Abstract
The role of ethanol-inducible cytochrome P450-2E1 (CYP2E1) in promoting aging-dependent hepatic disease is unknown and thus was investigated in this study. Young (7 weeks) and aged female (16 months old) wild-type (WT) and Cyp2e1-null mice were used in this study to evaluate age-dependent changes in liver histology, steatosis, apoptosis, fibrosis and many nitroxidative stress parameters. Liver histology showed that aged WT mice exhibited markedly elevated hepatocyte vacuolation, ballooning degeneration, and inflammatory cell infiltration compared to all other groups. These changes were accompanied with significantly higher hepatic triglyceride and serum cholesterol in aged WT mice although serum ALT and insulin resistance were not significantly altered. Aged WT mice showed the highest rates of hepatocyte apoptosis and hepatic fibrosis. Further, the highest levels of hepatic hydrogen peroxide, lipid peroxidation, protein carbonylation, nitration, and oxidative DNA damage were observed in aged WT mice. These increases in the aged WT mice were accompanied by increased levels of mitochondrial nitroxidative stress and alteration of mitochondrial complex III and IV proteins in aged WT mice, although hepatic ATP levels seems to be unchanged. Consistently, the aging-related nitroxidative changes were very low in aged Cyp2e1-null mice. These results suggest that CYP2E1 is important in causing aging-related hepatic steatosis, apoptosis and fibrosis possibly through increasing nitroxidative stress and that CYP2E1 could be a potential target for translational research in preventing aging-related liver disease.
Keywords: Liver, aging, CYP2E1, steatohepatitis, apoptosis, fibrosis, nitroxidative stress
Graphical Abstract
Introduction
Aging is a physiological process, that is characterized by a progressively decreased capacity to maintain internal homeostasis, with decreased ability to respond to various endogenous and exogenous stresses and increased risk of morbidity and mortality [1]. Many mechanistic theories for aging-related pathophysiological changes were proposed earlier [2] and have still been debated till to date [3, 4]. The prevailing mechanistic explanation for the aging process is “the free radical theory of aging” [5], despite being controversial [6]. This theory states that the increased damage in aged tissues is caused by the reaction of the free radicals with cellular macromolecules such as lipids, proteins, and nucleic acid, leading to compromised cellular functions and tissue injury [7]. Indeed, reactive oxygen species (ROS) and reactive nitrogen species (RNS) were reported to increase with aging. Consequently, accumulation of oxidized and/or nitrated proteins as well as lipid peroxides and oxidatively-damaged DNA are frequently observed in the aged tissues compared to the young counterparts [8, 9].
The liver was thought to exhibit minimal aging-dependent changes compared to other tissues [10]. This may be due to high contents of antioxidants and other defensive enzymes in the liver. However, recent reports showed that the liver also undergoes significant changes during aging process, since aged livers, especially in rodents after 12 months of age and older, contain less volume and regenerative capacity with elevated accumulation of insoluble oxidized proteins (e.g., lipofuscin) in the cytoplasm, altered nuclear shapes, and increased apoptosis, steatosis, fibrosis, and carcinoma, compared to the young counterparts [11, 12]. Interestingly, many studies showed a positive relationship between increased oxidative stress levels and deterioration of liver functions during the aging process [13, 14].
The cytochrome P450-2E1 (CYP2E1) enzyme, constitutively expressed in many tissues including the liver and extrahepatic organs, can oxidize a variety of small molecule substrates such as alcohol (ethanol), drugs, solvents, procarcinogens, and fatty acids [15–17]. It is induced by ethanol, acetone, and other small molecule substrates. CYP2E1 can also be regulated by age, gender, genetic factors, nutrition, hormones, as well as pathophysiological conditions such as diabetes and obesity [18, 19]. The superoxide anion can be produced from the CYP2E1-related catalytic activity even in the absence of its substrates [15–17]. The superoxide anion can become a potent oxidative radical that can cause severe tissue damage through interaction with other free radicals such as nitric oxide (NO) to produce a more potently toxic peroxynitrite [20]. It can be also converted to a more toxic hydroxyl radical in the presence of transition metals [21].
CYP2E1 levels have been evaluated both in aged people (usually ≥ 55 years) and animals (usually ≥ 12 months) and through the entire life span in animals [22–25]. The results of these studies revealed that CYP2E1 protein levels were unchanged or decreased, or CYP2E1 activities were decreased with aging. Furthermore, other reports showed increased levels of hepatocyte apoptosis and liver fibrosis in the aged mice [11, 26, 27]. However, to the best of our knowledge, the role of CYP2E1 in promoting hepatic steatosis, apoptosis, and fibrosis observed in aged rodents has never been evaluated. Thus, this study was aimed to investigate the role of CYP2E1 in promoting nitroxidative stress and hepatic changes by comparing the various parameters in young versus aged wild-type (WT) mice. To further verify the causal role of CYP2E1 in aging-related hepatic changes, we also evaluated the histological and biochemical characteristics of the livers in young and aged Cyp2e1-null mice compared to those of the WT counterparts.
Materials and methods
Materials
All chemicals used in this study were from Sigma Chemical (St. Louis, MO, USA), unless indicated otherwise. Specific antibodies against CYP2E1, inducible nitric oxide synthase (iNOS), 3-nitrotyrosine (3-NT), and β-actin were from Abcam Inc. (Cambridge, MA, USA). Respective antibodies against collagen 1A1 and 4-hydroxynonenal (HNE) were from Millipore (Billerica, MA, USA) and the sources of other primary antibodies are listed in Table 2. Anti-heat shock protein 90 (HSP90) antibody was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Secondary antibodies conjugated with horse radish peroxidase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Table 2.
Summary of primary antibodies used
| Antigen | Species | Dilution | Buffer | Catalogue number | Source |
|---|---|---|---|---|---|
| collagen1a1 | Rabbit | 1:1000 | 4% milk | Ab765P | Millipore |
| 3-Nitrotyrosine | Mouse | 1:2000 | 4% milk | Ab7048 | Abcam |
| HSP90 | Rabbit | 1:2000 | 4% BSA | C45G5 | Cell Signaling |
| NADPH oxidase | Rabbit | 1:50,000 | 5% milk | Ab133303 | Abcam |
| Xanthine oxidase | Rabbit | 1:50,000 | 5% milk | Ab109235 | Abcam |
| Total OXPHOS | Mouse | 1:2000 | 5% BSA | Ab110413 | Abcam |
| HO-1 | Rabbit | 1:500 | 4% milk | Sc-10789 | Santa Cruz Biotechnology |
| Prx | Goat | 1:500 | 4% milk | Sc23969 | Santa Cruz Biotechnology |
| iNOS | Rabbit | 1:500 | 4% milk | Sc651 | Santa Cruz Biotechnology |
| CYP2E1 | Rabbit | 1:25,000 | 5% milk | Ab19140 | Abcam |
| CYP2B | Rabbit | 1:25,000 | 5% milk | Gift | * Dr. James Hardwick |
| CYP4F | Rabbit | 1:5,000 | 5% milk | Gift | * Dr. James Hardwick |
| CYP1A | Rabbit | 1:20,000 | 5% milk | Gift | * Dr. James Hardwick |
| CYP2A | Rabbit | 1:20,000 | 5% milk | Gift | * Dr. James Hardwick |
| CYP4A | Rabbit | 1:20,000 | 5% milk | Gift | * Dr. James Hardwick |
| β-Actin | Rabbit | 1:2000 | 5% BSA | 4967S | Cell Signaling |
| VDAC | Goat | 1:2000 | 5% milk | Sc8829 | Santa Cruz Biotechnology |
Biochemistry and Molecular Pathology, Department of Integrative Medical Sciences, Northeastern Ohio University College of Medicine, Rootstown, Ohio, USA
Animals and tissue collection for various histology analyses
Age-matched young (approximately 7 weeks) and old female (approximately 16 months) inbred WT and Cyp2e1-null mice on a 129/Svj background [28] were used in this study. Mice were assigned to four groups (n≥10/group): (1) young WT; (2) old WT; (3) young Cyp2e1-null; and (4) old Cyp2e1-null. All mice were fed with the NIH31 autoclavable rodent diet, which contains 5.5% total fat, where animal fat (exclusively derived from menhaden fish oil) is 15% and vegetable oil (mostly from soy) represents 85%. Part of the large lobe of the livers was fixed in 10% neutral formalin for histological analysis and the rest of the liver was snap-frozen for further characterizations. Histological and biochemical analyses of liver tissues were performed as previously described [29, 30]. The histological evaluation and fibrosis scoring were performed blindly by an experienced pathologist. Each section was evaluated for hepatocyte vacuolation, ballooning degeneration, inflammatory cell filtration, hepatic fibrosis and other lesions. For evaluation of hepatic fibrosis, formalin-fixed liver tissue sections were stained with Sirius red and the image was captured by Olympus BX-51 microscope equipped with Digital Camera DP-70 (Olympus, Japan). Fibrotic and total areas were determined and the percentage of fibrotic area was calculated using Image J software (National Institutes of Health, MD, USA) from various image fields per slide. Animal experiments were performed in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee.
Measurements of serum aminotransferase, total hepatic iron, hepatic lipid peroxides and glutathione levels in young and aged mice
Plasma alanine aminotransferase (ALT) and cholesterol contents in each mouse were measured by using the clinical IDEXX Vet Test Chemistry Analyzer System (West Brook, ME, USA) [29–31]. The amount of intrahepatic triglyceride (TG) in 50 mg liver tissues was evaluated using a commercial kit (cat. # ETGA -200) (BioVision Research products, Mountain View, CA, USA), and normalized per gm tissue. Intrahepatic levels of lipid peroxidation marker malondialdehyde (MDA) + hydroxyalkenal (HAE) (μM) were evaluated using a commercial available kit (cat. # FR22) (Oxford Biomedical Research, Oxford, MI, USA). Total hepatic iron Fe(II+III) contents were evaluated by using the iron colorimetric assay kit (cat. # K390-100) (BioVision Research Products, Mountain View, CA, USA), containing a specific chemical chelator to block interference by copper. Intrahepatic reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) (in 50 mg liver extracts) were determined by using commercially available kits (cat. # 703002) (Cayman Chemical, Ann Arbor, Michigan, USA). The manufacturer’s protocols were followed whenever commercial kits were used.
