Abstract
Given the critical role of mucosal surfaces in susceptibility to infection, it is imperative that effective mucosal responses are induced when developing efficacious vaccines and prevention strategies for infection. Modulating the microbiota in the gastrointestinal (GI) tract through the use of probiotics (PBio) is a safe and well-tolerated approach to enhance mucosal and overall health. We assessed the longitudinal impact of daily treatment with the VSL#3 probiotic on cellular and humoral immunity and inflammation in healthy macaques. PBio therapy resulted in significantly increased frequencies of B cells expressing IgA in the colon and lymph node (LN), likely due to significantly increased LN T follicular helper cell (Tfh) frequencies and LN follicles. Increased frequencies of IL-23+ antigen presenting cells (APCs) in the colon were found post-PBio treatment, which correlated with LN Tfh. Finally, VSL#3 significantly down-modulated the response of TLR2, TLR3, TLR4 and TLR9-expressing HEK293 cells to stimulation with Pam3CSK4, Poly(I:C), LPS and ODN2006, respectively. These data provide a mechanism for the beneficial impact of PBio on mucosal health and implicates the use of PBio therapy in the context of vaccination or preventative approaches to enhance protection from mucosal infection by improving immune defenses at the mucosal portal of entry.
Introduction
Mucosal tissues are particularly vulnerable to infection as they are the major interface between the outside world and the internal environment. Often only a single layer of epithelial cells serves as a physical barrier between the host and the external environment. Protection from infection by pathogens at mucosal sites is facilitated by a complex interaction of multiple subsets of the innate and adaptive immune systems, leading to the production of soluble factors such as cytokines, chemokines, immunoglobulins and antimicrobial peptides. Protection from infection is also achieved through the integrity of a mucus layer that protects the epithelial barrier, and the presence of diverse microbial communities, collectively termed the microbiome (1). These protective mechanisms are critically important in preventing acquisition of sexually transmitted infections (STIs) (2, 3). In particular, mucosal immune integrity is important in preventing new human immunodeficiency virus (HIV) infections and HIV exposure at mucosal surfaces, such as the rectum or the vaginal tract, constitutes the major route for HIV transmission. Furthermore, alterations in the genital or gastrointestinal (GI) mucosa, including increased inflammation, changes in the host mucosa-associated microbiota, and damage to the mucosal epithelial barrier all contribute to increased risk of HIV transmission and pathogenesis (4). Thus, it is critical that future preventative strategies against HIV and other mucosal infections include methods that enhance mucosal immunity, as maintaining adequate protection of mucosal tissues could potentially increase resistance to initial infection and improve health in infected individuals (5).
One possible method to enhance the mucosal immune response is through modulation of the microbiota in the GI tract through probiotic (PBio) therapy. PBio treatment, described by the World Health Organization as “live microorganisms which when administered in adequate amounts confers a health benefit on the host”, is a safe and well-tolerated approach to enhancing mucosal and overall health (6–9). The use of PBio therapy has gained momentum given the numerous studies demonstrating the efficacy of PBio to enhance mucosal immune function and decrease GI-related diseases (6, 10, 11). In particular, probiotic therapy has been suggested to prevent recurrence or maintain remission from such inflammatory bowel diseases and complications as pouchitis, ulcerative colitis and Crohn’s disease (10–13).
PBio therapy has been shown to exert its robust effects on the immune system through modulation of pattern recognition receptors (PRRs), especially Toll-like Receptors (TLRs). For example, the genomes of Bifidobacterium spp. and Lactobacillus spp., common bacteria utilized in PBio therapies, are rich in unmethylated CpG motifs which can interact with TLR2 and TLR9 to enhance NF-kB signaling and epithelial barrier function by negating TLR4-induced epithelial disruption (14–16). PBio has also been implicated in the protection from viral infections, as demonstrated by the ability of Lactobacillus spp. to protect against respiratory syncytial virus and rotavirus infections through a TLR2 and TLR3-dependent manner (17–19).
In the context of simian immunodeficiency virus (SIV) infection of non-human primates, a critical and highly related animal model of HIV infection, we have previously demonstrated that PBio treatment induced several beneficial alterations in anti-retroviral therapy (ART)-treated SIV-infected pigtail macaques (20). Specifically, PBio-treated SIV-infected animals exhibited increased functionality of immune cells, decreased levels of cellular immune activation, and increased frequency and function of antigen presenting cells (APCs) and APC genes. Furthermore, recent work has demonstrated that treatment with PBio in conjunction with recombinant interleukin (IL)-21 improves intestinal Th17 frequencies and decreases the incidence of non-AIDS co-morbidities in ART-treated SIV-infected macaques (21). Several studies have also assessed the use of probiotics in HIV-infected individuals (22–24). Most recently, a study conduced in ART-treated HIV-infected individuals demonstrated that treatment with Saccharomyces boulardii, a probiotic yeast, for 12 weeks lead to reduced systemic levels of bacterial translocation and the pro-inflammatory cytokine IL-6 (25). Taken together, these data suggest that PBio therapy elicits a positive impact on mucosal immunity, especially in the context of a chronic viral infection. However, the mechanism through which PBio therapy exerts these effects has remained unclear, especially in the absence of a confounding lentiviral infection.
The goal of this study was to assess the longitudinal impact of PBio therapy on various aspects of mucosal immunity in healthy macaques in order to better understand if augmenting the microbiome may benefit mucosal and lymphoid immunity. To do this, we treated healthy macaques with VSL#3, a probiotic that has been shown in several clinical and pre-clinical studies to be highly beneficial (10, 11). We found that this treatment induced short term and long-term beneficial effects to mucosal and systemic immunity, and provides rationale for potential use of PBio therapy as an immunomodulator for new strategies for prevention of mucosal infections.
Materials and Methods
Study animals
Two pigtail macaques (PTM) and three rhesus macaques (RM) received PBio VSL#3, 2.25×1011 bacteria daily (2 capsules) for 80 days mixed with food. No adverse events due to PBio therapy were observed in any of the animals. Colon (all animals) and jejunum (RM) biopsies were obtained via endoscope, and inguinal or axillary LN were biopsied via surgical removal at baseline (day −7), day 28 and day 77/80 post-PBio treatment. Animals were housed and cared for in Association for the assessment and accreditation of Laboratory Animal Care international (AAALACi) accredited facilities, and all animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of University of Washington and Washington National Primate Research Center.
