Abstract
Background & Aims
Although chronic cough is a common, its etiology is often elusive, making patient management a challenge. Gastroesophageal reflux and airway hypersensitivity can cause chronic cough. We explored the relationship between reflux, phonation, and cough in patients with idiopathic chronic cough.
Methods
We performed a blinded, cross-sectional study of non-smoking patients with chronic cough (duration > 8 weeks) refractory to reflux treatment referred to the Digestive Disease Center at Vanderbilt University. All underwent 24-hour acoustic recording concurrently and temporally synchronized with ambulatory pH-impedance monitoring. Cough, phonation, and pH-impedance events were recorded. We evaluated the temporal relationship between cough and phonation or reflux events using Poisson and logistic regression.
Results
Seventeen patients met the inclusion criteria (88% female; 100% Caucasian; median age, 63 years and interquartile age range, 52–66 years; mean body mass index, 30.6 and interquartile range 27.9–34.0); there were 2048 analyzable coughing events. The probability of subsequent coughing increased with higher burdens of preceding cough, reflux, or phonation. Within the first 15 min after a cough event, the cough event itself was the main trigger of subsequent cough events. After this period, de novo coughing occurred with increases of 1.46-fold in association with reflux alone (95% confidence interval, 1.17–1.82; P<.001) and 1.71-fold in association with the combination of phonation and reflux events.
Conclusion
Antecedent phonation and reflux increased the rate of cough events in patients with idiopathic chronic cough. Reflux events were more strongly associated with increased rate of coughing. Our findings support the concept that airway hypersensitivity is a cause of chronic cough, and that the vocal folds may be an effector in chronic cough. ClinicalTrials.gov number, NCT01263626.
Keywords: Cough, GERD, Reflux, Hypersensitivity, Phonation
INTRODUCTION
Cough is among the most common symptoms for which medical attention is sought.1,2 It represents 10 – 38% of outpatient practice visits3,4; totaling over 26 million medical visits in the U.S. annually,5 and has a point prevalence of 9 – 33% in U.S. and European populations.6 Most cough results from viral upper respiratory infection and is self-limited. Chronic cough, however, is a refractory condition attributable to a variety of pulmonary and non-pulmonary etiologies.
Evaluation and management of chronic cough is resource intensive with significant economic burden.7 Varied etiology results in affected patients undergoing extensive diagnostic testing associated with pulmonary, allergy, otolaryngology, and gastroenterology consultations, which further balloons costs.8 Unfortunately, even with coordinated multi-specialty evaluation, chronic cough often defies categorization or empiric treatment and is termed idiopathic.
Several hypotheses are proposed to explain “idiopathic” chronic cough, estimated to represent 46% of cough patients.1 Among the most common are gastroesophageal reflux disease (GERD) and airway hypersensitivity syndrome.9 The former is thought to mediate cough through noxious refluxate stimulation of the distal esophagus via the esophagobronchial reflex or activation of sensory nerve receptors in the upper airway, which trigger the afferent loop of the cough reflex. The proposed pathophysiology of airway hypersensitivity is abnormal, repeated stimulation of sensory receptors causing neuroplastic changes in brainstem laryngeal controlling networks, inducing them into a perpetually hyperexcitable state, which increases the epithelial sensitivity to sensory stimuli.10,11
The vocal folds are uniquely exposed to frequent physical stimulation. Phonation involves 100 – 220 Hz adductory vocal fold contact cycles,12 causing low-grade, repeated stimuli. This rarely incites cough in normal physiologic circumstances, but is increasingly recognized as a trigger for cough in chronic cough patients due to a presumed upregulation of laryngeal sensory receptors.13,14 In this setting, phonation-induced cough is considered a clinical correlate for upper airway hypersensitivity.
Understanding the relative contribution of reflux and upper airway hypersensitivity as triggers for chronic cough could help direct therapeutic decisions and improve treatment effectiveness. The aim of this prospective, blinded longitudinal study was to explore the relationship between reflux, phonation, and cough events over time in a cohort of idiopathic chronic cough patients using a novel synchronized, continuous recording device.
