Abstract
S-nitrosothiols (SNOs) such as S-nitroso-L-cysteine (L-cysNO) are endogenous compounds with potent vasodilatory activity. During circulation in the blood, the NO moiety can be exchanged among various thiol-containing compounds by S-transnitrosylation, resulting in SNOs with differing capacities to enter the cell (membrane permeability). To determine whether the vasodilating potency of SNOs is dependent upon membrane permeability, membrane-permeable L-cysNO and impermeable S-nitroso-D-cysteine (D-cysNO) and S-nitroso-glutathione (GSNO) were infused into one femoral artery of anesthetized adult sheep while measuring bilateral femoral and systemic vascular conductances. L-cysNO induced vasodilation in the infused hind limb, whereas D-cysNO and GSNO did not. L-cysNO also increased intracellular NO in isolated arterial smooth muscle cells, whereas GSNO did not. The infused SNOs remained predominantly in a low molecular weight form during first-passage through the hind limb vasculature, but were converted into high molecular weight SNOs upon systemic recirculation. At systemic concentrations of ~0.6 μmol/L, all three SNOs reduced mean arterial blood pressure by ~50%, with pronounced vasodilation in the mesenteric bed. Pharmacokinetics of L-cysNO and GSNO were measured in vitro and in vivo and correlated with their hemodynamic effects, membrane permeability, and S-transnitrosylation. These results suggest local vasodilation by SNOs in the hind limb requires membrane permeation, whereas systemic vasodilation does not. The systemic hemodynamic effects of SNOs occur after equilibration of the NO moiety amongst the plasma thiols via S-transnitrosylation.
Keywords: S-nitrosothiol, nitric oxide, vasodilation, S-transnitrosylation, membrane permeability, in sheep
Graphical Abstract
Introduction
Low molecular weight S-nitrosothiols (SNOs) such as S-nitroso-L-cysteine (L-cysNO) and S-nitroso-glutathione (GSNO) are endogenous compounds formed by replacing the hydrogen of a thiol group with a nitroso group. This can occur by reaction of the thiol with products of the oxidation of nitric oxide (NO) free radical. Alternatively, SNOs can be formed by a process known as S-transnitrosylation, whereby the nitroso group from one SNO is transferred to another thiol according to the following reaction[1, 2]:
where R1SH and R2SH represent different thiol containing molecules.
SNOs have NO-like bioactivity, including potent vasodilation[3–5]. In blood, many SNOs have elimination half lives that are several orders of magnitude longer than that of free NO[1, 5, 6], raising the possibility that SNOs may serve as a circulating reservoir of NO bioactivity contributing to cardiovascular homeostasis[1].
SNOs can cause vasodilation by the traditional NO/cGMP-dependent pathway, or by less-characterized cGMP-independent pathways. In the cGMP-dependent pathway, SNOs are thought to enter the cell via the L-type amino acid transporter (LAT)[7–9], where they then release the NO moiety to activate soluble guanylate cyclase (sGC)[10–12]. Proposed cGMP-independent pathways to vasodilation may involve the S-transnitrosylation of functional proteins in the contractile signaling pathways[3, 4, 13, 14].
One experimental approach to test whether the vasoactivity of SNOs requires their cellular uptake is to compare the vasodilatory effects of membrane permeable L-cysNO, which enters cells via the selective L-type amino acid transporter[7, 8, 15, 16], with those of its stereoisomer D-cysNO and also with GSNO, both of which are membrane-impermeable[7, 8]. Previous studies comparing the hemodynamic effects of different SNOs were performed by intravenous administration of the SNOs[3, 4, 13]. However, SNOs injected into the circulation will confront either rapid enzymatic conversion to other species or S-transnitrosylation reactions with plasma thiols that may transfer the NO moiety to a molecule with distinctly different membrane permeability and bioactivity[17]. For example, major portions of L-cysNO and GSNO added to blood are rapidly consumed by S-transnitrosylation reaction with albumin [1, 18, 19]. Although the kinetics of the consumption of L-cysNO and GSNO in the circulation are not well defined, their expected half lives based on bench measurements performed in the presence of excess thiols [20, 21] would be shorter than the time needed for blood to circulate from the venous to arterial circulation. As a result, a majority of intravenously infused L-cysNO and GSNO would be converted to other types of SNOs via S-transnitrosylation prior to the infusate reaching the resistance vessels of the systemic arterial vasculature. Thus, it cannot be assumed that the effects of intravenously administered SNOs on arterial vascular tone are specific to the SNO that was infused as opposed to its S-transnitrosylation products. In contrast, if a SNO is infused into an artery and reaches resistance vessels within three seconds, a reasonable estimate of the time needed to reach the downstream resistance vessels, the chances of it remaining in the original infused form are greatly increased and local vascular responses are more likely to be specific to the SNO being infused.
We reasoned that the effects of an intra-arterial infused SNO on resistance to blood flow in the local downstream vasculature would be a more specific measure of the vasoactivity of the infused SNOs. We also reasoned that considerable S-transnitrosylation would occur prior to systemic recirculation of the infused SNO, and thus comparison of local and systemic responses would enable us to detect differences in vasoactivity of the infused SNO versus its S-transnitrosylation products.
This study tested the hypothesis that L-cysNO would have greater local vasodilatory activity than D-cysNO and GSNO due to its ability to enter the vascular smooth muscle cells. We tested this hypothesis by infusing these SNOs into one femoral artery of adult sheep while measuring the difference between ipsilateral and contralateral vascular conductances, and systemic hemodynamic responses (mean arterial blood pressure (MAP)). In addition, to determine whether the differential hemodynamic effects of these SNOs correlated with their rates of in vivo S-transnitrosylation, we characterized the kinetics of L-cysNO and GSNO metabolism in vitro and in vivo. Finally, we also compared the abilities of L-cysNO and GSNO to increase intracellular NO in isolated vascular smooth muscle cells.
Materials and Methods
Surgical procedures and intra-arterial infusion protocol
The surgical procedures were reported before[22] and described in the supplemental methods. The conductances of both ipsilateral and contralateral (non-infused) femoral artery were recorded during a stable baseline period and then while L-cysNO, D-cysNO, or GSNO were infused into one (ipsilateral) femoral artery at rates of 0.2, 0.4, 0.6, and 0.8 ml/min, increasing every three minutes, Blood samples were collected from the ipsilateral femoral vein and the brachial artery before and at the end of each infusion period. To compare the effects of SNOs between mesenteric and femoral arteries, in a subset of experiment, the conductances of superior mesenteric artery and contralateral femoral artery were recorded while ipsilateral infusion.