Tissue processing
LN tissues were dissected and a section was paraformaldehyde (PFA) fixed and paraffin embedded for immunohistochemistry analysis. Remaining LN tissue was processed into a single-cell suspension in R10 media (RPMI 1640 with 2.05mM L-glutamate, supplemented with 10% fetal bovine serum [FBS], 100U/ml Penicillin, 100μg/ml Streptomycin [all from GE Healthcare, Logan, UT] and 0.5mg/ml gentamicin [Corning, Manassas, VA]), as described previously (26). One to two gut biopsies were removed and fixed in PFA for immunohistochemistry use, and the remaining biopsies were digested with Liberase (40μg/ml, Sigma-Aldrich, St. Louis, MO) and DNAse (4μg/ml, Sigma-Aldrich) in R10 media without FBS for 1hr at 37°C with vigorous stirring, then ground through a 70μm cell strainer into a single cell suspension in R10 media. Single cell suspensions were stained immediately for flow cytometric analysis.
Flow cytometry
Multi-color flow cytometric analysis was performed on isolated LN and colon cells according to standard procedures using optimized anti-macaque or anti-human monoclonal antibodies that cross-react with PTM and RM. Predetermined optimal concentrations were used of the following antibodies: anti-CD3 PerCP (clone SP34-2, BDPharmigen, San Jose, CA), anti-CD4 eFluor605NC (clone OKT4, eBioscience, San Diego, CA), anti-CD8 APC-H7 (clone SK1, BDBiosciences, San Jose, CA), anti-CD28 ECD (clone CD28.2, Beckman Coulter, Brea, CA), anti-CD95 eFluor450 (clone DX2, eBioscience), anti-CXCR5 PE-Cy7 (Clone MU5UBEE, eBioscience), anti-PD-1 PerCP-eFluor710 (clone eBioJ105, eBioscience), anti-Ki-67 AF700 (clone B56, BDPharmigen), anti-HLA-DR BV711 (clone L243, BioLegend, San Diego, CA), anti-NKp44 APC (Miltenyi Biotec, San Diego, CA), anti-CD20 BV570 (clone 2H7, BioLegend), anti-IgA (polyclonal, Jackson, West Grove, PA), anti-IgG PE-Cy5 (clone G18-145, BDPharmigen), anti-IL-23 eFluor660 (clone 23dcdp, eBioscience). All cells were stained with the Live/Dead Fixable Aqua Dead Cell Stain Kit (ThermoFisher Scientific, Grand Island, NY), to exclude dead cells. All samples were permeabilized and fixed using CytoFix/Perm Kit (BD Pharmingen) and intracellularly stained to detect either Ki-67 or IL-23. For intracellular cytokine staining, cells were stimulated with phorbol myristate acetate (PMA; 5ng/ml, Sigma-Aldrich) and ionomycin (1μM/ml, Thermo-Fisher Scientific) for 10–14hrs with Brefeldin A (1μg/ml, Sigma-Aldrich), added after 1hr of stimulation. Flow cytometric acquisition was performed on a BD LSRII cytometer using FACS Diva software (Version 8, BD Pharmingen). Analysis of the acquired data was performed using FlowJo software (Version 10.0.8, TreeStar, Ashland, OR).
HEK TLR cell lines
HEK-Blue™ cells individually expressing TLR2, TLR3, TLR4, TLR9 and an NF-κB-inducible secreted embryonic alkaline phosphatase (SEAP) reporter gene, and the Null-1 control cell line were purchased from InvivoGen (San Diego, CA). Cell lines were initially expanded in growth medium (GM; DMEM supplemented with 4.5g/l glucose and L-glutamine [Corning], 10% fetal bovine serum, 100U/ml Penicillin, 100ug/ml Streptomycin and 100ug/ml Normocin™ [InvivoGen]), as per the manufacturer instructions. Cells were passaged every 2–3 days when cells were 70–80% confluent. After 4 passages in GM, each cell line was passaged in the presence of various selective antibiotics, including HEK-Blue™ Selection, Blasticidin and Zeocin (all from InvitroGen), as per the manufacturer instructions. After 10 passages, cells were used in PBio stimulation experiments. Cells were resuspended at 280,000 cells/ml in HEK-Blue™ Detection medium (InvivoGen) and plated at 50,400 cells/condition. Cells were cultured for 19hrs in the presence of positive controls appropriate for each cell line (hTLR2: 10ng/ml Pam3CSK4; hTLR3: 1ug/ml Poly (I:C); hTLR4: 100ng/ml LPS-EK Ultrapure; hTLR9: 10ug/ml ODN2006) (all from InvivoGen), VSL#3 re-suspended in either 100ml, 200ml or 500ml of molecular biology grade water (22.5×106, 11.25×106, and 4.5×106 bacteria cells/condition, respectively), combinations of the positive control and the three VSL3 doses or endotoxin free H2O (negative control; GE Healthcare). Positive signaling through TLRs was assessed by measuring activity of SEAP, an enzyme that hydrolyzes a substrate included in the HEK-Blue™ Detection Medium, resulting in a colorimetric change quantifiable by a spectrophotometer at 655nm. All conditions were performed in triplicate. Background signaling (results from the Null-1 control cell line) was subtracted from each of the TLR expressing cell lines.
Statistical analysis
Statistical analysis was performed using GraphPad Prism statistical software (Version 6, GraphPad Software, San Diego, CA). Statistical significance between baseline (day 7) and post-PBio treatment time points (days 28 and 77/80) was evaluated using a paired t test. Note that samples from day 77 or day 80 were considered one time point and grouped for statistical testing. Correlations were completed using a Pearson’s test. Statistical significance between different stimulation conditions of the HEK-Blue TLR expressing cell lines was evaluated using a paired t test. P values of <0.05 were considered significant.
Results
Experimental design
To evaluate the longitudinal impact of PBio therapy in the absence of a confounding lentiviral infection, healthy pigtail macaques (PTM, n=2) and rhesus macaques (RM, n=3) were treated orally with the medical grade probiotic VSL#3 (2 capsules; 2.25×1011 bacteria daily) for 77–80 days. The VSL#3 probiotic consists of Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophiles. Colon and lymph node samples were obtained from each animal at day −7 pre-PBio treatment, and days 28 and 77 or 80 post-PBio therapy (Figure 1). No adverse events due to PBio therapy were observed in any of the animals.
Fig. 1.
