Abstract
Exposures to particulate matter with diameter of 2.5 µm or less (PM2.5) may influence risk of birth defects. We estimated associations between maternal exposure to prenatal traffic-related air pollution and risk of cardiac, orofacial, and neural tube defects among Massachusetts births conceived 2001 through 2008. Our analyses included 2,729 cardiac, 255 neural tube, and 729 orofacial defects. We used satellite remote sensing, meteorological and land use data to assess PM2.5 and traffic-related exposures (distance to roads and traffic density) at geocoded birth addresses. We calculated adjusted odds ratios (OR) and confidence intervals (CI) using logistic regression models. Generalized additive models were used to assess spatial patterns of birth defect risk. There were positive but non-significant associations for a 10µg/m3 increase in PM2.5 and perimembranous ventricular septal defects (OR = 1.34, 95% CI: 0.98, 1.83), patent foramen ovale (OR = 1.19, 95% CI: 0.92, 1.54) and patent ductus arteriosus (OR = 1.20, 95% CI: 0.95, 1.62). There was a non-significant inverse association between PM2.5 and cleft lip with or without palate (OR = 0.76, 95% CI: 0.50, 1.10), cleft palate only (OR= 0.89, 95% CI: 0.54, 1.46) and neural tube defects (OR= 0.77, 95% CI: 0.46, 1.05). Results for traffic related exposure were similar. Only ostium secundum atrial septal defects displayed significant spatial variation after accounting for known risk factors.
Keywords: air pollution, birth defects, near-roadway pollution, satellite-based PM2.5, traffic-density
1. Introduction
Birth defects are prevalent in 3% of US live births (1), with cardiac, orofacial, and neural tube defects among the most common defects observed (2). Exposure to air pollution during pregnancy has been suggested to increase risk of birth defects (3–8) in some studies. The time between conception and birth is a sensitive and critical time for fetal development due to rapid cell proliferation and rapid development of various organ systems, thus understanding the influence of ambient exposures on fetal development may elucidate the mechanisms behind abnormal fetal development. Studies of fetal exposure to traffic-related air pollution including particulate matter with a diameter of 2.5 µm or less (PM2.5) have shown associations with adverse birth outcomes such as intrauterine growth retardation and preterm births (9–10), but investigations of the association of PM2.5 on birth defect risk have been inconclusive (11–18).
Exposure estimates for earlier studies were constrained to individuals living near air monitoring stations without daily assessments, limiting both spatial and temporal resolution of the exposure assessment resulting in exposure misclassification (19, 20). Earlier studies were unable to adjust for important confounders such as individual-level socioeconomic status (SES) and may have been limited by case ascertainment over a short study period (19, 20). Only one other study (17) to our knowledge has accounted for road density and residential distance to roadways, local measures of traffic-related air pollution, in addition to PM2.5 estimates when assessing risk of birth defects and exposure to ambient air pollution. Satellite-based PM2.5 prediction models can provide additional spatial and temporal information for exposure assessment. Models have evolved from using one single predictor (21) to multiple predictors (22–24) and from one-stage models (25) to multi-stage non-linear models (26–29).
The objective of this study is to examine the relationship between cardiac, orofacial, and neural tube defects and traffic-related air pollution using satellite-based PM2.5 exposure estimates and eight years of birth defects data for Massachusetts. To further assess the influence of PM2.5 on birth defect risk, our study includes an analysis of geographic patterns of birth defects across Massachusetts.
2. Methods
2.1 Study population
We obtained all live and still births from the Massachusetts state birth registry with an estimated conception date from January 1, 2001 through December 31, 2008. All births in the Massachusetts Birth Defects Registry having cardiac, orofacial, and neural tube defects (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 740.0–743.0, 745.0–748.0 and 749.0–749.3) were identified as cases. The Massachusetts Birth Defects Monitoring Program conducts active surveillance to collect diagnoses made before age one. We randomly selected 1,000 infants conceived each year to serve as a common control group among all live births without the defects of interest. We excluded birth addresses that could not be successfully geocoded to x and y coordinates (2%) and we excluded syndromic births (5%) that were associated with the outcomes of interest (eTable 1) among cases and controls. The Institutional Review Boards of the University of California at Irvine and the Massachusetts Department of Public Health approved this research.
