Abstract
Amyotrophic lateral sclerosis is a late-onset and terminal neurodegenerative disease. The majority of cases are sporadic with unknown causes and only a small number of cases are genetically linked. Recent evidence suggests that post-transcriptional regulation and epigenetic mechanisms, such as microRNAs, underlie the onset and progression of neurodegenerative disorders; therefore, altered microRNA expression may result in the dysregulation of key genes and biological pathways that contribute to the development of sporadic amyotrophic lateral sclerosis. Using systems biology analyses on postmortem human spinal cord tissue, we identified dysregulated mature microRNAs and their potential targets previously implicated in functional process and pathways associated with the pathogenesis of ALS. Furthermore, we report a global reduction of mature microRNAs, alterations in microRNA processing, and support for a role of the nucleotide binding protein, TAR DNA binding protein 43, in regulating sporadic amyotrophic lateral sclerosis-associated microRNAs, thereby offering a potential underlying mechanism for sporadic amyotrophic lateral sclerosis.
Keywords: Amyotrophic lateral sclerosis, Epigenetics, MicroRNA
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neuromuscular disease characterized by motor neuron degeneration (de Carvalho and Swash, 2011; Robberecht and Philips, 2013). While some (~15%) cases are familial (fALS), the majority (~85%) are sporadic (sALS) and several cell types and mechanisms contribute to the pathogenesis of the disease (Siddique and Ajroud-Driss, 2011; van Blitterswijk et al., 2012). This complexity has hindered therapeutic development, and Riluzole, the only approved therapy for ALS, exerts a modest benefit at best (Bensimon et al., 1994; Gordon, 2013). Thus, identification of the molecular mechanisms underlying ALS is critical to support effective therapy development.
Evidence suggests that environmental exposures, physical stress, and altered immunity may promote epigenetic changes and contribute to ALS (Ahmed and Wicklund, 2011; Callaghan et al., 2011; Qureshi and Mehler, 2013). Epigenetic mechanisms, including DNA methylation, histone remodeling, and microRNAs (miRNAs), reversibly regulate gene expression without altering the basic genetic code (Qureshi and Mehler, 2011). These mechanisms are induced by the same environmental/occupational risk factors associated with ALS (Callaghan et al., 2011; Cox et al., 2009; Furby et al., 2010), can accumulate throughout life, and their reversibility supports their potential utility as therapeutic targets. Our group and others previously examined how DNA methylation/hydroxymethylation and chromatin modifications contribute to the altered regulation of gene expression in spinal cord tissue, brain tissue, and whole blood from sALS subjects (Chestnut et al., 2011; Figueroa-Romero et al., 2012; Morahan et al., 2009; Tremolizzo et al., 2014). We found that only a subset of altered genes were regulated by methylation and hydroxymethylation (Figueroa-Romero et al., 2012), and while these modifications have been recently shown to play an important role in the development of neurodegeneration and mental disorders (Kato and Iwamoto, 2014), our data further suggest that other epigenetic mechanisms may contribute to altered gene expression in ALS.
miRNAs are small non-coding RNAs that negatively regulate up to 60% of the human genome by destabilizing mRNA or inhibiting translational efficiency. Canonical miRNA biogenesis begins in the nucleus where long primary miRNAs (pri-miRNAs) are encoded by the genome and cleaved by the microprocessor complex comprised of Drosha and DiGorge syndrome critical region gene 8 (DGCR8) to generate precursor miRNAs (pre-miRNAs) (Gregory et al., 2006). The pre-miRNAs are then transported to the cytoplasm by exportin 5 (Lund et al., 2004)and further processed by the endoribonuclease DICER-1 into mature 17-22-nucleotide duplex miRNAs. A single strand of the mature miRNA is incorporated into the RNA-induced silencing complex (RISC), which includes DICER, eukaryotic translation initiation factor 2C, 2/argonaute RISC catalytic component 2 (EIF2C2/AGO2), TAR (HIV-1) RNA binding protein 2 (TRBP), and protein kinase, interferon-inducible double-stranded RNA dependent activator (PACT) (Chendrimada et al., 2005; Emde et al., 2015; Lee et al., 2006). This complex facilitates binding of the mature miRNA to complementary nucleotide sequences in the 3’ untranslated region (UTR) of the mRNA targets.
Importantly, a single miRNA can regulate several hundred mRNA targets via RNA-dependent post-transcriptional silencing mechanisms, and mRNA transcripts can be regulated by multiple miRNAs (Paez-Colasante et al., 2015). Hence, altered expression of only a few miRNAs can impact many genes and promote aberrant effects on multiple biological pathways (Friedman et al., 2009; Liang, 2009). There are currently over 2,000 annotated human miRNAs that regulate many biological processes, including development, cell proliferation, and organ system homeostasis (Friedlander et al., 2014; Haramati et al., 2010). In the central nervous system, miRNAs are highly expressed, and their dysregulation is linked to several neurodegenerative diseases, including Parkinson’s disease (PD), Alzheimer’s disease (AD), and multiple sclerosis (Goodall et al., 2013; Hartl and Grunwald Kadow, 2013; Ksiazek-Winiarek et al., 2013; Maciotta et al., 2013). Notably, toxicants linked to sALS onset, such as heavy metals and pollution, are also associated with altered miRNA expression (Ahmed and Wicklund, 2011; Callaghan et al., 2011; Wang and Cui, 2012), and two fALS genes, fused in sarcoma (FUS) and transactive response DNA binding protein 43 (TARDBP) encoding the TDP-43 protein, are involved in RNA processing and miRNA biogenesis (Buratti et al., 2010; Kawahara and Mieda-Sato, 2012; Mackenzie et al., 2010).
Here, we investigated the contribution of miRNA dysregulation to ALS pathogenesis using a systems biology approach on postmortem spinal cord tissue from sALS and control subjects. We hypothesized that differential miRNA expression results in the dysregulation of key genes and biological pathways, contributing to sALS development. We identified differentially expressed mature miRNAs (DEmiRNAs) and their predicted mRNA targets in human postmortem spinal cord, and further examined biologically relevant pathways and functions affected by the miRNA-target pairs to gain insight into disease pathogenesis. Finally, we examined miRNA biogenesis and the role of TDP-43 as potential novel mechanisms underlying miRNA dysregulation in sALS.
Materials and Methods
Subjects and tissue
Frozen human spinal cord samples from 12 Caucasian ALS subjects without a family history of ALS and 12 age- and gender-matched neurologically-normal controls were obtained from the National Institute for Child Health and Human Development (NICHD) Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD (contract HHSN275200900011C, Ref. No NO1-HD-9-0011) and from the Michigan Brain Bank and Michigan ALS Consortium at the University of Michigan (Table 1). All autopsy participants at the University of Michigan signed a written informed consent under a protocol reviewed and approved by the University of Michigan Medical School Institutional Review Board (Protocol # HUM00028826). sALS samples were screened for abnormal GGGGCC hexanuclotide repeat expansions within the chromosome 9 open reading frame 72 (C9ORF72) gene, the most common genetic cause for fALS and sALS to date, using the repeat-primed PCR method as previously described (Meisler et al., 2013; Renton et al., 2011).
Table 1.
Clinical characteristics
| ALS | Control | p-value | ||
|---|---|---|---|---|
| n | 12 | 12 | - | |
| Age (years)(a) | 56 (35-71) | 55 (36-73) | NS | |
| Gender | Male | 10 | 10 | NS |
| Female | 2 | 2 | ||
| SC location | Cervical | 9 | 8 | NS |
| Thoracic | 3 | 4 | NS | |
| Onset | Bulbar | 2 | - | - |
| Limb | 8 | - | - | |
| NA | 2 | - | - | |
| Postmortem interval(b) | 14.5±7.0 h | 15.2±6.2 h | NS | |
| C9ORF72 | 1 | - | - | |
| Cause of death | ALS | 12 | - | <0.001 |
| Accident | - | 2 | - | |
| PC | - | 2 | - | |
| ASCVD | - | 5 | - | |
| Cancer | - | 1 | - | |
| Cardiac arrest | - | 1 | - | |
| NA | - | 1 | - |
median (range);
mean ± standard deviation; ALS, amyotrophic lateral sclerosis; ASCVD, arteriosclerotic cardiovascular disease; NA, no data available; NS, not significant; PC, pulmonary complications; SC, spinal cord
Nucleic acid extraction
Total RNA was extracted from postmortem human spinal cord with a miRNeasy kit and treated with RNAse-free DNAse1 according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). As previously reported, genomic DNA from ALS postmortem human spinal cord was isolated using the Promega Maxwell 16 Tissue DNA Purification kit and a Maxwell instrument (Promega Co, Madison, WI, USA) (Figueroa-Romero et al., 2012). Nucleotide concentration was assessed using a Nanodrop 2000 (Thermo Scientific, Wilmington, DE, USA).
miRNA array profiling and data analysis
High-throughput analysis of mature miRNAs in human spinal cord was performed using TaqMan OpenArray according to the manufacturer’s instructions (Applied Biosytems/Life Technologies, Carlsbad, CA, USA). Stem-loop reverse transcription (RT) was obtained with the TaqMan MicroRNA Reverse Transcription Kit and Megaplex RT primers and a pre-amplification utilized TaqMan PreAmp Master Mix and Megaplex PreAmp Primers. The RT and pre-amplification reactions were performed in a GeneAmp PCR System 9700 thermocycler (Applied Biosytems/Life Technologies). Diluted pre-amplification product was mixed with the TaqMan Open Array Real-Time PCR Master Mix and loaded in a 384-well OpenArray Plate. TaqMan OpenArray miRNA assays were performed at the University of Michigan's DNA Sequencing Core by the Affymetrix and Microarray Core Group to comprehensively measure miRNA abundance levels.
