Abstract
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells that promote tumor progression. Herein, we demonstrated that activation of a C-type lectin receptor, dectin-1, in MDSC differentially modulates the function of different MDSC subsets. Yeast-derived whole β-glucan particles (WGP), a ligand to engage and activate dectin-1, oral treatment in vivo significantly decreased tumor weight and splenomegaly in tumor-bearing mice with reduced accumulation of PMN-MDSC but not M-MDSC, and decreased PMN-MDSC suppression in vitro through the induction of respiratory burst and apoptosis. On a different axis, WGP-treated M-MDSC differentiated into F4/80+CD11c+ cells in vitro that served as potent antigen-presenting cells (APC) to induce Ag-specific CD4+ and CD8+ T cell responses in a dectin-1 dependent manner. In addition, ERK1/2 phosphorylation was required for the acquisition of APC properties in M-MDSC. Moreover, WGP-treated M-MDSC differentiated into CD11c+ cells in vivo with high MHC class II expression and induced decreased tumor burden when inoculated subcutaneously with LLC cells. This effect was dependent of the dectin-1 receptor. Strikingly, patients with non-small cell lung cancer (NSCLC) that had received WGP treatment for 10–14 days prior to any other treatment had a decreased frequency of CD14−HLA-DR−CD11b+CD33+ MDSC in the peripheral blood. Overall, these data indicate that WGP may be a potent immune modulator of MDSC suppressive function and differentiation in cancer.
Introduction
It is well appreciated that tumor cells produce a plethora of immune modulatory factors that constraint the tumor cytotoxic effects mediated by anti-tumor innate and adaptive immune responses (1–3). Not only tumor-derived factors drive angiogenesis for nutrient supply but also disrupt the rhythm of differentiation of bone marrow-derived immune cells towards the accumulation and expansion of a heterogenous population of immature immune-suppressive cells known collectively as myeloid-derived suppressor cells (MDSC) (4). In mice, two main subsets of MDSC have been identified according to their morphology and Gr-1, Ly6C, Ly6G and CD11b expression: monocytic MDSC (M-MDSC) resemble monocytes and are Gr1low/int CD11b+(Ly6ChighLy6G−CD11b+) (5) and polymorphonuclear MDSC (PMN-MDSC) resemble polymorphonuclear granulocytes and are Gr-1highCD11b+(Ly6GhighLy6ClowCD11b+) (6). In humans, MDSC lack the Gr-1 homolog and are defined as CD14− HLA-DR− CD11b+ CD33+ or CD14+HLA-DR−CD11b+CD33+ (7–10).
After the identification of MDSC as one of the major suppressors of T cell responses and inducers of T cell tolerance (11, 12), numerous studies have characterized their roles in cancer as suppressors of NK cells (13), inducers of regulatory T cells (Tregs) (14) , and precursors of tumor-associated macrophages (7). MDSC-mediated T cell suppression is mainly attributed to the expression of Arginase 1, iNOS, ROS (4) and cystine and cysteine deprivation (15). A main factor responsible for the accumulation of MDSC in cancer is the fact that MDSC are immature and do not subsequently differentiate to anti-tumor macrophages and dendritic cells (DCs) under the influence of tumor-derived factors (16). Therefore, the importance of targeting MDSC expansion, suppression and differentiation in combination with other therapies in cancer is being very well appreciated (17).
In an attempt to study a natural compound that targets MDSC, we studied the effect of the immunomodulator, particulate β-glucan on MDSC in tumor-bearing animals and non-small cell lung cancer (NSCLC) patients. Whole glucan particles (WGP) are micro-particles of 1,3-β-glucan extracted from the yeast Saccharomyces cerevisiae, that have been shown to activate immune cells through the stimulation of C-type lectin receptor, dectin-1 (18, 19). Previous studies have shown that β-glucan treatment activates dendritic cells (DC) and induces T cell responses in vivo (20). In addition, a recent report showed that WGP partially induces the differentiation of M-MDSC to F4/80+CD11c+ cells (21). However, the role of particulate β-glucan in the modulation of the function of different MDSC subsets in mice and its implementation in human patients needs further studies.
Herein, we delineated the effect of particulate β-glucan on the function of both PMN-MDSC and M-MDSC in mice. We demonstrated that particulate β-glucan induced a cytotoxic phenotype and subsequent apoptosis in PMN-MDSC, while converting M-MDSC to potent antigen-presenting cells (APCs) that uptake, process and present ovalbumin (OVA) Ag to OVA-specific CD4+ T cells and also cross-present Ag to prime OVA-specific CD8+ T cells. More importantly, NSCLC patients treated with particulate β-glucan for two weeks had a decreased accumulation of CD14− HLA-DR−CD11b+CD33+ MDSC in their peripheral blood as compared to its frequency in the peripheral blood before treatment, which was correlated with an increased trend in peripheral monocytes and MDSC Ag presentation function, and a decreased trend in Arginase 1 mRNA expression in peripheral PMN. Overall, these findings provide a further step in our understanding of the mechanisms by which particulate β-glucan enhances anti-tumor immunity in mice and how that findings can be translated in the benefit of cancer patients.
Materials and Methods
Mice and tumor models
Wildtype (WT) C57BL/6 mice were purchased from the National Cancer Institute (NCI). Dectin-1 KO mice were described previously (22, 23). OT-I Rag1−/− and OT-II CD4 ovalbumin TCR-Tg mice were purchased from Taconic.
Tumor models and particulate β-glucan therapeutic protocol were performed as described previously (20). Briefly, C57BL/6 WT or dectin-1 KO mice were implanted subcutaneously (s.c.) with Lewis Lung Carcinoma (LLC) (2x105/mouse) or mammary-cell carcinoma (E0771) cell lines (6x105/mouse). On day 8 post-implantation, mice with palpable tumors were orally administered daily by gavage with 100 µl particulate β-glucan (Biothera, 800 µg/mouse in PBS suspension) or with 100 µl PBS. Tumor diameters were measured with a caliper every three-four days, and mice were killed when the tumor diameter reached 15mm. The tumor volume was calculated based on the formula=length x width2/2. The protocols on murine tumor models were performed according to the institutional guidelines and laws, and were approved by the Institutional Animal Care and Use Committee at the University of Louisville.
Human subjects
NSCLC patients that were newly diagnosed, received particulate β-glucan treatment at the James Graham Brown Cancer Center, University of Louisville. These patients did not receive any other treatment prior or during β-glucan treatment. The clinicalpathological features of those patients were summarized in the Supplemental Table 1. The study was approved by the Institutional Ethical Board and blood samples were collected upon a written informed consent. Each NSCLC patient received 500 mg of particulate β-glucan for 10–14 days. Peripheral blood was collected before and after treatment. Whole blood cells were treated with ACK lysis buffer, washed 2X with PBS and stained with human anti-CD14, anti-HLA-DR, anti-CD11b and anti-CD33 Abs (Biolegend) for 30 min on ice. Samples were then washed 2X prior to acquisition on FACS Calibur. Neutrophils were purified with a histopaque density gradient and stored into Trizol at −80°C for RNA extraction (24).
Single cell suspensions from tumors
Mouse tumor tissues (12–15 mm in diameter) were excised and minced into small pieces. Additional mechanical digestion was performed with gentleMACS dissociator (Milteneyi Biotec). Tumors were enzymatically digested in RPMI 1640 medium containing 10% FBS, type IV collagenase (1 µg/ml) and hyaluronidase (10 ng/ml) for 45 minutes at 37°C on a rotator. A second step of mechanical dissociation was performed after enzymatic digestion. The digested cells were then filtered, pelleted and re-suspended in complete RPMI.
Flow Cytometry and Cell Sorting
Single-cell suspensions were treated with Fc-blocker for 10 min on ice and stained with the relevant fluorochrome-labeled mAbs for 30 min on ice. Cells were washed 2X with PBS or staining buffer (PBS+0.1%FBS) (for FACS analysis) or running buffer (Milteney) (for cell sorting). For FACS analysis, cells were acquired using FACS Calibur or FACS Canto II (BD biosciences). MDSC were sorted using BD FACSAria III cell sorter or MoFlo XDP (Beckman Coulter). The purity of sorted cells was >98% assessed by flow cytometry. For intracellular staining of IFN-γ and granzyme B, cells were first stained with either anti-CD4 or anti-CD8 Abs, fixed, permeabilized with Fixation/permeabilization buffer (Biolegend), washed with 1X permeabilization/washing buffer (Biolegend) and stained with the relevant cytokine Abs. Cells were then washed 2X with 1X perm/washing buffer and analyzed by flow cytometry. Data analysis was performed using FlowJo software (Tree Star). The following fluorochrome-labeled monoclonal antibodies were used: anti-mouse Gr-1, anti-mouse Ly6G, anti-mouse Ly6C, anti-mouse CD11b, anti-mouse CD45, anti-mouse F4/80, anti-mouse CD11c, anti-mouse CD40, anti-mouse CD86, anti-mouse CD80, anti-mouse IA/IE, anti-mouse H2-Kb, anti-CD8, anti-CD4, anti-IFN-γ, anti-GranzymeB and their corresponding isotype controls were purchased from Biolegend. The following anti-human monoclonal antibodies: anti-CD11b, anti-CD14, anti-CD33, anti-HLA-DR, anti-CD3 anti-IFN-γ with their corresponding isotype controls were also purchased from Biolegend.