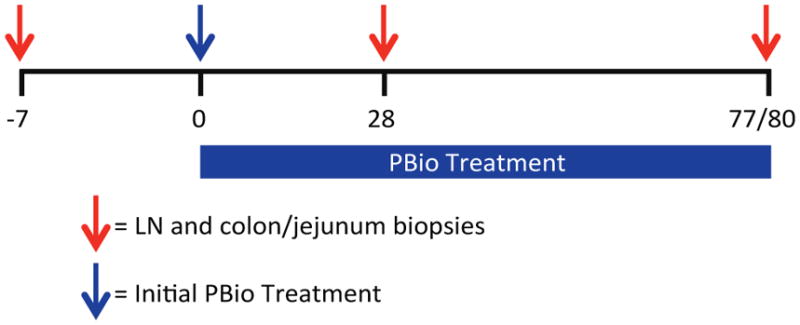
Experimental timeline. LN and colon/jejunum biopsies were collected from two PTM and three RM prior to (d−7) and post-PBio treatment (d28 and 77/80). Red arrows indicate days on which tissue was collected from each animal. Blue arrow indicates initiation of PBio therapy.
PBio therapy increases IgA in colon and LN
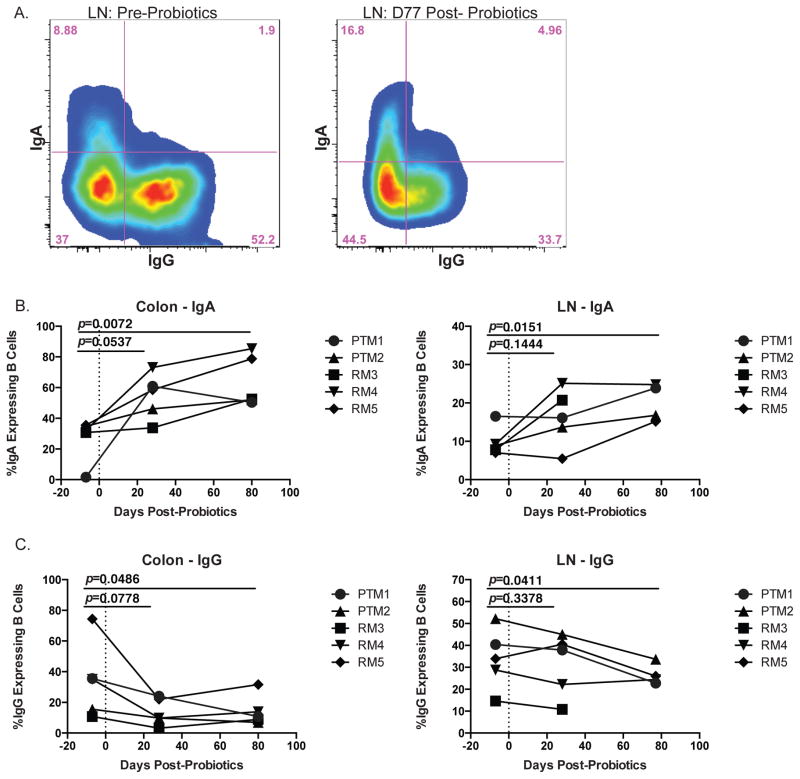
One of the major mechanisms utilized by the mucosal immune system to manage and maintain intestinal homeostasis is the production of mucosal immunoglobulin A (IgA), which not only aids in the defense against intestinal pathogens, but also helps to regulate the commensal intestinal microbiome (27). Indeed, previous work has shown that treatment of mice with various strains of PBio lactobacilli spp. induced an increase in the level of IgA in the intestine (28, 29) and an increase in the frequency of small intestine IgA-expressing B cells (30). In order to assess whether PBio treatment with VSL#3 resulted in commensurate changes to Ig phenotype, we measured the frequency of B cells that expressed IgA versus IgG before and after PBio therapy using flow cytometry (Figure 2A, representative staining). We found a trend towards increased IgA expression in both the colon and LN (p=0.0537 and 0.1444, respectively) at day 28 post-PBio and a significant increase in IgA expressing B cells at both sites by day 77 (p=0.0072 and 0.0151, respectively; Figure 2B). Conversely, we observed a trend towards decreased IgG expressing B cells in the colon and LN at day 28 post-PBio (p=0.0778 and 0.3379, respectively), and IgG was significantly decreased at both sites by day 77 post-PBio (p=0.0486 and 0.0411, respectively; Figure 2C). These data indicate that treatment with VSL#3 led to enrichment of B cells expressing IgA in mucosal and lymph node tissues that could have resulted from enhanced class switching from IgG to IgA, or an overall increase in IgA+ B cells.
Fig. 2.
Increased frequency of IgA expressing B cells in colon and LN post-PBio therapy. (A) Representative staining demonstrating the IgA and IgG expressing populations of B cells (CD20+) in the LN pre-PBio (left panel) and post-PBio (right panel). Cells were identified by first gating on lymphocytes and excluding doublets using forward scatter (FSC) and side scatter (SSC) properties and removing dead cells with an Aqua Live/Dead viability dye. The percentage of IgA+ and IgG+ cells was then determined within the CD3-CD20+ cell population. (B) Percentage of IgA expressing B cells in the colon (left panel) and LN (right panel) at all time-points. (C) Percentage of IgG expressing B cells in the colon (left panel) and LN (right panel) at all time-points. Each animal is represented by a different symbol (n=5). Each experiment was performed once per animal per time-point. Statistical significance between the two post-PBio time-points (d28 or d77/80) and the pre-PBio timepoint (d−7) was calculated using a paired t test.