Raw array data were analyzed using DataAssist™ v3.01 (Applied Biosystems/Life Technologies). Briefly, the PCR plate was comprised of 817 miRNAs, including multiple copies of endogenous controls. Samples with non-available values in more than 50% of data were excluded from further analyses. To identify additional poor-quality samples and to determine the most appropriate reference miRNA for normalization (RNU48, RNU44, and U6), a box-plot of un-normalized data with all endogenous control data marked was examined. Samples with high levels of variability in each endogenous control (cycle threshold (CT) value range > 3) were not included for further analyses. RNU48 was selected as the internal reference for calculating delta-CT, as it showed the least variation across all samples. The maximum CT value was set to 40 across all samples.
DEmiRNAs were determined using a two-sample t-test on the delta-CT data between the ALS and control samples. The False Discovery Rate (FDR) for each miRNA was obtained after adjusting the corresponding P-value with the Benjamini-Hochberg multiple testing correction method. A FDR < 0.05 was used as the cutoff for significant DEmiRNAs between ALS and control samples.
Identifying potential miRNA targets
Potential functional gene targets of the DEmiRNAs were identified by collecting a comprehensive list of both experimentally validated and predicted targets. Validated targets were obtained from TarBase, a comprehensive database of experimentally supported animal miRNA targets (Sethupathy et al., 2006). Predicted targets were obtained using both conserved and non-conserved sites from TargetScan (Score > −0.2) (Kawahara and Mieda-Sato, 2012; Lewis et al., 2005) and microRNA.org (mirSVR score < −0.1) (Betel et al., 2008; Meisler et al., 2013). To filter out potentially non-functional miRNA targets in sALS spinal cord samples, our previous microarray gene expression data from the same samples (Figueroa-Romero et al., 2012) were used to limit the targets to those among the differentially expressed genes (DEGs) that exhibited inversely correlated regulation. For each DEmiRNA-target gene pair, a Pearson correlation coefficient was calculated using the delta-CT values in the OpenArray and log2-transformed normalized intensity values in the microarray. Selection criteria for miRNA-target pairs included a Pearson correlation coefficient greater than 0.5 (P-value < 0.05) and a gene expression fold-change of at least 2.
Functional enrichment analysis and miRNA-target network analysis
miRNA-target pairs that passed the above filtering steps were subjected to functional enrichment analyses. For each miRNA, the collected high-quality targets were evaluated for functional enrichment, and a locally implemented version of the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (Huang da et al., 2009; Renton et al., 2011) was used to identify over-represented biological functions among the targets related to Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes pathways (Kanehisa and Goto, 2000). A FDR < 0.05 was used as the cutoff for significantly enriched terms. A heat map was generated to visualize the most significantly enriched terms, and the 10 most significantly enriched terms per gene set were collectively selected and their P-values were transformed by −log10(P-value) and color-indexed. Finally, a regulatory network that included the miRNA-target gene interactions identified above and the protein-protein interactions among the gene targets identified based on the Biological General Repository for Interaction Datasets (BioGRID) (Chatr-Aryamontri et al., 2013) was generated in Cytoscape (Huang da et al., 2009; Smoot et al., 2011).
Expression of miRNA and mRNA by quantitative PCR (qPCR)
Differential expression of selected miRNAs was confirmed by qPCR analysis using TaqMan universal PCR master mix (without Uracil-N glycosylase), TaqMan miRNA assays, and the pre-amplified product used for the array analysis (1:200 dilution in water) according to the manufacturer’s instructions and following the standard protocol for TaqMan using a StepOnePlus™ Real-Time PCR System (Applied Biosystems/Life Technologies). The fluorescence threshold CT value representing miRNA expression in sALS samples was calculated using StepOnePlus system software. miRNA levels were first normalized to RNU48 as the reference gene (delta-CT) and then relative to the control group (delta-delta-CT). Levels of miRNA PCR products are expressed as mean ± SEM and a two-sample equal variance t-test was performed using GraphPad Prism 5 to indicate significant miRNA differences between sALS and control.
To validate mRNA target expression levels, cDNA from the same total RNA extracted for the array was generated using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). qPCR was performed in triplicate using sequence-specific primers with Power SYBR® Green PCR Master Mix (Applied Biosystems/Life Technologies). Primer sequences for chemokine C-C Motif ligand 2 (CCL2) and cluster of differentiation 4 (CD4), TNF receptor superfamily, member 6 or Fas ligand (FAS), eukaryotic translation initiation factor 2C, 4/argonaute RISC catalytic component 4 (EIF2C4/AGO4), and aquaporin 1 (AQP1) are described in Table S1. The PCR amplification profile was as follows: 95°C for 5 min, 40 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 60 s, and extension at 72°C for 30 s, and a final phase of 72°C for 5 min. Differential expression of downstream targets was determined and analyzed as indicated for validation of miRNA expression using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene for normalization. Expression levels of pri-let-7e and pri-miR-577 were analyzed using TaqMan primary miRNA (pri-miRNA) assay probes using GAPDH for normalization (Applied Biosystems/Life Technologies, Carlsbad, CA) (Table S2).
Plasmid construction
To establish an inducible N-terminus (N)-Flag-tagged TDP-43 mouse embryonic stem (mES) cell line, we modified the pLox vector (Kyba et al., 2002) by inserting a Flag tag. Briefly, 6.25 nM each of the forward and reverse adaptor primers (Table S1) were annealed in 1X annealing buffer (100 mM Tris-HCl pH 8.0, 1 M NaCL) and incubated in boiling water that was gradually cooled to room temperature. The annealed adaptors were diluted 1:8000 in 0.1 X TE, pH 8.0, and 3 µl were cloned into the Sal1 site of the 5’-multicloning site (MCS) and the Xba1 site of the 3’MCS of the vector to introduce a Flag tag (MDYKDDDDK) followed by the restriction sites for Xho1, EcoR1, Kpn1, Xba1, Sal1/Acc1, Sma1, and Not1. This resulted in the elimination of the neo cassette and the eGFP gene sequence (the Xba1 site is not unique, as there is an Xba1 site upstream of the 5’ MCS). Human TDP-43 was PCR amplified using SH-SY5Y-derived cDNA as template and Phusion High-Fidelity DNA Polymerase (New England Biolabs, Inc., Ipswich, MA, USA) following the manufacturer’s protocol. TDP-43 was cloned into the Xho1-Xba1 sites of the CS2+ vector (generously provided by Dr. Dave Turner at the University of Michigan) and then subcloned into the Xho1-Not1 sites of the Flag-pLox vector.
To create the constructs required for the luciferase assays (pmirGlo-AGO4), the 3’ UTR of EIF2C4/AGO4 (nucleotides 75-929 3’ to the stop codon) was amplified in 25 µl reactions using 100 ng of spinal cord genomic DNA as template (Figueroa-Romero et al., 2012), specific primers (Table S1), and Q5® High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) following the manufacturer’s protocol. The reaction was performed in a Genemap PCR system 9700 thermocycler (Applied Biosystems/Life Technologies) with the following settings: 98°C for 30s, 35 cycles of denaturation at 98°C for 5 s, annealing at 70°C for 20 s, and extension at 72°C for 30 s, and a final phase of 72°C for 2 min. Amplicons were subcloned into the Xho1-Sbf1 sites of the pmirGlo Dual-Luciferase vector (Promega, Madison, WI, USA). All constructs were verified by sequencing at the University of Michigan Sequencing Core.