Preparation of SNOs
L-cysNO, D-cysNO, GSNO, and high molecular weight SNOs were prepared in a manner similar to that reported before[23, 24] and described in the supplemental methods. The chelator diethylene triamine pentaacetic acid (DTPA; 0.1 mmol/L) was added into all SNO preparations to avoid transition metal ion-mediated SNO decomposition.
Wire myography
Ovine mesenteric and femoral arterial rings (2 mm diameter and 5 mm long) were denuded of endothelium (verified with 1 μmol/L acetylcholine) and mounted in organ bath chambers as previously described[25]. Relaxation to 2.4 μmol/L high molecular weight SNOs was measured after constriction with 10 μmol/L 5-HT or 125 mmol/L KCl. Spontaneous changes in the tension of vessels that received blank control were subtracted from individual experiments before calculation of responses to SNOs.
Pharmacokinetics of SNOs
Results for concentration versus time were fit to one-phase decay curves to estimate half lives according to procedures described in supplemental methods.
Intracellular NO increase
The increase in intracellular NO and its byproducts[26] in isolated arterial smooth muscle cells following exposure to different concentrations of SNOs was measured by fluorescence as described in the supplemental methods.
Analytical methodology
Arterial blood gases were determined with an ABL-800 blood gas analyzer (Radiometer, Copenhagen). Concentrations of plasma nitrite and SNOs were determined by triiodide chemiluminescence (280i, Sievers, Boulder, CO) as previously described[27] and detailed in the online supplement. Briefly, plasma samples were divided into three aliquots: one used for direct injection (nitrite + SNOs), one for preincubation with 0.5% acid sulfanilamide (total SNOs), and another for preincubation with 5mmol/L HgCl2/0.5% acid sulfanilamide (mercury-resistant NO-adducts such as iron-nitrosyls and N-nitrosamines). An average accuracy of 92.5±1.4 % (calculated using the difference between the second and third injections) for GSNO standards spiked into plasma across the range of 10 nmol/L to 50 μmol/L was obtained, indicating acceptable accuracy and sensitivity of the method in the plasma matrix. The percentage of low versus high molecular weight SNOs in plasma was assessed by separating the fractions using a SephadexTM G-25 column (GE Healthcare, Sweden; exclusion limit=5000 Mr).
Statistics
Average values are given as mean ±SEM in the text and figures. Two-way ANOVA was used to compare the ipsilateral and/or mesenteric measurements with the contralateral side. One-way ANOVA with Bonferroni post hoc analysis was used to test significance of changes with time. Statistical analyses were carried out with Prism, v5.0c (Graphpad Software, La Jolla, CA) with significance accepted at p<0.05.
Results
Hemodynamic responses to intra-arterial infusion of SNOs
To test whether the vasoactivity of SNOs requires their cellular uptake, the hemodynamic effects of membrane-permeable L-cysNO were compared to those of the membrane-impermeable D-cysNO and GSNO. Representative tracings of femoral arterial blood flow, conductance, and MAP) during infusion of L-cysNO are shown in supplemental Figure S1. The baseline values of hemodynamic parameters before infusion of SNOs are given in supplemental Table S1.
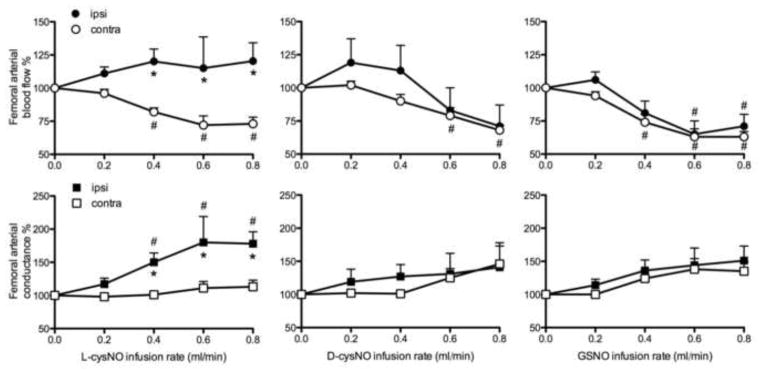
To test for the local vasodilation in femoral vessels, responses of ipsilateral arteries were compared to those of contralateral arteries (Figure 1). Blood flow and conductance of the ipsilateral femoral arteries increased in response to infusion of L-cysNO, becoming significantly higher than the contralateral side at infusion rates ≥0.4 ml/min. In contrast, neither D-cysNO nor GSNO infusion had a significant effect on ipsilateral blood flow or conductance.
Figure 1.
Responses of femoral arterial blood flow and conductance to infusions of L-cysNO, D-cysNO, and GSNO. Following baseline (0 ml/min infusion), each S-nitrosothiol (5 mmol/L) was infused continuously into the ipsilateral femoral artery with stepwise increases in infusion rates every 3 min. Only L-cysNO had local vasodilatory effects demonstrated by greater ipsilateral blood flow and conductance than contralateral. *p<0.05 versus contralateral; #p<0.05 versus baseline.
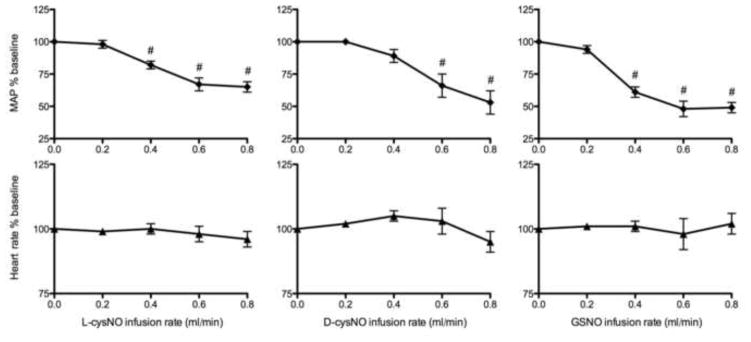
Although the infused SNOs had different local effects, and despite their dilution in the systemic circulation, each of the SNOs decreased MAP by about 50% (Figure 2). Femoral artery conductances in the contralateral leg during L-cysNO infusion and in both legs during D-cysNO and GSNO infusion tended to increase, but the change was not significant, and thus blood flow decreased commensurate with the fall in MAP (Figure 1). These results suggest that only the membrane permeable L-cysNO had rapid local vasodilatory effects, whereas all three SNOs tested had similar systemic vasodilatory effects.
Figure 2.