Apoptosis assays
Splenocytes from LLC-bearing C57BL/6 or Dectin-1−/−mice were stimulated with WGP for 3, 6, 14, or 18 hours and then stained with anti-Gr-1 and anti-CD11b Abs (Biolegend) and 7AAD and Annexin V dyes (BD biosciences). Cells were analyzed by flow cytometry.
Respiratory Burst assays
Sorted PMN-MDSC from WT or Dectin-1−/− mice were stained with dihydrorhodamine (DHR) 123 as described previously (25, 26). Briefly, cells were incubated with 1µM DHR and catalase for 5 min at 37°C. Particulate β-glucan was then added for 30 min, 60 min and 120 min. Rhodamine (RHO) was then detected on FL-1 channel on a FACS Calibur or FACS Canto II.
Western Blot analysis
Sorted M-MDSC or PMN-MDSC stimulated with or without particulate β-glucan (100 µg/ml) for indicated times were lysed in Triton X-100 lysis buffer in the presence of protease and phosphatase inhibitor. The whole cell extracts were subjected to SDS-PAGE and electro-transferred to PDVF membrane. The membranes were blocked and probed overnight at 4°C with the relevant primary and then incubated with the secondary Abs. The blots were developed with ECL Plus Western Blotting Detection Reagents (GE Healthcare). The primary Abs included: p-Erk1/2 (Thr202/Tyr204, Cell Signaling). Erk1/2 (MK1, Santa Cruz), p-Stat3 (Tyr705, Cell Signaling), p-AKT (Ser473, Cell Signaling), p-p38 (Thr180/Tyr182, Cell Signaling), p-Zap/Syk (Tyr319/Tyr352, Cell Signaling), STAT3 (C-20, Santa Cruz), p-SAPK/JNK (Thr183/Tyr185, Cell Signaling) and β-actin (Sigma-Aldrich).
Monocytic-MDSC (M-MDSC) differentiation assay
M-MDSC (CD11b+Ly6ChighLy6G−) were sorted from the spleens of LLC-bearing WT or dectin-1 KO mice and cultured for 7 days with 50 µg/ml particulate β-glucan in 48-well plates (corning). For in vivo differentiation assay, M-MDSC were sorted from C57Bl/6 LLC tumors (CD45.2) and treated with WGP (100 µg/ml) at 37 °C for overnight. Freshly isolated and WGP-treated M-MDSC were intratumorally injected into SJL LLC tumor-bearing mice (CD45.1). The mice were sacrificed 7 days later and single cell suspension from tumors was stained with anti-CD45.2, F4/80, CD11c, and MHC class II mAbs. The cells were analyzed by flow cytometry.
T cell proliferation and Ag-presentation assays
For T cell proliferation assay, M-MDSC and PMN-MDSC sorted from the spleens or Gr-1+CD11b+ MDSC from tumors of LLC-bearing mice, were co-cultured with 1µM carboxyfluorescin dye (CFSE)-labeled splenocytes from OT-II or OT-I mice in the presence of OVA (100 µg/ml in OT-II cultures, 50µg/ml in OT-I cultures, and 10 µg/ml in some splenic PMN-MDSC suppression experiments) and particulate β-glucan (50 µg/ml). Three days later, cells were harvested and stained. In addition, some T cell proliferation assays were performed by co-culturing sorted MDSC with CFSE-labeled splenocytes from C57BL/6 mice stimulated with plate-bound anti-CD3 (5 µg/ml) and soluble anti-CD28 (2 µg/ml).
For Ag-presentation assay, sorted M-MDSC from the spleens of LLC-bearing WT or dectin-1 KO mice were cultured in the presence or absence of particulate β-glucan (50µg/ml) for 7 days. In some experiments, MEK1/2 inhibitor (PD98059) (30 ng/ml) or DMSO was added to cultures during differentiation. Cells were washed and co-cultured with sorted and CFSE-labeled CD8+ or CD4+ T cells from OT-I and OT-II mice, respectively, in the presence or absence of whole OVA-Ag (50 µg/ml). T cell proliferation and IFN-γ or granzyme B production were assessed 4–5 days later by flow cytometry.
Tacking Ag-specific T cells by tetramer staining
To determine WGP treatment on Ag-specific T cell responses, WT mice were injected with OVA-expressing EG7 cells (3x106/mouse). After palpable tumors formed, mice were adoptively transferred with purified OT-1 CD8 T cells (1x106/mouse). Mice were treated with or without WGP treatment for 2 wks and sacrificed. Peripheral blood (PB) and tumor cells were used for tetramer staining. In brief, cells were blocked with Fc block (anti-CD16/32) for 10 min and then incubated with Alexa 488-labeled OVA H2-Kb MHC class I tetramer obtained from the NIH Tetramer Core Facility (1 to 1,000 dilution) for 30 min at room temperature. Cells were then washed twice with PBS and stained with CD8 and CD19 mAbs and analyzed by flow cytometry. Total CD8+CD19− cells were gated and analyzed for Tetramer binding.
RNA extraction and Quantitative Real-Time PCR (qRT-PCR)
RNA was extracted with Trizol reagent (Invitrogen) as described in the manufacturer protocols. Extracted RNA was transcribed to cDNA with a Reverse Transcription Kit (Bio-Rad). qRT-PCR reaction was performed using SYBR Green Supermix (Bio-Rad) with the relevant primers (Table S1) and the reaction was detected on MyiQ single color RT-PCR detection system (Bio-Rad). The change in gene expression was quantified by measuring the change in threshold (ΔΔCT), where ΔCt= Ct target gene-Ct House keeping gene and ΔΔCt= ΔCt induced- ΔCt reference. All primer sequences were listed in the Supplemental Table 2.
In vivo M-MDSC/LLC admixture experiments
Sorted splenic M-MDSC from WT or dectin-1 KO tumor-bearing mice were treated with or without particulate β-glucan for 18 hours and mixed with LLC cells (1x105) at a 1:1 ratio. Cells were mixed with Matrigel® Matrix Basement membrane (Corning) and implanted subcutaneously in the flanks of C57BL/6 mice. Tumor diameter was measured every two days and mice were sacrificed on day 14 or day 25.
Mixed lymphocyte reaction (MLR)
CD14+ HLA-DR+ CD3− or CD14−HLA-DR−CD11b+CD33+CD3− cells were sorted from the PBMCs of NSCLC patients and co-cultured with sorted and CFSE-labeled CD3+ cells from the PBMCs of allogeneic donor at 1:1 ratio for 5 days. Cells were then harvested and stained with human anti-CD3 and human anti-IFN-γ mAbs for subsequent flow cytometry analysis.
Statistical analysis
Data were analyzed using GraphPad Prism (5.0) software. Unpaired student t-test was used to calculate significance. Significance was assumed to be reached at p<0.05. All graph bars are expressed as mean ± SEM.
Results
Particulate β-glucan oral treatment diminishes tumor growth and differentially impacts the frequency of MDSC subsets in spleens and tumors
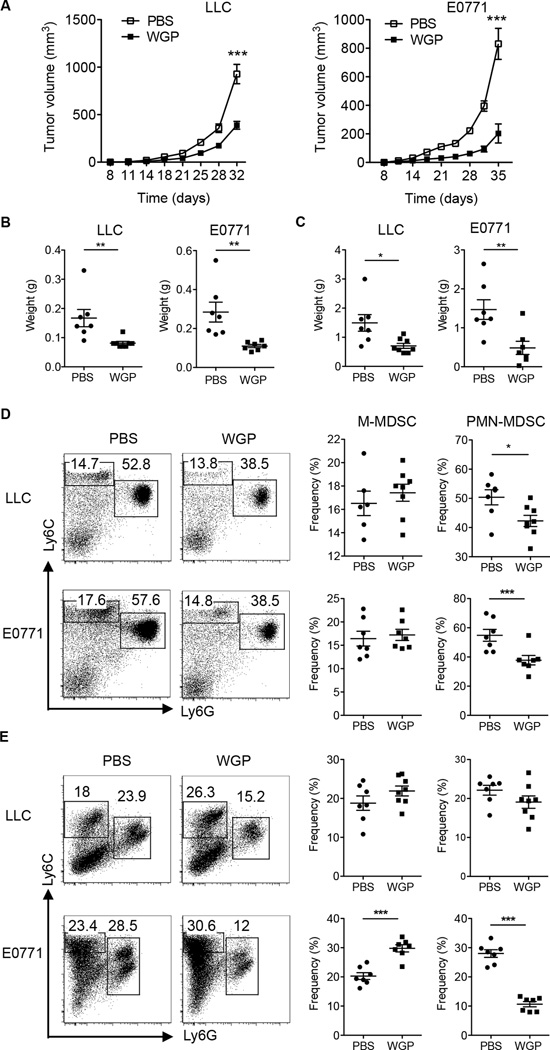
To delineate the effect of particulate β-glucan treatment on the composition of different MDSC subsets in spleens and tumors, C57BL/6 mice were challenged with Lewis Lung Carcinoma (LLC) and mammary carcinoma E0771 cell lines subcutaneously. Mice with palpable tumors were administered orally through gavage with PBS or particulate β-glucan (WGP) as previously established (20, 27). β-Glucan treatment significantly reduced tumor volumes (Fig. 1A), splenomegaly (Fig. 1B), and tumor weight (Fig. 1C) in both models. When analyzing the frequencies of MDSC subsets in the spleens and tumors, particulate β-glucan-treated mice had a significant decrease in PMN-MDSC frequencies in the spleen, but not M-MDSC, in both models (Fig. 1D). In addition, particulate β-glucan treatment caused a significant decrease in the frequencies of PMN-MDSC and increase in the frequencies of M-MDSC in the tumors of E0771 mice and trending in the tumors of LLC-bearing mice (Fig. 1E).