Establishment, culture, and transfection of Flag-TDP-43 inducible mES cells
The AinV15 cell line has been previously described (Kyba et al., 2002; Lunn et al., 2012; Reyes et al., 2008). Briefly, this mES cell line contains a reverse tet transactivator upstream of a puromycin selectable cassette and a truncated neomycin resistance cassette downstream of the interaction site allowing for puromycin and geneticin (G418) selection (Kyba et al., 2002; Lunn et al., 2012; Reyes et al., 2008). N-Flag-TDP-43 was integrated into the genome of the parental AinV15 cell line upstream of the tet operon to generate the tetracycline-inducible N-Flag-TDP-43 mES line as previously described (Lunn et al., 2012). Briefly, AinV15 cells were plated in 0.1% gelatin (from porcine skin Type A, Sigma-Aldrich, St. Louis, MO, USA)-coated 12-well plates (Corning/Cellgro, Manassas, VA, USA) at 8×104 cells/well in complete media [4.5 mM glucose DMEM (Gibco, Life Technologies), 10% heat inactivated mES-tested fetal bovine serum (FBS; University of Michigan Transgenic Core), 1000 units/mL human recombinant Leukemia inhibitory factor (Chemicon/Millipore, Billerica, MA, USA), and 1.5μg/mL puromycin (Sigma-Aldrich)] and maintained at 37 °C with 5% CO2. The cells were transfected with 1 µg of DNA and TransIT®-2020 transfection reagent (Mirus Bio LLC, Madison, WI) following the manufacturer’s protocol 24 h after plating. Three days later, the cells were expanded into a gelatin-coated 60 mm plate in complete media and incubated for 2 days. Selection of stably transfected cells was achieved by culturing the cells in complete media supplemented with 300 μg/mL G418 (Gibco/Life Technologies). A total of 6 colonies were picked from each selection plate and expanded for 10 passages. The N-Flag-TDP-43 mES cell lines were validated for N-Flag-TDP-43 insertion by incubating with increasing concentrations of doxycycline (Dox; 1-2 mg/ml) for 3 days prior to DNA and protein extraction for sequencing by the University of Michigan Sequencing Core and western blotting, respectively. One validated line was used for all subsequent experiments.
Luciferase reporter assays
N-Flag-TDP-43-expressing AinV15 cells were plated at ~2×105 cells/well in 0.1% gelatin-coated 6-well plates (Corning/Cellgro) in complete media on day 1. On day 2, media was changed to complete media ± 1μg/mL Dox to induce expression of TDP-43 (Kelloff and Sigman, 2005; Lunn et al., 2012). The cells were split into a 24-well plate on day 6 at ~7×104 cells/well and transfected using Lipofectamine 2000 reagent (Invitrogen/Thermo Fisher Scientific, Carlsbad, CA, USA) with 150 ng of pmirGlo (empty vector) or pmirGlo-AGO4 on day 7. Luciferase reporter activity was assayed 24 h after transfection in duplicate by using the Dual-Light system (Promega, Madison, WI, USA). Firefly luciferase activity was normalized to Renilla luciferase activity to account for transfection efficiency. Analysis of data was performed as previously reported (Van Etten et al., 2013) and expressed as mean ± SEM of percent repression from 3 independent experiments using a two-sample equal variance t-test with GraphPad Prism 5.
Western blot analysis
Laemmli sample buffer was added to luciferase assay protein extracts and the samples were boiled, centrifuged, and run in a SDS-polyacrylamide gel and the proteins were transferred to a PVDF membrane (EMB Millipore, Darmstadt, Germany). Immunoblot analysis was performed using rabbit polyclonal antibody for Flag (G400) (Cat# F1804; Sigma-Aldrich) and goat polyclonal antibody for Actin (1-19) (Cat# sc-1616; Santa Cruz Technology, Dallas, Texas, USA) as well as Santa Cruz Technology secondary antibodies: horseradish peroxidase-conjugated goat anti-mouse (sc-2005) and goat anti-rabbit (Cat# sc-2020).
Results
Mature miRNA dysregulation in postmortem human spinal cord
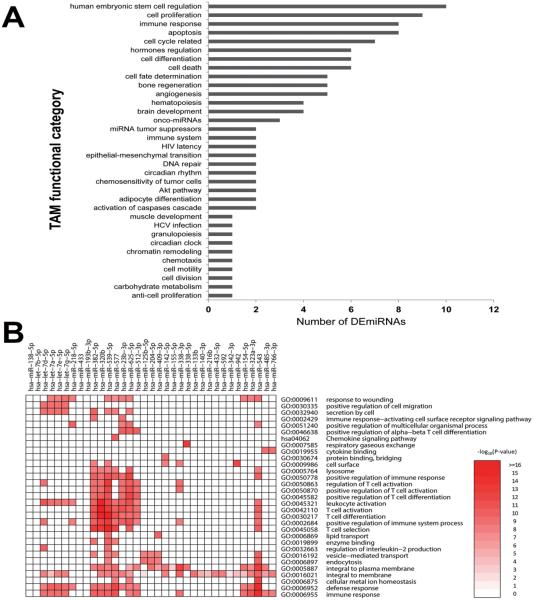
To determine whether altered miRNA regulation plays a role in the dysregulation of DEGs previously identified in postmortem human spinal cord tissue (Figueroa-Romero et al., 2012), we performed TaqMan OpenArray miRNA profiling on spinal cord tissue from 12 sALS and 12 control subjects (Table 1). Array profiling identified 90 mature DEmiRNAs in sALS, among which 98% were decreased and only miR-155 and miR-142-5p were increased (Table S3). Functional enrichment analyses provided insight into over-represented functions among the DEmiRNAs, identifying functional categories associated with functions known to be altered in ALS, such as cell death, the immune response, and brain development (Fig. 1A).
Fig. 1. Enriched biological functions of DEmiRNAs and targets in sALS spinal cord.
(A) To examine the distribution of functional categories for the DEmiRNAs, a bar chart was created based on the "Functions" categories of the tool for annotations of human miRNAs (TAM) database. The grey bars indicate the number of DEmiRNAs in each functional category. Only those functions with at least 5 DEmiRNAs are included. (B) For each DEmiRNA with a minimum of five target genes in the current study, we performed gene set enrichment analysis to identify the enriched biological functions among the collected high-quality targets (both validated and predicted). A heat map was generated to visualize the most significantly enriched terms. P-values were transformed by −log10(P-value) and color-indexed to indicate significance levels. Significance ranged from no significance (white) to highest significance (dark red).
The sALS postmortem spinal cord samples used in this study corresponded to subjects without a family history of ALS. Abnormal GGGGCC hexanuclotide repeat expansions within the C9ORF72 gene underlie the most common form of fALS, affecting about 40% of cases, and expansions are also found in 6-8% of sALS cases. Mutations in other genes, such as Cu2+/Zn2+ superoxide dismutase (SOD1), TARDBP, and FUS, are only found in 1-2% of sALS cases (Boylan, 2015). Based on the size of our cohort, we screened only for abnormal GGGGCC hexanuclotide repeat expansions within the C9ORF72 gene and identified one subject with an abnormal C9ORF72 expansion (Table 1; Fig. S1). Follow-up analyses on the array profiling results, however, revealed that the levels of selected dysregulated mature miRNAs and their predicted targets were similar across all samples, suggesting that the inclusion of the subject with an abnormal C9ORF72 expansion did not impact the outcomes of the present analyses.
DEmiRNA gene targets and affected biological functions in postmortem human spinal cord
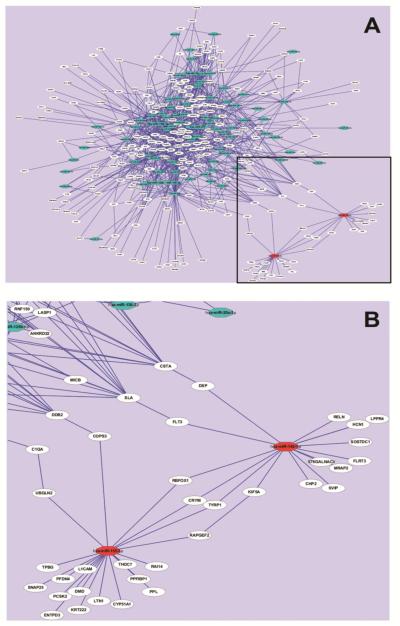
Potential relevant DEG targets of the 90 DEmiRNAs were obtained by compiling multiple miRNA prediction target databases that included both experimentally validated and predicted targets. Since miRNAs negatively regulate gene expression, pairs were limited by identifying inverse correlations between DEmiRNAs and mRNAs (1,182 DEGs from our previous work (Figueroa-Romero et al., 2012)). Examination of enriched biological functions among the potential DEmiRNA gene targets using heat map analysis indicated enrichment of defense and immune responses (Fig. 1B). Connections among the target genes and their corresponding miRNAs were next identified using target network analysis. This indicated that the increased (n=2) and decreased (n=88) DEmiRNAs and their targets (n=237) formed two large sub-networks (Fig. 2A) that are connected via protein-protein interactions between 8 targets (Fig. 2B).
Fig. 2. Association network of miRNA-targets in sALS human spinal cord.
(A) The complete miRNA-target network representing all 90 DEmiRNAs, and their targets. (B) Zoomed image of the boxed region in (A) depicting the two increased miRNAs and their predicted mRNA targets and interactions. Blue octagons represent decreased DEmiRNAs and red octagons represent increased DEmiRNAs. White circles represent the predicted miRNA target genes. Lines connecting two nodes represent miRNA-target or target-target interactions.