Due to differing etiologies, we divided cardiac defects into anatomical groupings based on ICD-9-CM groups. We only included groups with more than 70 cases to ensure sufficient numbers for model convergence. We also assessed the five most common single ICD-9-CM code cardiac defect diagnoses as their own outcome group. In total, there were 17 outcome groups for cardiac anomalies: transposition of great vessels, tetralogy of fallot, ventricular septal defect, ostium secundum atrial septal defect, endocardial cushion defect, pulmonary valve atresia and stenosis, aortic valve stenosis, hypoplastic left heart syndrome, patent ductus arteriosus, coarctation of aorta, pulmonary artery anomalies, insufficiency of the aortic valve, atrial septal defect- not otherwise specified (a subset of ostium secundum atrial septal defect), perimembranious ventricular septal defect (a subset of ventricular septal defect), muscular ventricular septal defect (a subset of ventricular septal defect), single common atrium (a subset of endocardial cushion defects), and patent foramen ovale (a subset of ostium secundum atrial septal defect). Cases of patent ductus arteriosus and patent foramen ovale were excluded if the infant was preterm (<36 weeks, 4% of patent ductus arteriosus and patent foramen ovale cases). Infants with more than one birth defect were categorized into multiple defect groups unless diagnoses were similar (eTable 2). Because the majority of cases had multiple birth defect diagnosis (74%), to assess if there was a difference between infants with multiple defects and isolated defects, these two groups were analyzed separately.
For neural tube defects, spina bifida was the most common defect and was analyzed separately; all other neural tube defects were analyzed together due to small numbers. Anencephaly was excluded (13% of neural tube defects) as those included in the registry may not be representative of all anencephaly cases due to early termination. Orofacial defects were divided into two categories: cleft lip with or without palate and cleft palate only.
2.2 Exposure assessment
Our primary analysis examined the relationship between birth defects and PM2.5 exposures modeled using satellite remote sensing, meteorological and land use data. Aerosol optical depth (AOD) is the integral of particle light extinction coefficients from the surface to the top of the atmosphere. It is a measure of the degree to which aerosols prevent light from penetrating the atmosphere and retrieved using wavelengths most sensitive to particles with sizes from 0.1 to 2 µm (30). Thus, AOD is related to the loadings of fine particles in the atmosphere and is a strong predictor of ground-level PM2.5 concentrations as most fine particles are emitted and confined in the boundary layer. The number of stationary ambient monitors is limited and the distribution is sparse, while AOD-estimated PM2.5 concentrations have the potential to expand the spatiotemporal coverage of ground networks and improve the accuracy of estimates of personal exposure to PM2.5 (31). The Geostationary Operational Environmental Satellite (GOES) is the major weather satellite operated by the National Oceanic and Atmospheric Administration (NOAA). GOES provides an aerosol and smoke product (GASP) with AOD retrievals every 30 minutes from sunrise to sunset at 4 km nominal spatial resolution. We obtained GASP AOD data from the NOAA National Environmental Satellite, Data, and Information Service. In this study, AOD measurements (available from 9 am to 3 pm local time) were averaged to generate daily AOD estimates (24).