METHODS
Patient population
Vanderbilt University Institutional Review Board approved this longitudinal observational trial (IRB #100706) (NCT01263626). The study population consisted of patients with the chief complaint of chronic cough (duration > 8 weeks) referred to the Digestive Disease Center for evaluation of GERD as a contributing factor and whose chronic cough was refractory to reflux treatment. Patients were current non-smokers with unremarkable chest radiographs who had undergone extensive testing for and exclusion of other common causes of chronic cough (e.g. angiotensin receptor inhibitors (ACEI) use, spirometry, methacholine challenge), and without voice complaints. Excluded were patients <18 years of age, pregnant, current smokers, had undergone surgery for reflux or peptic ulcer disease, or if they had a serious illness that would interfere with study participation. Once consented, patients were excluded if they did not cough or removed their recording monitor during the 24-h observation period. All acid-suppressive therapies were discontinued at least 10 days before any testing.
Protocol and study design
Patients underwent 24-hour acoustic recording concurrently and temporally synchronized with ambulatory pH-impedance monitoring at least 10 days off acid suppressive therapy. Encrypted recording collected by the acoustic monitor device were presence, frequency, and timing of phonation and cough events and time-synchronized pH-impedance data during both upright and supine states. Patients were instructed to push the symptom bottom in the pH-impedance recorder when they experienced any cough events in order to temporally compare true cough event occurrence (acoustic recording) to those reported by patients. The entire 24-hour recording for each patient was reviewed by 2 independent study personnel blinded to esophageal pH data in order to identify, record, and confirm all talk and cough event times. Esophageal motility testing and pH-impedance monitoring were performed as previously described.15
Acoustic monitoring system
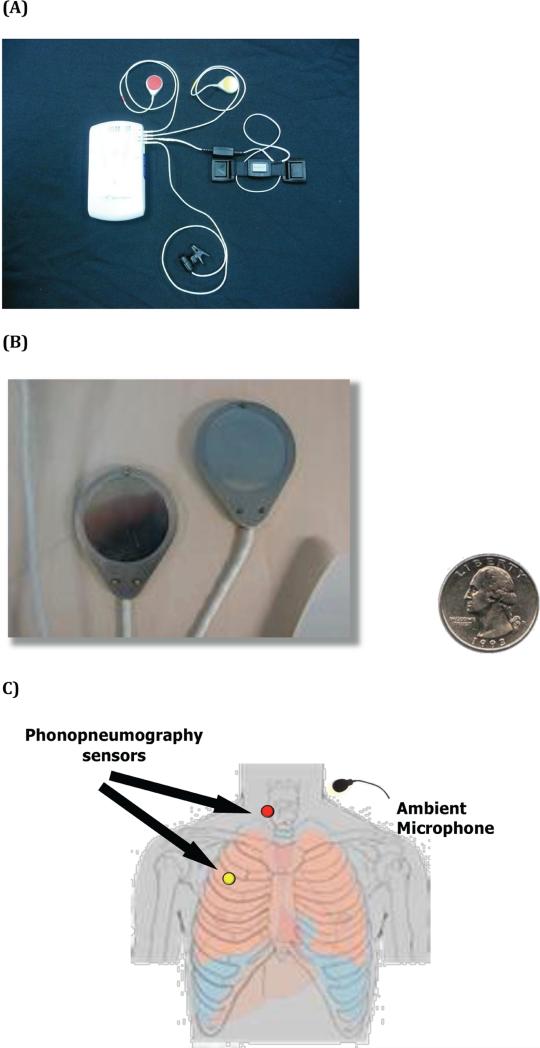
A novel and previously validated acoustic recording system with concurrent time-synchronized 24 hour ambulatory pH-impedance monitoring was used.15 Cough events were differentiated from throat clearing. Coughs were defined as events having an initial explosive phase involving pre-event inhalation, which contrasts with throat clearing in which no pre-event inhalation occurs.16 Cough events were detected via phonopneumography sensors positioned at two sites (Figure 1).15 An ambient microphone was affixed to the patient's shirt collar. Time synchronization of the separate acoustic and reflux monitoring recordings was achieved by an electronic signal delivered to both devices to align the signals in time. Study personnel reviewed and recorded all phonation events during the 24-hour study period. Phonation was defined as any talking event occurring in the minute before the cough event.
Figure 1.
A) Acoustic cough detection device composed of acoustic sensors, ambient microphone, and recorder device. B) Close up view of phonopneumography sensors and relative diameter compared to quarter. C) Illustration of chest wall and neck positioning of acoustic cough sensors.