Effects of L-cysNO, D-cysNO, and GSNO on heart rate and MAP (mean arterial blood pressure). Following baseline (0 ml/min infusion), each of the three S-nitrosothiols (5 mmol/L) was continuously infused into one femoral artery with stepwise increases in the infusion rate. All SNOs decreased MAP significantly with no significant effect on heart rate. #p<0.05 versus baseline.
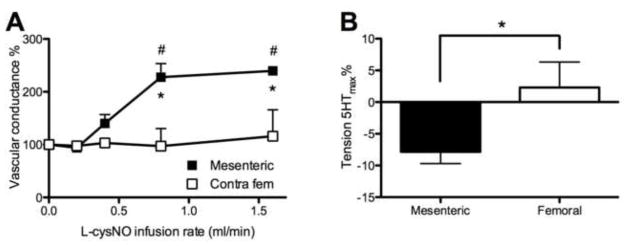
The decrease in MAP without increased femoral conductance suggested that significant dilation was occurring in vascular beds other than the hind limbs. Since the mesenteric arterial vasculature receives a large proportion of cardiac output (>20%) and thus is an important determinant of total peripheral vascular conductance [28, 29], mesenteric artery conductances were examined in a small separate group of sheep (n=3, 1, and 2 for L-cysNO, D-cysNO, and GSNO, respectively). In contrast to the lack of effect on the contralateral femoral artery, mesenteric artery conductance increased more than 2-fold during L-cysNO infusion into the ipsilateral femoral artery (Figure 3A). The effects of D-cysNO and GSNO infusion were similar to those of L-cysNO (data not shown).
Figure 3.
Comparison of vasodilating effects of S-nitrosothiols in mesenteric and femoral arteries. (A) Following baseline (0 ml/min infusion) L-cysNO (5 mmol/L) infused into the ipsilateral femoral artery at stepwise increasing rates, resulted in a much greater increase in conductance of the mesenteric artery than the contralateral femoral artery (n=3). (B) Responses of 5-HT pre-contracted mesenteric and femoral arteries to 2.4 μmol/L high molecular weight SNOs in vitro (n=5). High molecular weight SNOs caused relaxation in mesenteric arteries but not in femoral arteries. *p<0.05 mesenteric versus femoral; #p<0.05 versus baseline.
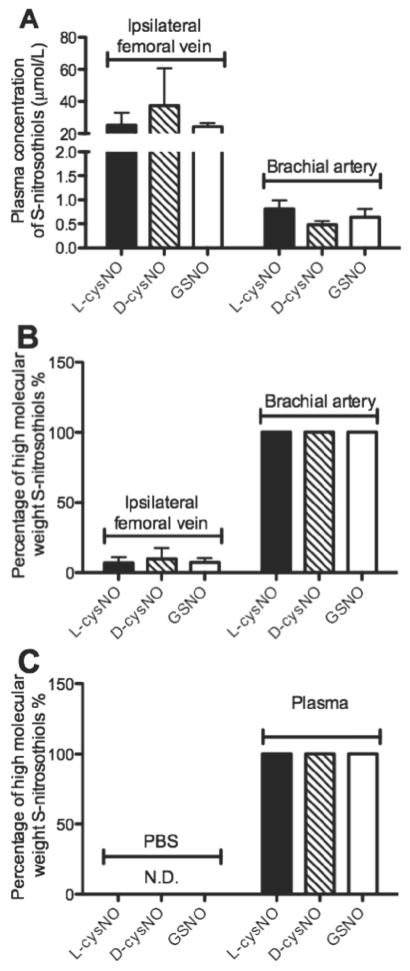
The concentrations of plasma SNOs as well as the proportion of low versus high molecular weight forms therein were determined before and after the infusions (Figure 4A–B). Before infusion, endogenous SNO concentrations were below the limit of detection in both ipsilateral femoral vein and brachial artery plasma. At the end of the 0.8 ml/min infusion period, plasma SNO concentrations in the ipsilateral femoral vein (assumed to closely approximate concentrations in the ipsilateral artery) were ~30 μmol/L (lower than 40 μmol/L, at which L-cysNO might inhibit rather than activate sGC[10]), over 90% of which were in the low molecular weight fraction. In contrast, plasma SNO concentrations in the brachial artery (assumed to closely approximate the concentrations in the mesenteric and contralateral femoral arteries) were ~0.6 μmol/L and were all in the high molecular weight fraction, suggesting virtually complete S-transnitrosylation from low to high molecular weight thiols during systemic recirculation. In addition, 40 seconds after the addition of L- and D-cysNO and GSNO into plasma in vitro, all SNOs (107.2±4.5% recovery on average) were only determined as high molecular weight SNO forms (Figure 4C), confirming the rapid and complete conversion of SNOs by S-transnitrosylation in plasma.
Figure 4.
Concentrations of S-nitrosothiols in plasma and the percentage of high molecular weight S-nitrosothiols therein after S-nitrosothiol infusion into the ipsilateral femoral artery (n=5). (A) Concentrations of plasma S-nitrosothiols in the ipsilateral femoral vein (left) and brachial artery (right). (B) Percentage of plasma S-nitrosothiols in high molecular weight form in the ipsilateral femoral vein (left) and brachial artery (right). (C) Percentage of S-nitrosothiols in high molecular weight form at 40 seconds after the addition of L- and D-cysNO and GSNO into PBS (left) and plasma (right). In our intra-arterial infusion model, concentration and molecular weight composition of plasma S-nitrosothiols in the ipsilateral femoral vein was assumed to closely approximate that of the ipsilateral femoral artery, while that in brachial artery was assumed to closely approximate that of the mesenteric and contralateral femoral arteries, where the dilution and S-transnitrosylation of the ipsilaterally applied S-nitrosothiols had already occurred during recirculation.
Wire myography
High molecular weight SNOs relaxed mesenteric arteries but not femoral arteries, after pre-contraction with 5-HT (Figure 3B) or KCl (data not shown). The dilation of mesenteric arteries was not affected by changing buffer immediately before the addition of SNOs, a procedure used to exclude the possible involvement of thiols or iron ion excreted from the vessels.