PBio therapy increases Tfh in LN
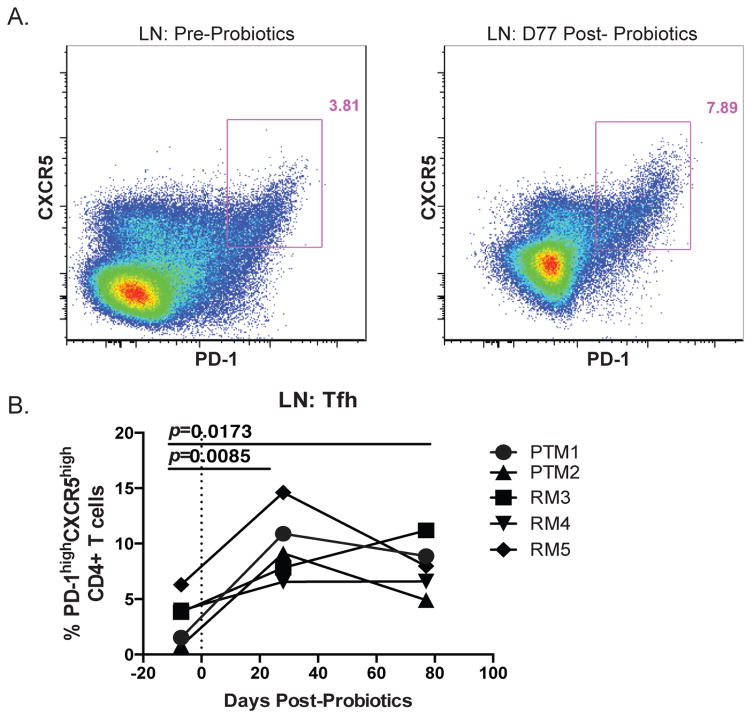
One mechanism to explain the expansion of IgA+ B cells in the colon and LN as a result of PBio therapy is increased frequencies of T follicular helper cells (Tfh cells). The major function of Tfh cells is to induce B cell responses in the LN (31, 32). Thus, the ability to alter and/or induce B cell antibody responses in vivo may rely on efficient Tfh responses. Given the observed alteration in B cell Ig production upon PBio treatment, we next measured Tfh cells from the LN of all animals by co-expression of PD-1 and CXCR5 on CD4+ T cells by flow cytometry (Figure 3A, representative staining). We found that there was a significant increase in the frequency of Tfh cells in the LN at day 28 post-PBio treatment (p=0.0085), which was maintained through day 77 post-PBio therapy (p=0.0173; Figure 3B).
Fig. 3.
Increased frequency of Tfh in the LN post-PBio therapy. (A) Representative staining demonstrating the Tfh population in the LN pre-PBio (left panel) and post-PBio (right panel). Cells were identified by first gating on lymphocytes and excluding doublets using FSC and SSC properties and removing dead cells with an Aqua Live/Dead viability dye. The frequency of Tfh cells within this subset was then determined by gating on CD3+CD4+PD-1hiCXCR5+ cells. (B) Percentage of Tfh in the LN at all time points. Each animal is represented by a different symbol (n=5). Each experiment was performed once per animal per time-point. Statistical significance between the two post-PBio time-points (d28 or d77/80) and the pre-PBio time-point (d−7) was calculated using a paired t test.
To determine if the increased Tfh cells were associated with increased LN follicles, we assessed LN morphology via H&E staining, as previously described (33). We quantified the number of follicles and found a trend towards increased overall follicles in the LN after PBio therapy (average follicle number pre-PBio was 17.6±5.03; post-PBio was 30.2±15.3; p=0.1130, data not shown; representative IHC, Supplemental Figure 1). Thus, PBio therapy led to an increased frequency of Tfh in the LN and increased LN follicles, which could subsequently influence B cell class switching to IgA and homing of these cells to the intestinal mucosa.
PBio therapy increases IL-23 production by antigen presenting cells
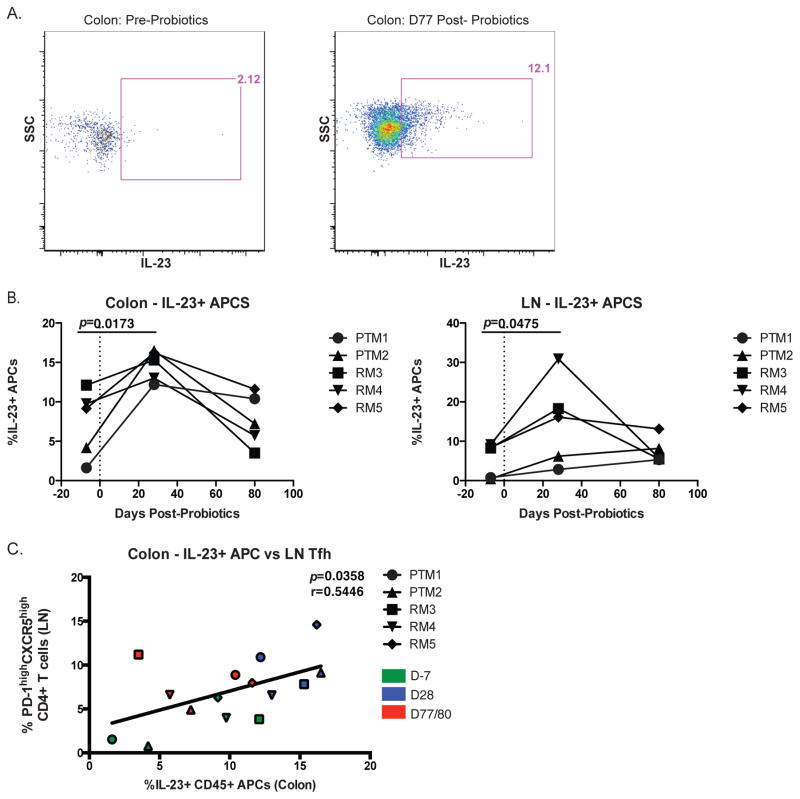
Tfh cells can be induced by their microenvironment, namely by key cytokines such as IL-23. IL-23 is produced mostly by APCs and is essential for induction of many essential CD4+ T cell subsets, including Tfh and Th17 cells (34). In addition, deficiency of the IL-12 receptor β1 (also the IL-23 receptor) results in decreased Tfh and B cells (35). Thus, we next measured the effect of PBio treatment on IL-23 production by APCs from the colon and LN after mitogen stimulation (Figure 4A). We found that there was an early increase (day 28) in IL-23 production by APCs in both the colon and LN (p=0.0173 and 0.0475, respectively; Figure 4B) after PBio therapy, but this increase did not persist to day 77 post-PBio treatment. Notably, we found a significant correlation between the frequency of IL-23 producing APCs in the colon and the frequency of LN Tfh cells (p=0.0358, r=0.5446; Figure 4C), suggesting that IL-23 production after PBio therapy could drive the expansion of Tfh cells in the LN.
Fig. 4.