Figure 1. Particulate β-glucan treatment in vivo reduces tumor burden and impacts the frequency of MDSC in spleens and tumors of LLC and E0771-bearing mice.
(A) C57BL/6 WT mice (n=7, 8) were injected subcutaneously (s.c) with LLC or E0771 tumor cell lines. Once palpable tumors were formed (day 8), mice were orally administered with particulate β-glucan (800 µg, daily) or PBS with a gavage needle at indicated time. Tumor diameters were measured every three days and tumor volumes were then calculated. (B) On day 32 (LLC model) or day 35 (E0771 model), mice were killed and spleens were excised and weighed. Each point in the data plot represents the spleen weight of each mouse in grams. PBS-treated group was compared to particulate β-glucan treated group (WGP) in both models (C) Tumor tissues were excised and weighted from WGP or PBS-treated mice. (D) Flow cytometry analysis of the frequencies of M-MDSC (Ly6G−Ly6Chigh) and PMN-MDSC (Ly6G+Ly6Cint) in the spleens of LLC and E0771-bearing mice treated with PBS or particulate β-glucan (WGP). Cells were gated on CD11b+ cells. (E) Frequencies of M-MDSC and PMN-MDSC in the tumors of LLC and E0771-bearing mice treated with PBS or particulate β-glucan (WGP). Cells were gated on CD45+CD11b+ cells. *p<0.05, ** p<0.01, *** p<0.001.
Next, we determined whether particulate β-glucan treatment stimulates Ag-specific T cell responses. To this end, C57Bl/6 mice were inoculated with OVA-expressing EG7 cells. Thus we can track OVA-specific CD8 T cells using tetramer staining. As shown in the Supplemental Fig. 1A, mice treated with WGP exhibited reduced tumor burden. OVA-specific CD8 T cells were significantly increased in the PB of WGP-treated tumor-bearing mice compared to those from untreated mice (Supplemental Fig. 1B). Furthermore, tumor-infiltrating OVA-specific CD8 T cells were also significantly increased in the tumor-bearing mice treated with WGP (Supplemental Fig. 1C). Those data suggest that particulate β-glucan treatment differentially regulate MDSC subsets with enhanced CD8 T cell responses.
Particulate β-glucan treatment abolishes splenic and tumor MDSC-mediated T cell suppression
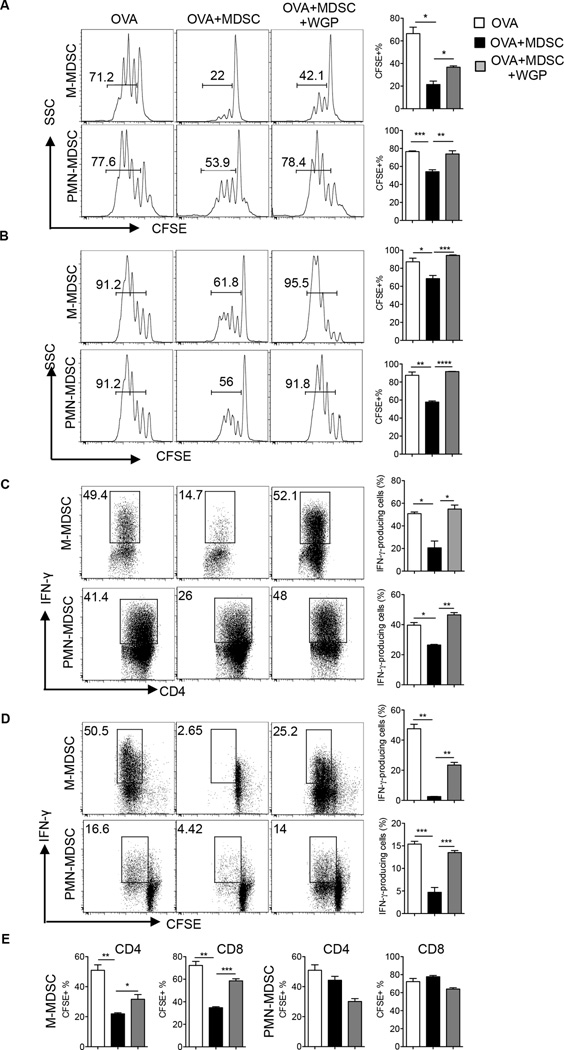
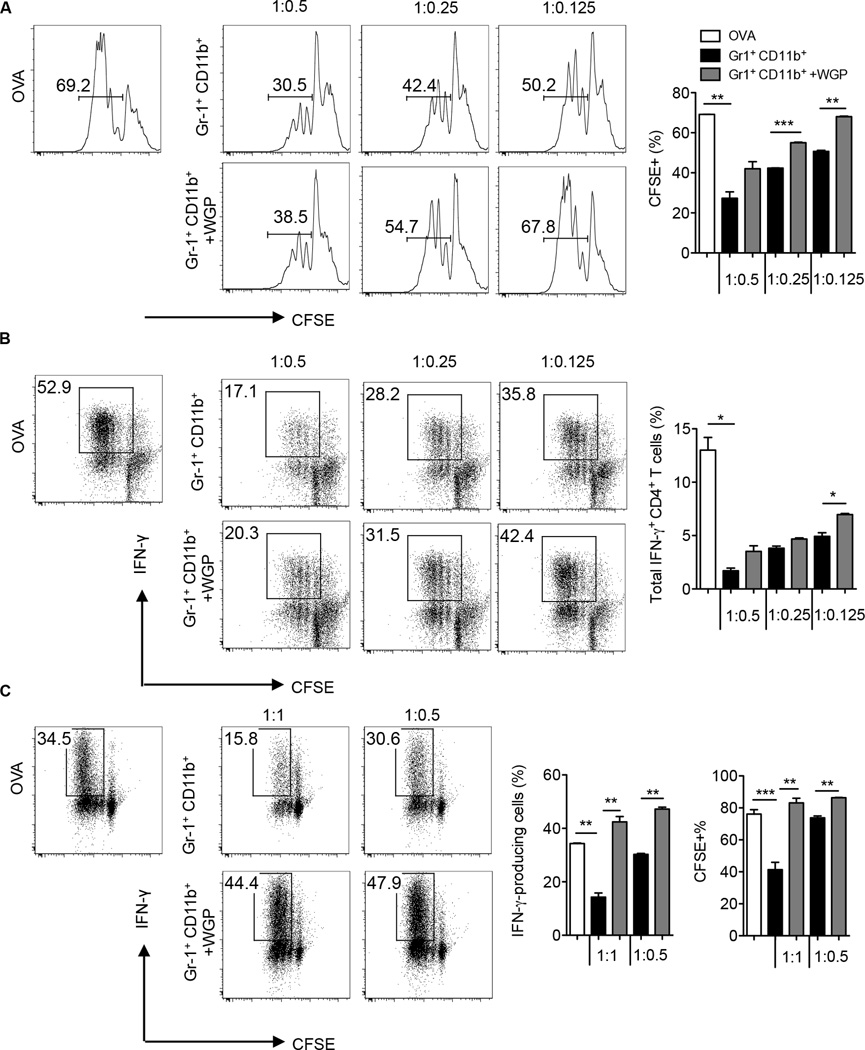
Since suppression of CD8+ effector T cells in tumor-bearing mice is largely attributed to MDSC (28, 29), we thought to determine whether particulate β-glucan impacts T cell suppression mediated by splenic and tumor MDSC. To this end, we studied the effect of particulate β-glucan treatment on MDSC-mediated inhibition of OVA-specific CD4+ and CD8+ T cells or Ag non-specific T cell proliferation induced by anti-CD3/CD28 stimulation. Suppression of OVA-specific CD4+ T cells mediated by splenic M-MDSC or PMN-MDSC was partially or completely obliterated upon particulate β-glucan treatment (Fig. 2A). Similarly, particulate β-glucan treatment significantly increased the proliferation of OVA-specific CD8+ T cells in the presence of suppressive M-MDSC or PMN-MDSC (Fig. 2B). Particulate β-glucan treatment also diminished the MDSC-mediated suppression of IFN-γ produced by OVA-specific CD4+ (Fig. 2C) and CD8+ T cells (Fig. 2D). In addition, particulate β-glucan treatment decreased the ability of M-MDSC to suppress CD4+ T cells and CD8+ T cells stimulated with anti-CD3/CD28 (Fig 2E). However, splenic PMN-MDSC did not suppress the proliferation of T cells stimulated with anti-CD3/CD28, as previously reported (5). To generalize the efficacy of particulate β-glucan on the modulation of MDSC suppression, tumor Gr-1+CD11b+ cells were also sorted from LLC tumors and co-cultured with OVA-specific CD4+ or CD8+ T cells in the presence of OVA at different ratios. While Gr-1+CD11b+ MDSC suppressed the proliferation and IFN-γ production of CD4+ T cells (Fig. 3A–B) and CD8+ T cells (Fig. 3C), particulate β-glucan treatment significantly enhanced T cell proliferation and IFN-γ production on CD4+ and CD8+ T cells (Fig. 3). Taken together, these data emphasize the ability of particulate β-glucan to reverse MDSC-mediated T cell suppression.