Hepatic tissue extraction for evaluating oxidized proteins
Total liver homogenate from each mouse was prepared in ice-cold extraction buffer (50 mM Tris–Cl, pH 7.5, 1 mM EDTA, and 1% CHAPS). Mitochondrial fraction was also prepared by differential centrifugation, as described previously [29, 31], and all extraction buffers were pre-equilibrated with nitrogen gas to remove the dissolved oxygen. Protein concentrations were determined using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA). Equal amounts of liver homogenates were resolved on 10 or 15% SDS–PAGE gels and electrically transferred to nitrocellulose membranes (Hercules, CA, USA). Membranes were initially blocked with 4 or 5% (w/v) nonfat milk proteins or bovine serum albumin (BSA), subsequently probed overnight at 4 °C with a specific primary antibody to each target protein as indicated in Table 2. After removal of the primary antibody by three separate steps of washing, membranes were incubated with a secondary antibody conjugated with horseradish alkaline phosphatase (Santa Cruz Biotechnology, Dallas, TX, USA) at room temperature for 1 h. Image detection was performed using a SuperSignal West Pico Kit (Pierce) according to the manufacturer’s instructions. β-Actin was used as a loading control for whole liver extracts or cytosol proteins. VDAC was used as a loading control for mitochondrial proteins. All other information including the antibody concentrations are presented in Table 2. For protein carbonylation, an aliquot of the hepatic lysates was derivatized with dinitrophenylhydrazine (DNPH) under acid denaturing conditions using Oxyblot protein oxidation detection kit (cat. # S7150) (Millipore, Billerica, MA). After separation by SDS-PAGE and transfer as explained, the membrane was subjected to western blotting with anti-dinitrophenyl primary antibody at 1:2000 x dilution. In order to assure specificity of the bands, separate aliquots of the hepatic lysates, that had been acid-denatured as well but not treated with DNPH, were used as a negative control.
Immunoprecipitation and immunoblot analysis
Hepatic cell lysates (40 μg proteins) were separated by 12% SDS-PAGE and subjected to immunoblot analyses, as previously described [31]. Specific antibodies to CYP2E1, iNOS, 3-NT and β-actin, as a loading control, were then incubated overnight at 4°C. After removal of the primary antibodies and followed by three separate steps of washing, the nitrocellulose membranes were incubated with the appropriate secondary antibodies conjugated with horseradish peroxidase. Protein bands were detected by enhanced chemiluminescence and their densities quantified using UN-SCAN-IT gel version 6.1 from Silk Scientific (Orem, Utah 84059 USA). For the immunoprecipitation experiment, 400 μg of hepatic cell lysates from each mouse was used to prepare a total of 2 mg proteins/sample (pooled from 5 mice/sample out of 10 mice/strain to prepare 2 samples/strain) and the specific antibody (5 μg) to collagen 1A1 or HSP90 was used with Pierce™ protein A/G magnetic beads from Life Technologies (Grand Island, NY, USA) following the manufacturer’s protocol. The immunoprecipitated proteins were subsequently analyzed by immunoblot analysis using the specific antibody against collagen 1A1, HSP90, or 3-NT (Table 2).
Immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Formalin-fixed liver samples were processed and 5-μm thick paraffin sections were used for immunohistochemistry. Briefly, deparaffinized liver sections were treated with 3% hydrogen peroxide followed by antigen retrieval. The sections were blocked with 2% non-fat skim milk solution, and incubated with the primary antibody against HNE, oxidized proteins, 3-NT, or collagen type 1A1. After incubation and subsequent washing steps, the attached primary antibody was then linked to the dextran polymer by following the manufacturer’s protocol (Envision kit, Dako, Carpinteria, CA, USA). The final reaction was performed by immersing the sections in a solution of 3,3′-diaminobenzidine (DAB). The sections were then counterstained with hematoxylin. Apoptotic cell death in liver tissues by detecting DNA strand breaks was determined using the ApopTag Peroxidase in situ apoptosis detection kit (cat. # S7100) (Millipore, Billerica, MA). Numbers of TUNEL-positive hepatocytes were counted in 10 high-power (200×) microscope fields.
Measurements of hepatic anti-oxidant enzyme activities, hydrogen peroxide levels, oxidative DNA damage, ATP, and proteasome activity
The catalytic activities of total hepatic superoxide dismutase (SOD) (cat. # 706002), catalase (CAT) (cat. # 707002), and glutathione peroxidase (Gpx) (cat. # 703102) were determined by using the kits from Cayman (Ann Arbor, MI, USA). Hydrogen peroxide (H2O2) levels were measured using the hydrogen peroxide assay kit (Abcam, cat. No. ab102500) following the colorimetric assay protocol (Abcam, cat. # ab102500). To prepare the hepatic total cell lysates, individual liver tissues (100 mg wet weight) were homogenized in the assay buffer contained in the kit by following the manufacturer’s protocol. The homogenates were then centrifuged at 10,000 × g for 2–5 min at 4°C, and the resulting supernatants were deproteinized by adding ice-cold 4 M perchloric acid (PCA) to a final concentration of 1 M and kept for 5 min on ice. The samples were centrifuged at 13,000 × g for 2 min at 4°C, and the excess PCA in the supernatant was precipitated by adding ice-cold 2 M KOH. The added volume of ice-cold 2 M KOH equals 34% of the supernatant of the sample (e.g., 34 μl 2 M KOH was added to 100 μl sample). The samples were then centrifuged at 13,000 × g for 15 min at 4°C, and the resulting supernatants were used to determine the H2O2 levels following the manufacturer’s protocol. In the presence of Horse Radish Peroxidase (HRP), the OxiRed Probe reacts with H2O2 to produce a color product which can be determined at 570 nm. The H2O2 level in each sample was determined from the standard curve using the H2O2 standard provided by the manufacturer. Hepatic ATP levels were evaluated using the ATP Assay kit (cat. # ab83355) (Abcam Inc), and the kit utilizes the phosphorylation of glycerol to generate a product which can be easily quantified by absorbance (O.D.max = 570 nm). Hepatic DNA was isolated using the Qiagen kit (Valencia, CA, USA) and oxidative DNA damage in 2 μg of isolated DNA was evaluated monitored with the ELISA kit (cat. # 589320) (Cayman Chemical, Ann Arbor, MI, USA) to measure 8-dihydro-2′-deoxyguanosine (8-OH-dG). Proteasome activity, particularly the chymotrypsin like activity in the functional proteasome assembly 20S, was measured utilizing an AMC-tagged peptide substrate by using the fluorometric proteasome activity assay kit (cat. # K245-100) (BioVision Research Products, Mountain View, CA, USA). The manufacturer’s instructions were followed when kits were used in evaluating various parameters.
Homeostatic model assessment of insulin resistance
All individual serum samples, collected from each mouse fasted overnight, were subjected to measurements of the levels of glucose and insulin by using the commercial kits (Cayman Chemical and Millipore), respectively. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the formula (glucose x insulin)/405, which provides a good estimation of insulin resistance, since it correlates very well with euglycemic clamp method (r = 0.88). [32].
Data evaluation
Data represent results from at least two separate measurements, unless otherwise stated. Each point represents the mean ± SEM, n=6–10. All statistical analyses were performed using Prism6 software. Two-way ANOVA was performed to assess the differences in all the results. When two-way ANOVA revealed the presence of genotype-age interaction, one-way ANOVA followed by post hoc Tukey’s studentized range test at a significance level of 0.05 was performed to determine the differences between the four groups. Liver weights were analyzed by analysis of covariance (ANCOVA) using body weight as a covariate. Other materials and methods not specified here were the same, as described [29–31, 33].
Results
More prominent hepatic histopathological changes in aged WT mice
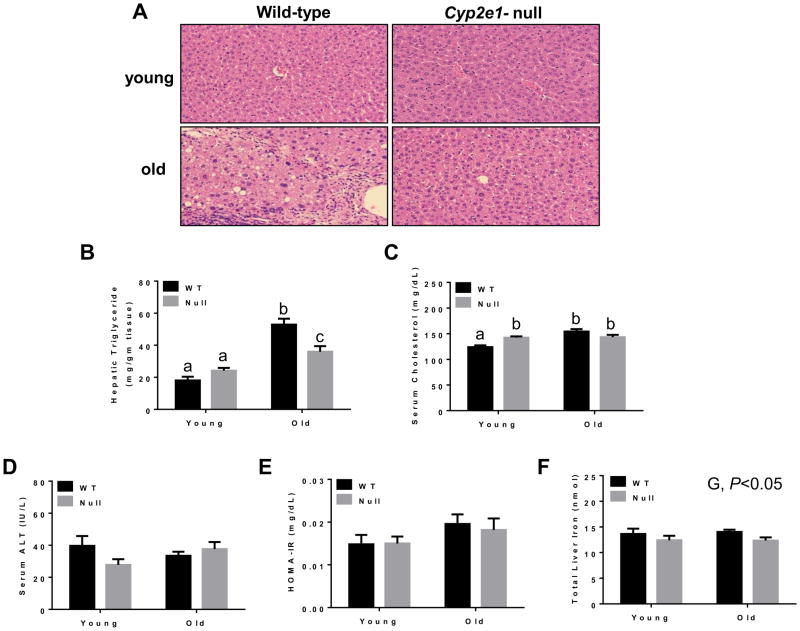
To investigate the age-related hepatic histological and/or structural changes, we compared the respective liver tissues of young (7-weeks) and aged (16-months) WT and Cyp2e1-null groups (n≥10/group). The livers of the aged WT mice were pale and the margins of the livers were blunt. The dilated gall bladders filled with bile were only observed in the aged WT. Hepatocyte vacuolation, ballooning degeneration, and inflammatory cell infiltration mostly with lymphocytes and neutrophils were markedly increased in aged WT compared to the corresponding Cyp2e1-null mice and young WT (Fig. 1A and Table 1). In addition, aged WT exhibited prominent karyomegaly, nuclear polyploidy, lipofuscin granules, and cholestasis while minimal changes were observed in aged Cyp2e1-null mice (Fig. 1 and Table 1). The morphologic features of the vacuolated hepatocyte observed in aged WT are consistent with microvesicular and macrovesicular steatosis, although some occasional fat deposits were observed in the aged Cyp2e1-null mice (Fig. 1A and Table 1). These histological results were supported by the measurements of hepatic TG contents and serum cholesterol levels. The hepatic TG levels (Fig. 1B) were significantly higher in aged mice compared with young animals (i.e. age effect) (P<0.005). In addition, a genotype-age interaction was observed (P<0.0001). Moreover, the hepatic TG level was highest in aged WT mice compared to all other groups although TG levels in aged Cyp2e1-null mice were slightly but significantly higher than those of the corresponding young group (Fig. 1B and Table 1). Similarly, serum cholesterol levels in aged mice were significantly higher compared to those of young mice (P<0.001) (Fig. 1C). A genotype-age interaction was also found (P<0.001). The levels of serum cholesterol in aged WT were significantly elevated from the corresponding young WT (Fig. 1C). None of these structural or histopathological changes were detected in the young mice of both genotypes. In contrast, plasma ALT activities (Fig. 1D) and homeostatic model assessment of insulin resistance (HOMA-IR) (Fig. 1E) did not exhibit significant changes by the genetic or aging differences. There was also a significant increase in the body weights and liver weights in aged mice when compared with young mice (age effect) (Supplementary Fig. 1). The differences in relative liver weight between the young and old WT or KO mouse groups were determined by using ANCOVA, with liver weight examined as the dependent variable and body weight as the covariate. For the WT groups, the normalized relative liver weights of the young and old mice were 3.84 ± 0.12 and 4.05 ± 0.115, respectively (p < 0.0001). For the KO mouse groups, the normalized relative liver weights of the young and old mice were 3.76 ± 0.062 g and 4.19 ± 0.077 g, respectively (p < 0.0001). ANCOVA revealed that liver weight was highly related to body weight for both WT and KO mouse groups. Slight but significant differences in total hepatic iron contents were observed in a genotype-dependent manner (P<0.05) as their levels in WT were significantly higher than those of their Cyp2e1-null counterparts (Fig. 1F).