Our analysis indicates that the two increased miRNAs in sALS spinal cord, miR-155 and miR-142, are predicted regulators of dysregulated sALS and neurodegeneration-related gene transcripts, which include ubiquilin2 (UBQLN2), RNA binding protein, fox-1 (RBFOX1), and reelin (RELN) (Fig. 2B). miR-155 and miR142 are also potential regulators of several genes, such as RBFOX1, crystallin-mu (CRYM), tyrosin kinase-related protein 1 (TYRP1), and indirectly through protein-protein interactions, with Rap guanine nucleotide exchange factor 2 (RAPGEF2) and kinesin family member 5A (KIF5A). These data suggest that several disease targets are likely regulated by these two miRNAs (Fig. 2B).
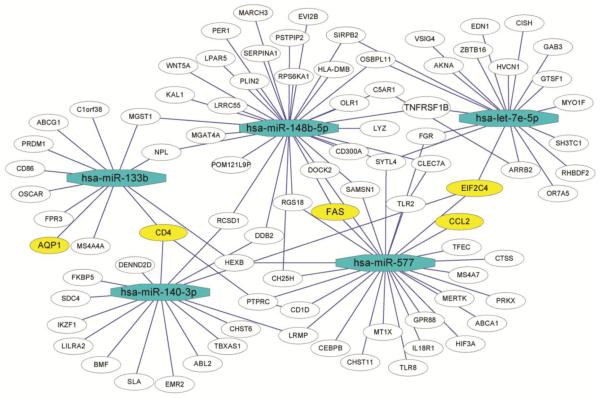
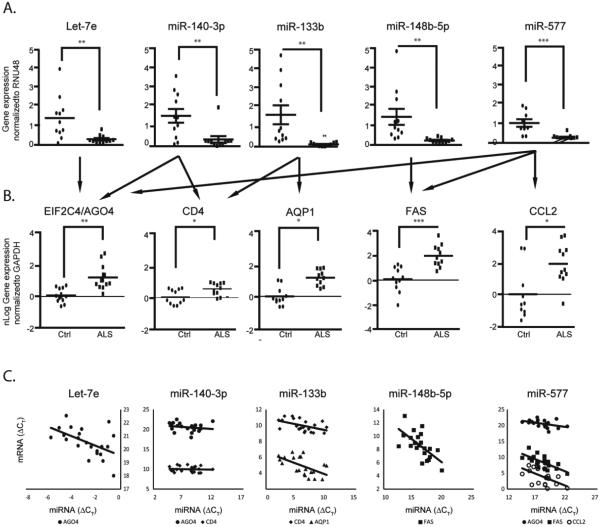
For the larger subnetwork of decreased miRNAs, we selected miRNAs to be further analyzed based on 1) high delta-CT (fold expression) in the array or high correlation expressions of miRNA and predicted mRNA pairs, or 2) an ALS candidate gene approach using the known biological function for the miRNA, the predicted mRNA candidate functions (focusing on ALS-related functions such as immune response, cell death, and neurodegeneration), or interconnection with ALS-related pathways in miRNA-mRNA predicted target networks. Based on these criteria, the miRNAs let-7e, miR-148b-5p, miR-577, miR-133b, and miR-140-3p (Fig. 3, blue octagons) were selected. These five DEmiRNAs collectively may regulate 86 different genes in human spinal cord (Fig. 3, white/yellow circles), among which we selected FAS, CD4, EIF2C4/AGO4, CCL2, and AQP1 for experimental validation, as they have been implicated in neuronal homeostasis and/or the pathogenesis of ALS (Fig. 3, yellow circles). Inverse correlation between the five selected miRNAs and the five predicted targets in sALS was confirmed by qPCR (Fig. 4). Together, these results demonstrate the identification and validation of differentially expressed miRNA-target pairs and provide insight into the potential biological implications of their dysregulation.
Fig. 3. Association network of select decreased miRNAs and their candidate mRNA targets in sALS human spinal cord.
Five decreased miRNAs were selected from the DEmiRNAs based on 1) array delta-CT (fold expression) or high correlation expression of miRNA and predicted mRNA pairs, or 2) by the known biological function for the miRNA or the predicted mRNA candidate (immune response, cell death, and neurodegeneration) or interconnection with several ALS related pathways by miRNA-mRNA predicted target network. Selected miRNA-mRNA interactions were then visualized using Cytoscape. Blue octagons represent decreased DEmiRNAs. White and yellow circles represent predicted candidate target genes, with the yellow circles representing genes selected for further investigation. Lines connecting two nodes represent miRNA-target or target-target interactions.
Fig. 4. Confirmation of selected DEmiRNAs and potential mRNAs targets in spinal cord.
(A) Preamplified miRNA cDNA from postmortem human spinal cord used for the array from sALS (n=11) and control (n=11) subjects was analyzed by qPCR using TaqMan technology. Results were normalized to RNU48 as the reference gene (delta-CT) and then relative to the control group (delta-delta-CT). Levels of miRNA PCR products are expressed as mean ± SEM. (B) For mRNA, cDNA was generated from total RNA extracted from the same postmortem human spinal cord samples as in (A) and analyzed by qPCR. mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and presented as fold change. Individual data points are plotted as nLog of mean ± SEM. Arrows indicate the predicted targets for the selected miRNAs. *P<0.05, ** P<0.01, ***P<0.001 compared to the control group (Ctrl). (C) X-Y scatter plot of miRNA and predicted target mRNA values from individual samples using their delta-Ct values referenced respectively from RNU48 miRNA (A) and GAPDH (B) mRNA levels indicate inverse correlation.
Expression of immature miRNA transcripts are not altered in sALS
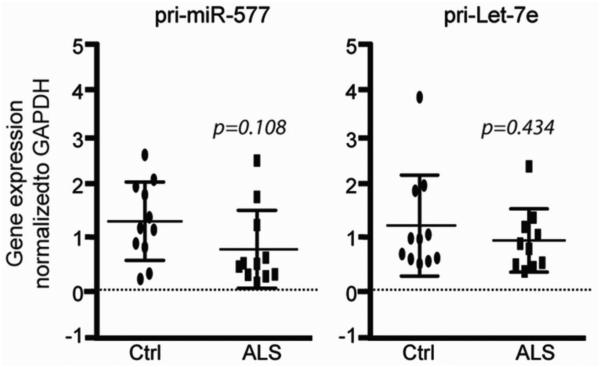
Given that the global decrease of most mature DEmiRNAs in sALS spinal cord may be explained by alterations either at the level of gene expression or at the level of miRNA processing, we examined intermediates in miRNA biogenesis for two of our selected DEmiRNAs. To determine if miRNA processing defects could explain the differences in mature miRNA levels, we examined the primary transcript expression levels of pri-let-7e, which is an evolutionarily conserved miRNA, and pri-miR-577, a predicted regulator of several genes. While the levels of the mature miRNAs for these two transcripts are differentially expressed in our array data (Fig. 4; Table S3), the levels of the two pri-miRNAs are the same between sALS and control samples (Fig. 5). These data suggest that differential expression of mature miRNAs in sALS is likely not secondary to alterations in gene expression, but instead, that miRNA processing is halted and/or premature miRNAs are “trapped” while undergoing processing.
Fig. 5. Let-7e and miR-577 primary transcripts are not altered in sALS.
Gene expression levels of pri-miR-577 and pri-Let-7e were analyzed in sALS and control samples by qPCR and normalized to GAPDH. The data are presented as fold change and plotted as mean ± SEM.
Over-expression of TDP-43 alters miRNA activity
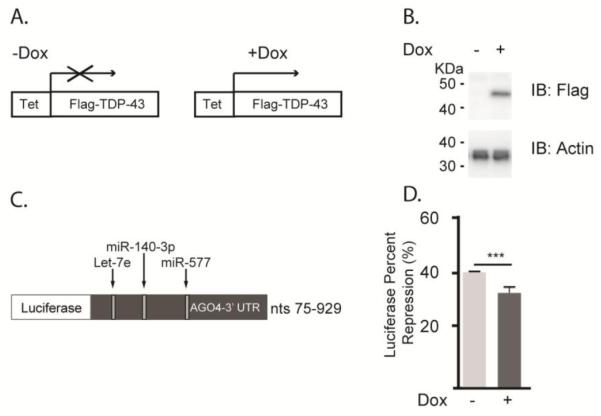
TDP-43 plays a role in miRNA biogenesis, has been linked to the inhibitory effect of miRNAs on their targets by interacting with mature and pri-miRNAs, and is known to prevent the incorporation of selected miRNAs into the RISC (Buratti et al., 2010; Emde et al., 2015; Kawahara and Mieda-Sato, 2012; King et al., 2014; Li et al., 2013b; Zhang et al., 2013); therefore, we were interested in determining whether TDP-43 plays a role in the miRNA-induced regulation of one of our selected target genes, EIF2C4/AGO4. AinV15 mES cells conditionally expressing N-Flag-TDP-43 under control of a Dox-inducible promoter were transiently transfected with a luciferase reporter expressing the 3’ UTR of EIF2C4/AGO4 in the presence or absence of Dox. While endogenous miRNAs negatively regulated the expression of the luciferase reporter, over-expression of TDP-43 attenuated the endogenous negative regulation of this reporter (Fig. 6). These data suggest that TDP-43 may alter the expression or function of endogenous miRNAs, offering a potential mechanism by which miRNA dysregulation occurs in ALS.