The 24-hour average PM2.5 concentrations from 2001 to 2008 collected from 35 U.S. Environmental Protection Agency (EPA) Federal Reference Monitors (FRM) were downloaded from the EPA’s Air Quality System Technology Transfer Network (http://www.epa.gov/ttn/airs/airsaqs/). Meteorological fields, including temperature and wind speed, were provided by the North American Land Data Assimilation System (NLDAS) Phase 2 and downloaded from the NLDAS website (http://ldas.gsfc.nasa.gov/nldas/). Elevation data were obtained from the National Elevation Dataset (NED) (http://ned.usgs.gov). Major roads were extracted from ESRI StreetMap USA (Environmental Systems Research Institute, Inc., Redland, CA). Forest cover data were derived from 2001 and 2006 land cover maps downloaded from the National Land Cover Database (NLCD) (http://www.mrlc.gov). Primary PM2.5 emissions were obtained from the 2002, 2005, and 2008 EPA National Emission Inventory (NEI) facility emissions reports. We developed a linear mixed effects model with 24-hour average EPA PM2.5 measurements from 2001 to 2008 as the dependent variable and AOD, meteorological fields and land use variables as predictors. The model incorporates day-specific random intercepts and slopes for AOD, temperature, and wind speed to account for the temporally varying relationship between PM2.5 (based on fixed ground monitors) and AOD. (32). This model was run annually for a 4 km modeling grid covering the spatial extent of Massachusetts to estimate daily PM2.5 concentrations from 2001 to 2008. The model structure can be expressed as
where PM2.5,st is the measured ground level PM2.5 concentration (µg/m3) at site s in day t; β0 and β0,t (day-specific) are the fixed and random intercept, respectively; AODst is the GASP AOD value (unitless) at site s in day t ; β1 and β1,t (day-specific) are the fixed and random slopes for AOD, respectively; Temperaturest is the air temperature (K) at site s in day t; β2 and β2,t (day-specific) are the fixed and random slopes for temperature, respectively; Wind Speedst is the 2 meters above ground wind speed (m/sec) at site s in day t; β3 and β3,t (day-specific) are the fixed and random slopes for wind speed, respectively; Elevations is elevation values (m) at site s; Major Roadss is road length (m) at site s; Forest Covers is percentage of forest cover (unitless) at site s; Point Emissionss is point emissions (tons per year) at site s; and Ψ is an unstructured variance-covariance matrix for the random effects. A ten-fold cross validation (CV) was conducted to evaluate the performance of the model, and statistical indicators including the coefficient of determination (R2) and square root of the mean squared prediction errors (RMSPE) were calculated between predicted PM2.5 concentrations and observations. The results show that CV RMSPE ranges from 2.42 to 3.50 µg/m3, and CV R2 ranges from 0.78 to 0.88 for years 2001 through 2008, indicating a good performance of the model.
The clinically estimated gestational age of infants was subtracted from date of birth to calculate conception date. The exposure assessment was performed for varying gestational weeks depending on the outcome of interest. For cardiac, neural tube, and orofacial defects, the windows of exposure are weeks 3–7, 1–4, and 6–12 of pregnancy, respectively, as these periods are considered to be the most critical exposure window for the development of the specified birth defects (33–35). Daily PM2.5 estimates were averaged for each exposure assessment interval. Infants were assigned a PM2.5 exposure measure if there was at least a single daily PM2.5 estimate for each week of the relevant gestational period. Satellite measures provide extensive spatial coverage throughout Massachusetts allowing us to assign exposure estimates to 95% of Massachusetts births included in our study. As a sensitivity analysis, we also assessed the effects of average daily PM2.5 over the first trimester of pregnancy.
To understand the influence of local traffic-related pollution on a near-roadway spatial scale, we considered the role of distance to major roadways and traffic density near birth residence (36–39). Using geographic information system software (ArcGIS, version 10.0; ESRI), we calculated the shortest distance (m) between each maternal address at birth and the nearest Class 1 (limited access highways) or 2 (multilane highways without limited access) road segment, and traffic density was calculated by summing the annual average daily traffic (AADT) for all Class 1 and 2 road segments within a 200 meter grid of Class 1 and 2 road segments (38), as air pollution from traffic reaches background levels around 200 meters (37). To estimate density for road segments with missing AADT counts, the AADT from the segments nearest to the missing segment of the same class with available data was used.