Figure 2. Particulate β-glucan treatment in vitro subverts splenic MDSC-mediated T cell suppression.
(A) CFSE-labeled OT-II splenocytes co-cultured with sorted M-MDSC or PMN-MDSC from the spleens of LLC-bearing mice in the presence or absence of particulate β-glucan (100 µg/ml in the M-MDSC cultures and 50 µg/ml in the PMN-MDSC cultures) and OVA (100 µg/ml) for 3 days at 1:1 ratio. Data represent the percentage of CFSE diluted cells gated on CD4+ T cells. The experiment was repeated two times with similar results. (B) CFSE-labeled OT-I splenocytes were co-cultured with sorted M-MDSC or PMN-MDSC from the spleens of LLC-bearing mice in the presence of OVA (50 µg/ml) and particulate β-glucan (50 µg/ml) for 3 days at 1:1 ratio. Data represent the percentage of CFSE diluted cells gated on CD8+ T cells. Data is representative of three independent experiments. (C) OT-II splenocytes co-cultured with sorted M-MDSC or PMN-MDSC from the spleens of LLC-bearing mice in the presence of OVA (100 µg/ml) with or without particulate β-glucan (50 µg/ml) for 3–4 days at 1:1 ratio and stimulated with PMA/Ionomycin for intracellular IFN-γ staining. Data represent the percentage of IFN-γ+ cells gated on CD4+ T cells. The experiment was repeated 3 times with similar results. (D) CFSE-labeled OT-I splenocytes co-cultured with sorted M-MDSC or PMN-MDSC from the spleens of LLC-bearing mice in the presence of OVA (50 µg/ml in M-MDSC cultures and 10 µg/ml in PMN-MDSC cultures) and particulate β-glucan (50 µg/ml) for 3 days at 1:1 ratio and then stimulated with PMA/Ionomycin for intracellular IFN-γ staining. Data represent the percentage of IFN-γ+ CFSE diluted cells gated on CD8+ T cells. Results are representative of three independent experiments. (E) CFSE-labeled splenocytes from C57BL/6 mice stimulated with plate-bound anti-CD3 (5 µg/ml) and soluble anti-CD28 (2 µg/ml) (white bar) and co-cultured with sorted splenic M-MDSC or PMN-MDSC from LLC-bearing mice for 3 days with (black bar) or without particulate β-glucan (50 µg/ml) (grey bar). Data represent the frequency of CFSE diluted cells gated on CD4+ or CD8+ T cells. The experiment was repeated twice with similar results. * p<0.05, **p<0.01, ***p<0.001.
Figure 3. Particulate β-glucan reduces tumor Gr-1+CD11b+ MDSC-mediated T cell suppression.
(A) Tumor Gr-1+CD11b+CD45+ MDSC sorted from LLC-bearing mice were co-cultured with CFSE-labeled OT-II splenocytes at indicated ratios, in the presence of OVA (100 µg/ml) with or without particulate β-glucan (50 µg/ml) for 3–4 days. Data represent the frequency of CFSE diluted cells gated on CD4+ T cells. The experiment was repeated twice with similar results. (B) Same cell cultures as (A) were further stimulated with PMA/Ionomycin for intracellular IFN-γ staining. The experiment was repeated twice with similar results. (C) Splenocytes of OT-I mice were co-cultured with sorted Gr-1+CD11b+CD45+ tumor MDSC from LLC-bearing mice at indicated ratios, in the presence of OVA (50 µg/ml in M-MDSC cultures and 10 µg/ml in PMN-MDSC cultures) with or without particulate β-glucan (50 µg/ml). Data represent the percentage of IFN-γ+ cells and CFSE diluted cells gated on CD8+ T cells. Results are representative of two independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Dectin-1 stimulation with particulate β-glucan induces PMN-MDSC respiratory burst and apoptosis
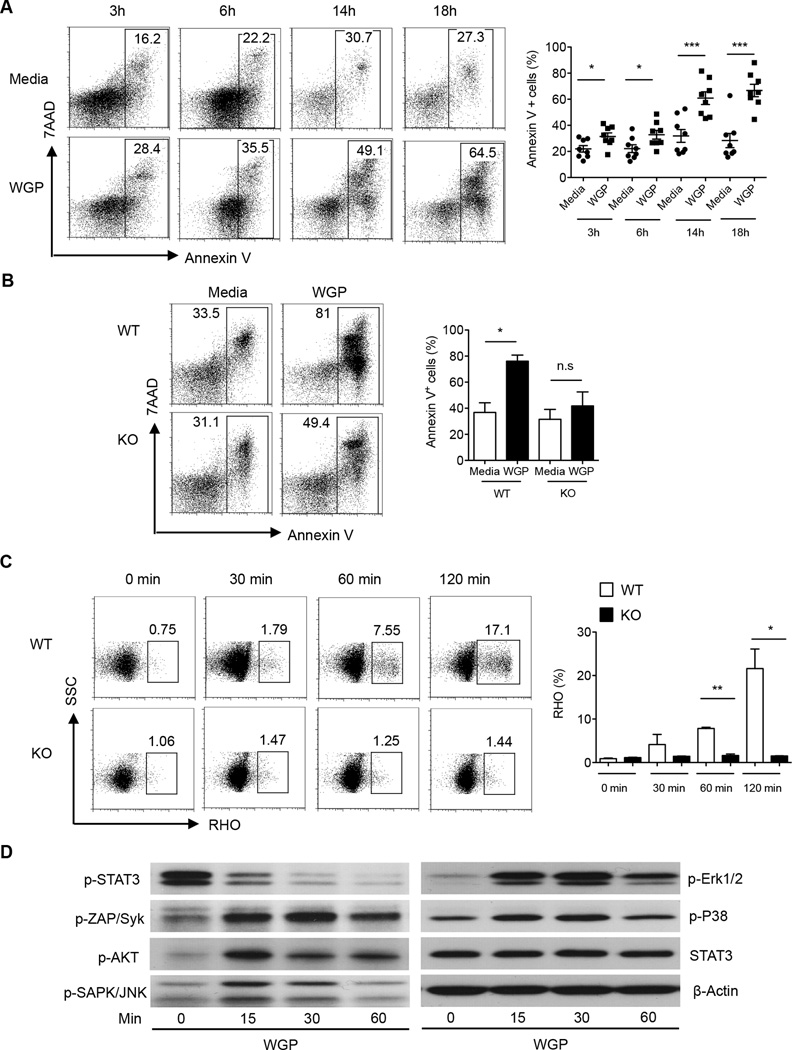
Given that WGP treatment reduced PMN-MDSC-mediated T cell suppression and decreased PMN-MDSC frequency in the spleens and tumors of tumor-bearing mice, we tested whether particulate β-glucan treatment has a further impact on PMN-MDSC viability. Particulate β-glucan treatment enhanced PMN-MDSC apoptosis at 3 hours, 6 hours and more drastically at 14 and 18 hours post-stimulation (Fig. 4A). PMN-MDSC apoptosis was mediated by dectin-1 receptor since particulate β-glucan did not induce PMN-MDSC apoptosis in PMN-MDSC sorted from the spleens of LLC-bearing dectin-1 knockout mice (Fig. 4B). Since particulate β-glucan-induced apoptosis is more drastic at late-time points, we asked whether particulate β-glucan treatment enhances PMN-MDSC respiratory burst, a hallmark of neutrophil-mediated cytotoxicity. We detected PMN-MDSC respiratory burst by dihydrorhodamine 123 (DHR) dye that oxidizes to fluorescent rhodamine 123 (RHO) in the presence of intracellular ROS (26). Upon particulate β-glucan stimulation, PMN-MDSC respiratory burst was significantly enhanced upon 1 hour and 2 hours post-stimulation (Fig. 4C). To exclude the effect of dectin-1 independent phagocytosis on the induction of PMN-MDSC respiratory burst, we stimulated PMN-MSDC from dectin-1 knockout mice with particulate β-glucan. As expected, particulate β-glucan stimulation did not induce respiratory burst in dectin-1 knockout PMN-MDSC (Fig. 4C).
Figure 4. Particulate β-glucan induces respiratory burst and apoptosis in PMN-MDSC in a dectin-1 dependent manner.
