Abstract
Mediolateral balance control during walking is a challenging task in post-stroke hemiparetic individuals. To detect and treat dynamic balance disorders, it is important to assess balance using reliable methods. The Berg Balance Scale (BBS), Dynamic Gait Index (DGI), margin-of-stability (MoS), and peak-to-peak range of angular-momentum (H) are some of the most commonly used measures to assess dynamic balance and fall risk in clinical and laboratory settings. However, it is not clear if these measures lead to similar conclusions. Thus, the purpose of this study was to assess dynamic balance in post-stroke hemiparetic individuals using BBS, DGI, MoS and the range of H and determine if these measure are correlated. BBS and DGI were collected from 19 individuals post-stroke. Additionally, kinematic and kinetic data were collected while the same individuals walked at their self-selected speed. MoS and the range of H were calculated in the mediolateral direction for each participant. Correlation analyses revealed moderate associations between all measures. Overall, a higher range of angular-momentum was associated with a higher MoS, wider step width and lower BBS and DGI scores, indicating poor balance control. Further, only the MoS from the paretic foot placement, but not the nonparetic foot, correlated with the other balance measures. Although moderate correlations existed between all the balance measures, these findings do not necessarily advocate the use of a single measure as each test may assess different constructs of dynamic balance. These findings have important implications for the use and interpretation of dynamic balance assessments.
Keywords: Berg balance scale, dynamic gait index, margin of stability, angular momentum, biomechanics
1. Introduction
Balance control is a challenging task in many patient populations including individuals with post-stroke hemiparesis. More than 50% of stroke survivors experience falls within one year post stroke (e.g., Ashburn et al., 2008). Lack of balance control can lead to physical injuries and long-term disabilities (e.g., Weerdesteyn et al., 2008). In addition, a recent study has shown that discordance was present between measured and perceived balance in over one third of the post-stroke individuals and that falls were more closely associated with measured balance than perceived balance (Liphart et al., 2015). Thus, it is important to assess dynamic balance using reliable methods in order to detect and treat balance disorders.
Various methods have been used to evaluate balance performance. These methods range from simple clinical scores such as Berg Balance Scale (BBS) (Berg et al., 1992) and Dynamic Gait Index (DGI) (Shumway-Cook and Woollacott, 1995) to more comprehensive laboratory-based measures such as margin-of-stability (MoS) (Hof et al., 2007) and whole-body angular momentum (H) (e.g., Silverman and Neptune, 2011). Further, clinical balance scores are based on discrete score assignments while completing a series of movement tasks, whereas the laboratory-based measures are continuous and obtained using kinematic and kinetic data during walking, often on a treadmill.
A survey study among 655 physical therapists has shown that BBS was the most commonly used measure to assess balance in stroke rehabilitation (Korner-Bitensky et al., 2006). A review study suggested that BBS is an effective and sound method for balance assessment in post-stroke individuals although a few studies observed floor and ceiling effects (Blum and Korner-Bitensky, 2008). Note that BBS is not a measure of dynamic balance, but is used through the use of a cut-off score (< 42) that relates to a higher risk of falls (e.g., Tilson et al., 2012). Another clinical measure that is widely used for assessing dynamic balance during gait activities is DGI, which has shown high reliability and validity in ambulatory post-stroke individuals (Jonsdottir and Cattaneo, 2007). Similar to the BBS, the DGI utilizes a cut-off score of 19 to indicate increased risk of falls (Shumway-Cook et al., 1997; Wrisley and Kumar, 2010). However, others assessing dynamic balance in 117 patients with balance and vestibular disorders also reported ceiling effects, suggesting the need for assessments capable of measuring a broader range of gait abilities (Dye et al., 2013).