Pharmacokinetics
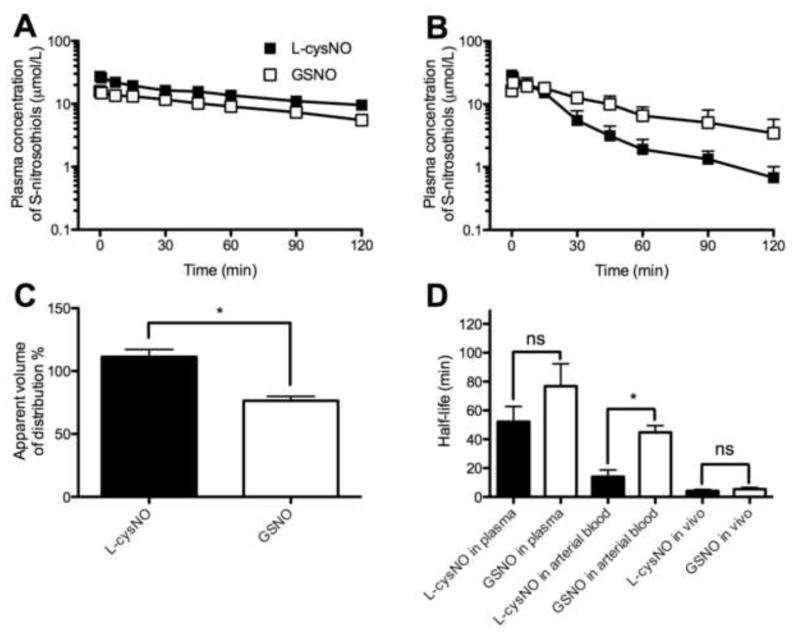
The metabolism of L-cysNO and GSNO, measured as the disappearance of SNOs (regardless of molecular weight), was slower in isolated plasma than in whole blood (Figure 5A–B). The half-lives of L-cysNO (52±11 min) and GSNO (77±16 min) were similar (p=0.24) in plasma. In contrast, in whole blood the half-life of L-cysNO (14±5 min) was significantly shorter than that of GSNO (45±5 min, Figure 5D).
Figure 5.
Pharmacokinetics of L-cysNO and GSNO presented as plasma concentration of total S-nitrosothiols (n=5 except for n=3 for L-cysNO in vivo). (A) L-cysNO and GSNO added to plasma in vitro. (B) L-cysNO and GSNO added to whole arterial blood in vitro. (C) Apparent volume of distribution of L-cysNO and GSNO 40 s after their addition to whole blood in vitro (normalized to concentrations measured after equimolar addition to PBS). The calculated apparent volume of distribution was compared to the plasma volume (~70%) of sheep whole blood. (D) Half-life of L-cysNO and GSNO after addition to plasma and whole blood in vitro, and intra-arterial infusion in vivo. *p<0.05 L-cysNO versus GSNO.
To estimate the membrane permeabilities of L-cysNO and GSNO during their first passage through the femoral artery, the apparent volume of distribution (dose/plasma concentration of SNOs) across the red blood cell membrane at 40 s after addition to whole blood was calculated and normalized to that determined after equimolar addition of the SNOs to PBS (Figure 5C). The apparent volume of distribution of L-cysNO was 111±6 % of the actual blood volume, indicating equilibrium across the red blood cell membrane was closely approached in less than 40 seconds, whereas that of GSNO was 76±4 % and similar to the plasma volume (~70%) of sheep whole blood, consistent with poor membrane permeation confining GSNO to the plasma space.
In vivo pharmacokinetics were studied by measuring rates of plasma SNO disappearance following discontinuation of an 18-min infusion of 5 mmol/L SNO in the sheep hind limb. Concentrations of plasma SNOs after 15 min of infusion were similar to 18 min (p=0.28 for L-cysNO; p=0.74 for GSNO; paired t-test), indicating steady state concentrations. The half-lives of L-cysNO (4.2±0.9 min) and GSNO (5.4±1.2 min) were similar (p=0.97) and both were markedly shorter than those measured in vitro (Figure 5D).
Intracellular NO increase
To test whether L-cysNO is more effective at increasing intracellular NO concentrations than GSNO, additional experiments were performed with smooth muscle cells isolated from femoral arteries. As shown in Figure 6 and Figure S2, intracellular NO concentration increased following exposure to both the NO donor ProliNO and L-cysNO. In contrast, exposure to GSNO did not affect intracellular NO concentration.
Figure 6.
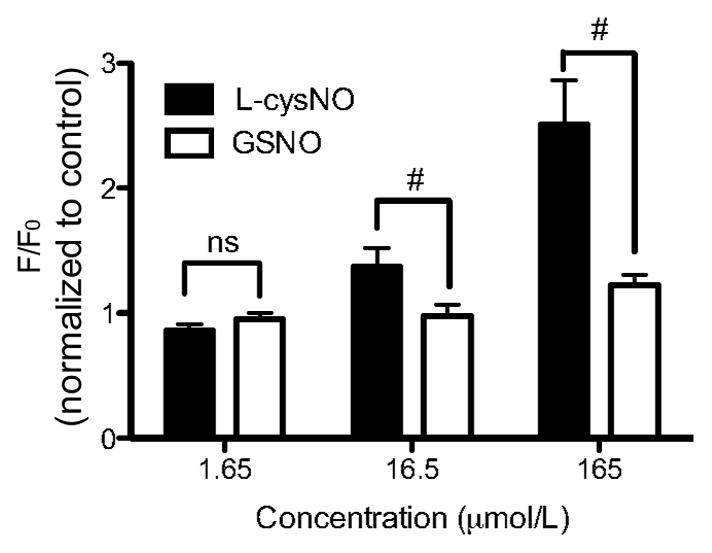
Changes in intracellular NO concentrations in isolated vascular smooth muscle cells exposed to L-cysNO and GSNO (n=5). L-cysNO induced a larger intracellular NO concentration increase (normalized to control) than GSNO at concentrations higher than 16.5 μmol/L. #p<0.05 L-cysNO versus GSNO.