2.3 Statistical analysis
We used logistic regression models to calculate crude and adjusted odds ratios (ORs) and 95% confidence intervals (CI) for each birth defect outcome group and PM2.5 exposure. Exposure-response relations were also investigated using cubic splines. We considered adjustment for the following covariates obtained from Massachusetts birth records: plurality, maternal race/ethnicity, maternal education, maternal language preference, delivery payment source, smoking during pregnancy, alcohol consumption during pregnancy, adequacy of prenatal care (measured by the Adequacy of Prenatal Care Utilization Index), marital status, and maternal age. Infant covariates included birth year, parity, and season of conception. We used geocoded addresses to determine the median household income and median home value of census block groups. Analyses that included local traffic measures (traffic density and distance to roadways) were modeled continuously using cubic spline models. For each defect group, we used the change of estimate criterion (10%) to determine which covariates would be included in the model (40). We excluded observations with missing covariates in our analysis. Although the percent of missing information was less than 5% for each variable of interest, we applied multivariate imputation for variables with missing values using the predictive mean matching, logistic regression, and polytomous logistic regression imputation method for continuous, binary, and categorical variables, respectively, to evaluate the influence of missing data on our estimates. We also assessed effect modification of PM2.5 exposure by maternal education. All analyses were conducted using R (R Software Version 3.0.3). The R packages mgcv and mice were used for the cubic spline models and multiple imputations, respectively.
We examined spatial patterns of birth defects using generalized additive models (GAMs) for each outcome group (41, 42) to determine the residual influence of geographic location after accounting for PM2.5 as a confounder and potential mediator. The optimal amount of smoothing was determined by minimizing the Akaike Information Criterion and permutations were used to test for the significance of the smooth term for location. The underlying spatial pattern of the defect was first assessed using an unadjusted model with only the smooth term for location. Two adjusted GAMS were also modeled. The first was adjusted for potential confounders including maternal education, age, race, adequacy of prenatal care, and season of conception. The second included all the indicated covariates from the first model with the addition of satellite-based PM2.5 measures to assess residual spatial patterns after accounting for the contribution of PM2.5 and other risk factors. The R package MapGAM was used to run the spatial GAMs and create the maps.
3. Results
We obtained records for 2,729 cardiac, 726 orofacial, 255 neural tube defect cases, and 7,816 controls geocoded, non-syndromic births from the 611,854 births in Massachusetts conceived from January 1, 2001 to December 31, 2008. The percentage of cases with mothers 30–34 years old ranged from 27–31%, depending on the defect. Between 61–72% of mothers were of non-Hispanic White race/ethnicity, and between 56–74% of mothers reported education levels greater than high school (Table 1). Eight percent of control mothers reported smoking during pregnancy, while up to 10% of mothers with orofacial defect reported smoking. The proportion of mothers that reported drinking during pregnancy ranged from 0.5–2% among the defect groups. Approximately 75% of all cases reported “adequate” prenatal care.
Table 1.