Margin-of-stability, a commonly used laboratory-based measure for assessing balance, is the minimum distance between the base of support and the extrapolated center-of-mass (CoM) (Hof, 2008). This measure is based on foot placement while accounting for body CoM position and velocity and has been used to assess dynamic balance in young healthy individuals in destabilizing environments (e.g., McAndrew Young et al., 2012), older adults while stepping to targets (Hurt and Grabiner, 2015), amputees (e.g., Bolger et al., 2014; Gates et al., 2013; Hof et al., 2007) and post-stroke individuals (e.g., Hak et al., 2013; Kao et al., 2014). Similarly, whole-body angular-momentum has been used to assess dynamic balance in a number of patient populations including post-stroke hemiparetic individuals (Nott et al., 2014), amputees (e.g., Pickle et al., 2014; Sheehan et al., 2015; Silverman and Neptune, 2011) and older adults (e.g., Pijnappels et al., 2005b). The regulation of whole-body angular-momentum is essential for maintaining dynamic balance during walking (e.g., Herr and Popovic, 2008) and can be achieved through proper foot placement and generation of the appropriate ground-reaction-forces (GRFs) (e.g., Pijnappels et al., 2005a).
Prior studies have suggested that balance control in the mediolateral direction is more challenging than in the anterior-posterior direction and that active control is needed to regulate mediolateral balance (Bauby and Kuo, 2000). A recent study investigated the relationship between clinical balance scores and the time rate of change of frontal-plane H in post-stroke individuals and found that a higher rate of change of H during the paretic leg stance was associated with poorer BBS and DGI scores (Nott et al., 2014). However, no study to our knowledge has investigated whether assessment methods that include MoS provide consistent findings, implying a similar construct between measures, or whether different measures provide somewhat different information regarding dynamic balance in post-stroke individuals. Thus, the purpose of this study was to assess mediolateral balance using BBS, DGI, MoS and peak-to-peak range of H in post-stroke hemiparetic individuals and determine the strength and direction of the relationships between these variables.
2. Methods
Nineteen post-stroke hemiparetic individuals (14 left hemiparesis; age: 62 ± 11 years; 6 female) walked on a split-belt instrumented treadmill (Techmachine, Andrezieux Boutheon, France) at their self-selected walking speed (Table 1). Subject inclusion criteria were previously described in detail (Bowden et al., 2013). In summary, individuals experienced stroke within the past 6 months to 5 years of the data collection, had lower extremity hemiparesis and were able to walk at least 10 meters with the assistance of maximum one person. The Fugl-Meyer Assessment scores of lower extremity motor recovery were less than 34 (Table 1). The study protocol and consent form were approved by an Institutional Review Board and all participants provided informed, written consent prior to study participation.
Table 1.
Participant characteristics: time since stroke, affected side, overground self-selected (SS) walking speed and lower extremity Fugl-Meyer (FMA) assessment score. The last row lists the mean values (±SD) across the participants. ‘L’ and ‘R’ indicate left and right, respectively.
| Subject # | Months Since Stroke | Affected Side | SS Walking Speed (m/s) | Lower Extremity FMA |
|---|---|---|---|---|
| 1 | 26 | L | 0.43 | 21 |
| 2 | 63 | R | 0.57 | 25 |
| 3 | 11 | L | 0.71 | 25 |
| 4 | 8 | R | 1.05 | 33 |
| 5 | 12 | L | 1.08 | 29 |
| 6 | 9 | L | 0.93 | 23 |
| 7 | 26 | L | 0.64 | 24 |
| 8 | 35 | L | 0.93 | 26 |
| 9 | 46 | R | 0.59 | 31 |
| 10 | 12 | L | 0.97 | 22 |
| 11 | 14 | R | 1.00 | 19 |
| 12 | 18 | L | 0.75 | 27 |
| 13 | 10 | L | 0.47 | 26 |
| 14 | 27 | L | 0.82 | 14 |
| 15 | 17 | L | 0.33 | 22 |
| 16 | 21 | R | 0.96 | 18 |