(A) Representative dot plots showing the frequency of annexin V+ cells gated on Gr-1highCD11b+ PMN-MDSC in splenocytes of LLC-bearing mice cultured with media only or with particulate β-glucan (100 µg/ml) for indicated time. Summarized data are also shown (n=8). (B) Frequencies of annexinV+ cells gated on Gr-1highCD11b+ PMN-MDSC from WT or dectin-1 KO LLC-bearing mice cultured for 18 hours with or without particulate β-glucan (100 µg/ml) (n=2). (C) Respiratory burst in PMN-MDSC sorted from spleens of LLC-bearing WT or dectin-1 KO mice stimulated with particulate β-glucan (100 µg/ml) for indicated time. Reduction of dihydrorhodamine 123 (DHR) to fluorescent rhodamine 123 (RHO) was assessed by flow cytometry. Data is representative of 6 independent experiments (WT) and two independent experiments (KO). (D) Western blot analysis of p-STAT3, p-Zap/Syk, p-Akt, p-SAPK/JNK, p-Erk1/2, p-p38, STAT3 and β-actin in PMN-MDSC sorted from the spleens of LLC-bearing mice and treated with particulate β-glucan (100 µg/ml) for 0, 15, 30 and 60 minutes. Results are representative of at least three independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Previous studies have highlighted the importance of STAT3 phosphorylation in the cascade events mediating PMN-MDSC survival and suppression (4). Since particulate β-glucan treatment abrogated PMN-MDSC mediated T cells suppression and induced PMN-MDSC apoptosis, we measured STAT3 phosphorylation at 15, 30 and 60 min post-stimulation. STAT3 phosphorylation was inhibited after 15 min and completely abrogated after 30 min upon β-glucan stimulation (Fig. 4D). This effect was mediated by dectin-1 signaling, since particulate β-glucan stimulation enhanced Syk phosphorylation. Moreover, particulate β-glucan stimulation enhanced phosphorylation of molecules downstream of the dectin-1 receptor such as Akt, JNK and Erk1/2 kinases but not p38 (Fig. 4D).
Particulate β-glucan treatment fully converts M-MDSC to potent APC in vitro dependent of the dectin-1 receptor signaling
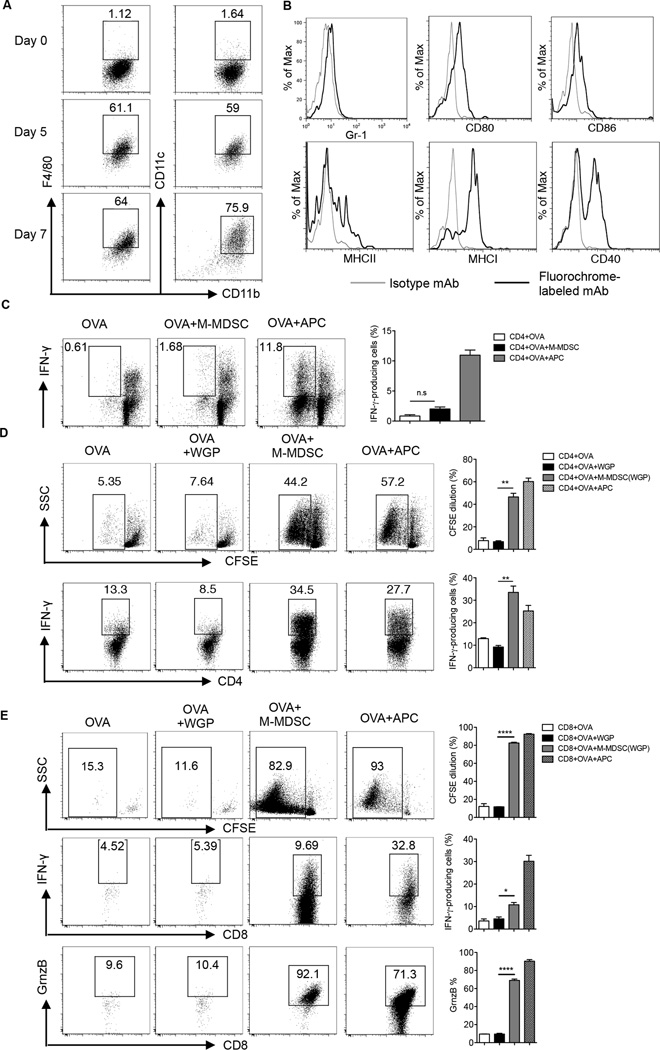
Previous studies have demonstrated the ability of particulate β-glucan to enhance dendritic cell Ag-presenting capability (27), and that 8–13% of M-MDSC cultured with particulate β-glucan and GM-CSF are F4/80+CD11c+ (21). To further determine the effect of particulate β-glucan on M-MDSC, we solely treated M-MDSC with particulate β-glucan in the absence of GM-CSF for 5 days or 7 days and assessed the expression of F4/80, CD11c, CD11b, Gr-1, CD80, CD86, MHC class I, MCH class II and CD40. The majority of M-MDSC cultured with particulate β-glucan for 7 days differentiated to F4/80lowCD11c+CD11b+Gr1− cells and expressed CD86, CD80, MHC class II, MHC class I and CD40 (Fig. 5A–B), cells were viable (trypan-blue exclusion) and 7AAD− (data not shown).
Figure 5. Particulate β-glucan induces the differentiation of M-MDSC to antigen-presenting cells in vitro.
(A) Expression of CD11c, F4/80 and CD11b surface markers on M-MDSC cultured with particulate β-glucan (50 µg/ml) for 0, 5 and 7 days. Results were repeated at least three times with similar results. (B) Expression of Gr-1, CD80, CD86, MHC class II, MHC class I, and CD40 surface markers on M-MDSC cultured with particulate β-glucan (50 µg/ml) for 7 days. Histograms represent the results of three independent experiments. (C) Frequency of IFN-γ+ CFSE− gated on CD4+ T cells in cultures where freshly sorted M-MDSC from the spleens of LLC-bearing mice were incubated for 5 days with CFSE-labeled CD4+ T cells in the presence of OVA (100µg/ml). Irradiated splenocytes were used as APC positive control. (D) Sorted and CFSE-labeled CD4+ OT-II T cells were co-cultured with splenic M-MDSC pre-cultured with particulate β-glucan for 7 days in the presence of OVA (50 µg/ml) for 4–5 days. The frequencies of CFSE diluted cells and IFN-γ+ CD4+ T cells were shown. Irradiated splenocytes were used as APC positive control. The results are representative of at least four independent experiments. (E) Sorted and CFSE-labeled CD8+ OT-I T cells were co-cultured for 4–5 days with OVA (50 µg/ml) and splenic M-MDSC pre-cultured with particulate β-glucan for 7 days. The frequencies of CFSE diluted cells, IFN-γ+ cells and Granzyme B+ cells gated on CD8+ T cells are demonstrated. Results are representative of two independent experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
It has been proposed that MDSC can uptake, process and present Ag, establishing a stable synapse with T cells required for Ag-specific T cell suppression (30). Freshly isolated splenic M-MDSC cultured with sorted OVA-specific CD4+ T cells in the presence of OVA did not induce CD4+ T cell proliferation or IFN-γ production (Fig. 5C). To test whether M-MDSC co-cultured with particulate β-glucan for 7 days differentiated to potent APC, we co-cultured M-MDSC with particulate β-glucan for 7 days and then cultured with sorted and CFSE-labeled OVA-specific CD4+ T cells in the presence of whole OVA Ag for 4–5 days. Differentiated M-MDSC induced CD4+ T cell proliferation and IFN-γ production (Fig. 5D). Moreover, differentiated M-MDSC cross-presented Ag to OVA-specific CD8+ T cells and immensely induced CD8+ T cell proliferation and molecules associated with effector functions such as IFN-γ and Granzyme B (Fig. 5E). Taken together, these data clearly demonstrate that particulate β-glucan converts suppressive M-MDSC to potent APC that can promote Th1 differentiation and Ag cross-presentation to CD8+ effector T cells.