Discussion
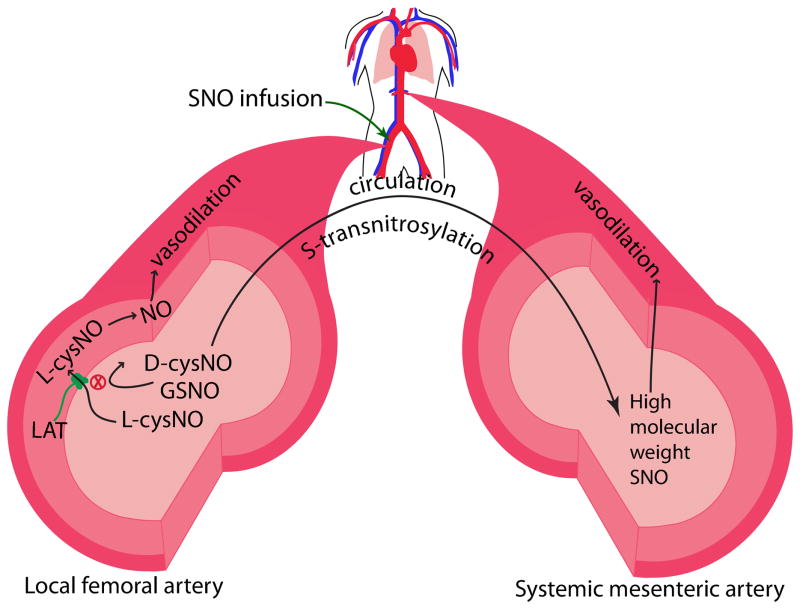
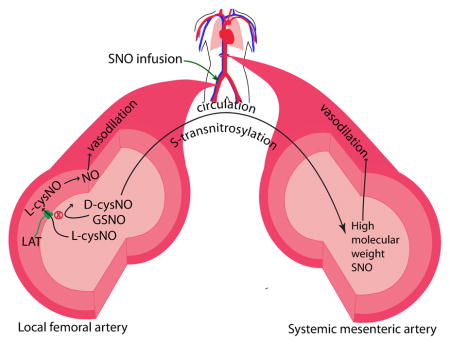
To our knowledge, this study is the first to use an intra-arterial infusion method to investigate the local and systemic hemodynamic effects of low molecular weight SNOs with different membrane permeabilities[7, 8]. The results of this study show that membrane-permeable L-cysNO has local vasodilatory effects in the vasculature served by the femoral artery, whereas membrane-impermeable D-cysNO and GSNO do not. We also show that L-cysNO can effectively induce increases in intracellular NO in vascular smooth muscle cells isolated from femoral arteries, whereas GSNO cannot. Together these findings are consistent with the idea that the vasodilatory effects of SNOs depend upon transmembrane passage of the SNO to transfer the NO moiety into smooth muscle cells. Despite varying local vasodilatory activity and membrane permeabilities, all three tested SNOs have potent and similar hypotensive effects on the systemic vasculature, particularly due to pronounced dilation in vascular beds such as the mesenteric circulation. Sampling of local and peripheral circulating blood showed that the low molecular weight SNOs that had been infused into the femoral artery remained in a low molecular weight form during their first passage through the hind limb, but were converted virtually completely into high molecular weight SNO species in the systemic circulation. These results support the argument that the local vasodilatory effects, or lack thereof, of the infused low molecular weight SNOs were mediated by their original low molecular weight forms and were dependent upon membrane permeability. These results, together with the in vitro finding that high molecular weight SNOs cause relaxation of mesenteric arteries, also indicate that the systemic vasodilatory effects were mediated by the high molecular weight S-transnitrosylation products derived from the infused low molecular weight SNOs. These findings suggest that the hemodynamic effects of SNOs are dependent upon S-transnitrosylation that can occur within the time available during circulation of blood through the body. The experimental approach and findings are summarized in Figure 7.
Figure 7.
Working model of results of the present study. Upon infusion into the femoral artery, L-cysNO elicits ipsilateral vasodilation while D-cysNO and GSNO do not, likely due to their lack of membrane permeability. During transit in the circulation, by S-transnitrosylation, all three SNO species are converted to high molecular weight SNOs with potent vasodilation in the systemic vasculature, including the mesenteric artery.
LAT is L-type amino acid transporter.
Mechanisms for SNO-mediated vasodilation
We observed local vasodilation with infusion of L-cysNO but not with D-cysNO or GSNO (Figure 1). These results are consistent with a previous report that L-cysNO has stronger hypotensive effects than D-cysNO in the conscious rat[3]. SNOs are proposed to mediate vasodilation by activation of sGC within vascular smooth muscle cells[12, 30–33], although direct effects of SNOs on ion channels such as large-conductance Ca2+-activated K+ channels have also been reported[14, 34]. sGC activation requires intracellular NO[12, 30–33], and several pathways by which extracellular SNOs are converted into intracellular NO have been proposed. First, SNOs may release free NO outside the cell, which would diffuse into the cell to activate sGC[35]. Our results are inconsistent with this possibility since no difference has been observed between the rates of decomposition or release of NO from L-cysNO and D-cysNO in either blood or cell cultures, even in the absence of metal chelators[3, 4]. Second, there is the possibility of stereoselective S-transnitrosylation of extracellular membrane thiols that then transport the NO moiety across the cell membrane[3, 4, 13]. While stereoselective reactivity might explain the difference we observed between responses to L-cysNO and D-cysNO[3], the lack of vasodilation by GSNO would be surprising given that this tripeptide consists of L-cysNO combined with glutamate and glycine. We acknowledge that GSNO[17] is often considered bioactive and sometimes even used as a NO donor. The lack of vasodilation by GSNO possibly results from the ineffectiveness of decomposition, S-transnitrosylation, and enzymolysis of GSNO to produce NO, and permeable and active SNOs such as L-cysNO or S-nitroso-cysteinyglycine within the transit time of passage through the sheep hindlimb vasculature[17, 36, 37], as will be discussed below. Third, it has been proposed that SNOs are rapidly processed by the red blood cell, resulting in the release of vasodilating SNOs [38, 39]. Thus, it is possible that the L-cysNO was taken up by the red blood cell and released again as a different SNO that mediated vasodilation, all within the transit time of the femoral artery circulation. The identity of the vasoactive SNO released from the red blood cell is not known. However, GSNO, which has been proposed elsewhere[38], would seem to be eliminated as a possible candidate by the present experiments due to its lack of vasodilatory activity. Fourth, and most consistent with the present findings, L-cysNO may have entered the vascular smooth muscle cell via the L-type amino acid transporter, which has been reported to exclude the movement of D-cysNO and GSNO across the cell membrane[7, 8, 15–17, 36]. Attempts to test this possibility in vivo with co-infusion of the L-type amino acid transporter blockers L-leucine and BCH were not successful due to the significant dilution of these compounds limiting the ability to achieve effective concentrations[8].