| 17 | 56 | L | 0.99 | 30 |
| 18 | 28 | L | 0.20 | 20 |
| 19 | 17 | L | 0.59 | 27 |
|
| ||||
| Mean(±SD) | 24(±16) | - | 0.74(±0.27) | 24.3(±4.8) |
BBS and DGI data were collected for each participant according to standard procedures (Berg et al., 1992; Shumway-Cook and Woollacott, 1995). Three-dimensional kinematics were collected at 100 Hz using a 12-camera motion capture system (VICON, Los Angeles, USA) and GRFs were recorded at 2000 Hz while participants walked at their self-selected walking speed during multiple 30-second trials (Bowden et al., 2013). The kinematic and GRF data were low pass filtered using a fourth-order Butterworth filter with cutoff frequencies of 6 Hz and 20 Hz, respectively. A 13-segment inverse dynamics model (C-Motion, Inc., Germantown, MD) was used to calculate body CoM position and velocity as well as angular-momentum for each segment. Center-of-pressure (CoP) was obtained using the force plates embedded in the treadmill. Margin-of-stability (MoS) in the mediolateral direction was calculated as the minimum distance between CoP and extrapolated center-of-mass (XcoM) (Hof et al., 2007). The XcoM was calculated as:
where Z and Ż are the body CoM position and velocity, respectively. l′ is the equivalent pendulum height (calculated as 1.34 × leg length (Hof et al., 2007)) and is the gravitational acceleration. MoS was calculated at each step for each foot placement and was normalized with respect to body height. In addition, to further understand MoS and its relationship to other measures, step width and step width variability (i.e., standard deviations normalized by body height) were calculated for each participant.
At each time step, whole-body angular-momentum (H) about the CoM was calculated as:
where and are the position and velocity vectors of the i-th segment’s CoM, respectively. and are the position and velocity vectors of the whole-body CoM. , mi and Ii are the angular velocity vector, and mass and moment of inertia of the i-th segment, respectively, and n is the number of segments. Angular-momentum was normalized by the product of subject mass, height and , where g is the gravitational acceleration and l is the subject height. The term has units of m/s and is independent of walking speed. The peak-to-peak range of angular-momentum in the mediolateral direction was calculated as the difference between the minimum and maximum values of frontal-plane angular-momentum over the entire gait cycle.
To determine whether MoS, range of H, BBS and DGI provide consistent assessments of balance performance, correlation analyses were performed between the various measures. Pearson’s correlation analyses were performed between MoS and the range of H using both the mean values across all participants and the individual step data as suggested by McAndrew Young et al. (2012). Further, non-parametric Spearman’s correlation analyses were performed between the clinical balance scores (BBS and DGI) and the laboratory-based measures (MoS and range of H) and between BBS and DGI.
3. Results
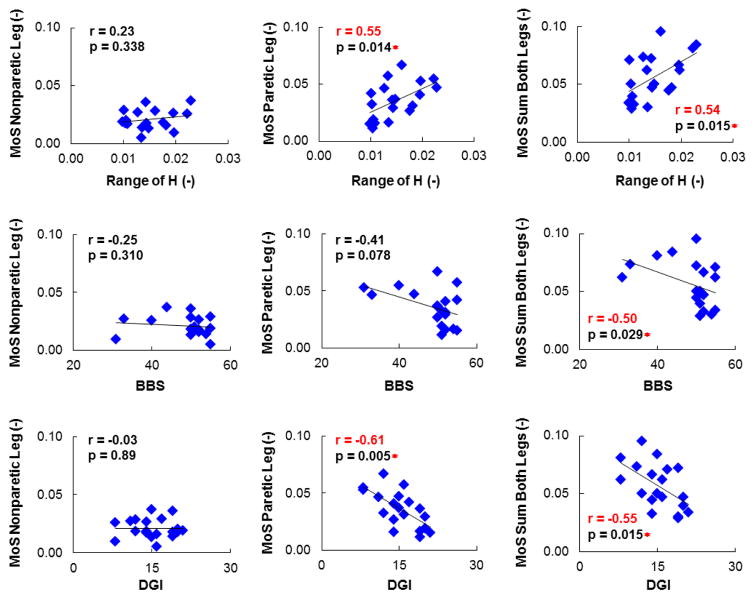
Significant (p < 0.05) moderate correlations were found among all balance measures (Table 2). BBS was positively correlated with DGI, while MoS (sum from both legs) was positively correlated with the range of H. The correlation between MoS and the range of H was significant for both the mean values across all the participants (Table 2) and the individual step data (r = 0.52, p < 0.001). However, both laboratory-based measures (MoS and the range of H) were inversely correlated with the clinical scores (BBS and DGI) (Table 2). In other words, individuals with a higher range of H and MoS (sum from both legs) had lower BBS and DGI scores (indicating poorer balance and gait performance). Further, examining MoS for each foot revealed that only the MoS corresponding to the paretic foot placement was correlated with the other balance measures (Fig. 1).