Description of Birth Defect Cases and Randomly Selected Controls in Massachusetts, Conceived 2001–2008
| n(%)a | Controls (n=7,816) |
Cardiac (n=2,729) |
Orofacial (n=726) |
Neural Tube (n=255) |
|---|---|---|---|---|
| Infant Sex | ||||
| Male | 4,032 (51.6) | 1,412 (51.7) | 411 (56.7) | 107 (42.0) |
| Female | 3,784 (48.4) | 1,317 (48.3) | 314 (43.3) | 148 (58.0) |
| Maternal Age | ||||
| <20 years | 462 (5.9) | 148 (5.4) | 46 (6.3) | 19 (7.4) |
| 21–24 years | 1,227 (15.7) | 417 (15.2) | 118 (16.2) | 38 (14.9) |
| 25–29 years | 1,878 (24.0) | 586 (21.4) | 181 (24.9) | 71 (27.8) |
| 30–34 years | 2,418 (30.9) | 853 (31.2) | 211 (29.1) | 70 (27.4) |
| 35+ years | 1,831 (23.4) | 725 (26.5) | 169 (23.3) | 57 (22.3) |
| Parity | ||||
| 0 | 3,521 (45.0) | 1,225 (44.8) | 320 (44.1) | 123 (48.2) |
| 1 | 2,665 (34.1) | 884 (32.3) | 247 (34.0) | 84 (32.9) |
| ≥ 2 | 1,612 (20.6) | 617 (22.6) | 157 (21.6) | 48 (18.8) |
| Adequacy of Prenatal Care | ||||
| Adequate | 6,098 (78.0) | 2,121 (77.7) | 529 (72.9) | 190 (74.5) |
| Intermediate | 1,372 (17.5) | 462 (16.9) | 149 (20.5) | 45 (17.6) |
| Inadequate | 247 (3.1) | 103 (3.7) | 36 (4.9) | 14 (5.4) |
| Unknown | 74 (0.9.0) | 34 (1.2) | 10 (1.3) | 5 (1.9) |
| None | 25 (0.3) | 9 (0.3) | 1 (0.1) | 1 (0.3) |
| Smoking During Pregnancy | ||||
| Yes | 623 (7.9) | 205 (7.5) | 74 (10.2) | 22 (8.6) |
| No | 7193 (92.0) | 2524 (92.4) | 651 (89.7) | 233 (91.3) |
| Drinking During Pregnancy | ||||
| Yes | 137 (1.7) | 36 (1.3) | 11 (1.5) | 1 (0.4) |
| No | 7,674 (98.1) | 2,691 (98.6) | 713 (98.3) | 254 (99.6) |
| Season of Conception | ||||
| Winter | 1,883 (24.0) | 646 (23.6) | 158 (21.7) | 55 (21.5) |
| Spring | 1,869 (23.1) | 651 (23.8) | 209 (28.8) | 62 (24.3) |
| Summer | 2,008 (25.6) | 657 (24.0) | 151 (20.8) | 63 (24.7) |
| Fall | 2,056 (26.3) | 775 (28.4) | 207 (28.5) | 75 (29.4) |
| Gestational Age | ||||
| <37 weeks | 692 (8.8) | 646 (23.7) | 99 (13.6) | 66 (25.8) |
| ≥37 weeks | 7,124 (91.1) | 2,083 (76.3) | 626 (86.4) | 189 (74.1) |
| Small for Gestational Age | ||||
| Yes | 845 (10.8) | 651 (23.8) | 153 (21.1) | 95 (37.2) |
| No | 6,961 (89.1) | 2,076 (76.0) | 572 (78.9) | 159 (62.3) |
| Maternal Race/Ethnicity | ||||
| Non-Hispanic White | 5,379(68.8) | 1,837 (67.3) | 522 (72.0) | 156 (61.1) |
| Non-Hispanic Black | 682(8.7) | 284 (10.4) | 35 (4.8) | 33 (12.9) |
| Hispanic | 1,059(13.5) | 375 (13.7) | 102 (14.0) | 46 (18.0) |
| Asian/Pacific Islander | 530 (6.7) | 157 (5.7) | 49 (6.7) | 11 (4.3) |
| Other | 162 (2.0) | 75 (2.7) | 17 (2.3) | 9 (3.5) |
| Maternal Education | ||||
| <12th grade | 793 (10.1) | 302 (11.1) | 86 (11.8) | 30 (11.8) |
| High school graduation | 2,010 (25.7) | 710 (26.0) | 2,15 (29.6) | 81 (31.8) |
| Some college | 5,002 (64.0) | 1,712 (62.7) | 424 (58.4) | 144 (56.4) |
| Maternal Language Preference | ||||
| English | 6,945 (88.8) | 2,407 (88.2) | 642 (88.5) | 209 (81.9) |
| Spanish | 400 (5.1) | 164 (6.0) | 39 (5.3) | 22 (8.6) |
| Portuguese | 181 (2.3) | 59 (2.1) | 15 (2.0) | 10 (3.9) |
| Other | 275 (3.5) | 86 (3.1) | 25 (3.4) | 13 (5.1) |
| Household Income | ||||
| <$20,000 | 339 (4.4) | 126 (4.6) | 32 (4.4) | 11 (4.3) |
| $20,000–$69,999 | 3899 (49.8) | 1431 (52.4) | 386 (39.4) | 158 (62.0) |
| ≥$70,000 | 3578 (45.8) | 1172 (42.9) | 307 (42.3) | 86 (33.7) |
| Delivery Source of Payment | ||||
| HMO | 1,365 (17.4) | 437 (16.0) | 125 (17.2) | 51 (20.0) |
| Medicaid/Common Health | 1,988 (25.4) | 741 (27.1) | 207 (28.5) | 79 (30.9) |
| Other | 4,463 (57.1) | 1,550 (56.8) | 392 (54.0) | 125 (49.0) |
Percentages may not sum to 100% due to rounding.