Statistical analysis
The unit of measurement for analysis was cough events measured repeatedly on the same subject over 24 hours. The repeated measures design provided adequate precision to estimate robust models incorporating time-varying interactions between reflux, phonation, and cough in these patients with idiopathic chronic cough.
Initial analysis involved dividing each patient's 24-hour observation period into disjoint 2-minute intervals. Within each, the presence or absence of phonation events, objective pH-impedance and cough events were identified and recorded to summarize the total number of 2-minute intervals in which the 8 combinations of events did or did not occur. Resultant data provided the probability of coinciding cough, phonation, and pH-impedance events. Symptom index (SI) and symptom association probability (SAP) based on true cough (detected on acoustic monitoring), reflux or phonation events, were then independently calculated. Reflux, phonation, and cough burden in the 60 minutes prior to a cough event was measured. The primary analysis used generalized estimating equations (GEE) with robust sandwich estimator was used to create a logistic regression model of the association between cough events (outcome) and reflux, phonation, and cough burden.
To model event temporality and the relative frequency of phonation, pH-impedance events, and coughing, we considered phonation and pH-impedance events in the minute prior to cough events. Poisson regression was employed to estimate whether an association between coughing rate (outcome) and the presence/absence of phonation and/or pH-event events in the minute preceding the cough (time varying predictors). Time to next cough was measured either from the start of study recording or from the time of the previously recorded cough event. Poisson regression facilitated the inclusion of pH-impedance events and phonation as time-varying covariates. These covariates were included in the model as indicators of occurrence within the prior minute and thus changed over time within a subject. The time axis was divided into disjoint 6-second intervals to approximate a continuous time to event process. Cough rate was flexibly modeled over time using natural splines (20 degrees of freedom) with degrees of freedom evaluated by AIC to check for overfitting. Correlation from taking repeated observations on the same subject was modeled using GEE with an independence working correlation matrix and robust Huber-White sandwich estimator clustering by subject. All statistical analyses were performed using the R version 3.1.0 statistical package.
RESULTS
Demographics and Reflux Parameters
Of the 21 patients with chronic cough who underwent testing, 4 were excluded due to either lack of cough events (N=3) or removal of the recording monitoring (N=1). Thus, 17 patients met inclusion criteria [88% female; 100% Caucasian; 6% prior smokers; 41% alcohol users; median (IQR) age 63 (52 – 66); body mass index 30.6 (27.9 – 34.0]. In this cohort, the median (IQR) total time pH < 4 was 4.0% [(1.7 – 6.1), 4.5% upright (2.1 – 8.9) and 0.0% in the supine position (0.0 – 1.9)]. Acid and non-acid reflux events occurred a median 24 (18 – 55) and 14 times (10 – 26) over the 24-hour observation period. In all, 45% had secondary symptoms of heartburn with or without regurgitation.
Patient-reported and acoustic recording of cough events
Overall, the median (IQR) number of cough events detected by audio recording were significantly (p< 0.001) higher than those reported by patients: 216 (90-275) and 34 (22-60), respectively. Patients were more likely to record a cough event as the number of coughs in series increased (1 cough: 10%, 2 coughs: 18%, 3+ coughs: 30%).