Table 2.
Correlations between various balance measures in individuals with post-stroke hemiparesis. Significant correlations (p < 0.05) are shown with ‘*’. The acronyms are as follows: (H): range of whole-body angular momentum, (MoS): margin-of-stability sum from both legs, (BBS): Berg Balance Score, (DGI): Dynamic Gait Index.
| H & MoS | H & BBS | H & DGI | MoS & BBS | MoS & DGI | BBS & DGI | |
|---|---|---|---|---|---|---|
| r | 0.54 | −0.55 | −0.48 | −0.50 | −0.55 | 0.64 |
| p | 0.015* | 0.014* | 0.036* | 0.029* | 0.015* | 0.003* |
Figure 1.
Top row shows the Pearson correlations between normalized mean margin-of-stability (MoS) and range of whole-body angular momentum (H). Middle and bottom rows show the Spearman correlations between mean MoS & Berg Balance Score (BBS) and mean MoS & Dynamic Gait Index (DGI), respectively. The left, middle and right columns correspond to the mean MoS for the nonparetic leg, the paretic leg, and the sum of both legs, respectively. Significant correlations (p < 0.05) are shown in red with ‘*’.
4. Discussion
The purpose of this study was to investigate whether assessing mediolateral balance in post-stroke hemiparetic individuals using BBS, DGI, MoS and range of H provides consistent results. All balance measures were moderately correlated with each other (Table 2) for participants in this study (Table 1). BBS was positively correlated with DGI. That is, individuals with a lower BBS also had a lower DGI, indicating poorer overall mobility and balance control. This finding was in agreement with previous studies where positive correlations were reported between BBS and DGI in post-stroke individuals (e.g., Jonsdottir and Cattaneo, 2007; Nott et al., 2014), older adults (Park et al., 2013) and individuals with vestibular dysfunction (Whitney et al., 2003). Also, MoS (sum from both legs) and the range of H were positively correlated. This was true both for the mean values across all the participants as well as the individual step data. The positive correlation between MoS and the range of H in the frontal plane can be related to the mediolateral foot placement, which is the common factor between the two measures. Mediolateral MoS is simply the ML distance between the body CoM and CoP while accounting for body CoM velocity. Further, whole-body H in the frontal plane is regulated by the external moment associated with the ML and vertical GRFs and distances between the body CoM and CoP. Post-hoc analysis showed that both MoS (r = 0.76, p < 0.001) and the range of H (r = 0.46, p = 0.04) are positively correlated with mean step width, and only the range of H (r = 0.61, p = 0.005) is correlated with step width variability. However, both of the laboratory-based measures (MoS and the range of H) were inversely correlated with the clinical scores (BBS and DGI). That is, individuals with a higher range of H had a higher MoS, wider step width and lower BBS and DGI scores, indicating poor balance control. Note that out of the 19 participants in this study, only 3 participants had a DGI score greater than the cut off score of 19 indicating that the majority of the participants in this study would be identified as having a higher risk of falls.