Fig. 1.
Histological and biochemical hepatic changes in aged WT mice. (A) Representative photomicrographs (200×) following H&E staining from the indicated mouse livers are presented (n=8/group). The levels of (B) hepatic triglycerides, (C) serum cholesterol, (D) serum ALT, (E) HOMA-IR, and (F) Total liver iron are shown for all four groups (n=6–10/group). G, genotype. Labeled means without a common letter differ, P<0.05.
Table 1.
Summary of histopathological evaluation hepatic aging in WT and Cyp2e1-null mice
| Young WT | Old WT | Young Null | Old Null | |
|---|---|---|---|---|
| Number of animals | 8 | 8 | 8 | 8 |
| Hepatocyte vacuolation/steatosis | 0 | 8 | 0 | 4 |
| Slight | 3 | 4 | ||
| Moderate | 2 | |||
| Severe | 3 | |||
| Ballooning degeneration | 0 | 7 | 0 | 4 |
| Slight | 2 | 4 | ||
| Moderate | 3 | |||
| Severe | 2 | |||
| Inflammatory cell infiltration | 0 | 7 | 0 | 3 |
| Slight | 2 | 3 | ||
| Moderate | 2 | |||
| Severe | 3 | |||
| Fibrosis (Sirius Red) | 0 | 7 | 0 | 3 |
| Slight | 1 | 1 | ||
| Moderate | 4 | 2 | ||
| Severe | 2 | |||
| Necrosis | 0 | 2 | 0 | 0 |
| Cholestasis | 0 | 6 | 0 | 2 |
Grading System: slight, few hepatocytes affected; moderate, multiple foci of hepatocytes or the cell surroundings affected; severe, diffuse pan-lobular damage.
Increased hepatic apoptosis and fibrosis in aged WT mice
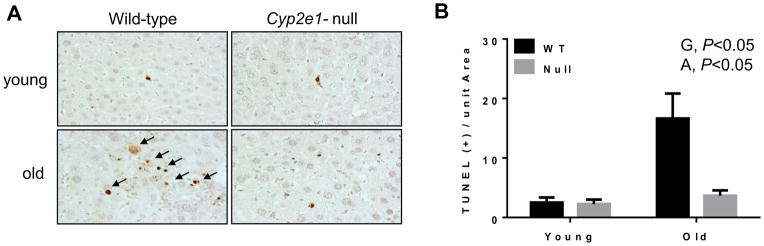
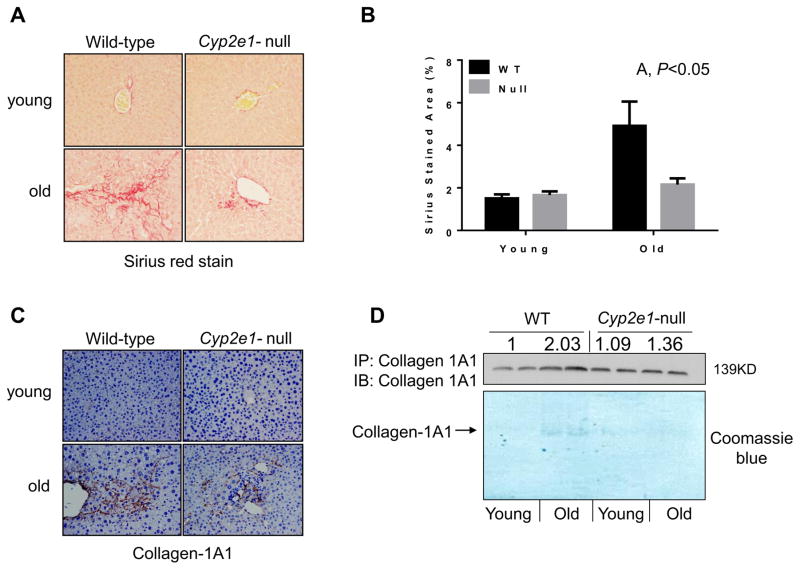
The increased levels of hepatocyte apoptosis and liver fibrosis in the aged mice have been previously reported [11, 26, 27], although the role of CYP2E1 and the underlying mechanism(s) were not studied. To evaluate any difference in hepatocyte apoptosis among the four groups, the TUNEL assay was performed. TUNEL-positive apoptotic hepatocytes were readily observed in the aged WT (Fig. 2A). Significant differences were observed in the levels of apoptotic hepatocytes resulting from genotype (P<0.05) and age effects (P<0.05) where aged WT mice showed the highest levels of apoptotic cells (Fig. 2B). In addition, hepatic fibrosis, as evidenced by Sirius red staining, was significantly increased in aged WT, compared to the other groups (Figs. 3A, B and Table 1). Two-way ANOVA revealed a significant aging-dependent differences between the groups (P<0.05). The accumulation of collagen fiber and development of hepatic fibrosis in aged WT mice were further confirmed by immunohistochemistry for collagen formation using the specific anti-collagen 1A1 antibody (Fig. 3C), and immunoprecipitation of collagen 1A1 followed by immunoblotting with the same antibody (Fig. 3D). Collectively, these results (Figs. 1–3) unequivocally demonstrate that the WT mice undergo more progressive aging-dependent hepatic histopathological and structural changes. In contrast, the corresponding aged Cyp2e1-null mice seem protected from the histopathological changes, suggesting an important role of CYP2E1 in aging-related liver disease.
Fig. 2.
Increased levels of apoptotic hepatocytes in livers of aged WT mice. (A) TUNEL-positive hepatocytes were identified by black arrows and (B) quantified in 10 high-power fields (×200) for the indicated mouse groups (n=5–6/group). G, genotype; A, age.
Fig. 3.
Increased hepatic fibrosis in aged WT mice. (A and B) Representative histological analysis of hepatic sections for all indicated mouse groups (n=8/group) are provided to show collagen Sirius red staining and percentage of fibrotic areas, respectively. The results of (C) immunohistochemical analysis, and (D) immunoprecipitation followed by immunoblot analysis are provided and relative ratio between the groups are provided following densitometric analysis. Specific anti-collagen 1A1 antibody was used for both C and D.
Elevation of different oxidative stress parameters in aged WT
Oxidative stress has been suggested to play a contributing role in the aging process and cell senescence [13, 14]. Oxidative stress may result from the imbalance between oxidative radical production and their removal by the antioxidant defense system including GSH and antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (Gpx), heme oxygenase 1 (HO-1), peroxiredoxin (Prx), thioredoxin (Trx), etc. Many proteins including CYP2E1, iNOS, NADPH-dependent oxidase, and xanthine oxidase, as well as mitochondrial dysfunction with potential alteration of the mitochondrial electron transfer chain (ETC) [34] are known to contribute to increased nitroxidative stress in the cells.
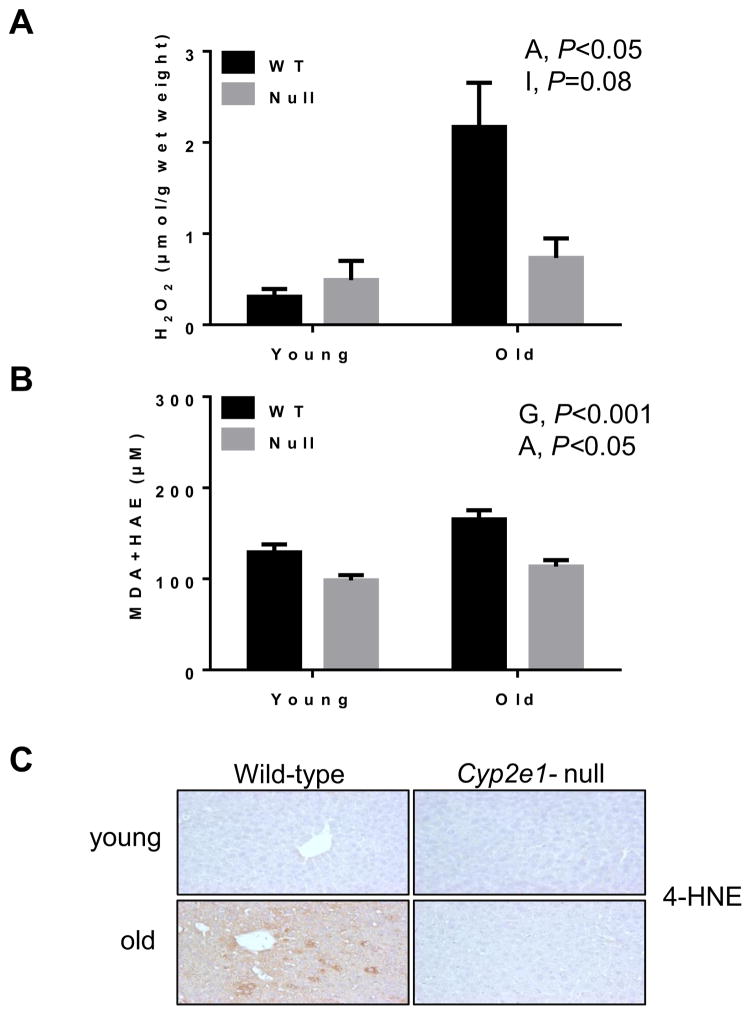
We first determined whether oxidative stress levels were increased in aged WT and correlated with the signs of hepatic aging. Thus, we evaluated the levels of hepatic H2O2 and other markers of oxidative stress including lipid peroxidation, oxidative protein modification and DNA damage, as well as the amounts of the mitochondrial complex proteins, since all these parameters are closely associated with the aging-related oxidative stress and consequences [35–38]. The levels of H2O2 (Fig. 4A) were higher in aged mice than young mice (P<0.05), and lipid peroxidation, evaluated by both the measurement of MDA + HAE (Figs. 4B and C) and immunohistochemistry (Fig. 4D), were increased in WT than the corresponding Cyp2e1-null mice (i.e., genotype effect. P<0.001) and in old mice than young mice (i.e., age effect, P<0.05).