Figure 6. Over-expression of TDP-43 reduces miRNA regulation of EIF2C4/AGO4 stability.
(A) Schematic representation of protein expression when N-Flag-TDP-43 AinV15 cells containing an inducible tetracycline response element (Tet) are grown in the presence or absence of Dox for 5 days. (B) Total cell lysates were immunoblotted for the Flag tag to confirm the expression of Flag-TDP-43 in the presence or absence of Dox. Actin levels were assessed to ensure equal protein loading. (C) At day 3 of Dox induction, the cells were transiently transfected with a luciferase reporter fused to the 3’ UTR of EIF2C4/AGO4 (75-929 nt). The reporter harbors seed sequences recognized by three of the 5 selected DEmiRNAs, including miR-140-3p, miR-577, and Let-7e. (D) Relative luciferase activity was quantified in duplicate to determine the effect of endogenous miRNAs on luciferase reporter expression in the presence or absence of TDP-43 over-expression. Data are presented as the mean ± SEM of three independent experiments. ***P<0.001.
Discussion
The lack of an effective treatment for ALS and the emerging association of epigenetic alterations stemming from environmental exposures has fueled increased interest in understanding miRNA regulation in the nervous system in ALS, especially since miRNAs may be potential therapeutic targets (Buratti et al., 2010; Kawahara, 2010; Mackenzie et al., 2010; Williams et al., 2009). Here, we identified 90 DEmiRNAs in sALS postmortem spinal cord that associate with the differential expression of 237 predicted gene targets. These DEmiRNA:DEG pairs affect functional processes and pathways including cell death, defense responses, immune responses, and inflammation, thereby supporting their biological relevance and providing insights into potential pathogenic mechanisms. Furthermore, we demonstrate global reduction of mature miRNAs but no changes in two pri-miRNA transcripts, indicating that miRNA biogenesis and/or miRNA turnover are likely compromised in sALS. Finally, our data support for a role of TDP-43 in miRNA regulation and offers insight into a potential pathogenic mechanism for sALS.
The potential contribution of epigenetic mechanisms, including DNA methylation, chromatin remodeling, and miRNAs, to neurodegeneration is an emerging area of intense investigation. Of particular interest, miRNA dysregulation is linked to aging and neurodegenerative disorders, including PD, AD, and multiple sclerosis (Goodall et al., 2013; Li et al., 2011; Ma et al., 2014). miRNA alterations are also present in microglial and neuronal cells in rodent ALS models and in peripheral blood mononuclear cells (PBMCs), fibroblasts, cerebrospinal fluid, and postmortem tissue from ALS subjects (Butovsky et al., 2012; Campos-Melo et al., 2013; De Felice et al., 2012; Freischmidt et al., 2014; Koval et al., 2013a; Kye and Goncalves Ido, 2014; Shinde et al., 2013), results which all support our observed DEmiRNAs in postmortem ALS spinal cord tissue. DEmiRNAs are likewise present in serum from pre-manifest fALS mutation carriers, suggesting both a role for miRNAs in ALS pathogenesis and as potential diagnostic biomarkers (Freischmidt et al., 2014). Therapeutic strategies targeting miRNAs (DeVos and Miller, 2013; Koval et al., 2013a; Nolan et al., 2014) further endorse their role in ALS pathogenesis; both intraventricular delivery of a miR-155 inhibitor as well as intracerebroventricular injection of an antagomir to miR-29a increases lifespan in ALS transgenic mice. We contend that understanding the contribution of miRNAs to sALS has the potential to enhance our understanding of disease mechanisms and offer novel therapeutic targets.
The majority of the identified DEmiRNAs in postmortem ALS spinal cord are decreased and may collectively promote the up-regulation of over 200 gene targets. This is consistent with recent reports demonstrating overall decreases of miRNAs in ALS spinal cord tissue (Campos-Melo et al., 2013; Emde et al., 2015). Interestingly, functional analysis of our DEmiRNA:DEGs reveals enriched biological functions, including cell death, defense and immune responses, and brain development, suggesting alterations in the neuronal microenvironment. Notably, human ALS research is restricted by the unavailability of nervous tissue to study during disease development, and while postmortem tissue provides a direct way to analyze spinal cord and brain, it is difficult to differentiate whether molecular alterations are due to pre-mortem events, post-mortem handling of the tissue, or tissue region (Ferrer et al., 2008) rather than the pathogenic mechanisms responsible for disease onset and progression. Our study most likely represents alterations in miRNA levels within the neuronal microenvironment rather than in motor neurons themselves. ALS is a non-cell autonomous multifactorial disease, and end-stage ALS spinal cord presents considerable reactivity and proliferation of astrocytes and microglia, respectively, in concert with a significant reduction of motor neurons and oligodendrocytes (Philips and Rothstein, 2014). Interestingly, Emde et al recently reported a similar global reduction of mature miRNAs in laser-captured microdissected ALS postmortem spinal cord motor neurons. Thus, although our study may reflect a lack of motor neurons at end-stage, the high representation of dysregulated miRNAs involved in the regulation of immune and inflammation response-related genes strongly argues that our profiling embraces mostly non-neuronal cells (Jiang et al., 2005) or that the miRNA profile of non-neuronal cells is similar to that of motor neurons that have survived degeneration. Previous studies assessing miRNA alterations in ALS mouse microglia similarly identified immune-related miRNAs (Parisi et al., 2013), and a number of groups are investigating the immunological aspects underlying ALS pathogenesis (Evans et al., 2013; Zhao et al., 2013). Whether our observed findings reflect alterations in endogenous or infiltrating immune cells requires additional investigation; however, these data do support continued research on understanding a potential immunological component to neurodegeneration in ALS.
In our dataset, only miR-155 and miR-142-5p were increased. These miRNAs show the same trend in multiple sclerosis PBMCs, ALS mouse spinal cord, and human ALS monocytes (Butovsky et al., 2012; Koval et al., 2013b; Ma et al., 2014); however, a decrease in miR-142-5p levels was reported in ALS subject fibroblasts (Raman et al., 2014), indicating potential tissue-specific regulation. Interestingly, miR-155 and miR-142-5p are predicted to regulate UBQLN2 and RELN, respectively, genes down-regulated among our DEGs (Figueroa-Romero et al., 2012). UBQLN2 mutations are associated with fALS and its malfunction is associated with altered clearance of protein aggregates (Zhang et al., 2014). RELN is an extracellular matrix glycoprotein important for neural circuit formation and maintenance and cortical neuron migration. RELN dysfunction or loss is associated with neurodegeneration in AD, epilepsy, and autism (Folsom and Fatemi, 2013), and reports demonstrate RELN regulation by heavy metals and methylation (Zhubi et al., 2014). Given the previous association of both targets with neurodegeneration in conjunction with the loss of connectivity along the corticospinal tract in ALS, additional investigations into potential pathogenic pathways affected by these two miRNAs are warranted.
For further analyses, we next selected five decreased DEmiRNAs and five gene targets based on both fold change/high correlation between the DEmiRNA:DEG pairs and an ALS candidate gene approach. The miRNAs let-7e, miR-148b-5p, miR-577, miR-133b, and miR-140-3p, as well as the targets FAS, CD4, EIF2C4/AGO4, CCL2, and AQP1, were selected. Interestingly, we observed decreased levels of several members of the let-7 miRNA family in sALS spinal cord. Alterations in let-7 are present in animal models of PD and serum from subjects with multiple sclerosis and AD (Asikainen et al., 2010; Gandhi et al., 2013; Gehrke et al., 2010; Tan et al., 2014). Let-7b processing is also regulated by TDP-43 and its expression is altered in lymphoblast cell lines as well as cerebrospinal fluid, serum, and PBMCs from sALS patients (Buratti et al., 2010; Butovsky et al., 2012; Freischmidt et al., 2013; Kawahara and Mieda-Sato, 2012). These observations provide further support for a potential pathological role for let-7 family members in ALS.
Similarly, the predicted gene targets FAS, CCL2, and CD4 are implicated in ALS and neurodegeneration. Microglia express increased CCL2 leading up to disease onset in ALS mice, and CD4+ T cells mediate dopaminergic toxicity in a mouse model of PD and are present in the spinal cord of ALS subjects (Brochard et al., 2009; Butovsky et al., 2012; Engelhardt et al., 1993; Kawamata et al., 1992). Importantly, the association of EIF2C4/AGO4 is also of particular interest given its role as a member of the argonaute family of proteins, a component of the RISC in miRNA function (Modzelewski et al., 2012; Sasaki et al., 2003; Winter and Diederichs, 2011). Indeed, recent studies demonstrate that argonaute proteins contribute to miRNA processing and function and are dysregulated in ALS and Huntington’s disease (Raman et al., 2014; Savas et al., 2008; Tan et al., 2014), suggesting that miRNA biogenesis may be altered in neurodegeneration.