3.1 PM2.5 and traffic-related exposures
Our PM2.5 analysis included 2,610 (95.6%) cardiac, 692 (95.3%) orofacial, and 247 (96.8%) neural tube defects with a measure of prenatal PM2.5 exposure for the relevant gestational period. Of the 7,816 controls, 278 (4%), 352 (5%), and 254 (3%) were excluded from the cardiac, orofacial, and neural tube analyses, respectively, due to missing PM2.5 data during their respective critical window of exposure. Traffic density and residential distance to nearest major roadway were successfully calculated for all cases and controls.
Cubic splines relating continuous satellite-based PM2.5 exposure to log odds of birth defects were approximately linear. As such, we report results from logistic regression models of the association between birth defects and PM2.5 exposure modeled continuously for a 10 µg/m3 increase of PM2.5 (Table 2). Non-imputed results are presented since estimates were not altered when missing values were imputed. The adjusted ORs for perimembranous ventricular septal defects (OR = 1.34, 95% CI: 0.98, 1.83), patent foramen ovale (OR = 1.18, 95% CI: 0.91, 1.53) and patent ductus arteriosus (OR = 1.24, 95% CI: 0.94, 1.62) were elevated and approaching significance. Odds ratios for the remaining cardiac defects were generally null with wide confidence intervals. Results for the general neural tube defects group suggested an inverse association (OR = 0.70, 95% CI: 0.46, 1.05) whereas for spina bifida the OR was slightly elevated (OR = 1.22, 95% CI: 0.61, 2.30), although neither was statistically significant. Non-significant inverse associations were also observed for orofacial defects (cleft lip with or without palate: OR = 0.76, 95% CI: 0.50, 1.10; cleft palate: OR = 0.89, 95% CI: 0.54, 1.46). We found that crude estimates were similar to adjusted estimates for all defects except for endocardial cushion and all orofacial defects (Table 2). When assessing a wider exposure window of the first trimester of pregnancy, we found similar results for most defects (eTable3). Compared to estimates using narrow exposure windows, estimates obtained using first trimester exposure windows were closer to the null for patent ductus arteriosus and atrial septal defect and further away from the null for tetralogy of fallot and endocardial cushion defect. We found evidence for effect modification of PM2.5 exposure by maternal education level for endocardial cushion defects (P = 0.017), perimembranous VSD (P = 0.030), and single common atrium (P = 0.020) (eTable 4).
Table 2.