Increased frequency of IL-23+ APC in the colon and LN post-PBio therapy. (A) Representative staining demonstrating the IL-23+ APC population in the colon pre-PBio (left panel) and post-PBio (right panel). Cells were identified by first gating on lymphocytes and excluding doublets using FSC and SSC properties and removing dead cells with an Aqua Live/Dead viability dye. The frequency of IL-23+ APCs within this subset was then determined by gating on CD45+HLA-DR+ cells. (B) Percentage of IL-23+ APC in the colon (left panel) and LN (right panel) at all time-points. Each animal is represented by a different symbol. Statistical significance between the two post-PBio time-points (d28 or d77/80) and the pre-PBio time-point (d−7) was calculated using a paired t test. (C) Correlation between colon IL-23+ APC frequencies and LN Tfh frequencies at all time-points. Each animal is represented by a different symbol (n=5). Each experiment was performed once per animal per time-point. D−7 time-points are in green, d28 time-points are blue and d77/80 time-points are red. Statistical significance of the correlation was calculated using a Pearson’s test.
PBio therapy decreases frequency of proliferating and activated CD4+ T cells in the colon
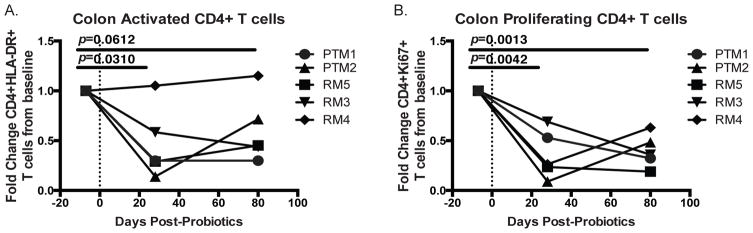
A concern in any mucosal intervention is balancing the benefits of initiating essential immune responses against the potential risk of excessive inflammation. Indeed, inflammatory bowel disease (IBD), which is classically defined as chronic uncontrolled intestinal inflammation, can be characterized by elevated frequencies of activated T cells, including Th17 cells, in the intestine (36, 37) and increased percentages of activated and proliferating T cells in the periphery (38). In addition, it is well established that activated CD4+ T cells are the preferential targets of HIV (39) and the high prevalence of these cells in the GI tract causes this tissue to experience the greatest CD4+ T cell loss during progressive HIV infection and become a reservoir for HIV replication (40, 41). Therefore we investigated whether treatment with PBio could impact the frequency of activated or proliferating cells. We found that the frequency of activated CD4+ T cells, as measured by HLA-DR expression, in the colon was significantly decreased at day 28 post-PBio therapy (p=0.0310, Figure 5A) and trended towards a sustained decrease to day 80 post-PBio treatment (p=0.0612, Figure 5A). In addition, the frequency of proliferating CD4+ T cells, as measured by Ki-67 expression, in the colon was significantly reduced 28 days post-PBio treatment (p=0.0042, Figure 5B) and this decrease was sustained to day 77/80 post-PBio therapy (p=0.0013, Figure 5B). This indicates that treatment with PBio therapy may decrease harmful excessive activation of adaptive immunity at mucosal sites, and for HIV infection in particular, could aid in reducing the availability of potential HIV target cells.
Fig. 5.
Decreased frequency of activated and proliferating CD4+ T cells in the colon post-PBio therapy. Percentage of HLA-DR expressing (A) and Ki-67 expressing (B) CD4+ T cells at all time-points. Cells were identified by first gating on lymphocytes and excluding doublets using FSC and SSC properties and removing dead cells with an Aqua Live/Dead viability dye. The frequency of CD3+CD4+ HLA-DR+ or CD4+Ki-67+ cells were then identified within these cells. Each animal is represented by a different symbol (n=5). Each experiment was performed once per animal per time-point. Statistical significance between the two post-PBio timepoints (d28 or d77/80) and the pre-PBio time-point (d−7) was calculated using a paired t test.
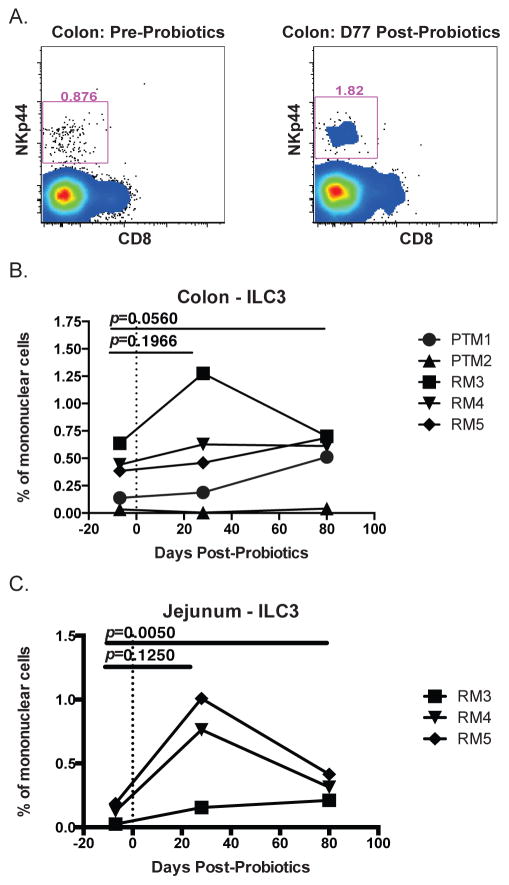
PBio therapy may alter frequency of ILC3
In addition to the well documented loss of CD4+ T cells from the mucosa of HIV infected individuals, recent work has shown that a subset of innate lymphoid cells (ILC), known as ILC3, are depleted in the mucosa of SIV infected macaques (42–44). ILC3s play an important role in maintaining intestinal integrity through their production of homeostatic cytokines, including IL-22 and IL-17, and loss of these cells during SIV infection contributes to loss of epithelial integrity (42, 45), which could contribute to gut dysfunction. We therefore examined whether PBio therapy would have a beneficial impact on ILC3 frequency by gating NKp44+ ILCs by flow cytometry as described previously (42) (Figure 6A, representative staining). We found that PBio therapy resulted in a trend towards increased ILC3 frequency in the colon post-PBio treatment (day 28: p=0.1966; day 77/80: p=0.0560; Figure 6B).
Fig. 6.