These results are consistent with Nott et al. (2014) who showed the time rate of change of frontal-plane angular-momentum during paretic leg stance was inversely correlated with BBS (r = −0.54, p < 0.001) and DGI (r = −0.57, p < 0.001). However, the significant correlations between higher MoS and lower BBS and DGI are not as clear. Individuals with higher MoS had a wider step width, which was also associated with a poor cancellation of segmental angular-momenta about the CoM. Pijnappels et al. (2004) found an insufficient reduction of angular-momentum was associated with higher rate of falls in the older adults. Further, in a study by Sheehan et al. (2015) assessing the effects of lateral perturbations on balance control during amputee walking, they noted that their findings support the interpretation that greater angular momentum is associated with greater fall risk. Thus, given the positive correlation between the range of angular momentum and MoS, it is not surprising that higher MoS is associated with poor clinical balance scores. However, others (e.g., Hurt and Grabiner, 2015) have interpreted higher MoS in older adults as an indication of better dynamic stability compared to younger adults, and a similar interpretation of MoS was made in post-stroke individuals (e.g., Hak et al., 2015). Based on the results in the present study and the higher rate of falls in both older adults (Woollacott and Tang, 1997) and post-stroke individuals (Forster and Young, 1995), an alternative interpretation is that individuals with higher MoS may be at a higher risk of falling. It is not clear if the higher MoS is adopted as a compensatory mechanism for those with poor balance control to improve their balance performance, or if an increased level of MoS may paradoxically represent decreased stability during walking. In addition, examining MoS for each foot revealed that only the MoS corresponding to the paretic foot placement was correlated with the other balance measures (Fig. 1). This finding highlights the importance of the paretic mediolateral foot placement in dynamic balance control, suggesting that in post-stroke individuals it may be more suitable to examine the MoS for each foot individually rather than the average sum of both legs, which has been used in previous studies (e.g., Hak et al., 2015; Hak et al., 2013). Together, these findings suggest that the use and interpretation of MoS to assess dynamic balance performance should be carefully considered.
The moderate correlations between the various balance measures do not necessarily advocate the use of a single measure. Dynamic balance is multidimensional and previous studies have suggested the lack of perfect correlations may indicate that each test measures different aspects of balance (Jonsdottir and Cattaneo, 2007; Whitney et al., 2003). The advantage of clinical balance scores is that they provide a simple global assessment which does not require the collection and processing of more complex body-segment kinematic and kinetic data. However, contrary to the laboratory-based measures, the clinical balance scores cannot provide insight into the mechanisms for maintaining dynamic balance or the mechanical contributors to the loss of balance. The clinical balance scores are analogous to walking speed, which is a global indicator of gait dysfunction, but it does not identify potential contributing factors. In addition, the assessment floor and ceiling effects previously described may be another important limitation of clinical balance scores (e.g., Blum and Korner-Bitensky, 2008; Dye et al., 2013). Similarly, MoS can provide some insight into foot placement, but not the GRFs, which are critical to maintaining dynamic balance. Further, it does not account for the segmental moment of inertia and rotational motions that influence dynamic balance. Although a more complex measure, whole-body angular momentum can provide insight into how the sum of linear and angular momenta of all the body segments (about the body CoM) can be controlled by adjustments in foot placement and generation of appropriate GRFs (e.g., Silverman and Neptune, 2011; Silverman et al., 2012). Further, since angular-momentum is primarily regulated by muscle forces (Neptune and McGowan, 2011), assessing corresponding changes in the GRFs as reflected in angular momentum measures can provide an objective method for monitoring changes in balance control and assessing the effectiveness of balance training programs.