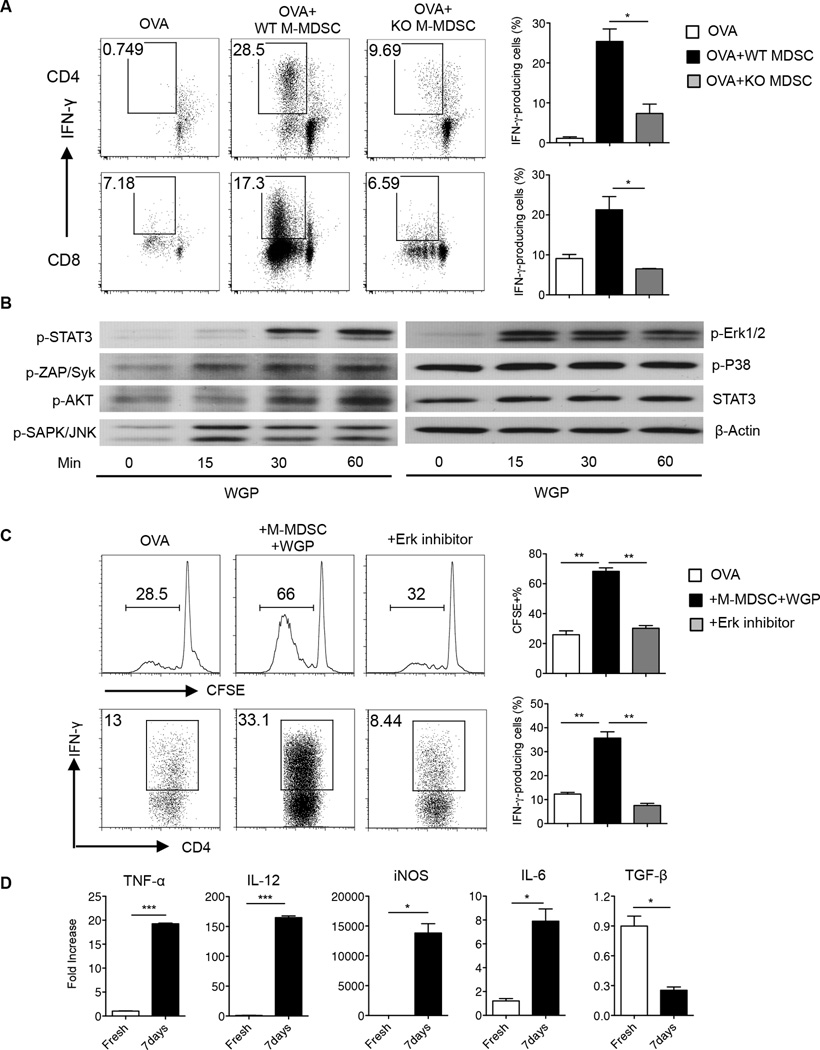
To exclude the possibility of any artifacts in M-MSDC differentiation that might be promoted by particulate β-glucan phagocytosis alone and to test whether it is directed by dectin-1 receptor, we performed Ag presentation assays with wild-type or dectin-1 KO M-MDSC pretreated with particulate β-glucan for 7 days. WT M-MDSC but not dectin-1 KO M-MDSC induced OVA-specific CD4+ T and CD8+ T cell proliferation and IFN-γ production (Fig. 6A). In addition, particulate β-glucan stimulation enhanced the phosphorylation of Syk, Akt, JNK and Erk1/2 but not p38 (Fig. 6B). Surprisingly, STAT3 phosphorylation was enhanced after particulate β-glucan stimulation (Fig. 6B), which is different from PMN-MDSC (Fig. 4D). Pretreatment of M-MDSC with WGP in the presence of MEK1/2 inhibitor (PD98059) abrogated the acquired Ag-presenting capability of differentiated M-MDSC (Fig. 6C). In addition, M-MDSC treated with particulate β-glucan for 7 days increased the expression of TNF-α, IL-12, iNOS and IL-6, and decreased TGF-β compared to freshly sorted M-MDSC (Fig. 6D). Enhancement of TNF-α and IL-12 mRNA expression was completely abrogated in dectin-1 KO M-MDSC upon particulate β-glucan stimulation (data not shown).
Figure 6. Particulate β-glucan-induced M-MDSC antigen-presenting function is dectin-1 and Erk1/2 dependent.
(A) CD4+ and CD8+ T cells were sorted from OT-II and OT-I mice, respectively, CFSE-labeled and co-cultured for 4–5 days with M-MDSC sorted from the spleens of WT or dectin-1 KO LLC-bearing mice. M-MDSC were pre-treated with particulate β-glucan for 7 days prior to co-culture with CD4+ or CD8+ T cells. The frequency of CFSE−IFN-γ+, gated on CD4+ T cells (upper panel) or CD8+ T cells (lower panel), are represented in the dot plots. Results are representative of two-independent experiments. (B) Western blot analysis of p-STAT3, p-Zap/Syk, p-Akt, p-SAPK/JNK, p-Erk1/2, p-p38, STAT3 and β-actin in M-MDSC sorted from the spleens of LLC-bearing mice and stimulated with particulate β-glucan (100 µg/ml) for 0, 15, 30 and 60 minutes. (C) CD4+ T cells sorted from OT-II mice were CFSE-labeled and co-cultured with OVA and M-MDSC pre-treated with particulate β-glucan in the presence of MEK1/2 inhibitor (PD98059) (30 ng/ml) or DMSO for 7 days. Data demonstrate the frequencies of CFSE diluted cells and IFN-γ+ cells gated on CD4+ T cells. Results are representative of two independent experiments. (D) The relative mRNA expression of TNF-α, IL-12, iNOS, IL-6, and TGF-β in MDSC, sorted from the spleens of LLC-bearing mice, and incubated with particulate β-glucan for 7 days compared to its expression in freshly sorted M-MDSC. *p<0.05, **p<0.01.
M-MDSC treated with β-glucan differentiate into CD11c+MHCclassII+ cells in vivo and induce decreased tumor burden
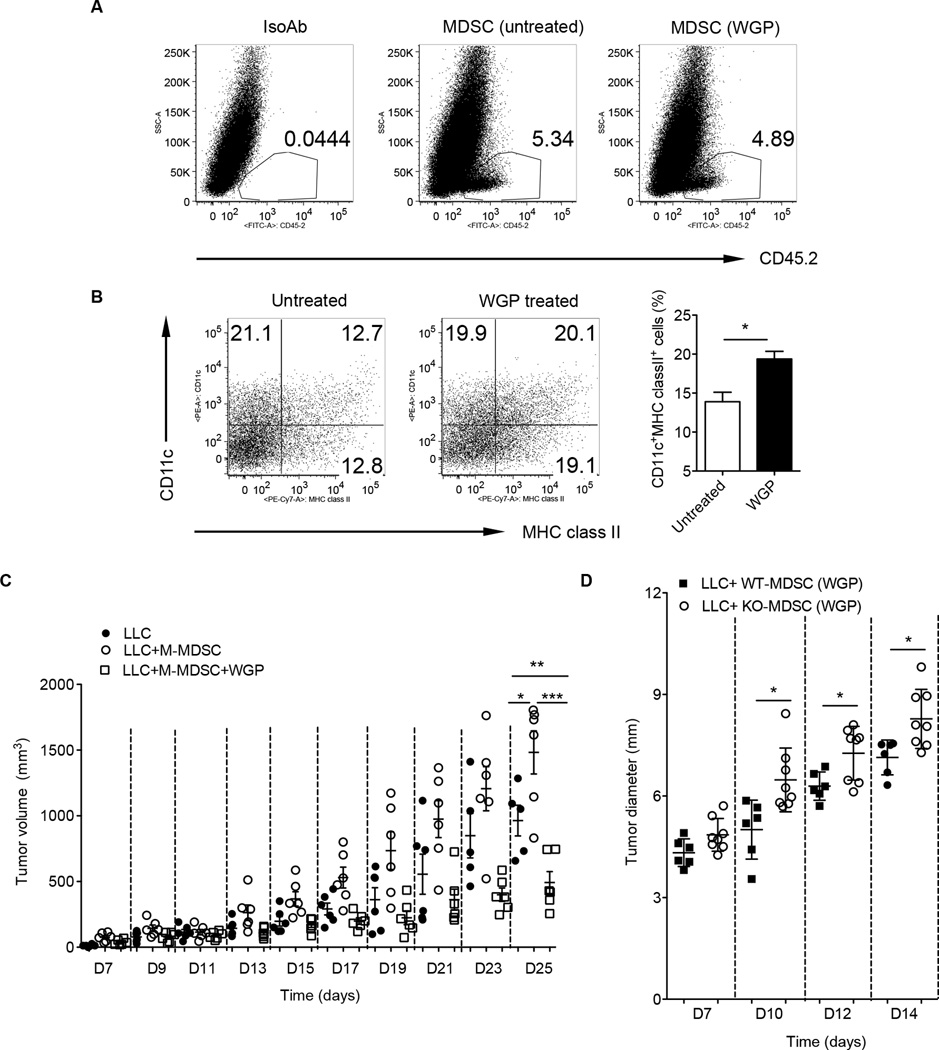
Next, we examined whether particulate β-glucan treatment also promotes M-MDSC differentiation in vivo. M-MDSC sorted from tumors of C57Bl/6 mice (CD45.2) were treated with or without WGP for overnight and then injected into tumors of congenic SJL mice (CD45.1). Tumors were excised 7 days after injection. As shown in Fig. 7A, transferred cells were readily seen in the tumors. WGP-treated M-MDSC had significantly more CD11c+ cells compared to untreated M-MDSC. In addition, those CD11c+ cells expressed high level of MHC class II molecule (Fig. 7B). They also co-expressed low level of F4/80 (data not shown). To evaluate the effect of particulate β-glucan-treated M-MDSC on tumor development in vivo, we performed admixture experiments with M-MDSC/tumor cells. M-MDSC or M-MDSC treated with particulate β-glucan for 18 h were mixed with LLC cells at a 1:1 ratio, and injected subcutaneously into C57BL/6 mice. Untreated M-MDSC induced enhanced tumor progression and growth compared to LLC alone (Fig. 7C). In contrast, particulate β-glucan-treated M-MDSC did not promote tumor growth. Mice implanted with LLC/ particulate β-glucan-treated M-MDSC had significantly decreased tumor burden compared to LLC alone (Fig. 7C), suggesting particulate β-glucan-treated M-MDSC stimulate anti-tumor immune response. This effect was dependent of the dectin-1 receptor (Fig. 7D).
Figure 7. Particulate β-glucan treatment induces M-MDSC differentiation in vivo with reduced tumor growth.