Fig. 4.
Increased levels of oxidative stress and lipid peroxidation in aged WT. Equal amounts of liver tissue lysates were used to determine the levels of (A) H2O2 and (B) MDA+HAE for the indicated group (n=6/young groups and n=10/old groups). (C) Representative immunohistochemical analysis of hepatic lipid peroxidation determined with the anti-4-HNE antibody in all indicated mouse groups (n=5–6/group) are presented. G, genotype; A, age; I, genotype-age interaction.
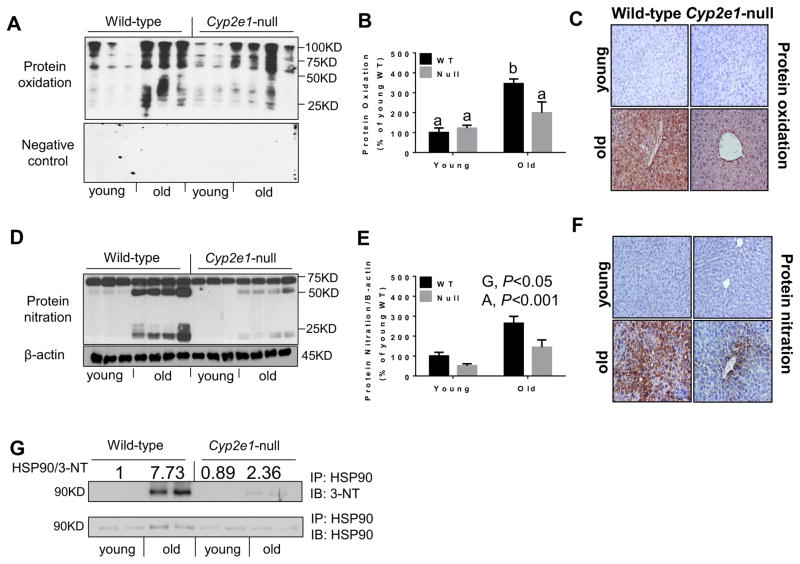
Furthermore, Oxyblot (Fig. 5A and B) followed by two-way ANOVA showed that the levels of hepatic oxidized proteins were significantly higher in aged mice than young mice (i.e., age effect, P<0.005). The specificity of the oxidized proteins was confirmed by disappearance of the bands when they were exposed to 1× derivatization control solution (Fig. 5A). Furthermore, a genotype-age interaction was observed with oxidized protein levels in aged WT mice, which showed ~3.5-fold higher levels than the young Cyp2e1-null mice (Fig. 5A). When one-way ANOVA was employed, oxidized protein levels in aged WT were significantly higher than all other groups (Fig. 5B). Immunohistochemical analysis further revealed that the levels of oxidized proteins were markedly increased in the aged WT compared to those of the other three groups (Fig. 5C). Consistently, immunoblot analysis with anti-3-NT antibody showed higher levels of hepatic protein nitration in WT than Cyp2e1-null mice (i.e., genotype effect, P<0.05) and in aged mice than young counterparts (i.e., age effect, P<0.001) (Figs. 5D and E). The highest levels of protein nitration in aged WT were also observed by immunohistochemical analysis (Fig. 5F). In order to further confirm the remarkable differences in protein nitration between the two aged mouse groups, we also evaluated the nitration levels of heat shock protein 90 (HSP90), a heat-shock protein abundantly expressed in hepatocytes and shown to cause cell death when it is nitrated [39]. The levels of nitration in the immunoprecipitated HSP90 was remarkably greater in aged WT compared to all other three groups (Fig. 5G, top panel), while there was only a modest increase in the levels of HSP90 protein in the aged WT (bottom panel). This result confirms the remarkable difference in the nitrated protein levels between the aged WT and the corresponding Cyp2e1-null mice, however the role of nitrated HSP90 in mediating hepatocytes damage remains to be determined.
Fig. 5.
Increased levels of oxidatively-modified proteins in aged WT. (A, D) Representative results of immunoblot (B, E) and densitometric analysis (n=8–10/group). (C, F) Immunohistochemistry for the indicated target proteins (n=5–6/group) are shown. (A, C) Oxidized proteins using the commercially available kit for protein oxidation analysis and (D, F) nitrated proteins and β-actin, used as a loading control (D), were determined by immunoblot and immunohistochemistry analyses, as indicated. (G) Results of immunoprecipitation of HSP90 followed by immunoblot analysis are presented. Specific anti-HSP-90 and 3-NT antibodies were used, as indicated. G, genotype; A, age. Labeled means without a common letter differ, P<0.05.
Since the hepatic levels of hydrogen peroxide was significantly elevated in the aged WT (Fig. 4) and it is a known precursor of hydroxyl radical which causes oxidative DNA damage, generating 8-oxo-deoxyguanosine (8-OH-dG) [36], we next evaluated the levels of oxidatively-modified DNA in our mouse groups (Fig. 6). Indeed, significantly higher contents of 8-OH-dG in hepatic DNA were observed in WT compared to Cyp2e1-null mice (i.e., genotype effect, P<0.005). Because there was a significant genotype-age interaction (P<0.005), one-way ANOVA was further performed. Significantly higher levels of oxidative DNA damage were observed in aged WT compared to the young WT and aged Cyp2e1-null mice, providing additional evidence for the significant oxidative stress-mediated events in the aged WT than the corresponding Cyp2e1-null mice.
Fig. 6.
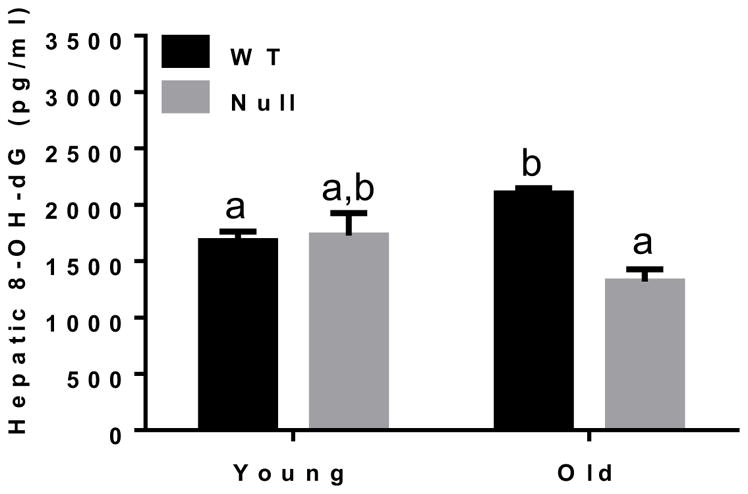
Increased levels of oxidatively-modified hepatic DNA in aged WT. Equal amounts of liver tissues from all four mice groups (n=6/young groups and n=10/old groups) were used to isolate DNA. Same amounts of isolated DNA (2 μg/sample) were used to measure the hepatic levels of 8-OH-dG, a marker of oxidative DNA damage. Labeled means without a common letter differ, P<0.05.
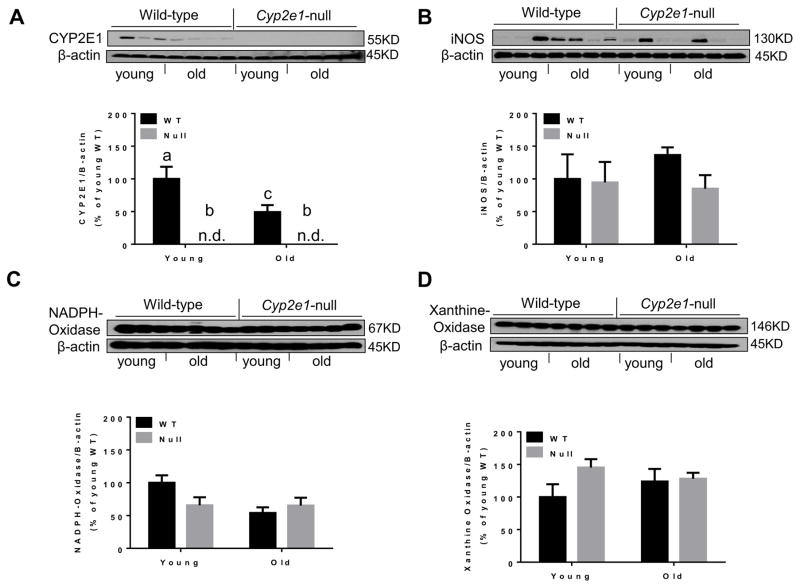
We also investigated some potential sources of the nitroxidative stress that might result from the imbalance between oxygen radical production and its removal, as mentioned previously. Therefore, we evaluated the levels of some potential sources of hepatic oxygen radical production in the cytosol or mitochondria. As expected, there was a significant difference in the levels of CYP2E1 in WT mice compared to Cyp2e1-null mice (i.e., genotype effect, P<0.0001) and in aged mice compared to young mice (P<0.05) with an observation of a genotype-age interaction (P<0.05) (Fig. 7A). Interestingly, one-way ANOVA verified a significant decrease in the levels of CYP2E1 protein in aged WT compared to young WT. Additional analysis of other oxidative stress-inducing proteins revealed that none of iNOS (Fig. 7B), NADPH-oxidase (Fig. 7C), and xanthine oxidase (Fig. 7D) was increased in the aged WT compared to the other groups. Further, mitogen-activated protein kinases, involved in the stress signaling pathways such as p-JNK and p-P38 kinase, were not significantly changed in the aged WT and Cyp2e1-null mice (data not shown).
Fig. 7.
Little changes in the levels of some oxidative stress-inducing proteins eased levels of oxidative stress and lipid peroxidation in aged WT. Equal amounts of whole liver lysate proteins (40 μg/lane) were used for immunoblot analyses. Representative results of immunoblot analysis and densitometric analysis results (n=8–10/group) are presented for the levels of: (A) CYP2E1, (B) iNOS, (C) NADPH-oxidase, and (D) xanthine oxidase. β-Actin was used as a loading control. Labeled means without a common letter differ, P<0.05.