Phonation, Reflux and Cough Events
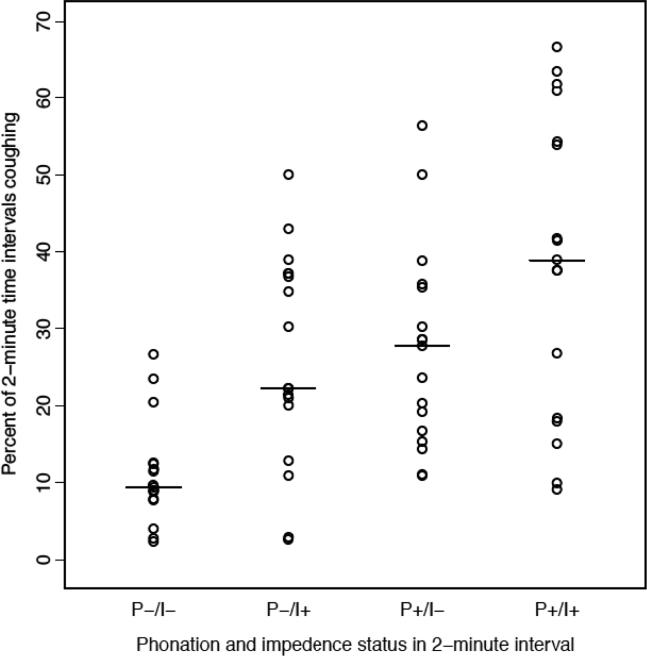
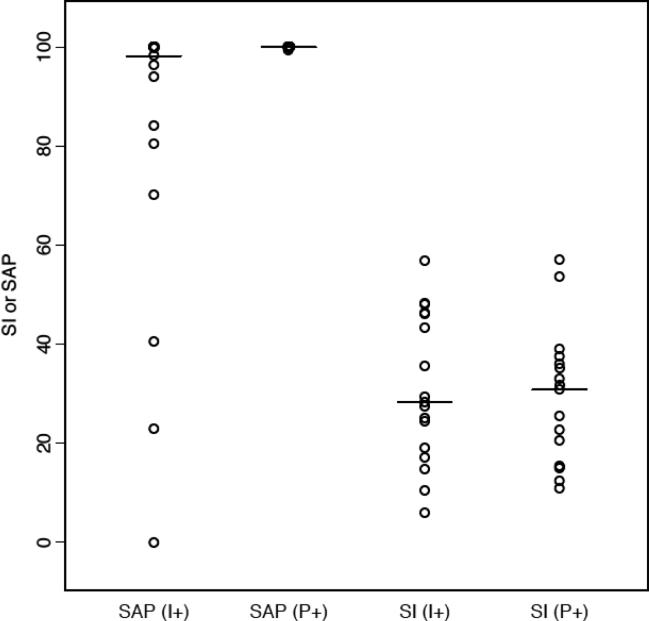
In all, patients had 2,048 analyzable coughing events. The percent of 2-minute intervals in which coughing occurred in the presence/absence of phonation and/or impedance event is shown in Figure 2. Coughing occurred in 11.4% of intervals that were devoid of either phonation or impedance events (P−/I−). Coughing was 2.25- and 2.14-fold more common in those who phonated without impedance events (P+/I−) and those who only had impedance events without phonation (P−/I+), respectively. Patients who had both phonation and impedance events (P+/I+) had the highest percentage of coughing events (37.4%). Median SI for phonation and impedance were similar (27.6% and 29.6%), while the median SAP was 100% for both conditions (Figure 3).
Figure 2.
Percent of 2-minute intervals that cough occurred in conjunction with phonation (P), pH-Impedance (I) event or both (+ presence; − absence)
Figure 3.
Median and distribution of objectively measured Symptom Associated Probability (SAP) and Symptom Index (SI) for Cough in relation to preceding pH-Impedance event (I) and phonation (P) events
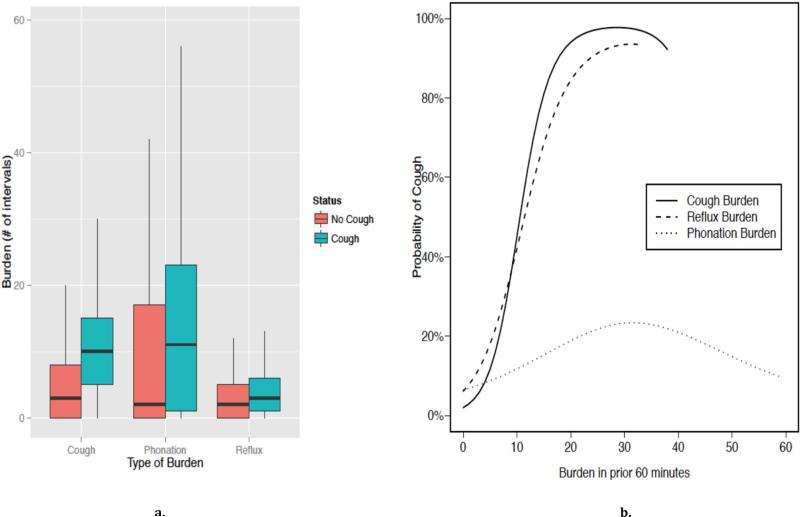
Burden were defined as the number of one-minute intervals that a cough, reflux, or phonation event occurred in the 60 minutes preceding a cough. For example, an individual's reflux burden would be 30 if a reflux event occurred in 30 of 60 (30/60) potential intervals. This was compared to the corresponding burden during 60-minute intervals in which there was no cough event (i.e., no events). We found that the cough, reflux, and phonation burden were each significantly higher prior to cough episodes (Figure 4). In the 60 minutes prior to cough events (n=2048), median phonation burden was 11 compared to intervals with no events (n=19487) when the median phonation burden was 2 (Figure 4a). Similarly, median cough and reflux burdens were 10/60 and 3/60 of one-minute intervals. This contrasts with median cough and reflux burdens of 3/60 and 2/60 prior to no events. Repeated measures logistic regression modeling showed the probability of coughing increased with phonation (p=0.007), reflux (p=0.011), and cough (p=0.005) burden (Figure 4b). For cough and reflux burden, the probability of cough increased rapidly to a burden of 20. Rate of increase was less pronounced for increases in phonation burden. Specifically, rate changed from 6% to 12% when phonation burden increased from 0 to 10. However, probability of cough increased from 2% to 45% and from 6% to 42% when cough and reflux burden increased from 0 to 10, respectively. At a burden of 40, the probability of cough was much higher for cough burden (87%) and reflux burden (89%) than phonation burden (21%).
