-1358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 51.Movita D, Kreefft K, Biesta P, van Oudenaren A, Leenen PJ, Janssen HL, et al. Kupffer cells express a unique combination of phenotypic and functional characteristics compared with splenic and peritoneal macrophages. J Leukoc Biol. 2012;92:723–733. doi: 10.1189/jlb.1111566. [DOI] [PubMed] [Google Scholar]
- 52.Palma G, De Laurenzi V, De Marco M, Barbieri A, Petrillo A, Turco MC, et al. Plasmacytoids dendritic cells are a therapeutic target in anticancer immunity. Biochim Biophys Acta. 2012;1826:407–414. doi: 10.1016/j.bbcan.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 54.Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):21. doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Murphy KA, Church CH, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013;36:477–489. doi: 10.1097/01.cji.0000436722.46675.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sommariva M, De Cecco L, De Cesare M, Sfondrini L, Menard S, Melani C, et al. TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. 2011;71:6382–6390. doi: 10.1158/0008-5472.CAN-11-1285. [DOI] [PubMed] [Google Scholar]
- 57.Mason KA, Neal R, Hunter N, Ariga H, Ang K, Milas L. CpG oligodeoxynucleotides are potent enhancers of radio- and chemoresponses of murine tumors. Radiother Oncol. 2006;80:192–198. doi: 10.1016/j.radonc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, et al. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2009;59:137–148. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Proietti E, Greco G, Garrone B, Baccarini S, Mauri C, Venditti M, et al. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moschella F, Proietti E, Capone I, Belardelli F. Combination strategies for enhancing the efficacy of immunotherapy in cancer patients. Ann N Y Acad Sci. 2010;1194:169–178. doi: 10.1111/j.1749-6632.2010.05464.x. [DOI] [PubMed] [Google Scholar]
- 61.Hong SH, Yoon IH, Kim YH, Yang SH, Park MJ, Nam HY, et al. High-dose cyclophosphamide-mediated anti-tumor effects by the superior expansion of CD44(high) cells after their selective depletion. Immunobiology. 2009;215:182–193. doi: 10.1016/j.imbio.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Salem ML, Diaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, et al. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2011;61:353–362. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, et al. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 65.Tongu M, Harashima N, Monma H, Inao T, Yamada T, Kawauchi H, et al. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother. 2012;62:383–391. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 67.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 68.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, et al. Trial watch: toll-like receptor agonists for cancer therapy. Oncoimmunology. 2013;2:e25238. doi: 10.4161/onci.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 70.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 71.Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, et al. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523–532. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 75.Mohos A, Sebestyen T, Liszkay G, Plotar V, Horvath S, Gaudi I, et al. Immune cell profile of sentinel lymph nodes in patients with malignant melanoma –FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome. J Transl Med. 2013;11:43. doi: 10.1186/1479-5876-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.