Increased frequency of ILC3 in colon and jejunum post-PBio therapy. (A) Representative staining demonstrating ILC3 frequency in the colon pre-PBio (left panel) and post-PBio (right panel). Cells were identified by first gating on CD45+ lymphocytes and excluding doublets using FSC and SSC properties and removing dead cells with an Aqua Live/Dead viability dye. The frequency of ILC3s were then identified by gating on CD3-CD8-HLA-DR-/low NKp44+ cells. Percentage of ILC3 in the colon (B) and jejunum (C) at all time-points. Each animal is represented by a different symbol (n=5). Each experiment was performed once per animal per time-point. Statistical significance between the two post-PBio time-points (d28 or d77/80) and the pre-PBio timepoint (d−7) was calculated using a paired t test.
Previous work has shown that ILC3 are persistently depleted in the jejunum of SIV-infected macaques (44). Therefore, in a subset of animals (only three animals with jejunum data available), we investigated whether ILC3 frequency in the jejunum would be impacted by PBio therapy. We found that in this small group, ILC3s trended towards increasing in the jejunum 28 days post-PBio treatment and were significantly increased by 77/80 days post-PBio treatment (p=0.1250 and 0.0050, respectively; Figure 6C). These data suggest that PBio therapy enhances ILC3 frequencies in the small intestine and could potentially enhance mucosal homeostasis in HIV-infected individuals.
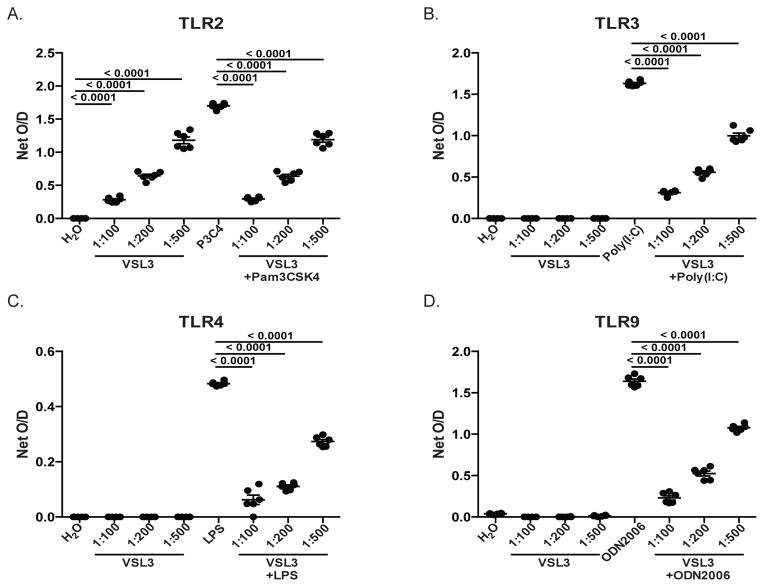
PBio modulates signaling through TLRs
Previous work has demonstrated that PBio therapy exerts its immunomodulatory effect by regulating signaling through pattern recognition receptors (PRR), including Toll-like receptors (TLRs) (14, 46, 47). In order to better understand the mechanism by which PBio therapy elicits the observed immune changes we describe here, we utilized HEK-293 T cells that had been stably transfected with individual TLRs and a colorimetric reporter gene. TLR2-, TLR3-, TLR4-, TLR9 expressing and Null-1 control cells were cultured in the presence of various doses of PBio, positive controls specific for each TLR, or combinations of the PBio doses and the positive controls. Activation of TLR signaling was assessed after 18hrs of culture. PBio at all doses was able to elicit positive signaling through TLR2 (p<0.0001 for all doses, Figure 7A), but induced no positive signaling through TLR3, TLR4 or TLR9 (Figure 7B–C). Compared to the level of signaling in response to the positive control alone, co-culture of TLR2-, TLR3-, TLR4- and TLR9-expressing cells with PBio at all doses significantly reduced signaling in response to the positive controls: Pam3CSK4, Poly (I:C), LPS and ODN2006, respectively (p<0.0001 for all comparisons, Figure 7A–D). Taken together, these data suggest that PBio are able to modulate signaling through TLRs, thus providing a mechanism by which PBio can regulate and contribute to intestinal homeostasis.
Fig. 7.
PBio down-modulates signaling through TLRs. Culture of TLR2- (A), TLR3- (B), TLR4- (C) and TLR9-expressing (D) HEK-293 cells with various doses of PBio (1 capsule in 100ml water [1:100], 1 in 200ml [1:200] or 1 in 500ml [1:500]) alone or in conjunction with positive controls specific for each TLR (TLR2: Pam3CSK4 10ng/ml; TLR3: Poly (1:C) 1ug/ml; TLR4: Ultrapure LPS-EK 100ng/ml; TLR9: ODN2006 10ug/ml). Data are representative of six independent experiments. Statistical significance between either the PBio alone and the negative control or the PBio+positive control and the positive control alone was calculated using a paired t test.
Discussion
Here we demonstrated that modulating the intestinal microbiome in healthy primates induces alterations to systemic and mucosal immunity and provides potential mechanistic insight into why probiotic therapy has profound benefits when routinely used. We observed that PBio administration resulted in elevated frequencies of colon and LN IL-23+ APCs. IL-23 is critical in inducing Tfh responses, which in turn are necessary for inducing B cell responses. Indeed, we found that the post-PBio frequency of colon IL-23+ APCs correlated positively with elevated frequencies of LN Tfh. This elevation in Tfh frequency likely contributed to the increased percentage of IgA producing B cells we observed in the colon and the LN post-PBio. Furthermore, we identified significant PBio-induced increases in the frequency of jejunum and colon ILC3s. Although IL-23+ cells and ILC3 did not correlate (data not shown), IL-23 is associated with ILC development and overall increases in the mucosae could contribute to increased ILC3s. As mucosal IgA and intestinal ILC3s are essential in maintaining intestinal homeostasis, our data provide a model by which PBio promotes mucosal health through the modulation of the intestinal and peripheral immune functions.
Our results show that there is some variation over time in the response to PBio therapy. Notably, the significant increase in IL-23+ APCs at day 28 post-PBio therapy is not sustained to day 77 post-PBio treatment, despite continual treatment. A possible explanation for this decrease could be that the APCs become “tolerant” of PBio therapy over time, thereby decreasing their cytokine response to the treatment. Alternatively, the APCs could be trafficking to various lymphatic sites, lowering the overall frequencies of these cells in the colon and LN, and thereby contributing to the systemic effects of PBio therapy observed in this study. Further studies are necessary in order to determine the exact cause of this observation.