In contrast to the variable local vasodilatory effects, and despite their varying membrane permeabilities, all three tested SNOs caused potent and comparable systemic vasodilation (Figure 2). In the present study, all three infused SNOs were converted to high molecular weight species within one circulation time, indicating the NO moiety of the infused SNOs was rapidly transferred to more stable high molecular weight SNOs. This fast approach to equilibrium could explain the similar systemic hemodynamic effects of the three different SNOs, as each could produce the same S-transnitrosylation products. Even though we detected only high molecular weight SNOs in systemic circulation, we cannot exclude the possibility that the systemic vasodilation was mediated by trace amounts of membrane-permeable low molecular weight SNOs (e.g. L-cysNO, HSNO (thionitrous acid)[16, 24], or S-nitroso-cysteinyglycine[36, 37]), or dinitrosyl-iron complexes[40] not detected by our assay methodology. However, the involvement of small molecules seems unlikely since the isolated high molecular weight SNOs dilated mesenteric arteries in the absence of small thiols and iron ion in the organ bath (Figure 3B). Although we did not characterize the identity of the high molecular weight SNOs, it is likely that they are membrane impermeable by virtue of their large size. The high molecular weight SNOs may cause vasodilation by releasing NO during S-transnitrosylation of thiols on the cell surface, such as PDI (protein disulfide isomerase)[41]. Alternatively, the systemic vasodilation may have been mediated by a NO-cGMP independent pathway, such as S-nitrosation of functionally important thiols in the extracellular portions of large-conductance Ca2+-activated K+ channels on the plasma membrane[14, 34].
Sensitivity of SNO-mediated vasodilation
Local vasodilation by L-cysNO became apparent only with infusion rates higher than 0.4 ml/min, which corresponds to ≥15 μmol/L in the ipsilateral femoral artery (Figures 1 and 4). Given that circulating concentrations of SNOs are typically measured to be < 50 nmol/L[42, 43], this result suggests that the sensitivity of the hind limb vessels to L-cysNO is low, and that it is not a physiological regulator of femoral vascular tone. However, systemic MAP was decreased by ~40% with GSNO infusions of 0.4 ml/min, corresponding to estimated plasma SNO concentration of <300 nmol/L (50% of the 600 nmol/L measured at the 0.8 ml/min infusion rate). These results indicate that SNOs may play an important role in the regulation of MAP under physiological conditions, and are even more likely play a role during pathophysiological states such as sepsis, when circulating SNO concentrations are elevated to >500 nM[44–46].
Reasons for hypotension include decreased cardiac output as well as increased total vascular conductance. We did not measure cardiac output in this study, but heart rate remained unchanged (Figure 2), and diminished cardiac output seems unlikely because SNOs are known to increase stroke volume[47]. Therefore, it is more likely that the hypotension resulted from increases in total vascular conductance rather than cardiac changes. Nonetheless, the increase in femoral conductance was much less than would be anticipated based on the decrease in MAP. Thus, the magnitude of hypotension observed must have required disproportionately greater dilation in vascular beds other than the hind limbs. Indeed, a more sensitive relaxation response to SNOs was observed in mesenteric arteries than in femoral arteries, both in vivo and in vitro (Figure 3A–B). However, assuming the mesenteric vasculature accounts for 20% in total vascular conductance[28, 29], the increase in mesenteric conductance (130%; Figure 3A) alone is not enough to account for the 50% fall in MAP and thus conductance was likely increased in other vascular beds as well. The finding of organ-specific vascular responses to SNOs throughout the body differs from earlier reports in rats[3, 48]. Further studies to determine the mechanism underlying the organ-specific vascular responses (both in vivo and in vitro) to SNOs (both low and high molecular weight) are underway in our lab.
Pharmacokinetics
L-cysNO and GSNO were relatively stable in isolated plasma. In contrast, in isolated whole blood, L-cysNO disappeared more quickly than GSNO, consistent with its greater membrane permeability compared to GSNO, and suggesting the red blood cell participates in SNO metabolism. In contrast, when introduced into the circulation the half-lives of L-cysNO and GSNO were similar (both ~5 minutes; Figure 5D), consistent with that reported for high molecular weight SNOs[49]. These findings are in agreement with a model of distinctly different local responses to the SNO infusions based on membrane permeabilities, yet common systemic responses mediated by products common to both L-cysNO and GSNO (Figure 7).
To be vasoactive in its own right in the ipsilateral femoral artery, cellular uptake of L-cysNO must outpace S-transnitrosylation of plasma thiols[1, 2], by which L-cysNO can be converted into membrane-impermeable high molecular weight SNOs. On the other hand, although GSNO itself is membrane-impermeable, it may transfer its NO moiety into cells via membrane-permeable S-transnitrosylation products such as L-cysNO and/or HSNO[16, 24], or enzymolysis product S-nitroso-cysteinyglycine[36, 37]. At 40 seconds after addition to whole blood (Figure 5C), L-cysNO demonstrated homogeneous distribution between the plasma and red blood cells, which, like smooth muscle cells, also have L-type amino acid transporters[8]. This observation suggests that S-transnitrosylation is not fast enough to out-compete the local cellular uptake of L-cysNO. In contrast, GSNO remained predominantly in the plasma phase after 40 seconds, suggesting that plasma thiols and enzymes cannot facilitate the local membrane permeation of GSNO. Both of these results indicate that the membrane permeabilities of L-cysNO and GSNO were not altered by the plasma components during their first passage through the ipsilateral femoral artery. It is worth noting that the possibility of enzymatic conversion of the infused SNOs, as opposed to S-transnitrosation reactions, may have played a significant role in the vasoactive responses we observed. A number of enzyme-catalyzed pathways for low molecular weight SNOs have been described[17], some inactivating the NO moiety entirely, and others facilitating S-transnitrosylation or release of an NO-bioactive species. Although a majority of the low molecular weight SNOs infused in our experiments appear to have resulted in high molecular weight SNOs, the possible role of enzyme-mediated reactions facilitating this process or producing minor products with potent vasodilatory activity cannot be excluded.
In the systemic circulation, the impermeable plasma high molecular weight SNOs might equilibrate with thiols in red blood cells (such as hemoglobin and glutathione) via permeable PDI[41, 50], L-cysNO, HSNO[16, 24], or S-nitroso-cysteinyglycine[36, 37]. As a major sink and/or source of SNO[51, 52], red blood cells may provide further buffer to SNOs than plasma[53].
In addition to producing other SNOs by S-transnitrosylation, SNOs also form nitrite, which can serve as a vasodilator[54, 55]. Indeed, in this study, approximately half of the L-cysNO and GSNO disappearance was accounted for by increases in plasma nitrite to concentrations of ~0.4 μmol/L. We have recently reported, however, that in sheep nitrite is a poor vasodilator even at concentrations orders of magnitude greater than those observed in the present study[22, 56]. Thus it seems more likely that the vasodilation observed in this study was not due to increases in circulating nitrite.