Figure 4.
a) Burden of 1-minute intervals in which cough, phonation, or reflux events occurred in 60 minutes before cough event or no cough event; b) probability of cough event based on burden of cough, reflux or phonation in preceding 60 minutes
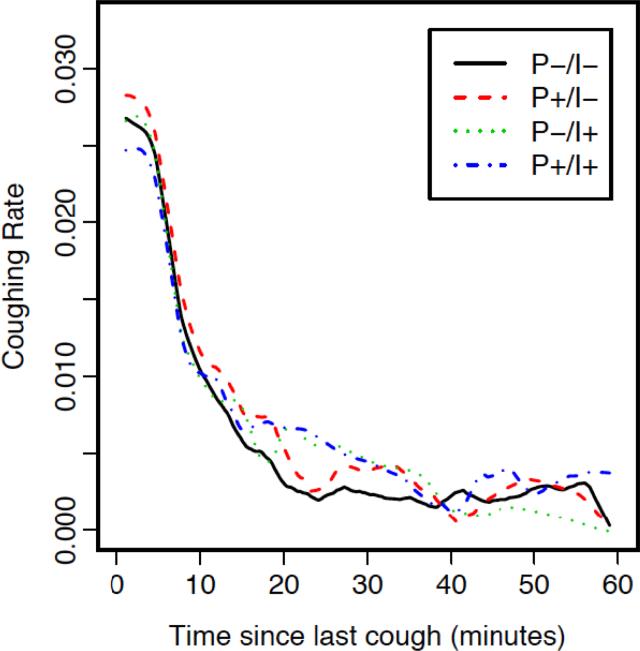
Figure 5 shows coughing rate and time since last cough based on whether it was preceded by phonation and/or pH-impedance events in the previous minute. Coughing rates were not significantly different for the four strata (i.e. P+/I+, P+/I−, P−/I+, P−/I−) within 15-minutes of the previous cough. The highest rate of coughing occurred immediately after the preceding cough (left-hand side of Figure 5) irrespective of whether or not phonation and/or pH-impedance events occurred. Thus, a prior cough increased the likelihood of further coughing sufficiently to mask any differential association with phonation and/or impedance events in the ensuing 15 minutes.
Figure 5.
Cough rate and time since last cough based on whether it was preceded by phonation (P) and/or pH-impedance (I) events
However, a de novo cough occurring greater than 15 minutes after a patient's previous cough (right side of Figure 5) appeared more likely to be preceded by phonation and/or impedance events. Specifically, de novo coughing during this interval occurred at a 1.46-fold (CI 1.17 – 1.82, p<0.001) and 1.71-fold (CI 1.25 – 1.82, p<0.001) increased rate if preceded by P−/I+ or P+/I+ compared to P−/I−. Despite a similar rate ratio magnitude (RR 1.45, CI 0.89 – 2.35), the association between cough and P+/I− did not reach statistical significance (p=0.14).