Along these lines, our results identified a global reduction in levels of mature DEmiRNAs and altered regulation of their corresponding targets in ALS that may underlie a potential defect in miRNA biogenesis or RNA stability. We found that the levels of two immature pri-miRNAs, pri-miR-577 and pri-miR-let-7e, were not altered in postmortem sALS spinal cord tissue compared to their mature forms. Recent evidence suggests that alterations of miRNA biogenesis lead to motor neuron degeneration, in part, due to the aberrant catalytic activity of the cytoplasmic RNAse Dicer and protein-protein interactions between molecular components responsible for stress granule formation and miRNA biogenesis (Emde et al., 2015; Haramati et al., 2010). We argue that dysregylation of non-canonical miRNA biogenesis may also play a role in sALS pathogenesis, as we observe increased levels of miR-155 and miR-142-5p in sALS while the rest of the dysregulated miRNAs are decreased (Abdelfattah et al., 2014).
Mutations in genes encoding the nucleotide binding proteins FUS and TDP-43 and abnormal hexanucleotide repeat expansions in C9ORF72, as well as the pathological aggregation of FUS and TDP-43 in stress granules and processing bodies (p-bodies) in the cytoplasm (Li et al., 2013a) or disruption of nuclear paraspeckle formation (Shelkovnikova et al., 2014) in neurons and glia, further highlights the role of altered RNA processing in ALS pathogenesis (Belzil et al., 2013; Duan et al., 2014). Of interest, TDP-43 binds to miRNAs and components of the miRNA-processing pathway at different steps, exhibiting direct interactions with mature miRNAs, nuclear pri-miRNAs, the nuclear RNAse Drosha, and the cytoplasmic RNAse Dicer (Buratti et al., 2010; Emde et al., 2015; Kawahara and Mieda-Sato, 2012; King et al., 2014; Li et al., 2013b; Zhang et al., 2013). In addition, TDP-43 also affects how miR-206, a skeletal muscle-specific miRNA involved in neuromuscular junction re-innervation in ALS animal models and following nerve injury, incorporates into the RISC (King et al., 2014). Our data suggests that TDP-43, in part, may regulate the function of miRNAs decreased in sALS spinal cord as well as their downstream targets, perhaps by the sequestration of miRNA biogenesis components to stress granules upon stress (Emde et al., 2015). Furthermore, it is important to note that post-transcriptional RNA modifications could also play a role in the global reduction of dysregulated RNAs by altering the stability of the premature miRNAs. Although miRNAs are thought to be more stable than mRNAs, global and rapid turnover of miRNAs is more striking in neuronal cells compared to other cell types (Krol et al., 2010). Post-transcriptional O-methylation is a well-known mechanism that increases the stability of small RNAs (Ji and Chen, 2012), while on the other hand, O-methylation of the 5’ mono-phosphate end of pre-miR-145 by the BCDIN3 domain-containing methyltransferase (BCDIN3D) interferes with further maturation by Dicer (Xhemalce et al., 2012). Collectively, our data and these studies suggest that global reduction of miRNA levels in ALS may result from their sequestration within RNA-binding protein aggregates, accelerated RNA turn over, or disruption of miRNA biogenesis (Paez-Colasante et al., 2015).
We acknowledge that the size of the cohort analyzed in this study was relatively small due to the limited availability of human postmortem samples. Nevertheless, others have reported a similar global decrease in mature miRNAs using smaller cohorts (Campos-Melo et al., 2013; Emde et al., 2015), suggesting that this observation is relevant to the disease, as it has been reported in three different cohorts. With the advent of multicenter biobanks, these results should be validated in larger cohorts in the future. Moreover, the rapid development of stem cell technology to generate human motor neurons from patient-derived induced pluripotent stem cells will allow us to determine whether the drastic decline in miRNAs is responsible for the onset of the disease, facilitates the progression of the disease, or whether it results from end-stage complications.
Conclusions
This study combines gene and miRNA expression profiling/bioinformatics analyses to address the hypothesis that miRNA dysregulation promotes aberrant effects on biologically relevant pathways that underlie ALS. Our data suggest a potential mechanism for altered miRNA expression in ALS and justify further studies examining the mechanisms and consequences of altered miRNA and target gene expression, as well as the utility of miRNA profiling as a potential diagnostic, pathogenic, and prognostic biomarker for ALS.
Supplementary Material
Highlights.
Mature miRNA are globally dysregulated in postmortem sporadic ALS spinal cord
Altered genes affect neuronal homeostasis, ALS pathogenesis, and miRNA biogenesis
Altered processes include cell death, defense/immune responses, and inflammation
Primary transcripts of a subset of dysregulated mature mRNAs are unchanged in sALS
Acknowledgements
We are grateful to the participants in this study and their families. We also thank Dr. Dave Turner at the University of Michigan for generously providing the CS2+ vector, as well as Dr. Fang He for expert consultation and intellectual discussion, Mr. Michael D. Cataldo, M. S. for technical assistance, and Mrs. Judith Bentley for excellent administrative support during the preparation of this manuscript. This work was supported in part by the University of Michigan A. Alfred Taubman Medical Research Institute (http://www.taubmaninstitute.org); the Katherine Rayner Fund; the Program for Neurology Research and Discovery; the National Institutes of Health (NIH) AG020628, AI110502, AG024824 and AG013283 (to R.Y.), ES017885 and P30 U035448 (to R.Y and E.L.F.), and T32 NS007222 (Postdoctoral Fellowship to D.E.B.); the Michigan Institute for Clinical & Health Research (supported by NIH UL1RR024986); the Geriatrics Research, Education and Clinical Care Center (GRECC) of the VA Ann Arbor Healthcare System; and the Juvenile Diabetes Research Foundation (Postdoctoral Fellowship to J.H.).
Abbreviations
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- AQP1
aquaporin 1
- BCDIN3D
BCDIN3 domain-containing methyltransferase
- BioGRID
Biological Genomic Repository for Interaction Datasets
- C9ORF72
chromosome 9 open reading frame 72
- CCL2
chemokine C-C motif ligand 2
- CD4
cluster of differentiation 4
- CRYM
crystallin-mu
- CT
cycle threshold
- DAVID
Database for Annotation, Visualization, and Integrated Discovery
- DEG
differentially expressed gene
- DEmiRNA
differentially expressed microRNA
- DGCR8
DiGorge syndrome critical region gene 8
- EIF2C4/AGO2
eukaryotic translation initiation factor 2C,2/argonaute 2
- EIF2C4/AGO4
eukaryotic translation initiation factor 2C,4/argonaute 4
- fALS
familial amyotrophic lateral sclerosis
- FAS
TNF receptor superfamily member 6/Fas ligand
- FDR
false discovery rate
- FUS
fused in sarcoma
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GO
gene ontology
- KIF5A
kinesin family member 5A
- miRNA
microRNA
- PACT
protein kinase, interferon-inducible double-stranded RNA dependent activator
- PBMC
peripheral blood mononuclear cell
- PD
Parkinson’s disease
- qPCR
quantitative polymerase chain reaction
- RAPGEF2
Rap guanine nucleotide exchange factor 2
- RBFOX1
RNA binding protein, fox 1
- RELN
reelin
- RISC
RNA induced silencing complex
- RT
reverse transcription
- sALS
sporadic amyotrophic lateral sclerosis
- TAM
tool for annotations of human miRNAs
- TDP-43
TAR DNA binding protein 43
- TYRP1
tyrosinase-related protein 1
- UBQLN2
ubiquilin 2
- UTR
untranslated region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALSoD: ALS Online Genetic Database Available from: http://alsod.iop.kcl.ac.uk/
- microRNA.org Available from http://microRNA.org/
- Abdelfattah AM, Park C, Choi MY. Update on non-canonical microRNAs. Biomolecular concepts. 2014;5:275–287. doi: 10.1515/bmc-2014-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Wicklund MP. Amyotrophic lateral sclerosis: what role does environment play? Neurologic clinics. 2011;29:689–711. doi: 10.1016/j.ncl.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Asikainen S, Rudgalvyte M, Heikkinen L, Louhiranta K, Lakso M, Wong G, Nass R. Global microRNA expression profiling of Caenorhabditis elegans Parkinson's disease models. Journal of molecular neuroscience : MN. 2010;41:210–218. doi: 10.1007/s12031-009-9325-1. [DOI] [PubMed] [Google Scholar]