Intracellular NO increase
The DAF-FM experiments indicated that incubation of vascular smooth muscle cells with L-cysNO increased intracellular NO more than GSNO (Figure 6 and Figure S2). While it should be noted that the DAF-FM measures not only NO but also its byproducts, the difference noted between L-cysNO and GSNO is consistent with the greater membrane permeability of L-cysNO than GSNO, providing further support of the hypothesis that local vasodilation was brought about by the cellular uptake of L-cysNO.
Conclusions
Our results indicate that the vasodilatory potency of SNOs on the infused hind limb arteries is dependent upon their membrane permeability. However, the findings also illustrate a rapid disappearance of the infused low molecular weight SNOs within one circulatory transit, with considerable conversion to a high molecular weight form. This conversion was associated with potent systemic vasodilation, likely caused by the high molecular weight SNO products via a membrane permeation-independent pathway in vascular beds such as the mesentery. These results suggest that the hemodynamic effects of SNOs depend upon an equilibrium of S-transnitrosylation in blood.
Supplementary Material
Highlights.
SNOs were infused intra-arterially while measuring local and systemic vasodilation.
L-cysNO caused local dilation, but D-cysNO and GSNO did not.
Despite varying membrane permeabilities, all three SNOs caused systemic vasodilation.
SNOs dilated the sheep mesenteric vasculature more potently than that of the hindlimb.
Hemodynamic effects of SNOs depend on an S-transnitrosylation equilibrium in blood.
Acknowledgments
The authors acknowledge the expert technical assistance of Shannon L. Bragg.
Source of Funding
These experiments were supported by NIH grants R01HL95973 (ABB), PO1 HD-31226 (LDL), R03HD069746 (SMW), and MRI-DBI 0923559 (SMW).
Abbreviations
- SNOs
S-nitrosothiols
- L-cysNO
S-nitroso-L-cysteine
- GSNO
S-nitroso-glutathione
- NO
nitric oxide
- sGC
soluble guanylate cyclase
- MAP
mean arterial blood pressure
- DTPA
diethylene triamine pentaacetic acid
- HSNO
thionitrous acid
- PDI
protein disulfide isomerase
Footnotes
Disclosure
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jourd’heuil D, Hallen K, Feelisch M, Grisham MB. Dynamic state of S-nitrosothiols in human plasma and whole blood. Free Radic Biol Med. 2000;28:409–417. doi: 10.1016/s0891-5849(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 2.Tsikas D, Sandmann J, Rossa S, Gutzki FM, Frolich JC. Measurement of S-nitrosoalbumin by gas chromatography-mass spectrometry. I. Preparation, purification, isolation, characterization and metabolism of S-[15N]nitrosoalbumin in human blood in vitro. J Chromatogr B Biomed Sci Appl. 1999;726:1–12. [PubMed] [Google Scholar]
- 3.Davisson RL, Travis MD, Bates JN, Lewis SJ. Hemodynamic effects of L- and D-S-nitrosocysteine in the rat. Stereoselective S-nitrosothiol recognition sites. Circ Res. 1996;79:256–262. doi: 10.1161/01.res.79.2.256. [DOI] [PubMed] [Google Scholar]
- 4.Travis MD, Davisson RL, Bates JN, Lewis SJ. Hemodynamic effects of L- and D-S-nitroso-beta,beta-dimethylcysteine in rats. Am J Physiol. 1997;273:H1493–1501. doi: 10.1152/ajpheart.1997.273.3.H1493. [DOI] [PubMed] [Google Scholar]
- 5.Keaney JF, Jr, Simon DI, Stamler JS, Jaraki O, Scharfstein J, Vita JA, Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993;91:1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Whorton AR. Identification of stereoselective transporters for S-nitroso-L-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J Biol Chem. 2005;280:20102–20110. doi: 10.1074/jbc.M413164200. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Whorton AR. Functional characterization of two S-nitroso-L-cysteine transporters, which mediate movement of NO equivalents into vascular cells. Am J Physiol Cell Physiol. 2007;292:C1263–1271. doi: 10.1152/ajpcell.00382.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci U S A. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riego JA, Broniowska KA, Kettenhofen NJ, Hogg N. Activation and inhibition of soluble guanylyl cyclase by S-nitrosocysteine: involvement of amino acid transport system L. Free Radic Biol Med. 2009;47:269–274. doi: 10.1016/j.freeradbiomed.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmajothi MV, Sun NZ, Auten RL. S-nitrosothiol transport via PEPT2 mediates biological effects of nitric oxide gas exposure in macrophages. Am J Respir Cell Mol Biol. 2013;48:230–239. doi: 10.1165/rcmb.2012-0305OC. [DOI] [PubMed] [Google Scholar]
- 12.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis SJ, Hoque A, Bates JN. Differentiation of L- and D-S-nitrosothiol recognition sites in vivo. J cardiovasc Pharmacol. 2005;46:660–671. doi: 10.1097/01.fjc.0000181714.94827.5d. [DOI] [PubMed] [Google Scholar]
- 14.Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–565. doi: 10.1038/sj.ki.5002340. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Li S, Marshall ZM, Whorton AR. A cystine-cysteine shuttle mediated by xCT facilitates cellular responses to S-nitrosoalbumin. Am J Physiol Cell Physiol. 2008;294:C1012–1020. doi: 10.1152/ajpcell.00411.2007. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto A, Gow AJ. Membrane transfer of S-nitrosothiols. Nitric Oxide. 2011;25:102–107. doi: 10.1016/j.niox.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broniowska KA, Diers AR, Hogg N. S-nitrosoglutathione. Biochim Biophys Acta. 2013;1830:3173–3181. doi: 10.1016/j.bbagen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandmann J, Schwedhelm KS, Tsikas D. Specific transport of S-nitrosocysteine in human red blood cells: Implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett. 2005;579:4119–4124. doi: 10.1016/j.febslet.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 19.Tsikas D, Sandmann J, Luessen P, Savva A, Rossa S, Stichtenoth DO, Frolich JC. S-Transnitrosylation of albumin in human plasma and blood in vitro and in vivo in the rat. Biochim Biophys Acta. 2001;1546:422–434. doi: 10.1016/s0167-4838(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 20.Tsikas D, Sandmann J, Rossa S, Gutzki FM, Frolich JC. Investigations of S-transnitrosylation reactions between low- and high-molecular-weight S-nitroso compounds and their thiols by high-performance liquid chromatography and gas chromatography-mass spectrometry. Anal Biochem. 1999;270:231–241. doi: 10.1006/abio.1999.4084. [DOI] [PubMed] [Google Scholar]