5. Conclusions
In summary, we found in individuals with post-stroke hemiparesis that a higher range of angular-momentum was associated with a higher MoS, wider step width and lower BBS and DGI scores, indicating poor balance control. These findings are contrary to previous studies that interpret a higher MoS with increased balance control. We found a higher MoS was related to wider steps, which increases the time rate of change of frontal-plane angular-momentum and was associated with poorer clinical balance scores. Thus, although higher MoS scores may be associated with improved static balance, higher MoS scores in dynamic tasks such as walking may be indicative of decreased balance control and a higher risk of falling. It is unknown whether decreasing the MoS by taking narrower steps would lead to increased or decreased balance control in these individuals and is a topic of great interest. Since dynamic balance is multidimensional, each measure may provide insight into different aspects of balance. While clinical balance scores can provide a simple global assessment of balance performance during various tasks (many of which are not related to walking), the laboratory-based measures can provide more insight into the underlying biomechanical mechanisms for maintaining dynamic balance. Specifically, MoS can provide insight into foot placement mechanisms while the analysis of angular-momentum provides information about both foot placement and the generation of appropriate GRFs for maintaining dynamic balance. Thus, the findings from this study can provide insight for selecting a suitable assessment measure based on the depth and extensiveness of the assessment as well as interpreting the assessment results with respect to the other balance measures. Further research is needed to provide clinicians with quantifiable tools (e.g., using pressure sensitive mats and accelerometry) to assist with clinical decision making.
Acknowledgments
The authors would like to acknowledge Ms. Leah Carter for her valuable contributions to this study. This work was supported by an American Heart Association SouthWest Affiliate pre-doctoral fellowship (12PRE12030414), the Rehabilitation Research and Development Service of the Department of Veteran’s Affairs (N0787-W) and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM109040.
Footnotes
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the AHA, the VA or the NIH.
Conflict of interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age Ageing. 2008;37:270–276. doi: 10.1093/ageing/afn066. [DOI] [PubMed] [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(Suppl 2):S7–11. [PubMed] [Google Scholar]
- Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–566. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- Bolger D, Ting LH, Sawers A. Individuals with transtibial limb loss use interlimb force asymmetries to maintain multi-directional reactive balance control. Clin Biomech (Bristol, Avon) 2014;29:1039–1047. doi: 10.1016/j.clinbiomech.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA. Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders. Arch Phys Med Rehabil. 2013;94:856–862. doi: 10.1016/j.apmr.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Dye DC, Eakman AM, Bolton KM. Assessing the validity of the dynamic gait index in a balance disorders clinic: an application of Rasch analysis. Phys Ther. 2013;93:809–818. doi: 10.2522/ptj.20120163. [DOI] [PubMed] [Google Scholar]
- Forster A, Young J. Incidence and Consequences of Falls Due to Stroke - a Systematic Inquiry. Brit Med J. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates DH, Scott SJ, Wilken JM, Dingwell JB. Frontal plane dynamic margins of stability in individuals with and without transtibial amputation walking on a loose rock surface. Gait Posture. 2013;38:570–575. doi: 10.1016/j.gaitpost.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak L, Houdijk H, van der Wurff P, Prins MR, Beek PJ, van Dieen JH. Stride frequency and length adjustment in post-stroke individuals: influence on the margins of stability. J Rehabil Med. 2015;47:126–132. doi: 10.2340/16501977-1903. [DOI] [PubMed] [Google Scholar]
- Hak L, Houdijk H, van der Wurff P, Prins MR, Mert A, Beek PJ, van Dieen JH. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin Biomech (Bristol, Avon) 2013;28:1041–1048. doi: 10.1016/j.clinbiomech.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Herr H, Popovic M. Angular momentum in human walking. J Exp Biol. 2008;211:467–481. doi: 10.1242/jeb.008573. [DOI] [PubMed] [Google Scholar]