Previous work has suggested that the method by which PBio exerts its beneficial effect is through modulation of TLR signaling. In particular, TLR9 has been suggested as a mediator of the beneficial impact of PBio, as treatment with PBio was unable to decrease the severity of DSS-induced colitis in TLR9-deficient mice (48). Our data utilizing individual TLR expressing cell lines suggests that VSL#3 PBio do not specifically signal through TLR3, 4 or 9, but by TLR2. However, regardless of the ability to positively signal through TLRs, VSL#3 PBio are able to down-modulate the response of TLR2-, TLR3-, TLR4- and TLR9-expressing cell lines to stimulation with their specific ligands. These results indicate that VSL#3 may play a regulatory role in the intestine by limiting TLR-induced immune activation via reducing the amount of signaling through TLRs. These findings have particular implications for inflammatory diseases such as HIV, in which breakdown of the intestinal epithelial barrier results in high levels of translocation of bacterial products, particularly LPS, from the lumen of the gut into the systemic periphery. Pro-inflammatory responses to translocated microbes and microbial products, such as via TLR4 signaling after LPS stimulation, contribute to the elevated immune activation and inflammation that are characteristic of HIV infection (49). Thus, our findings suggest that the use of PBio therapy to down-modulate inflammatory signaling through TLRs could help to reduce immune activation in the GI tract in response to HIV-associated bacterial translocation. Limiting inflammation in this tissue could not only promote intestinal homeostasis and health, but also potentially reduce residual viral replication and eventually diminish the intestinal viral reservoir. In addition, inflammation has been highly correlated with transmission of STIs and HIV, and thus decreasing overt inflammation at mucosal surfaces may lead to increased protection from infections. Future studies are necessary to determine whether this mechanism determined using an in vitro culture system would be useful in minimizing inflammation in vivo, given the complexity of TLR signaling in tissues.
The alterations we observed here have several implications for improving vaccine responses or therapeutics, especially in the context of HIV and other mucosal infections such as STIs. The robust immunity this therapy induced could potentially be protective when combined with vaccines by increasing vaccine-induced mucosal immunity. Indeed, testing PBio therapy as a persistent “adjuvant” for mucosal vaccination strategies is warranted. However, a concern when eliciting mucosal immunity is the fear of unintentionally inducing targets for infection, especially in the context of HIV, where CD4+ T cells are the major targets for infection. This was of particular concern here given the increased frequencies of Tfh cells, which have been shown to be preferential targets for HIV infection (50, 51). However, our data suggest that treatment with PBio leads to a reduction in mucosal frequencies of activated and proliferating CD4+ T cells. Thus, even though Tfh cells are increased in lymphoid tissues, the decreased frequency of activated and proliferating CD4+ T cells at the mucosal portals of entry may be protective, warranting further investigation of these mechanisms.
One question that remains unanswered is whether the beneficial effects of PBio therapy are due to bacteria themselves or bacterial products. It has been hypothesized that probiotics do not persistently colonize the GI tract and as such long-term daily treatment is required for beneficial effects. This indicates that it is more likely the bacterial products that are important for immunity. Indeed, our data demonstrate alterations to TLR signaling with probiotics even in short term co-cultures, which suggests that bacterial products capable of stimulating or inhibiting TLR responses can result in modulated immunity. Future studies to determine mechanisms to improve colonization of probiotic bacteria or alternatively sustained delivery of bacterial products that does not require daily intake of probiotics would be highly beneficial.
A caveat of this study is that a relatively small number of macaques (n=5) were utilized and some animal-to-animal variation was observed. However, even within this small subset of animals, PBio therapy had a statistically significant impact and we expect that with a larger sample size we would see similar, if not more striking differences. Additional studies with larger animal cohorts are currently being planned to further investigate the immunological impact of PBio therapy. A further caveat of this study is that we utilized both PTM and RM simultaneously in order to understand the impact of PBio treatment. However, because no differences in the results obtained with either type of macaque were found, we believe that this in fact strengthens our findings, as our results are consistent across species and are not just limited to a particular subset.
In summary, these data provide a comprehensive analysis of the effects of PBio therapy in healthy primates. We found that PBio therapy results in increases in many immune cell types that could be extraordinarily protective in the context of mucosal immunity and infections. In addition, given that probiotics are extremely well tolerated with virtually no adverse side effects, use of PBio in mucosal protection could be very advantageous. These data demonstrate that future studies to exploit microbiome manipulation as a tool to improve mucosal vaccine responses or decrease mucosal inflammation and infections should be performed.
Supplementary Material
Acknowledgments
We would like to thank all veterinary staff of the Washington National Primate Research Center for animal studies. We also thank Michael Koday for assistance with tissue processing. We would like to thank Sigma-Tau for their generous contribution of VSL#3 probiotics for these studies. We thank Dr. Jason Brenchley for helpful advice in preparing this manuscript. We thank the histology and immunohistochemistry core of the UW Department of Comparative Pathology for assistance with tissue preparation.
Footnotes
These studies were supported by start-up funds to NRK from University of Washington and Washington National Primate Research Center. Follow-up studies were supported by 1R01AI120712-01 to NRK and 1R21AI120795-01 to RKR.