DISCUSSION
The current blinded, cross-sectional study used a concurrent time-synchronized audio and pH-impedance device to measure gastroesophageal reflux and phonation triggers in patients with idiopathic chronic cough. Phonation involves repeated vocal fold contact (~100 – 220 Hz); however, in normal physiologic conditions this process does not trigger cough. There is recent evidence, however, that even minimal stimulation caused by routine vocal fold vibration is sufficient to instigate cough in those with hypersensitivity syndrome.17,18 Complicating this association is that GERD is similarly implicated.19 It is important to study the differential effects of these two potential triggers to advance the understanding of idiopathic chronic cough pathophysiology and to identify potential methods and targets for prevention and treatment.
Our novel pathophysiological study offers several clinically relevant results. First, the probability of cough is increased with higher burdens of preceding phonation, reflux and cough. Second, it objectively demonstrated that a patient's cough is nearly universally accompanied by additionally coughing within a 15-minute window independent of whether there was an antecedent phonation or pH-impedance event. Third, cough rate was highest immediately following the prior cough and diminished with time from initial cough. Thus, cough was the trigger for subsequent cough events within the ensuing 15-minute interval. Fourth, after the first 15 minutes interval, de novo cough was more likely if preceded by either a phonation or pH-impedance event. While both event types increased risk of cough, only immediately antecedent pH-impedance events were significantly associated with an increased rate of de novo cough.
These findings extend the results from a recent study.16 In that study, 70% of chronic cough patients exhibited a temporal relationship between reflux and cough. Their finding that ½ of patients had positive objectively measured SAP for cough preceded by reflux was higher than previously reported.20 Our study further assessed whether phonation was an additional trigger for chronic cough. We demonstrated robust associations between both phonation and reflux and cough. It also demonstrated that “cough begets cough” providing further evidence for the central neuronal sensitization hypothesis that links repeated irritation to cough. Furthermore, these data suggest that idiopathic chronic cough may be multifactorial, providing some explanation for the unpredictable efficacy of proton pump inhibitors.
Interestingly, few areas besides the vocal folds routinely experience repeated physical stimulation. Trauma from repeated coughing increases airway sensitivity to less noxious substances. As such, changes in environmental temperature (e.g. cold)21, smells and fumes22, dryness and change in upper airway secretion viscosity21, and phonation17,18 can sufficiently irritate the noci- and chemoreceptors in the vocal folds to activate the cough reflex.23,24 Further supporting the hypothesized role of phonation in idiopathic chronic cough are results from several studies25,26 including one randomized trial27 that found that behavioral cough suppressive treatment with speech language pathologists can effectively treat these patients. Thus, reflux-negative patients may be good candidates for laryngeal desensitization therapy.
Our results confirmed that reflux is a significant trigger in patients with idiopathic chronic cough. Specifically, rate of cough was increased 47% if immediately preceded by a pH-impedance event alone and even more so if combined with a phonation event (RR 1.71). The role of GERD in chronic cough is challenging to fully characterize. It is proposed that gastric reflux contributes to cough either directly in the form of laryngeal penetration and aspiration or indirectly through vagal irritation, Despite these suppositions, it remains difficult to directly implicate reflux as the causative factor in idiopathic chronic cough without the existence of a definitive diagnostic test.
Physiologically, phonation rarely triggers coughs. However, in this cohort of chronic cough patients, antecedent phonation was non-significantly associated with increased rate of a cough event. This is an important finding because phonation events were substantially more common than pH-impedance events both in this cohort and in the general population with chronic cough. It is recommended that clinicians carefully question patients on whether phonation is a trigger for their chronic cough. If recognized as a stimulus, it may etiologically implicate laryngeal hypersensitivity and offer an alternative therapeutic target using voice therapy17,28 or neuromodulators including gabapentin and tricyclic antidepressants.29
Coincidentally, visceral hypersensitivity has also been associated with reflux disease.30 The mechanism is similarly uncertain, but is thought to occur secondary to peripheral and central neurologic sensitization and psychoneuroimmune interactions.31 In the absence of objectively measured pathologic GERD, patient symptom indices have been proposed as surrogate diagnostic tests to identify patients with this reflux-variant.32 It is important to dissociate patient reported cough events during reflux monitoring from those in this study, which were based on actual cough events detected via acoustic monitoring. The findings that most (71%-91%) patients do not push the event marker on the reflux monitor should caution against the use of symptom association parameters.33
Study limitations deserve mention. First, the current investigation had a small sample size of patients with idiopathic chronic cough; however, the unit of measurement for analysis was cough events not patients; thus, the study was adequately powered. Second, event data was collected over a 24-hour period. Thus, results may not be generalizable to the broader population of patients with chronic cough or if measured over a different or longer time interval. Four of 21 patients were excluded: one removed the device and three had insufficient cough events. Lack of cough events is likely due to day-to-day cough variability in this condition. Furthermore, there is potential for observer effect bias; that is, patients knew they were being monitored, which may have changed behavior and affected results. In spite of limitations, our findings have important clinical implications and increase understanding of factors that stimulate cough in this population.