Tumor M-MDSC sorted from C57Bl/6 LLC tumor-bearing mice (CD45.2) were treated with or without WGP for overnight and then intratumorally injected into SJL tumor-bearing mice (CD45.1). Mice were sacrificed after 7 days and single cell suspensions from tumors were stained with anti-CD45.2 or isotype control mAb (A) and CD11c and MHC class II. Cells were gated on CD45.2+ cells (B). The percentage of CD11c+MHC class II+ cells was summarized. (C) M-MDSC sorted from LLC tumor-bearing mice were treated with or without WGP and then mixed with LLC cells for injection. LLC alone was used as control. Tumor growth was monitored and recorded. (D) M-MDSC sorted from LLC tumor-bearing wildtype or dectin-1 KO mice were treated with WGP and mixed with LLC cells for injection. Tumor growth was monitored. *p<0.05, **p<0.01, ***p<0.001.
Particulate β-glucan treatment reduces the frequency of HLA-DR−CD14−CD11b+CD33+ MDSC in the peripheral blood of NSCLC patients
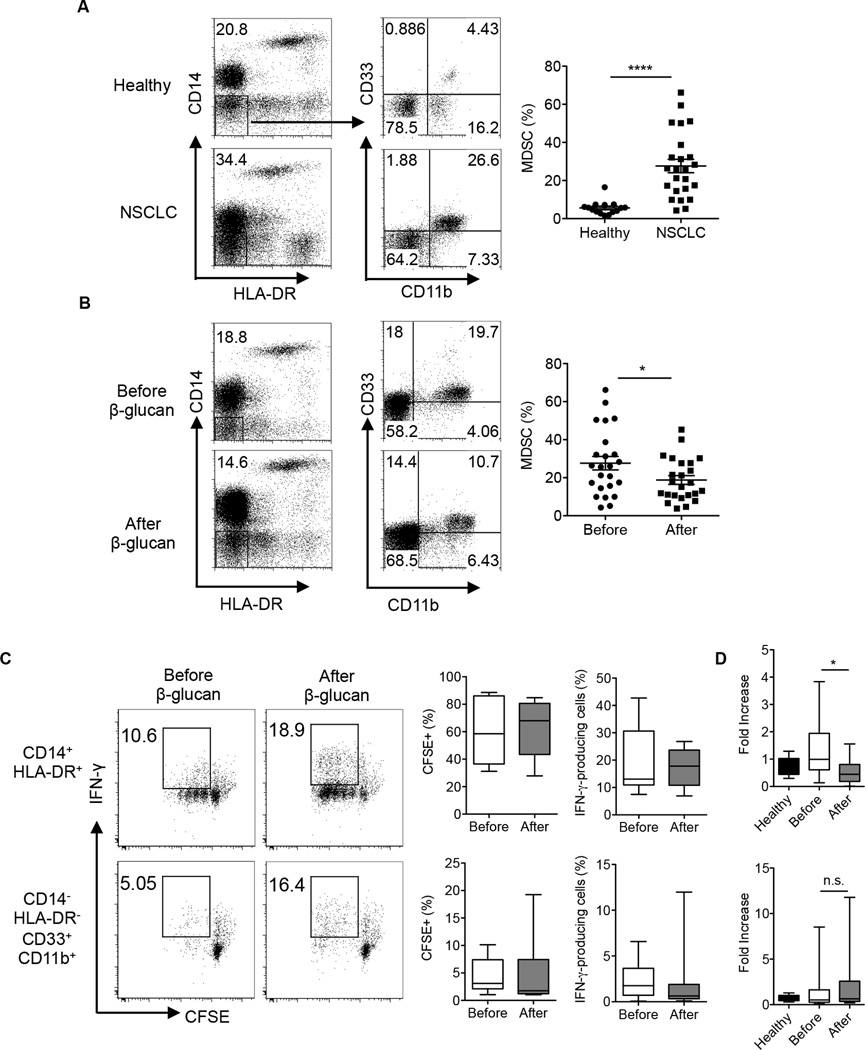
To imply the significance of the current findings into human patients, we conducted a particulate β-glucan clinical trial in patients with non-small cell lung cancer (NSCLC) that were newly diagnosed and had not received any other treatment such as chemotherapy. Particulate β-glucan was administered orally for 10–14 days at 500 mg dose and blood was withdrawn before and after treatment. Consistent with previous studies (8, 9), the frequency of HLA−CD14− CD33+CD11b+ MDSC was substantially increased in the peripheral blood of patients with NSCLC compared to those in age and sex matched healthy donors (Fig. 8A). Particulate β-glucan treatment significantly reduced the percentage of HLA-DR−CD14−CD33+CD11b+ MDSC in the peripheral blood of NSCLC patients when compared to its frequency in the peripheral blood before treatment (Fig. 8B).
Figure 8. Particulate β-glucan treatment in vivo decreased the frequency of CD14−HLA-DR−CD33+CD11b+ MDSC in the peripheral blood of NSCLC patients.
(A) Frequency of CD33+CD11b+ MDSC gated on CD14−HLA-DR− cells in the peripheral blood of NSCLC patients (n=23) compared to age and sex matched healthy donors (n=13). (B) Frequency of CD33+CD11b+ MDSC gated on CD14−HLA-DR− cells in the peripheral blood of NSCLC patients before and after particulate β-glucan treatment for 10–14 days (n=23). (C) IFN-γ production and proliferation (CFSE diluted cells) of allogeneic T cells (CD3+) cultured at 1:1 ratio with CD14+HLA-DR+CD11b+CD33+ or CD14−HLA-DR−CD11b+CD33+ isolated from the peripheral blood of NSCLC patients before and after particulate β-glucan treatment (n=11) (D) Relative Arginase1 mRNA expression levels in neutrophils (PMN) isolated from the peripheral blood of healthy controls, and patients with NSCLC before and after particulate β-glucan treatment. Arginase 1 relative mRNA expression significantly decreased in a cohort of 15 patients, whereas no significant change was reported in the other 20 patients (total n=35). *p<0.05, **p<0.01, ***p<0.001.
Next, we sought to determine the Ag-presenting capability of CD14+HLA-DR+ monocytes and CD14−HLA-DR−CD11b+CD33+ MDSC in a mixed lymphocyte reaction as previously described (31). Particulate β-glucan treatment induced an enhanced trend in proliferation and IFN-γ production by allogeneic T cells in both populations (Fig. 8C). In addition, IFN-γ production by T cells from patients after treatment were also trending enhanced although it did not reach statistically significance (data not shown).
In NSCLC, Arginase 1 expression was reported to be expressed by CD14−CD11b+CD33+ SSChigh PMN-MDSC (9, 32). To assess the effect of particulate β-glucan treatment on the function of PMN-MDSC, we compared the expression of Arginase 1 mRNA in the PMN-isolated from the peripheral blood of NSCLC patients before and after particulate β-glucan treatment. The expression of Arginase 1 mRNA was significantly decreased in a cohort of 15 patients, and became comparable with Arginase 1 mRNA expression in healthy controls (Fig. 8D upper panel), whereas it did not significantly change in the other cohort of 20 patients (Fig. 8D lower panel). Overall, NSCLC patients have a decreased frequency of CD14−HLA-DR−CD11b+CD33+ MDSC with improved effector function after oral particulate β-glucan treatment.
Discussion
Herein, we demonstrated that treatment with yeast-derived particulate β-glucan reduces tumor growth and differentially modulates PMN-MDSC and M-MDSC frequencies in tumor-bearing mice. This differential modulation was also reflected in its effect on the functions of both subsets. Firstly, we demonstrated that particulate β-glucan reverses PMN-MDSC suppression, induces respiratory burst and enhances apoptosis. On the second axis, particulate β-glucan skews the immature suppressive phenotype of M-MDSC towards a potent APC phenotype that drives the differentiation of Th1 CD4+ T cells and induces cytotoxic CD8+ T cells. On a third and most prevalent axis, particulate β-glucan was tested in patients newly diagnosed with NSCLC as an immunomodulator in cancer. This is the first clinical trial with particulate β-glucan in patients newly diagnosed with NSCLC that have not yet been subjected to any other treatment since chemotherapy significantly impacts on MDSC frequency and function. It is important to note that particulate β-glucan is a natural compound with no reported off target effects, with a low cost and can be administered orally.
Particulate β-glucan induced PMN-MDSC respiratory burst early after activation and largely enhanced PMN-MDSC apoptosis, which positively correlates with the decreased frequency of PMN-MDSC in tumors and spleens upon particulate β-glucan treatment. Although ROS have been implied as one of the main mechanisms for T cell suppression (33) which is regulated by STAT3 activation (34), other reports suggest that PMN-MDSC can acquire a tumor cytotoxic phenotype in the absence of TGF-β (35). That goes in line with what we previously showed that TGF-β mRNA expression is reduced in tumors of mice treated with particulate β-glucan (20). In addition, activation of neutrophils with β-glucan, enhanced neutrophil cytotoxicity against Candida albicans (36). Interestingly, STAT3 phosphorylation levels in PMN-MDSC decreased after dectin-1/Syk activation with particulate β-glucan that might have led to PMN-MDSC apoptosis, and correlates with the findings that treatment of splenic Gr-1+CD11b+ from tumor-bearing mice with the JAK2/STAT3 inhibitor JSI-I24 in vitro for 7 days in the presence of GM-CSF and tumor-conditioned medium enhanced cell death compared to untreated cells (37).