Crude and Adjusted Odds Ratiosa and 95% Confidence Interval for Prenatal Exposure to PM2.5 in Massachusetts, and Cardiac, Neural Tube, and Orofacial Defects Among Infants Conceived Between 2001–2008.
| Defect Typeb | n | Crude OR(95% CI)c | Adjusted OR (95% CI)c |
|---|---|---|---|
| Isolated Birth Defects | 890 | 0.92 (0.71, 1.21) | 0.97 (0.94, 1.00) |
| Multiple Birth Defects | 2571 | 1.01 (0.84, 1.20) | 1.01 (0.85, 1.21) |
| Total Cases | 3,461 | ||
| Cardiac | |||
| Transposition of the Great Vessel | 233 | 0.90 (0.59, 1.37) | 0.92 (0.60, 1.41) |
| Tetralogy of Fallot | 153 | 0.99 (0.58, 1.68) | 1.00 (0.59, 1.71) |
| Ostium Secundum ASD | 1457 | 0.96 (0.80, 1.16) | 0.98 (0.81, 1.19) |
| Endocardial Cushion Defect | 139 | 0.99 (0.58, 1.68) | 1.18 (0.67, 2.09) |
| Pulmonary Valve Atresia/Stenosis | 436 | 1.01 (0.73, 1.39) | 1.05 (0.76, 1.45) |
| Aortic Valve Stenosis | 93 | 1.15 (0.58, 2.30) | 1.18 (0.58, 2.38) |
| Hypoplastic Left Heart Syndrome | 69 | 0.70 (0.34, 1.45) | 0.73 (0.35, 1.42) |
| Patent Ductus Arteriosus | 675 | 1.24 (0.95, 1.62) | 1.24 (0.94, 1.62) |
| Coarctation of Aorta | 205 | 1.03 (0.65, 1.63) | 1.03 (0.65, 1.64) |
| Pulmonary Artery Anomalies | 172 | 1.10 (0.67, 1.83) | 1.04 (0.64, 1.68) |
| VSD | 864 | 1.08 (0.86, 1.37) | 1.09 (0.86, 1.37) |
| Perimembranous VSD | 494 | 1.32 (0.98, 1.81) | 1.34 (0.98, 1.83) |
| Muscular VSD | 328 | 0.87 (0.61, 1.25) | 0.89 (0.62, 1.27) |
| Single Common Atrium, Cor Tiloculare | 335 | 1.12 (0.78, 1.62) | 1.19 (0.82, 1.72) |
| Atrial Septal Defect, NOS | 235 | 1.24 (0.80, 1.94) | 1.23 (0.78, 1.90) |
| Patent Foramen Ovale | 725 | 1.15 (0.89, 1.50) | 1.18 (0.91, 1.53) |
| Insufficiency of Aortic Valve | 262 | 1.11 (0.73, 1.68) | 1.16 (0.76, 1.76) |
| Neural Tube | |||
| Neural Tube Defects | 199 | 0.72 (0.47, 1.09) | 0.77 (0.46, 1.05) |
| Spina Bifida | 89 | 1.22 (0.63, 2.37) | 1.18 (0.61, 2.30) |
| Orofacial | |||
| Cleft Lip with & without Palate | 406 | 1.02 (0.71, 1.47) | 0.76 (0.50, 1.10) |
| Cleft Palate Only | 251 | 1.24 (0.78, 1.95) | 0.89 (0.54, 1.46) |
Abbreviations: ASD, atrial septal defect; CI, confidence interval; NOS, not otherwise specified; OR, odds ratio; PM2.5, particulate matter with a diameter of 2.5 µm or less; VSD, ventricular septal defect.
Control group n=7538,7464,7562 for cardiac, neural tube, and orofacial defect analysis, respectively.
All models adjusted for maternal race, education, median household income of block group, alcohol consumption during pregnancy, and plurality. Isolated and multiple birth defects further adjusted for maternal age, language preference, parity, and adequacy of prenatal care. Cardiac defects further adjusted for maternal age, language preference, parity, and adequacy of prenatal care. Neural tube defects further adjusted for maternal age, language preference, parity, adequacy of prenatal care, and smoking during pregnancy. Orofacial defects further adjusted for season of conception, infant sex, adequacy of prenatal care, and smoking during pregnancy.