Conclusion
Idiopathic chronic cough is a diagnostic challenge. This study objectively demonstrated that both antecedent phonation and reflux increased the rate of a cough event in a cohort of patients with idiopathic chronic cough. Of these two triggers, reflux events were more strongly associated with increased rate of coughing. There is also evidence that “cough begets cough”, providing evidence for airway hypersensitivity syndrome and implicating the vocal folds as a potential instigator in idiopathic chronic cough.
Acknowledgments
Grant Support: NIH (1K23DC013559) – salary support for first author (D.O.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: no author has any financial or personal disclosures or conflicts of interest
References
- 1.McCrory DC, et al. Assessment and Management of Chronic Cough AHRQ Comparative Effectiveness Reviews. 2013 [Google Scholar]
- 2.Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med. 2000;343:1715–1721. doi: 10.1056/NEJM200012073432308. doi:10.1056/NEJM200012073432308. [DOI] [PubMed] [Google Scholar]
- 3.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. The American review of respiratory disease. 1990;141:640–647. doi: 10.1164/ajrccm/141.3.640. doi:10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 4.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. The American review of respiratory disease. 1981;123:413–417. doi: 10.1164/arrd.1981.123.4.413. [DOI] [PubMed] [Google Scholar]
- 5.National Ambulatory Medical Care Survey: 2008 Summary Tables. < http://www.cdc.gov/nchs/ahcd.htm>.
- 6.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. doi:10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 7.Morice AH, McGarvey L, Pavord I. British Thoracic Society Cough Guideline, G. Recommendations for the management of cough in adults. Thorax. 2006;61(Suppl 1):i1–24. doi: 10.1136/thx.2006.065144. doi:10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis DO, et al. High economic burden of caring for patients with suspected extraesophageal reflux. The American journal of gastroenterology. 2013;108:905–911. doi: 10.1038/ajg.2013.69. doi:10.1038/ajg.2013.69. [DOI] [PubMed] [Google Scholar]
- 9.Gibson PG, Simpson JL, Ryan NM, Vertigan AE. Mechanisms of cough. Current opinion in allergy and clinical immunology. 2014;14:55–61. doi: 10.1097/ACI.0000000000000027. doi:10.1097/ACI.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 10.Pacheco A. Chronic cough: from a complex dysfunction of the neurological circuit to the production of persistent cough. Thorax. 2014;69:881–883. doi: 10.1136/thoraxjnl-2014-205661. doi:10.1136/thoraxjnl-2014-205661. [DOI] [PubMed] [Google Scholar]
- 11.Morice AH, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. The European respiratory journal. 2014 doi: 10.1183/09031936.00218613. doi:10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- 12.Titze IR, Hunter EJ. Feasibility of measurement of a voice range profile with a semi-occluded vocal tract. Logopedics, phoniatrics, vocology. 2011;36:32–39. doi: 10.3109/14015439.2010.548828. doi:10.3109/14015439.2010.548828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, et al. Capsaicin-sensitive cough receptors in lower airway are responsible for cough hypersensitivity in patients with upper airway cough syndrome. Medical science monitor : international medical journal of experimental and clinical research. 2013;19:1095–1101. doi: 10.12659/MSM.889118. doi:10.12659/MSM.889118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison M, Rammage L, Emami AJ. The irritable larynx syndrome. Journal of voice : official journal of the Voice Foundation. 1999;13:447–455. doi: 10.1016/s0892-1997(99)80049-6. [DOI] [PubMed] [Google Scholar]
- 15.Kavitt RT, et al. Symptom reports are not reliable during ambulatory reflux monitoring. The American journal of gastroenterology. 2012;107:1826–1832. doi: 10.1038/ajg.2012.342. doi:10.1038/ajg.2012.342. [DOI] [PubMed] [Google Scholar]