- Belzil VV, Gendron TF, Petrucelli L. RNA-mediated toxicity in neurodegenerative disease. Mol Cell Neurosci. 2013;56:406–419. doi: 10.1016/j.mcn.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan K. Familial Amyotrophic Lateral Sclerosis. Neurologic clinics. 2015;33:807–830. doi: 10.1016/j.ncl.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. The Journal of clinical investigation. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, De Conti L, Stuani C, Romano M, Baralle M, Baralle F. Nuclear factor TDP-43 can affect selected microRNA levels. FEBS J. 2010;277:2268–2281. doi: 10.1111/j.1742-4658.2010.07643.x. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. The Journal of clinical investigation. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B, Feldman D, Gruis K, Feldman E. The association of exposure to lead, mercury, and selenium and the development of amyotrophic lateral sclerosis and the epigenetic implications. Neurodegener Dis. 2011;8:1–8. doi: 10.1159/000315405. [DOI] [PubMed] [Google Scholar]
- Campos-Melo D, Droppelmann CA, He Z, Volkening K, Strong MJ. Altered microRNA expression profile in amyotrophic lateral sclerosis: a role in the regulation of NFL mRNA levels. Molecular brain. 2013;6:26. doi: 10.1186/1756-6606-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatr-Aryamontri A, Breitkreutz BJ, Heinicke S, Boucher L, Winter A, Stark C, Nixon J, Ramage L, Kolas N, O'Donnell L, Reguly T, Breitkreutz A, Sellam A, Chen D, Chang C, Rust J, Livstone M, Oughtred R, Dolinski K, Tyers M. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2013;41:D816–823. doi: 10.1093/nar/gks1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PA, Richer R, Metcalf JS, Banack SA, Codd GA, Bradley WG. Cyanobacteria and BMAA exposure from desert dust: a possible link to sporadic ALS among Gulf War veterans. Amyotroph Lateral Scler 10 Suppl. 2009;2:109–117. doi: 10.3109/17482960903286066. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, Swash M. Amyotrophic lateral sclerosis: an update. Curr Opin Neurol. 2011;24:497–503. doi: 10.1097/WCO.0b013e32834916a9. [DOI] [PubMed] [Google Scholar]
- De Felice B, Guida M, Guida M, Coppola C, De Mieri G, Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene. 2012;508:35–40. doi: 10.1016/j.gene.2012.07.058. [DOI] [PubMed] [Google Scholar]
- DeVos SL, Miller TM. Antisense oligonucleotides: treating neurodegeneration at the level of RNA. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10:486–497. doi: 10.1007/s13311-013-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Sharma S, Xia Q, Garber K, Jin P. Towards Understanding RNA-Mediated Neurological Disorders. Journal of genetics and genomics = Yi chuan xue bao. 2014;41:473–484. doi: 10.1016/j.jgg.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Emde A, Eitan C, Liou LL, Libby RT, Rivkin N, Magen I, Reichenstein I, Oppenheim H, Eilam R, Silvestroni A, Alajajian B, Ben-Dov IZ, Aebischer J, Savidor A, Levin Y, Sons R, Hammond SM, Ravits JM, Moller T, Hornstein E. Dysregulated miRNA biogenesis downstream of cellular stress and ALS-causing mutations: a new mechanism for ALS. The EMBO journal. 2015;34:2633–2651. doi: 10.15252/embj.201490493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50:30–36. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Martinez A, Boluda S, Parchi P, Barrachina M. Brain banks: benefits, limitations and cautions concerning the use of post-mortem brain tissue for molecular studies. Cell and tissue banking. 2008;9:181–194. doi: 10.1007/s10561-008-9077-0. [DOI] [PubMed] [Google Scholar]
- Figueroa-Romero C, Hur J, Bender DE, Delaney CE, Cataldo MD, Smith AL, Yung R, Ruden DM, Callaghan BC, Feldman EL. Identification of epigenetically altered genes in sporadic amyotrophic lateral sclerosis. PloS one. 2012;7:e52672. doi: 10.1371/journal.pone.0052672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom TD, Fatemi SH. The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology. 2013;68:122–135. doi: 10.1016/j.neuropharm.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freischmidt A, Muller K, Ludolph AC, Weishaupt JH. Systemic dysregulation of TDP-43 binding microRNAs in amyotrophic lateral sclerosis. Acta neuropathologica communications. 2013;1:42. doi: 10.1186/2051-5960-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freischmidt A, Muller K, Zondler L, Weydt P, Volk AE, Bozic AL, Walter M, Bonin M, Mayer B, von Arnim CA, Otto M, Dieterich C, Holzmann K, Andersen PM, Ludolph AC, Danzer KM, Weishaupt JH. Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain : a journal of neurology. 2014 doi: 10.1093/brain/awu249. [DOI] [PubMed] [Google Scholar]
- Friedlander MR, Lizano E, Houben AJ, Bezdan D, Banez-Coronel M, Kudla G, Mateu-Huertas E, Kagerbauer B, Gonzalez J, Chen KC, Leproust EM, Marti E, Estivill X. Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome biology. 2014;15:R57. doi: 10.1186/gb-2014-15-4-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furby A, Beauvais K, Kolev I, Rivain JG, Sebille V. Rural environment and risk factors of amyotrophic lateral sclerosis: a case-control study. J Neurol. 2010;257:792–798. doi: 10.1007/s00415-009-5419-5. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Healy B, Gholipour T, Egorova S, Musallam A, Hussain MS, Nejad P, Patel B, Hei H, Khoury S, Quintana F, Kivisakk P, Chitnis T, Weiner HL. Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Annals of neurology. 2013;73:729–740. doi: 10.1002/ana.23880. [DOI] [PubMed] [Google Scholar]
- Gehrke S, Imai Y, Sokol N, Lu B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 2010;466:637–641. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall EF, Heath PR, Bandmann O, Kirby J, Shaw PJ. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front Cell Neurosci. 2013;7:178. doi: 10.3389/fncel.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH. Amyotrophic Lateral Sclerosis: An update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013;4:295–310. doi: 10.14336/AD.2013.0400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RI, Chendrimada TP, Shiekhattar R. MicroRNA biogenesis: isolation and characterization of the microprocessor complex. Methods in molecular biology (Clifton, N.J.) 2006;342:33–47. doi: 10.1385/1-59745-123-1:33. [DOI] [PubMed] [Google Scholar]
- Haramati S, Chapnik E, Sztainberg Y, Eilam R, Zwang R, Gershoni N, McGlinn E, Heiser PW, Wills AM, Wirguin I, Rubin LL, Misawa H, Tabin CJ, Brown R, Jr., Chen A, Hornstein E. miRNA malfunction causes spinal motor neuron disease. Proc Natl Acad Sci U S A. 2010;107:13111–13116. doi: 10.1073/pnas.1006151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M, Grunwald Kadow IC. New roles for "old" microRNAs in nervous system function and disease. Frontiers in molecular neuroscience. 2013;6:51. doi: 10.3389/fnmol.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Chen X. Regulation of small RNA stability: methylation and beyond. Cell research. 2012;22:624–636. doi: 10.1038/cr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YM, Yamamoto M, Kobayashi Y, Yoshihara T, Liang Y, Terao S, Takeuchi H, Ishigaki S, Katsuno M, Adachi H, Niwa J, Tanaka F, Doyu M, Yoshida M, Hashizume Y, Sobue G. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Annals of neurology. 2005;57:236–251. doi: 10.1002/ana.20379. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Iwamoto K. Comprehensive DNA methylation and hydroxymethylation analysis in the human brain and its implication in mental disorders. Neuropharmacology. 2014;80:133–139. doi: 10.1016/j.neuropharm.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Kawahara Y. Implications of microRNA dysfunction in the pathogenesis of ALS. Rinsho Shinkeigaku. 2010;50:979–981. doi: 10.5692/clinicalneurol.50.979. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Mieda-Sato A. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc Natl Acad Sci U S A. 2012;109:3347–3352. doi: 10.1073/pnas.1112427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- Kelloff GJ, Sigman CC. New science-based endpoints to accelerate oncology drug development. European journal of cancer (Oxford, England : 1990) 2005;41:491–501. doi: 10.1016/j.ejca.2004.12.006. [DOI] [PubMed] [Google Scholar]