- Hof AL. The ‘extrapolated center of mass’ concept suggests a simple control of balance in walking. Hum Mov Sci. 2008;27:112–125. doi: 10.1016/j.humov.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25:250–258. doi: 10.1016/j.gaitpost.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Hurt CP, Grabiner MD. Age-related differences in the maintenance of frontal plane dynamic stability while stepping to targets. J Biomech. 2015;48:592–597. doi: 10.1016/j.jbiomech.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil. 2007;88:1410–1415. doi: 10.1016/j.apmr.2007.08.109. [DOI] [PubMed] [Google Scholar]
- Kao PC, Dingwell JB, Higginson JS, Binder-Macleod S. Dynamic instability during post-stroke hemiparetic walking. Gait Posture. 2014;40:457–463. doi: 10.1016/j.gaitpost.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner-Bitensky N, Wood-Dauphinee S, Teasell R. Best versus actual practices in stroke rehabilitation: results of the Canadian National Survey [abstract] Stroke. 2006;37:631. [Google Scholar]
- Liphart J, Gallichio J, Tilson JK, Pei Q, Wu SS, Duncan PW. Concordance and discordance between measured and perceived balance and the effect on gait speed and falls following stroke. Clin Rehabil. 2015 doi: 10.1177/0269215515578294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew Young PM, Wilken JM, Dingwell JB. Dynamic margins of stability during human walking in destabilizing environments. J Biomech. 2012;45:1053–1059. doi: 10.1016/j.jbiomech.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, McGowan CP. Muscle contributions to whole-body sagittal plane angular momentum during walking. J Biomech. 2011;44:6–12. doi: 10.1016/j.jbiomech.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott CR, Neptune RR, Kautz SA. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture. 2014;39:129–134. doi: 10.1016/j.gaitpost.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Ko YM, Park JW. The Correlation between Dynamic Balance Measures and Stance Sub-phase COP Displacement Time in Older Adults during Obstacle Crossing. Journal of Physical Therapy Science. 2013;25:1193–1196. doi: 10.1589/jpts.25.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickle NT, Wilken JM, Aldridge JM, Neptune RR, Silverman AK. Whole-body angular momentum during stair walking using passive and powered lower-limb prostheses. J Biomech. 2014;47:3380–3389. doi: 10.1016/j.jbiomech.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieen JH. Contribution of the support limb in control of angular momentum after tripping. J Biomech. 2004;37:1811–1818. doi: 10.1016/j.jbiomech.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieen JH. How early reactions in the support limb contribute to balance recovery after tripping. J Biomech. 2005a;38:627–634. doi: 10.1016/j.jbiomech.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieen JH. Push-off reactions in recovery after tripping discriminate young subjects, older non-fallers and older fallers. Gait Posture. 2005b;21:388–394. doi: 10.1016/j.gaitpost.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sheehan RC, Beltran EJ, Dingwell JB, Wilken JM. Mediolateral Angular Momentum Changes in Persons With Amputation During Perturbed Walking. Gait Posture. 2015;41:795–800. doi: 10.1016/j.gaitpost.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M. Motor control: theory and practical applications. Williams & Wilkins; Baltimore: 1995. pp. 323–324. [Google Scholar]
- Silverman AK, Neptune RR. Differences in whole-body angular momentum between below-knee amputees and non-amputees across walking speeds. J Biomech. 2011;44:379–385. doi: 10.1016/j.jbiomech.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Silverman AK, Wilken JM, Sinitski EH, Neptune RR. Whole-body angular momentum in incline and decline walking. J Biomech. 2012;45:965–971. doi: 10.1016/j.jbiomech.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Tilson JK, Wu SS, Cen SY, Feng Q, Rose DR, Behrman AL, Azen SP, Duncan PW. Characterizing and identifying risk for falls in the LEAPS study: a randomized clinical trial of interventions to improve walking poststroke. Stroke. 2012;43:446–452. doi: 10.1161/STROKEAHA.111.636258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195–1213. [PubMed] [Google Scholar]
- Whitney S, Wrisley D, Furman J. Concurrent validity of the Berg Balance Scale and the Dynamic Gait Index in people with vestibular dysfunction. Physiother Res Int. 2003;8:178–186. doi: 10.1002/pri.288. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Tang PF. Balance control during walking in the older adult: research and its implications. Phys Ther. 1997;77:646–660. doi: 10.1093/ptj/77.6.646. [DOI] [PubMed] [Google Scholar]
- Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90:761–773. doi: 10.2522/ptj.20090069. [DOI] [PubMed] [Google Scholar]