- 16.Smith JA, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139:754–762. doi: 10.1053/j.gastro.2010.06.050. doi:10.1053/j.gastro.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Murry T, et al. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope. 2010;120:1576–1581. doi: 10.1002/lary.20985. doi:10.1002/lary.20985. [DOI] [PubMed] [Google Scholar]
- 18.Vertigan AE, Gibson PG, Theodoros DG, Winkworth AL. The role of sensory dysfunction in the development of voice disorders, chronic cough and paradoxical vocal fold movement. International journal of speech-language pathology. 2008;10:231–244. doi: 10.1080/17549500801932089. doi:10.1080/17549500801932089. [DOI] [PubMed] [Google Scholar]
- 19.Irwin RS, Madison JM, Fraire AE. The cough reflex and its relation to gastroesophageal reflux. Am J Med. 2000;108(Suppl 4a):73S–78S. doi: 10.1016/s0002-9343(99)00341-1. [DOI] [PubMed] [Google Scholar]
- 20.Sifrim D, et al. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut. 2005;54:449–454. doi: 10.1136/gut.2004.055418. doi:10.1136/gut.2004.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suadicani P, Hein HO, Meyer HW, Gyntelberg F. Exposure to cold and draught, alcohol consumption, and the NS-phenotype are associated with chronic bronchitis: an epidemiological investigation of 3387 men aged 53-75 years: the Copenhagen Male Study. Occupational and environmental medicine. 2001;58:160–164. doi: 10.1136/oem.58.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarlo SM. Cough: occupational and environmental considerations: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:186S–196S. doi: 10.1378/chest.129.1_suppl.186S. doi:10.1378/chest.129.1_suppl.186S. [DOI] [PubMed] [Google Scholar]
- 23.Sant'Ambrogio G, Sant'Ambrogio FB. Role of laryngeal afferents in cough. Pulmonary pharmacology. 1996;9:309–314. doi: 10.1006/pulp.1996.0040. [DOI] [PubMed] [Google Scholar]
- 24.Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology. 2013;18:948–956. doi: 10.1111/resp.12103. doi:10.1111/resp.12103. [DOI] [PubMed] [Google Scholar]
- 25.Patel AS, et al. Improvement in health status following cough-suppression physiotherapy for patients with chronic cough. Chronic respiratory disease. 2011;8:253–258. doi: 10.1177/1479972311422547. doi:10.1177/1479972311422547. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain S, Birring SS, Garrod R. Nonpharmacological interventions for refractory chronic cough patients: systematic review. Lung. 2014;192:75–85. doi: 10.1007/s00408-013-9508-y. doi:10.1007/s00408-013-9508-y. [DOI] [PubMed] [Google Scholar]
- 27.Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006;61:1065–1069. doi: 10.1136/thx.2006.064337. doi:10.1136/thx.2006.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vertigan AE, Gibson PG. The role of speech pathology in the management of patients with chronic refractory cough. Lung. 2012;190:35–40. doi: 10.1007/s00408-011-9333-0. doi:10.1007/s00408-011-9333-0. [DOI] [PubMed] [Google Scholar]
- 29.Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, Dupont LJ. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8:9. doi: 10.1186/1745-9974-8-9. doi:10.1186/1745-9974-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowles CH, Aziz Q. Visceral hypersensitivity in non-erosive reflux disease. Gut. 2008;57:674–683. doi: 10.1136/gut.2007.127886. doi:10.1136/gut.2007.127886. [DOI] [PubMed] [Google Scholar]
- 31.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. doi:10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 32.Aanen MC, et al. Effect of proton-pump inhibitor treatment on symptoms and quality of life in GERD patients depends on the symptom-reflux association. Journal of clinical gastroenterology. 2008;42:441–447. doi: 10.1097/MCG.0b013e318074dd62. doi:10.1097/MCG.0b013e318074dd62. [DOI] [PubMed] [Google Scholar]
- 33.Slaughter JC, et al. Caution about overinterpretation of symptom indexes in reflux monitoring for refractory gastroesophageal reflux disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:868–874. doi: 10.1016/j.cgh.2011.07.009. doi:10.1016/j.cgh.2011.07.009. [DOI] [PubMed] [Google Scholar]