- King IN, Yartseva V, Salas D, Kumar A, Heidersbach A, Ando DM, Stallings NR, Elliott JL, Srivastava D, Ivey KN. The RNA-binding Protein TDP-43 Selectively Disrupts MicroRNA-1/206 Incorporation into the RNA-induced Silencing Complex. The Journal of biological chemistry. 2014;289:14263–14271. doi: 10.1074/jbc.M114.561902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval ED, Shaner C, Zhang P, du Maine X, Fischer K, Tay J, Chau BN, Wu GF, Miller TM. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Human molecular genetics. 2013a;22:4127–4135. doi: 10.1093/hmg/ddt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval ED, Shaner C, Zhang P, du Maine X, Fischer K, Tay J, Chau BN, Wu GF, Miller TM. Method for Widespread microRNA-155 Inhibition Prolongs Survival in ALS-Model Mice. Human molecular genetics. 2013b doi: 10.1093/hmg/ddt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Duebel J, Bicker S, Fehling HJ, Schubeler D, Oertner TG, Schratt G, Bibel M, Roska B, Filipowicz W. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Ksiazek-Winiarek DJ, Kacperska MJ, Glabinski A. MicroRNAs as novel regulators of neuroinflammation. Mediators Inflamm. 2013;2013:172351. doi: 10.1155/2013/172351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Kye MJ, Goncalves Ido C. The role of miRNA in motor neuron disease. Front Cell Neurosci. 2014;8:15. doi: 10.3389/fncel.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. The EMBO journal. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li N, Bates DJ, An J, Terry DA, Wang E. Up-regulation of key microRNAs, and inverse down-regulation of their predicted oxidative phosphorylation target genes, during aging in mouse brain. Neurobiol Aging. 2011;32:944–955. doi: 10.1016/j.neurobiolaging.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. The Journal of cell biology. 2013a;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lu Y, Xu XL, Gao FB. The FTD/ALS-associated RNA-binding protein TDP-43 regulates the robustness of neuronal specification through microRNA-9a in Drosophila. Human molecular genetics. 2013b;22:218–225. doi: 10.1093/hmg/dds420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M. MicroRNA: a new entrance to the broad paradigm of systems molecular medicine. Physiological genomics. 2009;38:113–115. doi: 10.1152/physiolgenomics.00080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science (New York, N.Y.) 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Pacut C, Stern E, Sakowski SA, Velkey JM, O'Shea S, Feldman EL. Intraspinal transplantation of neurogenin-expressing stem cells generates spinal cord neural progenitors. Neurobiology of disease. 2012;46:59–68. doi: 10.1016/j.nbd.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, Singh N, Nagarkatti M, Nagarkatti P. Expression, regulation and function of microRNAs in multiple sclerosis. International journal of medical sciences. 2014;11:810–818. doi: 10.7150/ijms.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciotta S, Meregalli M, Torrente Y. The involvement of microRNAs in neurodegenerative diseases. Front Cell Neurosci. 2013;7:265. doi: 10.3389/fncel.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Grant AE, Jones JM, Lenk GM, He F, Todd PK, Kamali M, Albin RL, Lieberman AP, Langenecker SA, McInnis MG. C9ORF72 expansion in a family with bipolar disorder. Bipolar disorders. 2013;15:326–332. doi: 10.1111/bdi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewski AJ, Holmes RJ, Hilz S, Grimson A, Cohen PE. AGO4 regulates entry into meiosis and influences silencing of sex chromosomes in the male mouse germline. Developmental cell. 2012;23:251–264. doi: 10.1016/j.devcel.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan JM, Yu B, Trent RJ, Pamphlett R. A genome-wide analysis of brain DNA methylation identifies new candidate genes for sporadic amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:418–429. doi: 10.3109/17482960802635397. [DOI] [PubMed] [Google Scholar]
- Nolan K, Mitchem MR, Jimenez-Mateos EM, Henshall DC, Concannon CG, Prehn JH. Increased expression of microRNA-29a in ALS mice: functional analysis of its inhibition. Journal of molecular neuroscience : MN. 2014;53:231–241. doi: 10.1007/s12031-014-0290-y. [DOI] [PubMed] [Google Scholar]
- Paez-Colasante X, Figueroa-Romero C, Sakowski SA, Goutman SA, Feldman EL. Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nature reviews. Neurology. 2015;11:266–279. doi: 10.1038/nrneurol.2015.57. [DOI] [PubMed] [Google Scholar]
- Parisi C, Arisi I, D'Ambrosi N, Storti AE, Brandi R, D'Onofrio M, Volonte C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell death & disease. 2013;4:e959. doi: 10.1038/cddis.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Rothstein JD. Glial cells in amyotrophic lateral sclerosis. Experimental neurology. 2014;262:111–120. doi: 10.1016/j.expneurol.2014.05.015. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Advances in Epigenetics and Epigenomics for Neurodegenerative Diseases. Curr Neurol Neurosci Rep. 2011 doi: 10.1007/s11910-011-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Epigenetic mechanisms governing the process of neurodegeneration. Molecular aspects of medicine. 2013;34:875–882. doi: 10.1016/j.mam.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman R, Allen SP, Goodall EF, Kramer S, Ponger LL, Heath PR, Milo M, Hollinger HC, Walsh T, Highley JR, Olpin S, McDermott CJ, Shaw PJ, Kirby J. Gene expression signatures in motor neuron disease fibroblasts reveal dysregulation of metabolism, hypoxia-response and RNA processing functions. Neuropathology and applied neurobiology. 2014 doi: 10.1111/nan.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JH, O'Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nature reviews. Neuroscience. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shiohama A, Minoshima S, Shimizu N. Identification of eight members of the Argonaute family in the human genome. Genomics. 2003;82:323–330. doi: 10.1016/s0888-7543(03)00129-0. [DOI] [PubMed] [Google Scholar]
- Savas JN, Makusky A, Ottosen S, Baillat D, Then F, Krainc D, Shiekhattar R, Markey SP, Tanese N. Huntington's disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. Proc Natl Acad Sci U S A. 2008;105:10820–10825. doi: 10.1073/pnas.0800658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P, Corda B, Hatzigeorgiou AG. TarBase: A comprehensive database of experimentally supported animal microRNA targets. Rna. 2006;12:192–197. doi: 10.1261/rna.2239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelkovnikova TA, Robinson HK, Troakes C, Ninkina N, Buchman VL. Compromised paraspeckle formation as a pathogenic factor in FUSopathies. Human molecular genetics. 2014;23:2298–2312. doi: 10.1093/hmg/ddt622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S, Arora N, Bhadra U. A Complex Network of MicroRNAs Expressed in Brain and Genes Associated with Amyotrophic Lateral Sclerosis. Int J Genomics. 2013;2013:383024. doi: 10.1155/2013/383024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique T, Ajroud-Driss S. Familial amyotrophic lateral sclerosis, a historical perspective. Acta Myol. 2011;30:117–120. [PMC free article] [PubMed] [Google Scholar]
- Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Yu JT, Tan MS, Liu QY, Wang HF, Zhang W, Jiang T, Tan L. Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2014;40:1017–1027. doi: 10.3233/JAD-132144. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Messina P, Conti E, Sala G, Cecchi M, Airoldi L, Pastorelli R, Pupillo E, Bandettini Di Poggio M, Filosto M, Lunetta C, Agliardi C, Guerini F, Mandrioli J, Calvo A, Beghi E, Ferrarese C, Consortium E. Whole-blood global DNA methylation is increased in amyotrophic lateral sclerosis independently of age of onset. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2014;15:98–105. doi: 10.3109/21678421.2013.851247. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk M, van Es MA, Koppers M, van Rheenen W, Medic J, Schelhaas HJ, van der Kooi AJ, de Visser M, Veldink JH, van den Berg LH. VAPB and C9orf72 mutations in 1 familial amyotrophic lateral sclerosis patient. Neurobiol Aging. 2012;33:2950–2954. doi: 10.1016/j.neurobiolaging.2012.07.004. e2951. [DOI] [PubMed] [Google Scholar]
- Van Etten J, Schagat TL, Goldstrohm AC. A guide to design and optimization of reporter assays for 3' untranslated region mediated regulation of mammalian messenger RNAs. Methods (San Diego, Calif.) 2013;63:110–118. doi: 10.1016/j.ymeth.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Wang J, Cui Q. Specific Roles of MicroRNAs in Their Interactions with Environmental Factors. Journal of nucleic acids. 2012;2012:978384. doi: 10.1155/2012/978384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science (New York, N.Y.) 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Diederichs S. Argonaute proteins regulate microRNA stability: Increased microRNA abundance by Argonaute proteins is due to microRNA stabilization. RNA biology. 2011;8:1149–1157. doi: 10.4161/rna.8.6.17665. [DOI] [PubMed] [Google Scholar]
- Xhemalce B, Robson SC, Kouzarides T. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell. 2012;151:278–288. doi: 10.1016/j.cell.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KY, Yang S, Warraich ST, Blair IP. Ubiquilin 2: a component of the ubiquitin-proteasome system with an emerging role in neurodegeneration. The international journal of biochemistry & cell biology. 2014;50:123–126. doi: 10.1016/j.biocel.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Almeida S, Lu Y, Nishimura AL, Peng L, Sun D, Wu B, Karydas AM, Tartaglia MC, Fong JC, Miller BL, Farese RV, Jr., Moore MJ, Shaw CE, Gao FB. Downregulation of microRNA-9 in iPSC-derived neurons of FTD/ALS patients with TDP-43 mutations. PloS one. 2013;8:e76055. doi: 10.1371/journal.pone.0076055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:888–899. doi: 10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhubi A, Chen Y, Dong E, Cook EH, Guidotti A, Grayson DR. Increased binding of MeCP2 to the GAD1 and RELN promoters may be mediated by an enrichment of 5-hmC in autism spectrum disorder (ASD) cerebellum. Translational psychiatry. 2014;4:e349. doi: 10.1038/tp.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.