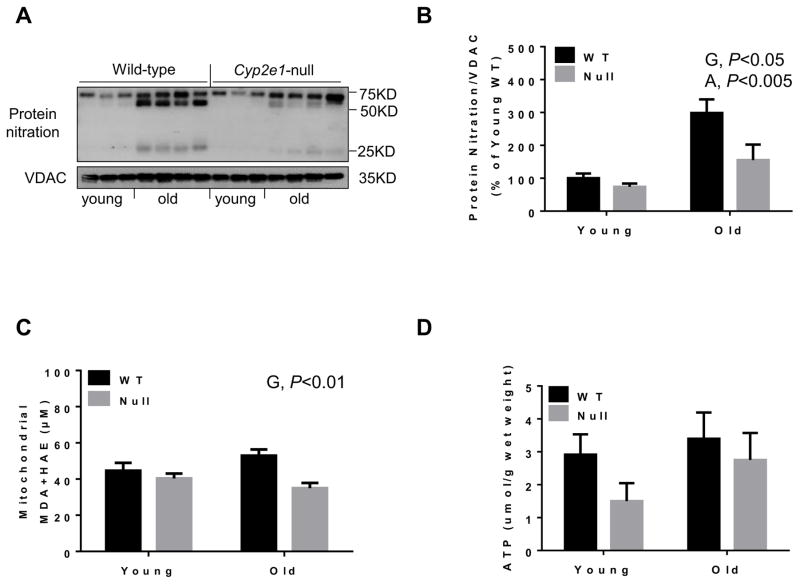
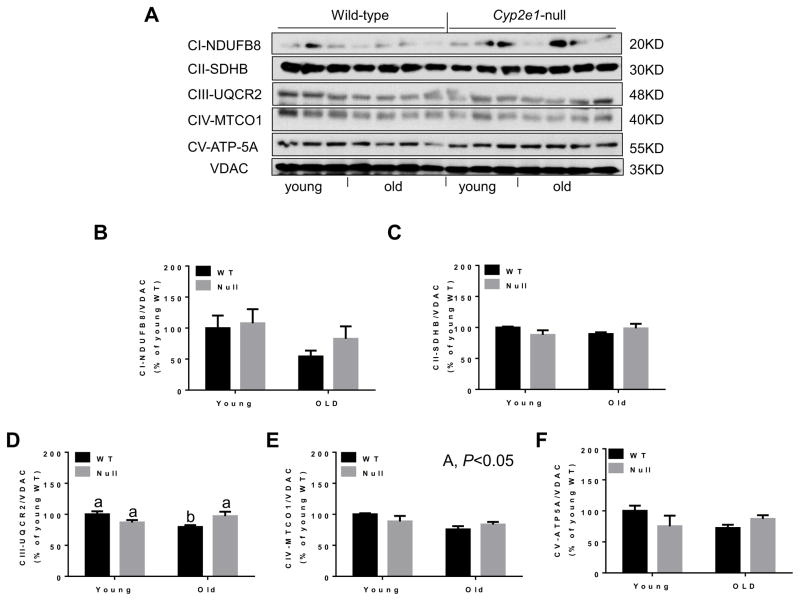
Other parameters of oxidative stress markers such as protein nitration and lipid peroxidation were evaluated with mitochondrial extracts. Higher levels of mitochondrial protein nitration were monitored in WT mice than Cyp2e1-null mice (i.e., genotype effect, P<0.05) and in aged mice relative to the corresponding young counterparts (i.e., age effect, P<0.005) (Figs. 8A and B). Furthermore, the levels of mitochondrial lipid peroxidation were higher in WT compared to Cyp2e1-null mice (i.e., genotype effect, P<0.01). However, the ATP levels in different groups were unchanged (Fig. 8D). Since mitochondrial complexes proteins have been reported to be altered during the aging process, leading to increased disturbance in the ETC with elevated oxygen radicals [35, 36], we analyzed the levels of mitochondrial complex proteins using the total OXPHOS rodent WB antibody cocktail, which contains 5 mouse monoclonal antibodies against CI subunit NDUFB8, CII-SDHB, CIII-Core protein 2 (UQCR2), CIV subunit 1 (MTCO1) and CV alpha subunit (ATP-5A). Immunoblot and statistical analyses showed no significant changes in the levels of the subunits of CI, CII, and CV despite the general trend of decreased levels of these proteins in aged WT compared to their young counterparts (Figs. 9A, B, C, and F). In contrast, a significant genotype-age interaction was observed for CIII subunit protein levels (P<0.01) (Fig. 9D). One-way ANOVA revealed that the amount of CIII subunit in aged WT was significantly lower than all other groups and that such alteration was absent in Cyp2e1-null mice (Fig. 9D). Further, the levels of CIV subunit in aged mice were lower than young counterparts (i.e., age effect, P<0.05) (Fig. 9E). Taken together, these data (Figs. 8 and 9) suggest that mitochondrial dysfunction via alteration of the ETC proteins might be potentially involved in the increased oxidative stress during the hepatic aging process and that this role is more prominent in aged WT than in aged Cyp2e1-null mice. However, additional studies are still needed to further establish the role of mitochondria in mediating the hepatic aging process.
Fig. 8.
Increased levels of mitochondrial oxidatively-modified proteins and lipid peroxidation with little changes in ATP levels in aged WT. (A) Representative results of immunoblot analysis of nitrated proteins and VDAC, used as a loading control, and (B) densitometric analysis for the levels of protein nitration in all mouse groups (n=8–10/group). (C) MDA+HAE for the indicated group (n=6–10/group). (D) Hepatic ATP levels (n=6–10/group). G, genotype; A, age.
Fig. 9.
Alteration in the levels of mitochondrial complex proteins in aged WT mice. Equal amounts of liver mitochondrial proteins from all mouse groups were used. (A) Representative results of immunoblot analysis of CI subunit NDUFB8, CII-SDHB, CIII-core protein 2 (UQCR2), CIV subunit 1 (MTCO1), and CV alpha subunit (ATP-5A) using specific total OXPHOS antibody, and VDAC, used as a loading control, and (B–F) densitometric analysis for all complexes as illustrated (n=8–10/group). A, age. Labeled means without a common letter differ, P<0.05.
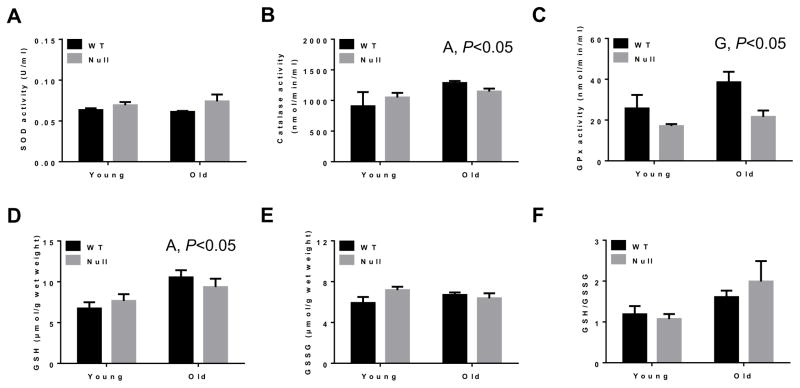
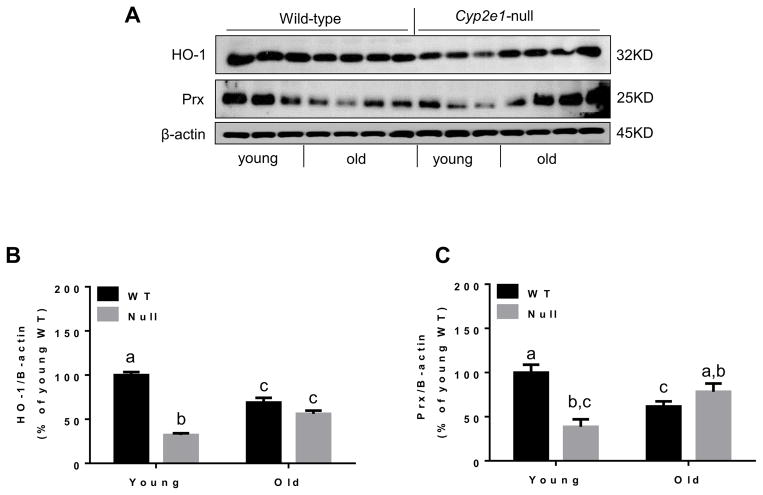
Following the evaluation of various sources of oxygen radical production, we next examined some cellular defense systems that handle and/or remove oxygen radicals. It has been suggested that lipid peroxidation and other parameters of oxidative stress, observed in animal models of nonalcoholic steatohepatitis, decrease hepatic antioxidant enzyme activities, their protein amounts, and GSH contents [41]. Although we did not recognize any significant alteration in SOD activity (Fig. 10A), there was significant increase in catalase activity in aged mice compared with young mice (i.e., age effect, P<0.05) (Fig. 10B) and GPx activity (Fig. 10C) in WT compared with the corresponding Cyp2e1-null mice. Further, increased levels of GSH in aged mice compared with young mice were observed (i.e., age effect, P<0.05) (Fig. 10D) although there was no significant difference in the levels of GSSG (Fig. 10E) or GSH/GSSG (Fig. 10F). Significant differences in the levels of HO-1 and Prx proteins in WT compared to Cyp2e1-null mice were detected (i.e., genotype effect, P<0.0001) and a genotype-age interaction was also recognized for these two proteins (P<0.0001) (Figs. 11A, B, and C). One-way ANOVA showed that both HO-1 and Prx protein levels were significantly lower in aged WT compared with their young control mice, while these protein levels were significantly elevated in aged Cyp2e1-null mice compared with their young counterparts (Figs. 11A, B, and C). Furthermore, the levels of Prx protein in aged Cyp2e1-null mice were significantly higher than the aged WT (Figs. 11A and C). It is noteworthy to mention the significantly lower levels of these two proteins in the young Cyp2e1-null mice when compared with the young WT counterparts. These results may reflect the difference in the basal levels of oxidative stress in these two mouse groups. We however did not observe any significant change in the levels of Trx protein (data not shown).
Fig. 10.
Changes in the activities of hepatic anti-oxidant enzymes and levels of glutathione in aged WT and Cyp2e1-null mice. (A–E) Equal amounts of whole liver lysates from all mouse groups were used to determine the hepatic activities of anti-oxidant enzymes, including (A) superoxide dismutase (SOD), (B) catalase (CAT), (C) glutathione peroxidase (Gpx) and the levels of (D) reduced GSH and (E) oxidized GSSH. (F) The levels of GSH/GSSH, as a marker for anti-oxidant capacity, for the indicated groups are presented (n=6/young groups and n=10/old groups). G, genotype; A, age.
Fig. 11.
Differential regulation of the antioxidants proteins HO-1 and Prx in aged WT and Cyp2e1-null mice. Equal amounts of whole liver lysates from all mouse groups were used for immunoblot analysis. (A) Representative results of immunoblot analysis of HO-1 and Prx, and β-actin, used as a loading control. Densitometric analysis results (n=8–10/group) are presented for the levels of: (B) HO-1, and (C) Prx. β-Actin was used as a loading control. Labeled means without a common letter differ, P<0.05.