Despite several studies reporting the anti-tumor effect of particulate β-glucan through the activation of innate immune cells and induction of Th1 T cell responses (20, 27, 38), little is known on the role of particulate β-glucan in the modulation of tumor-associated macrophages (TAMs), MDSC and regulatory T cells. It is reported that particulate β-glucan induces the differentiation of M-MDSC to F4/80+ CD11c+ cells with a decreased suppressive phenotype (21). However, this study showed that the differentiated F4/80+ CD11c+ cells represent only 8–13% of the gated M-MDSC population cultured for 48 hours in the presence of GM-CSF. Whereas, Youn et al showed that GM-CSF alone could induce the expression of CD11c to around 35% and F4/80 to around 60% in splenic M-MDSC at day 3 (5). In addition, expression of F4/80 or CD11c surface markers does not necessarily imply a non-suppressive phenotype, since it has been shown that MDSC do differentiate to suppressive F4/80+ cells in the tumor microenvironment(39) (7, 40). In our study, we showed that particulate β-glucan treatment induces differentiation of M-MDSC to potent APC both in vitro and in vivo. In vitro differentiated M-MDSC by β-glucan stimulate Ag-specific Th1 and CD8 T cell responses. Furthermore, WGP-treated M-MDSC differentiate into CD11c+ cells with high MHC class II expression in vivo and induce reduced tumor growth, suggesting that the function of these cells would be skewed towards an anti-tumor phenotype. Our data thus provide likely a direct functional link between M-MDSC differentiation induced by particulate β-glucan treatment and reduced tumor growth.
We also demonstrated that dectin-1 receptor signaling is required for the acquisition of Ag-presenting function, since M-MDSC from dectin-1 KO mice treated with particulate β-glucan for 7 days did not acquire such capability. Although STAT3 phosphorylation has been shown to be a key mechanism for MDSC-mediated suppression (41) , we found that activation of STAT3 in M-MDSC did not impact the reversal of suppression and acquisition of Ag-presenting phenotype, maybe largely due to the shift in the cytokine profile towards an ‘M1-like’ phenotype with enhanced expression of IL-12, TNF-α, iNOS and inhibition of TGF-β, in addition to the upregulation of MHC class II, MHC class I and the costimulatory markers such as CD86 and CD40. However, the Erk inhibitor completely abrogated the conversion of M-MDSC to APC mediated by particulate β-glucan.
It is worth noting that several previous studies have shown that β-glucan-containing fungal cell wall extracts such as zymosan can promote regulatory innate immune responses and modulate autoimmunity (42, 43). However, most of those studies used zymosan as β-glucan preparation and zymosan only contains approximately 14% of β-glucan. The particulate β-glucan WGP used in our study is a pharmaceutical grade and contains >99.9% of β-glucan. Therefore, the data from those studies cannot fully ascribe to β-glucan’s effect. In fact, zymosan-induced arthritis model has been widely used in the field (44). Furthermore, dose is another factor which may contribute to those seemingly contradictory data. In Karumuthil-Melethil S et al. study, they concluded that low dose of β-glucan induces regulatory innate immune responses (43).
It has been well documented that MDSC accumulate in different human cancers such as brain (45, 46), head and neck (47), breast (48), lung cancer (49, 50) and others (reviewed in (10, 16, 17)). In many of these studies, the accumulation of MDSC in patients was correlated with poor prognosis. In the current study, newly diagnosed patients with NSCLC were given particulate β-glucan orally for 10–14 days. Treated patients had a significant decrease in the percentage of CD14−HLA-DR−CD11b+CD33+ MDSC in the peripheral blood. Importantly, these patients were not exposed to any therapy during the administration of particulate β-glucan, which allowed the sole assessment of particulate β-glucan efficacy in patients. In addition, an enhanced trend in T cell allogeneic responses to CD14+HLA-DR+ and CD14−HLA-DR−CD11b+CD33+ cells was observed after treatment. The administration of particulate β-glucan for only 10–14 days may not have been enough to modulate APC function in vivo, however it was sufficient to reduce the percentages of CD14−HLA-DR−CD11b+CD33+ MDSC in the peripheral blood.
The expression of arginase I in PMN-MDSC has been reported in NSCLC patients and correlated with poor prognosis and CD8+ T cell suppression (9, 32). Interestingly, a cohort of 15 patients had a significant decreased expression of arginase I in PMN from the peripheral blood while no significant change was reported in the other 20 patients subjected to particulate β-glucan treatment. The patients with decreased arginase I after particulate β-glucan had a higher trend in arginase I expression compared to healthy controls before the treatment. Unlike the other patients, the level of arginase I was comparable to healthy controls, suggesting that patients’ variable responses to the treatment might have been due to different stages, tumor sizes, and progression. However, no correlation was found among those factors. This may be due to limited patient numbers in this trial. Nevertheless, this study provides a first insight towards introducing particulate β-glucan as an immunomodulator therapy against solid tumors in humans. Since MDSC play a critical role in tumor-mediated immune evasion, the findings from current study provide a rationale design to combine β-glucan treatment with other immunotherapeutic approaches such as cancer vaccines and immune checkpoint inhibitors therapy.
Supplementary Material
Acknowledgments
We thank M. Hall and A. Harper for recruiting human NSCLC patients. We also thank C. Worth for helping in the operation of Moflow sorter. The authors also wish to thank Drs. J. Suttles, H. Shirwan, and S. Uriarte for the constructive comments and suggestions for the study.
This work was supported by the NIH R01CA150947, P01CA163223 (to J. Y.), the Kentucky Lung Cancer Research Program, and the American Cancer Society Grant RSG-14-199-01 (to C. D).
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 7.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, Sarnaik A, Horna P, Sotomayor E, Gabrilovich DI. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nature immunology. 2013;14:211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AM, Groen HJ, Smit EF, Hoogsteden HC, Hegmans JP, Aerts JG. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung cancer (Amsterdam, Netherlands) 2013;81:468–474. doi: 10.1016/j.lungcan.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Annals of the New York Academy of Sciences. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 11.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. Journal of immunology (Baltimore, Md. : 1950) 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. Journal of immunology (Baltimore, Md. : 1950) 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. Journal of immunology (Baltimore, Md. : 1950) 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 14.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer research. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albeituni SH, Ding C, Yan J. Hampering immune suppressors: therapeutic targeting of myeloid-derived suppressor cells in cancer. Cancer journal (Sudbury, Mass.) 2013;19:490–501. doi: 10.1097/PPO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunological reviews. 2010;234:335–352. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 19.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, Qian K, Vasilakos J, Saijo S, Iwakura Y, Yannelli JR, Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian J, Ma J, Ma K, Guo H, Baidoo SE, Zhang Y, Yan J, Lu L, Xu H, Wang S. beta-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol. 2013;43:1220–1230. doi: 10.1002/eji.201242841. [DOI] [PubMed] [Google Scholar]
- 22.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 23.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Allendorf DJ, Hansen R, Marroquin J, Ding C, Cramer DE, Yan J. Yeast beta-glucan amplifies phagocyte killing of iC3b–opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J Immunol. 2006;177:1661–1669. doi: 10.4049/jimmunol.177.3.1661. [DOI] [PubMed] [Google Scholar]
- 25.Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. Journal of immunological methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 26.Walrand S, Valeix S, Rodriguez C, Ligot P, Chassagne J, Vasson MP. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clinica chimica acta; international journal of clinical chemistry. 2003;331:103–110. doi: 10.1016/s0009-8981(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Cai Y, Qi C, Hansen R, Ding C, Mitchell TC, Yan J. Orally administered particulate beta-glucan modulates tumor-capturing dendritic cells and improves antitumor T-cell responses in cancer. Clin Cancer Res. 2010;16:5153–5164. doi: 10.1158/1078-0432.CCR-10-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. Journal of immunology (Baltimore, Md. : 1950) 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. The Journal of clinical investigation. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer research. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 31.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer immunology, immunotherapy : CII. 2013;62:1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. Journal of cancer research and clinical oncology. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. Journal of immunology (Baltimore, Md. : 1950) 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 34.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr, Venkatesh D, Yun SH, Mayadas TN. The beta-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell host & microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer research. 2005;65:9525–9535. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karumuthil-Melethil S, Gudi R, Johnson BM, Perez N, Vasu C. Fungal beta-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J Immunol. 2014;193:3308–3321. doi: 10.4049/jimmunol.1400186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garaulet G, Alfranca A, Torrente M, Escolano A, Lopez-Fontal R, Hortelano S, Redondo JM, Rodriguez A. IL10 released by a new inflammation-regulated lentiviral system efficiently attenuates zymosan-induced arthritis. Mol Ther. 2013;21:119–130. doi: 10.1038/mt.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-oncology. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 47.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, Lang S. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. Journal of leukocyte biology. 2011;89:311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 48.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. Journal of immunology (Baltimore, Md. : 1950) 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 50.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer immunology, immunotherapy : CII. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.