- 21.Meyer DJ, Kramer H, Ozer N, Coles B, Ketterer B. Kinetics and Equilibria of S-Nitrosothiol-Thiol Exchange between Glutathione, Cysteine, Penicillamines and Serum-Albumin. FEBS Lett. 1994;345:177–180. doi: 10.1016/0014-5793(94)00429-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Schroeder HJ, Barcelo L, Bragg SL, Terry MH, Wilson SM, Power GG, Blood AB. Role of blood and vascular smooth muscle in the vasoactivity of nitrite. Am J Physiol Heart Circ Physiol. 2014;307:976–986. doi: 10.1152/ajpheart.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Hogg N. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L467–474. doi: 10.1152/ajplung.00350.2003. [DOI] [PubMed] [Google Scholar]
- 24.Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, Mansfield S, Hume JR. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol. 2005;144:252–264. doi: 10.1038/sj.bjp.0706077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortese-Krott MM, Rodriguez-Mateos A, Kuhnle GGC, Brown G, Feelisch M, Kelm M. A multilevel analytical approach for detection and visualization of intracellular NO production and nitrosation events using diaminofluoresceins. Free Radical Bio Med. 2012;53:2146–2158. doi: 10.1016/j.freeradbiomed.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd’Heuil D, Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. Faseb J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 28.Alexander N, DeQuattro V. Gastrointestinal and mesenteric hemodynamic patterns in neurogenic hypertensive rabbits. Circ Res. 1974;35:646–651. doi: 10.1161/01.res.35.4.646. [DOI] [PubMed] [Google Scholar]
- 29.Fiore G, Brienza N, Cicala P, Tunzi P, Marraudino N, de Schinosa LL, Fiore T. Superior mesenteric artery blood flow modifications during off-pump coronary surgery. Ann Thorac Surg. 2006;82:62–67. doi: 10.1016/j.athoracsur.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Batenburg WW, Popp R, Fleming I, de Vries R, Garrelds IM, Saxena PR, Danser AH. Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF? Br J Pharmacol. 2004;142:125–135. doi: 10.1038/sj.bjp.0705747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dierks EA, Burstyn JN. Nitric oxide (NO), the only nitrogen monoxide redox form capable of activating soluble guanylyl cyclase. Biochem Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Padron RI, Pham SM, Pang M, Li S, Aitouche A. Molecular dissection of mouse soluble guanylyl cyclase alpha1 promoter. Biochem Biophys Res Commun. 2004;314:208–214. doi: 10.1016/j.bbrc.2003.12.078. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AL, Berka V, Sharina I, Martin E. Dynamic ligand exchange in soluble guanylyl cyclase (sGC): implications for sGC regulation and desensitization. J Biol Chem. 2011;286:43182–43192. doi: 10.1074/jbc.M111.290304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- 36.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 37.Zaman K, Hanigan MH, Smith A, Vaughan J, Macdonald T, Jones DR, Hunt JF, Gaston B. Endogenous S-nitrosoglutathione modifies 5-lipoxygenase expression in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:387–393. doi: 10.1165/rcmb.2005-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Hemoglobin betaCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci U S A. 2015;112:6425–6430. doi: 10.1073/pnas.1502285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Phys. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 40.Severina IS, Bussygina OG, Pyatakova NV, Malenkova IV, Vanin AF. Activation of soluble guanylate cyclase by NO donors--S-nitrosothiols, and dinitrosyl-iron complexes with thiol-containing ligands. Nitric Oxide. 2003;8:155–163. doi: 10.1016/s1089-8603(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran N, Root P, Jiang XM, Hogg PJ, Mutus B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc Natl Acad Sci U S A. 2001;98:9539–9544. doi: 10.1073/pnas.171180998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi R, Giustarini D, Milzani A, Colombo R, Dalle-Donne I, Di Simplicio P. Physiological levels of S-nitrosothiols in human plasma. Circ Res. 2001;89:E47. [PubMed] [Google Scholar]
- 43.Tsikas D, Schmidt M, Bohmer A, Zoerner AA, Gutzki FM, Jordan J. UPLC-MS/MS measurement of S-nitrosoglutathione (GSNO) in human plasma solves the S-nitrosothiol concentration enigma. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:147–157. doi: 10.1016/j.jchromb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 45.Jourd’heuil D, Gray L, Grisham MB. S-nitrosothiol formation in blood of lipopolysaccharide-treated rats. Biochem Biophys Res Commun. 2000;273:22–26. doi: 10.1006/bbrc.2000.2892. [DOI] [PubMed] [Google Scholar]
- 46.Ottesen LH, Harry D, Frost M, Davies S, Khan K, Halliwell B, Moore K. Increased formation of S-nitrothiols and nitrotyrosine in cirrhotic rats during endotoxemia. Free Radical Bio Med. 2001;31:790–798. doi: 10.1016/s0891-5849(01)00647-5. [DOI] [PubMed] [Google Scholar]
- 47.Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A. 2012;109:4314–4319. doi: 10.1073/pnas.1113319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis SJ, Bhopatkar MY, Walton TM, Bates JN. Role of voltage-sensitive calcium-channels in nitric oxide-mediated vasodilation in spontaneously hypertensive rats. Eur J Pharmacol. 2005;528:144–149. doi: 10.1016/j.ejphar.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 49.Sonveaux P, Kaz AM, Snyder SA, Richardson RA, Cardenas-Navia LI, Braun RD, Pawloski JR, Tozer GM, Bonaventura J, McMahon TJ, Stamler JS, Dewhirst MW. Oxygen regulation of tumor perfusion by S-nitrosohemoglobin reveals a pressor activity of nitric oxide. Circ Res. 2005;96:1119–1126. doi: 10.1161/01.RES.0000168740.04986.a7. [DOI] [PubMed] [Google Scholar]
- 50.Kallakunta VM, Slama-Schwok A, Mutus B. Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells. Redox Biol. 2013;1:373–380. doi: 10.1016/j.redox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 52.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandmann J, Schwedhelm KS, Tsikas D. Specific transport of S-nitrosocysteine in human red blood cells: Implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett. 2005;579:4119–4124. doi: 10.1016/j.febslet.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 54.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 55.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 56.Truong GT, Schroder HJ, Liu T, Zhang M, Kanda E, Bragg S, Power GG, Blood AB. Role of nitrite in regulation of fetal cephalic circulation in sheep. J Physiol. 2014;592:1785–1794. doi: 10.1113/jphysiol.2013.269340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.