The accumulation of oxidative proteins has been attributed partly due to inhibition of proteasome activity during aging [42, 43], although this theory of inhibited proteasome activity during aging has been challenged due to rather complex process [43, 44]. As shown in Supplementary Fig. 2, our data revealed that trypsin-like activity of 20S proteasome was significantly elevated in WT mice compared to Cyp2e1-null mice (i.e., genotype effect, P<0.05), and in aged mice than young counterparts (i.e., age effect, P<0.0005), while there was a genotype-age interaction (P<0.05). One-way ANOVA analysis showed that trypsin-like activity of 20S proteasome was significantly higher in aged WT than all other groups. We did not detect a significant difference in autophagy between aged WT and Cyp2e1-null mice (data not shown).
TNF-α is a key pro-inflammatory cytokine, primarily secreted by monocytes and macrophages. It is well-established that it exhibits a broad range of biological effects including the induction of hepatocyte necrosis in vivo or in vitro [45] and implication in hepatic inflammation in aged mice [46]. Therefore, we evaluated the levels of hepatic TNF-α in all four groups (Supplementary Fig. 3). The levels of hepatic TNF-α as measured by ELISA in the WT mice were significantly different from those of Cyp2e1-null mice (i.e., genotype effect, P<0.005), and in aged mice compared to young mice (i.e., age effect, P<0.05). In addition, a genotype-age interaction was recognized (P<0.01) (Supplementary Fig. 3). Further one-way ANOVA revealed that its levels in aged Cyp2e1-null mice were significantly lower than all other groups including the aged WT.
We finally evaluated the levels of other cytochrome P450 proteins in order to evaluate the effect of aging on these proteins. Interestingly, with the exception of CYP4F, which did not exhibit any significant alteration in the protein levels between the groups (Supplementary Fig. 4B), the levels of all other examined proteins such as CYP2B, CYP1A, CYP2A, and CYP4A decreased in aged mice compared to young mice (i.e., age effect) and were significantly different between WT and Cyp2e1-null mice (i.e., genotype effect). Genotype-age interactions were observed in CYP2B (P<0.0005) and CYP1A (P<0.0005) (Supplementary Figs. 4A and C). One-way ANOVA for these two proteins showed similar patterns as the levels of these two proteins were significantly decreased in aged WT compared with their young controls. In addition, the levels of CYP2B and CYP1A exhibited significant decrease in young Cyp2e1-null mice when compared with young WT. However, the levels of both proteins were not significantly different from aged Cyp2e1-null mice (Supplementary Figs. 4A and C), suggesting a differential regulation of other cytochrome P450 proteins upon CYP2E1 deletion. It is noteworthy to mention that differences were observed in the basal levels of some proteins examined in young WT and Cyp2e1-null mice, as exemplified by many cytochromes P450 and antioxidant enzymes such as HO-1 and Prx. This variation might be explained in part by the potential changes in the normal physiological homeostasis in response to the deletion of CYP2E1, likely leading to different basal metabolism and oxidative stress.
Collectively, these results (Figs. 4–10) suggest that oxidative stress is increased in parallel with the hepatic aging process (Figs. 1–3) in WT mice. Furthermore, since all these changes were not prominent in aged Cyp2e1-null mice, CYP2E1 plays at least a partial role in the aging-related hepatic steatosis, apoptosis, and fibrosis.
Discussion
Mice have been widely used as a suitable model to study the underlying mechanism(s) of aged-related hepatic steatosis, apoptosis, and fibrosis since they are short-lived and share many similarities with human conditions [11, 26, 27]. Constitutive expression of CYP2E1, involved in ROS production, has been reported to be significantly higher in female mice than males, suggesting a more potentially pronounced pathophysiological outcomes in females than males possibly resulting from CYP2E1-mediated metabolisms [47]. Thus young and aged female mice were used to further study the role of CYP2E1 in aging-dependent hepatic changes in this study. However, it is still important to evaluate the role of CYP2E1 in mediating hepatic aging in male mice to compare the effect of gender-dependent difference in this process. It is reasonable to assume that the hepatic aging in male mice would appear later in life than in female mice, at least partially, due to the lower expression of CYP2E1 in male mice than females.
CYP2E1 is known to produce high levels of ROS [15–17] and plays a role in the formation of lipid peroxidation [48], protein oxidation [34, 49], and protein nitration [20, 30, 33]. It is also known to promote hepatic carcinogenesis by oxidizing DNA in alcohol-exposed rodents [50]. All these reports suggest that CYP2E1 in mice is likely to play an important role in promoting tissue injury after exposure to various hepatotoxic agents such as ethanol, acetaminophen, and high fat diet. The main aim of this investigation was to directly evaluate the role of CYP2E1 in age-related hepatic disorder by studying the levels of oxidative stress markers and the degrees of hepatic steatosis, apoptosis, and fibrosis in aged WT compared with those of the young WT. We also determined the biochemical and histological changes in the aged Cyp2e1-null mice and compared to those of the other groups including the aged WT to further confirm the critical role of CYP2E1 in aging-related hepatic disease.
Our results showed that the deletion of Cyp2e1 gene effectively decreased the features of liver aging [11, 26, 27] such as hepatic vacuolation, ballooning degeneration, inflammatory cell infiltration, apoptosis, and fibrosis, as observed in aged WT compared to other groups. However, there were no significant differences in the ALT levels among all four groups. These results are consistent with previous reports suggesting that the occurrence of hepatic disorder is not necessarily accompanied with the elevation of liver transaminases [29, 30, 51]. Aging process is associated or facilitated by increased production of free radicals with chronic oxidative stress [52] that might arise from normal metabolism of endogenous and exogenous compounds, leading to oxidative damage [27] to cellular macromolecules including lipids, proteins, and DNA. Indeed, lipid peroxidation and oxidative post-translational protein modifications, as well as oxidative DNA damage are well-established markers for oxidative stress during the aging process [27, 37, 38]. The first indication, that oxidative stress might be involved in age-related hepatocyte damage in our rodents, was through the detection of significantly higher amounts of lipofuscin granules (consisting of lipid peroxidation products and oxidatively-modified proteins) [27, 53] observed only in aged WT. Consistently, the levels of hepatic H2O2, lipid peroxidation, protein oxidation, and nitration, and oxidative DNA damage, all of which are well-established markers of oxidative stress during the aging process in many tissues including the liver [27, 53–56], were observed in aged WT compared to the aged Cyp2e1-null mice and other groups. Further, the protective role of CYP2E1 deletion has been in agreement with many reports, that verified the pathological role of CYP2E1 in the development of alcoholic steatohepatitis [48, 57, 58] and non-alcoholic steatohepatitis [30], via oxidative stress-mediated events since CYP2E1 is known to increase the levels of ROS and RNS even in the absence of its substrates [16, 17, 33, 50, 51]. Since the age-related hepatic changes are very prominent in the WT than Cyp2e1-null mice, it is reasonable to assume that the nitroxidative stress might have peaked at earlier time point(s) in the WT and that CYP2E1 is partially responsible for the increased oxidative stress-related hepatic steatosis, apoptosis, and fibrosis observed in aged WT mice.
Hepatic steatosis (fat accumulation) renders the liver more susceptible to other insults such as inflammation and oxidative/nitrosative stress. This increased sensitivity is particularly evident in the presence of oxidizable, unsaturated n-6 fatty acids in steatotic livers [29, 41]. Several molecular mechanisms have been proposed for the occurrence of aging-dependent hepatic steatosis via oxidative DNA damage, activation of p300-C/EBP dependent fat synthesis, telomere shortening, inhibition of autophagy, nuclear factor-κβ pathway activation, M1 macrophage inflammatory response activation, and oxidative stress [11]. Thus, it is intriguing to assume that the “two-hit” hypothesis that steatosis primes the liver to secondary insults, including ROS/RNS and inflammation [59], plays a role in the development and/or progression of non-alcoholic steatohepatitis. It is also possible that a vicious cycle may develop between oxidative stress and hepatic steatosis, particularly in WT mice more than in Cyp2e1-null mice. Thus, these results suggest that CYP2E1-mediated oxidative stress and subsequent changes [11 51, 20, 34, 60] play at least a partial role in the aging-related hepatic disease. However, IR, which can also be caused by oxidative stress, does not seem to be involved in promoting the early events of hepatic aging including steatosis since there was no significant increase in HOMA-IR in aged WT mice compared to the other groups (Fig. 1), although IR has been implicated in the development and progression of steatohepatitis (inflammation) [30].
Oxidative/nitrosative events are well-documented to cause DNA damage and oxidative post-translational modifications of Cys-residues and/or nitration of Tyr residue of cellular proteins [20, 31, 61–63], which negatively affect functions of the modified target proteins prior to the observation of a full-blown liver disease. Oxidative stress-induced modifications of cellular macromolecules might trigger mitochondrial dysfunction and hepatocyte apoptosis, which can be stimulated by nitrated Hsp90 (Fig. 5E), as demonstrated [39]. In addition, dysregulation of apoptosis may accelerate the process of aging and age-related diseases. Further, ROS/RNS, oxidatively-modified proteins, and lipid peroxidation, may increase inflammation and activate hepatic stellate cells, thus accelerating the development of liver fibrosis [41, 57]. Taken together, the deletion of CYP2E1 significantly prevented the development of oxidative stress and other characteristics of liver aging such as steatosis, apoptosis, and fibrosis, suggesting that CYP2E1 plays, at least a partial role, in promoting hepatic aging process most likely through increasing the levels of nitroxidative stress.