Disclosure/Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology letters. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Brotman RM, Ravel J, Bavoil PM, Gravitt PE, Ghanem KG. Microbiome, sex hormones, and immune responses in the reproductive tract: challenges for vaccine development against sexually transmitted infections. Vaccine. 2014;32:1543–1552. doi: 10.1016/j.vaccine.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushic C. The role of the local microenvironment in regulating susceptibility and immune responses to sexually transmitted viruses in the female genital tract. Journal of reproductive immunology. 2009;83:168–172. doi: 10.1016/j.jri.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Current opinion in immunology. 2015;36:22–30. doi: 10.1016/j.coi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Reeves RK, Burgener A, Klatt NR. Targeting the gastrointestinal tract to develop novel therapies for HIV. Clinical pharmacology and therapeutics. 2015;98:381–386. doi: 10.1002/cpt.186. [DOI] [PubMed] [Google Scholar]
- 6.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 7.Madsen KL. The use of probiotics in gastrointestinal disease. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2001;15:817–822. doi: 10.1155/2001/690741. [DOI] [PubMed] [Google Scholar]
- 8.Hummelen R, Vos AP, van’t Land B, van Norren K, Reid G. Altered host-microbe interaction in HIV: a target for intervention with pro- and prebiotics. International reviews of immunology. 2010;29:485–513. doi: 10.3109/08830185.2010.505310. [DOI] [PubMed] [Google Scholar]
- 9.Boirivant M, Amendola A, Butera A. Intestinal microflora and immunoregulation. Mucosal immunology. 2008;1(Suppl 1):S47–49. doi: 10.1038/mi.2008.52. [DOI] [PubMed] [Google Scholar]
- 10.Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflammatory bowel diseases. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 11.Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. BioMed research international. 2015;2015:505878. doi: 10.1155/2015/505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. Journal of clinical gastroenterology. 2006;40:260–263. doi: 10.1097/00004836-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 14.de Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Frontiers in immunology. 2014;5:60. doi: 10.3389/fimmu.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong Y, Huang J, Tang W, Chen B, Cai W. Effects of probiotics, probiotic DNA and the CpG oligodeoxynucleotides on ovalbumin-sensitized Brown-Norway rats via TLR9/NF-kappaB pathway. FEMS immunology and medical microbiology. 2012;66:71–82. doi: 10.1111/j.1574-695X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 16.Kant R, de Vos WM, Palva A, Satokari R. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. Journal of medical microbiology. 2014;63:293–308. doi: 10.1099/jmm.0.064220-0. [DOI] [PubMed] [Google Scholar]
- 17.Kitazawa H, Villena J. Modulation of Respiratory TLR3-Anti-Viral Response by Probiotic Microorganisms: Lessons Learned from Lactobacillus rhamnosus CRL1505. Frontiers in immunology. 2014;5:201. doi: 10.3389/fimmu.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villena J, Chiba E, Vizoso-Pinto MG, Tomosada Y, Takahashi T, Ishizuka T, Aso H, Salva S, Alvarez S, Kitazawa H. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC microbiology. 2014;14:126. doi: 10.1186/1471-2180-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlasova AN, Chattha KS, Kandasamy S, Liu Z, Esseili M, Shao L, Rajashekara G, Saif LJ. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PloS one. 2013;8:e76962. doi: 10.1371/journal.pone.0076962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quinones M, Deming CB, Perkins M, Hazuda DJ, Miller MD, Lederman MM, Segre JA, Lifson JD, Haddad EK, Estes JD, Brenchley JM. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. The Journal of clinical investigation. 2013;123:903–907. doi: 10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz AM, Klase ZA, DiNapoli SR, Vujkovic-Cvijin I, Carmack K, Perkins MR, Calantone N, Vinton CL, Riddick NE, Gallagher J, Klatt NR, McCune JM, Estes JD, Paiardini M, Brenchley JM. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal immunology. 2015 doi: 10.1038/mi.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hummelen R, Changalucha J, Butamanya NL, Koyama TE, Cook A, Habbema JDF, Reid G. Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microbes. 2014;2:80–85. doi: 10.4161/gmic.2.2.15787. [DOI] [PubMed] [Google Scholar]
- 23.Irvine SL, Hummelen R, Hekmat S. Probiotic yogurt consumption may improve gastrointestinal symptoms, productivity, and nutritional intake of people living with human immunodeficiency virus in Mwanza, Tanzania. Nutrition research. 2011;31:875–881. doi: 10.1016/j.nutres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Schunter M, Chu H, Hayes TL, McConnell D, Crawford SS, Luciw PA, Bengmark S, Asmuth DM, Brown J, Bevins CL, Shacklett BL, Critchfield JW. Randomized pilot trial of a synbiotic dietary supplement in chronic HIV-1 infection. BMC complementary and alternative medicine. 2012;12:84. doi: 10.1186/1472-6882-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A, Saenz D, Sorli L, Montero M, Horcajada JP, Knobel Freud H. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. Journal of acquired immune deficiency syndromes (1999) 2015;68:256–263. doi: 10.1097/QAI.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 26.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, Martin MA, Levine AD, Estes JD, Brenchley JM. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal immunology. 2010;3:387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal immunology. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobita K, Yanaka H, Otani H. Lactobacillus crispatus KT-11 enhances intestinal immune functions in C3H/HeN mice. Journal of nutritional science and vitaminology. 2010;56:441–445. doi: 10.3177/jnsv.56.441. [DOI] [PubMed] [Google Scholar]
- 29.Ya T, Zhang Q, Chu F, Merritt J, Bilige M, Sun T, Du R, Zhang H. Immunological evaluation of Lactobacillus casei Zhang: a newly isolated strain from koumiss in Inner Mongolia, China. BMC immunology. 2008;9:68. doi: 10.1186/1471-2172-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigon G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clinical and vaccine immunology : CVI. 2007;14:485–492. doi: 10.1128/CVI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 32.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans TI, Li H, Schafer JL, Klatt NR, Hao XP, Traslavina RP, Estes JD, Brenchley JM, Reeves RK. SIV-induced Translocation of Bacterial Products in the Liver Mobilizes Myeloid Dendritic and Natural Killer Cells Associated With Liver Damage. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nature immunology. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, Banchereau J, Casanova JL, Ueno H. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. The Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 37.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. The Journal of experimental medicine. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funderburg NT, Stubblefield Park SR, Sung HC, Hardy G, Clagett B, Ignatz-Hoover J, Harding CV, Fu P, Katz JA, Lederman MM, Levine AD. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140:87–97. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, Grovit-Ferbas K, Mackay CR, Chen ISY, Giorgi JV. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS (London, England) 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 41.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Richert-Spuhler LE, Evans TI, Gillis J, Connole M, Estes JD, Keele BF, Klatt NR, Reeves RK. Hypercytotoxicity and rapid loss of NKp44+ innate lymphoid cells during acute SIV infection. PLoS pathogens. 2014;10:e1004551. doi: 10.1371/journal.ppat.1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, Kuzmichev YV, Carville A, Johnson RP. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal immunology. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal immunology. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflammatory bowel diseases. 2008;14:1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 47.Kemgang TS, Kapila S, Shanmugam VP, Kapila R. Cross-talk between probiotic lactobacilli and host immune system. Journal of applied microbiology. 2014;117:303–319. doi: 10.1111/jam.12521. [DOI] [PubMed] [Google Scholar]
- 48.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 50.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klatt NR, Vinton CL, Lynch RM, Canary LA, Ho J, Darrah PA, Estes JD, Seder RA, Moir SL, Brenchley JM. SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood. 2011;118:5803–5812. doi: 10.1182/blood-2011-07-365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.