Oxidative stress-mediated cell damage is related to the balance between the production of oxidants and the defense capacity of the anti-oxidant system [32, 34, 64]. There are many sources of oxidative stress in the cell such as CYP2E1, iNOS, NADPH-oxidase, xanthine oxidase, and the mitochondrial electron transfer chain [32, 34, 64]. Another potential source of increased oxidative stress is hepatic iron, which has been reported to act as a priming or sensitizing factor for CYP2E1-induced injury in hepatocytes through increasing oxidative stress [21]. Interestingly, we did not observe a significant change in the amount of iNOS, NADPH oxidase, or xanthine oxidase. The levels of CYP2E1 in aged WT were actually significantly lower than their young controls in agreement with several reports with either a decrease or no change in CYP2E1 contents during the aging process [22–25]. Similarly, the amounts of several other hepatic cytochrome P450 proteins such as CYP2B, CYP1A, CYP2A, and CYP4A were also lower in aged WT compared to their young controls, suggesting that aged WT may have decreased hepatic drug metabolizing capacity compared to their young counterparts. However increased levels of total hepatic iron were observed in WT compared to Cyp2e1-null mice. Despite the fact that these iron levels do not seem to increase with aging (Fig. 1F), the higher exposure to iron in WT throughout the life span compared to Cyp2e1-null mice might contribute, at least partially, to the increased levels of oxidative stress, as previously mentioned [21]. We also observed changes in the levels of the mitochondrial ETC complexes, suggesting a potential role of mitochondrial impairment with increased production of ROS such as superoxide anion and hydrogen peroxide, further leading to increased levels of mitochondrial protein nitration and lipid peroxidation in aged WT. Indeed, it was previously shown that mitochondria isolated from old animals released elevated amounts of hydrogen peroxide than young animals [65]. In addition, there was an inverse relation between the increased ROS production by mitochondria and the life span [66]. The mitochondrial respiration is essential for producing ATP whereas oxidatively-damaged mitochondria may produce decreased amount of energy. However, in our study, the levels of ATP in aged WT were not significantly changed (Fig. 8D). This is not surprising since most of hepatic ATP production have been reported to remain unchanged during the aging process, possibly through an adaptive mechanism [67], although decreased ATP production during aging process was also documented [68]. The potential role of mitochondria in mediating the hepatic aging-related changes in WT mice needs additional investigation to further establish their importance in the aging process. Inflammation and oxidative stress are closely related to each other and inflammation has also been implicated in the hepatic aging process [26]. The anti-inflammatory adaptive response seems to be more intact or stronger in aged Cyp2e1-null mice since the amount of TNF-α in this aged group was significantly lower than all other groups.
In contrast, intracellular GSH system constitutes a major antioxidant defense system in the liver [69]. Further, the systematic elimination of oxygen radicals (O2·−, OH·−, H2O2, etc) requires the coordinated action by anti-oxidant enzymes such as SOD, CAT, and GPx. Disruption or suppression of the CAT, GPx, and SOD activities would intuitively contribute to the induction and/or enhancement of oxidative stress. In addition, decreased expression of other anti-oxidant defense systems such as HO-1 and Prx, can lead to increased oxidative stress. In fact, the levels of HO-1, which can protect HepG2 cells against CYP2E1-dependent toxicity potentially via production of CO following its upregulation [70], and Prx, a thioredoxin-dependent antioxidant protein, were decreased in aged WT compared those in young WT (Fig. 11). In contrast, these proteins were upregulated in a coordinate manner, as reported [71], in aged Cyp2e1-mull mice compared to the corresponding young mice. These changes may also contribute to increased oxidative stress in aged WT mice.
Interestingly, we observed an increased activities of catalase and Gpx in age- and gene-dependent manners, respectively, although SOD activity did not change significantly. In addition, although the amounts of GSSG or GSH/GSSG did not change, a significantly increased levels of GSH were observed in an age-dependent effect (Fig. 10). These results are not uncommon, since reports regarding the levels of antioxidant defense in aged animals are contradicting. The increases in catalase and Gpx activities and GSH might be considered as an adaptive mechanism against the increased levels of oxidative stress, as previously reported in aged rats [72], although the decrease in GSH levels were also documented in other studies [33]. Moreover, some reports showed that neither GSH nor GSSG exhibited significant changes in an aged mice model [73]. Nonetheless, in this model, the overall anti-oxidant response capacity seems lower in aged WT compared to aged Cyp2e1-null mice due to the differential regulation of HO-1 and Prx during aging since the levels of both anti-oxidant proteins HO-1 and Prx in aged WT were decreased while their levels were increased in aged Cyp2e1-null mice when compared to their corresponding young mice. Further, the aged WT mice would actually need considerably higher antioxidant defense, not even equal, response than aged Cyp2e1-null mice considering the higher levels of oxidative radical production in aged WT mice.
Another potential reason for the accumulation of oxidatively-modified proteins could be due to alteration in the proteasome system. It has been reported that the inhibition of proteasome activity has been reported to correlate with the accumulation of oxidatively-modified proteins, which are the substrates for proteasome [74, 75]. However, other reports also suggested that there is an age-dependent increment of 20S proteasome activity [76, 77], in agreement with our results (Supplementary Fig. 2), where proteasome activity was higher in aged WT mice than Cyp2e1-null mice. These data likely reflect an adaptive response to eliminate the oxidatively-modified proteins. However, it is reasonable to assume that the discrepancy in various reports on the levels of anti-oxidant status, energy production rate, and proteasome activity, may be due to different animal strains, source, duration, and severity of oxidative stress, duration of aging, gender of animals, environmental surroundings, and other factors.
Thus, it is clear that there is a significant difference in the cellular redox balances between the 16-month old aged WT and the corresponding Cyp2e1-null mice. The differences in oxidative stress levels could probably result from increased ROS production through the altered mitochondrial ETC function with decreased amounts or activities of Gpx, HO-1 and Prx. In addition, increased levels of oxidative stress in WT mice could have resulted from the increased nitroxidative stress through CYP2E1-mediated metabolism of endogenous substrates throughout the life span, although its levels can be decreased later as the mice aged, as shown in this study (Fig. 7A) and in other reports [22–24]. Similar examples of increased nitroxidative stress and oxidative modifications of cellular macromolecules, despite the decreased CYP2E1 levels, were observed in animals treated with acetaminophen [33, 63] or carbon tetrachloride [78]. These results also suggest that the ability of stress response in the aged hepatocytes might be reduced in the aged WT mice more profoundly. Consequently, elevated levels of lipid peroxidation, oxidized proteins and DNA along with histological changes in fat accumulation, apoptosis and fibrosis in aged WT mice. Since several reports suggested either a decreased or unchanged levels of CYP2E1 during the aging process and in this study (Fig. 7A) in agreement with other reports [22–25], it is reasonable to assume that the constitutive expression of CYP2E1 throughout the entire life-span is sufficient to promote the increased levels of nitroxidative stress even under normal physiological conditions. It is also possible that the constitutive expression of CYP2E1 plays a permissive role to other gene(s)/protein(s) that increase oxidative stress-mediated consequences. It is noteworthy to mention that the temporally different regulations and the consequence of events during the aging process in WT and Cyp2e1-null mice warrant more investigations since many genes, involved in causing nitroxidative stress, stress signaling pathways, and inflammation, as with TNF-α, could have been differentially regulated at earlier time points and returned to basal levels at the time of tissue collection. This is particularly important for the stress signaling pathways which can be activated by oxidative stress [64]. Since we did not observe activation of these pathways, they could have been activated at earlier time points before the development of the histopathological changes.
In summary, CYP2E1 appears to be involved in fat accumulation and inflammation in aged liver and the advancement to apoptosis and fibrosis. This can be explained by the fact that CYP2E1 increases the production of ROS/RNS, which likely increase lipid peroxidation, oxidative protein modifications, oxidative DNA damage and inflammation, all of which can negatively affect the vital functions of the liver and promote the development of hepatic injury, as observed in aged WT mice. The higher oxidative stress levels in aged WT than Cyp2e1-null mice seem to be attributed to increased oxidative radical production. Conceivably, the aging-associated hepatic changes were absent or significantly decreased in the corresponding aged Cyp2e1-null mice. Therefore, our results represent the first report to demonstrate the novel role of CYP2E1 in the hepatic aging process in rodents. Furthermore, CYP2E1 could be a potential target for translational research in preventing aging-related liver disease.
Supplementary Material
Highlights.
Despite the aging-related hepatic steatosis, apoptosis, and fibrosis via oxidative stress-related mechanisms, the role of cytochrome P450-2E1 (CYP2E1), a known oxidative stress inducer, in the hepatic aging process has never been evaluated.
Young (7 weeks) and aged female (16 months old) wild-type (WT) and Cyp2e1-null mice were used to characterize the potential role of CYP2E1 in mediating the aging-related hepatic disease.
The liver histology showed that aged WT mice exhibited markedly elevated hepatocyte vacuolation, ballooning degeneration, and inflammatory cell infiltration compared to all other groups.
Immunohistochemistry and biochemical data clearly showed that the aged WT mice also showed the highest levels of hepatic steatosis, hepatocyte apoptosis and hepatic fibrosis accompanied with the highest levels of hepatic hydrogen peroxide, lipid peroxidation, protein carbonylation, nitration, and oxidatively-modified DNA 8-oxo-2′-deoxyguanosine.
These aging-related oxidative changes observed in the aged WT were absent or very low in the aged Cyp2e1-null mice, suggesting the important role of CYP2E1 in promoting aging-related hepatic disease possibly through increasing nitroxidative stress.
Acknowledgments
This research was supported by the Intramural Research Program of National Institute on Alcohol Abuse and Alcoholism and by a grant to Youngshim Choi from the KRIBB Research Initiative Program (Korean Biomedical Scientist Fellowship Program), Korea Research institute of Bioscience and Biotechnology, Republic of Korea. We are thankful to Dr. Frank J. Gonzalez (National Cancer Institute, NIH, Bethesda, MD, USA) and Dr. James P. Hardwick (Northeastern Ohio University College of Medicine, Rootstown, OH) for providing the WT and Cyp2e1-null mice and the polyclonal antibodies to different P450 isoforms, respectively. We also thank Dr. Klaus Gawrisch for supporting this study.
Abbreviations
- ALT
alanine aminotransferase
- CAT
catalase
- CYP
cytochrome P450
- ETC
electron transfer chain
- Gpx
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulfide (oxidized)
- HO-1
heme oxygenase-1
- 4-HNE
4-hydroxynonenal
- HOMA-IR
homeostatic model assessment of insulin resistance
- HSP90
heat shock protein 90
- iNOS
inducible nitric oxide synthase
- MDA
malondialdehyde
- NO
nitric oxide
- 3-NT
3-nitrtotyrosine
- 8-OH-dG
8-dihydroxy-2′-deoxyguanosine
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SOD
superoxide dismutase
- TG
triglyceride
- TNF-α
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild-type
Footnotes
Conflict of interest
The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Medvedev ZA. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65:375–398. doi: 10.1111/j.1469-185x.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 3.Sergiev PV, Dontsova OA, Berezkin GV. Theories of aging: an ever-evolving field. Acta Naturae. 2015;7:9–18. [PMC free article] [PubMed] [Google Scholar]
- 4.Cefalu CA. Theories and mechanisms of aging. Clin Geriatr Med. 2011;27:491–506. doi: 10.1016/j.cger.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Gladyshev VN. The free radical theory of aging is dead. Long live the damage theory! Antioxid Redox Signal. 2014;20:727–731. doi: 10.1089/ars.2013.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free radical biology & medicine. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie B, Gan W, Shi F, Hu GX, Chen LG, Hayakawa H, Sekiguchi M, Cai JP. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid Med Cell Longev. 2013;2013:303181. doi: 10.1155/2013/303181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmucker DL. Aging and the liver: an update. J Gerontol A Biol Sci Med Sci. 1998;53:B315–320. doi: 10.1093/gerona/53a.5.b315. [DOI] [PubMed] [Google Scholar]
- 11.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31:184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mak KM, Kwong AJ, Chu E, Hoo NM. Hepatic steatosis, fibrosis, and cancer in elderly cadavers. Anat Rec (Hoboken) 2012;295:40–50. doi: 10.1002/ar.21525. [DOI] [PubMed] [Google Scholar]
- 13.Colantoni A, Idilman R, de Maria N, Duffner LA, Van Thiel DH, Witte PL, Kovacs EJ. Evidence of oxidative injury during aging of the liver in a mouse model. J Am Aging Assoc. 2001;24:51–57. doi: 10.1007/s11357-001-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebel M, de Souza-Pinto NC, Bohr VA. Metabolism, genomics, and DNA repair in the mouse aging liver. Curr Gerontol Geriatr Res. 2011;2011:859415. doi: 10.1155/2011/859415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20:138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 17.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 18.Novak RF, Woodcroft KJ. The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch Pharm Res. 2000;23:267–282. doi: 10.1007/BF02975435. [DOI] [PubMed] [Google Scholar]
- 19.Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cederbaum AI. Iron and CYP2E1-dependent oxidative stress and toxicity. Alcohol. 2003;30:115–120. doi: 10.1016/s0741-8329(03)00104-6. [DOI] [PubMed] [Google Scholar]
- 22.Hunt CM, Strater S, Stave GM. Effect of normal aging on the activity of human hepatic cytochrome P450IIE1. Biochem Pharmacol. 1990;40:1666–1669. doi: 10.1016/0006-2952(90)90470-6. [DOI] [PubMed] [Google Scholar]
- 23.Wauthier V, Verbeeck RK, Buc Calderon P. Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activities in Wistar rats. What role for oxidative stress? Arch Toxicol. 2004;78:131–138. doi: 10.1007/s00204-003-0526-z. [DOI] [PubMed] [Google Scholar]
- 24.Yun KU, Oh SJ, Oh JM, Kang KW, Myung CS, Song GY, Kim BH, Kim SK. Age-related changes in hepatic expression and activity of cytochrome P450 in male rats. Arch Toxicol. 2010;84:939–946. doi: 10.1007/s00204-010-0520-1. [DOI] [PubMed] [Google Scholar]
- 25.Kwak HC, Kim HC, Oh SJ, Kim SK. Effects of age increase on hepatic expression and activity of cytochrome P450 in male C57BL/6 mice. Arch Pharm Res. 2015;38:857–864. doi: 10.1007/s12272-014-0452-z. [DOI] [PubMed] [Google Scholar]
- 26.Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging cell. 2013;12:950–954. doi: 10.1111/acel.12128. [DOI] [PubMed] [Google Scholar]
- 27.Gregg SQ, Gutierrez V, Robinson AR, Woodell T, Nakao A, Ross MA, Michalopoulos GK, Rigatti L, Rothermel CE, Kamileri I, Garinis GA, Stolz DB, Niedernhofer LJ. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55:609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. The Journal of biological chemistry. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 29.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelmegeed MA, Moon KH, Hardwick JP, Gonzalez FJ, Song BJ. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free radical biology & medicine. 2009;47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song BJ, Abdelmegeed MA, Henderson LE, Yoo SH, Wan J, Purohit V, Hardwick JP, Moon KH. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Hocker J, Aust D, Rohrbach H, Napiwotzky J, Reith A, Link TA, Seibel P, Holzel D, Kadenbach B. Defects of the respiratory chain in the normal human liver and in cirrhosis during aging. Hepatology. 1997;26:709–719. doi: 10.1002/hep.510260324. [DOI] [PubMed] [Google Scholar]
- 36.Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annual review of biochemistry. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 37.Ortuno-Sahagun D, Pallas M, Rojas-Mayorquin AE. Oxidative stress in aging: advances in proteomic approaches. Oxid Med Cell Longev. 2014;2014:573208. doi: 10.1155/2014/573208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Li W, Chen R, McIntyre TM. Circulating biologically active oxidized phospholipids show on-going and increased oxidative stress in older male mice. Redox Biol. 2013;1:110–114. doi: 10.1016/j.redox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, Basso M, Melero Fernandez de Mera RM, Sparrow NA, Calingasan NY, Kiaei M, Rhoads TW, Ma TC, Grumet M, Barnes S, Beal MF, Beckman JS, Mehl R, Estevez AG. Nitration of Hsp90 induces cell death. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1102–1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki T, Tahara S, Shinkai T, Kuramoto K, Matsumoto S, Yanabe M, Takagi S, Kondo H, Kaneko T. Lifespan extension in the spontaneous dwarf rat and enhanced resistance to hyperoxia-induced mortality. Exp Gerontol. 2013;48:457–463. doi: 10.1016/j.exger.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing research reviews. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breusing N, Arndt J, Voss P, Bresgen N, Wiswedel I, Gardemann A, Siems W, Grune T. Inverse correlation of protein oxidation and proteasome activity in liver and lung. Mechanisms of ageing and development. 2009;130:748–753. doi: 10.1016/j.mad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Gohlke S, Mishto M, Textoris-Taube K, Keller C, Giannini C, Vasuri F, Capizzi E, D’Errico-Grigioni A, Kloetzel PM, Dahlmann B. Molecular alterations in proteasomes of rat liver during aging result in altered proteolytic activities. Age. 2014;36:57–72. doi: 10.1007/s11357-013-9543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JH, Redmond HP, Watson RW, Bouchier-Hayes D. Role of lipopolysaccharide and tumor necrosis factor-alpha in induction of hepatocyte necrosis. The American journal of physiology. 1995;269:G297–304. doi: 10.1152/ajpgi.1995.269.2.G297. [DOI] [PubMed] [Google Scholar]
- 46.Park JH, Ha H. Short-term Treatment of Daumone Improves Hepatic Inflammation in Aged Mice. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2015;19:269–274. doi: 10.4196/kjpp.2015.19.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konstandi M, Cheng J, Gonzalez FJ. Sex steroid hormones regulate constitutive expression of Cyp2e1 in female mouse liver. Am J Physiol Endocrinol Metab. 2013;304:E1118–1128. doi: 10.1152/ajpendo.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology; 2008:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 49.Suh SK, Hood BL, Kim BJ, Conrads TP, Veenstra TD, Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 50.Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR, Rusyn I. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 51.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 52.Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012;47:979–987. doi: 10.1016/j.exger.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Safwat MH, El-Sawalhi MM, Mausouf MN, Shaheen AA. Ozone ameliorates age-related oxidative stress changes in rat liver and kidney: effects of pre- and post-ageing administration. Biochemistry (Mosc) 2014;79:450–458. doi: 10.1134/S0006297914050095. [DOI] [PubMed] [Google Scholar]
- 54.Sobocanec S, Balog T, Kusic B, Sverko V, Saric A, Marotti T. Differential response to lipid peroxidation in male and female mice with age: correlation of antioxidant enzymes matters. Biogerontology. 2008;9:335–343. doi: 10.1007/s10522-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharya A, Leonard S, Tardif S, Buffenstein R, Fischer KE, Richardson A, Austad SN, Chaudhuri AR. Attenuation of liver insoluble protein carbonyls: indicator of a longevity determinant? Aging cell. 2011;10:720–723. doi: 10.1111/j.1474-9726.2011.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadowska-Bartosz I, Ott C, Grune T, Bartosz G. Posttranslational protein modifications by reactive nitrogen and chlorine species and strategies for their prevention and elimination. Free Radic Res. 2014;48:1267–1284. doi: 10.3109/10715762.2014.953494. [DOI] [PubMed] [Google Scholar]
- 57.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 58.Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, Song BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free radical biology & medicine. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free radical biology & medicine. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song BJ, Akbar M, Abdelmegeed MA, Byun K, Lee B, Yoon SK, Hardwick JP. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014;3:109–123. doi: 10.1016/j.redox.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mechanisms of ageing and development. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- 66.Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 67.Anantharaju A, Feller A, Chedid A. Aging Liver. A review. Gerontology. 2002;48:343–353. doi: 10.1159/000065506. [DOI] [PubMed] [Google Scholar]
- 68.Romano AD, Greco E, Vendemiale G, Serviddio G. Bioenergetics and mitochondrial dysfunction in aging: recent insights for a therapeutical approach. Curr Pharm Des. 2014;20:2978–2992. doi: 10.2174/13816128113196660700. [DOI] [PubMed] [Google Scholar]
- 69.Meister A, Anderson ME. Glutathione. Annual review of biochemistry. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 70.Gong P, Cederbaum AI, Nieto N. Heme oxygenase-1 protects HepG2 cells against cytochrome P450 2E1-dependent toxicity. Free radical biology & medicine. 2004;36:307–318. doi: 10.1016/j.freeradbiomed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Immenschuh S, Fahimi HD, Baumgart-Vogt E. Complementary regulation of heme oxygenase-1 and peroxiredoxin I gene expression by oxidative stress in the liver. Cellular and molecular biology. 2005;51:471–477. [PubMed] [Google Scholar]
- 72.Stojkovski V, Hadzi-Petrushev N, Ilieski V, Sopi R, Gjorgoski I, Mitrov D, Jankulovski N, Mladenov M. Age and heat exposure-dependent changes in antioxidant enzymes activities in rat’s liver and brain mitochondria: role of alpha-tocopherol. Physiol Res. 2013;62:503–510. doi: 10.33549/physiolres.932514. [DOI] [PubMed] [Google Scholar]
- 73.Jiang P, Sheng Y, Ji L. The age-related change of glutathione antioxidant system in mice liver. Toxicol Mech Methods. 2013;23:396–401. doi: 10.3109/15376516.2013.769655. [DOI] [PubMed] [Google Scholar]
- 74.Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. The international journal of biochemistry & cell biology. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 75.Goto S, Nakamura A. Age-associated, oxidatively modified proteins: A critical evaluation. Age (Omaha) 1997;20:81–89. doi: 10.1007/s11357-997-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussong SA, Kapphahn RJ, Phillips SL, Maldonado M, Ferrington DA. Immunoproteasome deficiency alters retinal proteasome’s response to stress. Journal of neurochemistry. 2010;113:1481–1490. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jang SH, Yu LR, Abdelmegeed MA, Gao Y, Banerjee A, Song BJ. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015;6:552–564. doi: 10.1016/j.redox.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.