ORs and 95% CIs correspond to a 10 µg/m3 increase of average PM2.5 measured at the 4 km grid cell of infant’s birth address during weeks 3–7,1–4, and 6–12 of pregnancy for cardiac, neural tube, and orofacial defects, respectively.
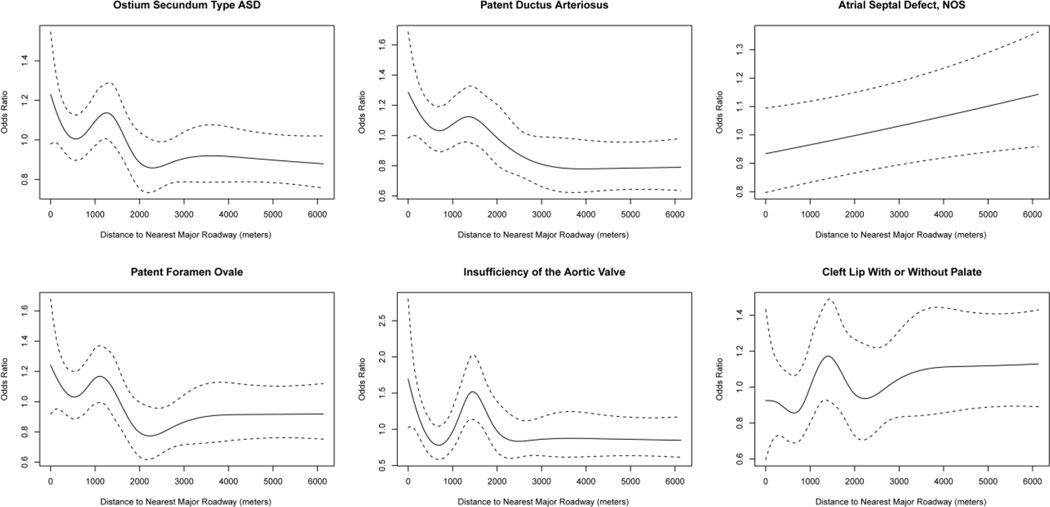
For comparison, we also examined the relationship between birth defects and local traffic measures. Results for residential proximity to major roadways, modeled continuously with cubic splines are shown in Figure 1 for defects that yielded significant associations (see eResults1 for further details). Cubic splines indicated that traffic density and risk of defects was approximately linear, therefore odds ratios are presented for an interquartile range increase of AADT (eTable 5). Results show similar associations with traffic density as many of the same defects associated with PM2.5 and distance to major roadways. Ostium secundum atrial septal defects (OR = 1.03, 95% CI: 1.00, 1.06), patent foramen ovale (OR =1.05, 95% CI: 1.01, 1.08), and insufficiency of the aortic valve (OR = 1.07, 95% CI: 1.01, 1.12) were positively associated with traffic density. Patent ductus arteriosus was positively associated with traffic density and approaching significance (OR = 1.03, 95% CI: 0.99, 1.07). Cleft lip with or without palate was negatively associated with traffic density (OR = 0.92, 95% CI: 0.85, 0.98). Results were similar for imputed datasets (data not shown).
Figure 1.
Association of Residential Distance to Major Roadways and Risk of Defects. Exposure response curve showing the adjusted odds ratio (solid line) and the 95% confidence interval (dashed line) using cubic splines to model the association of residential distance to a major roadway and birth defects among infants conceived in Massachusetts, 2001–2008. Only birth defects with significant associations are displayed. Estimates are only presented for residential addresses within continental Massachusetts. All models adjusted for maternal race, education, median household income of block group, alcohol consumption during pregnancy, and plurality. Cardiac defects further adjusted for maternal age, language preference, parity, adequacy of prenatal care. Orofacial defects further adjusted for season of conception, infant sex, adequacy of prenatal care, and smoking during pregnancy. Major roadways defined as limited access highways and multi-lane highways (class 1 and 2 roads). Abbreviations: ASD – atrial septal defect; NOS - not otherwise specified.
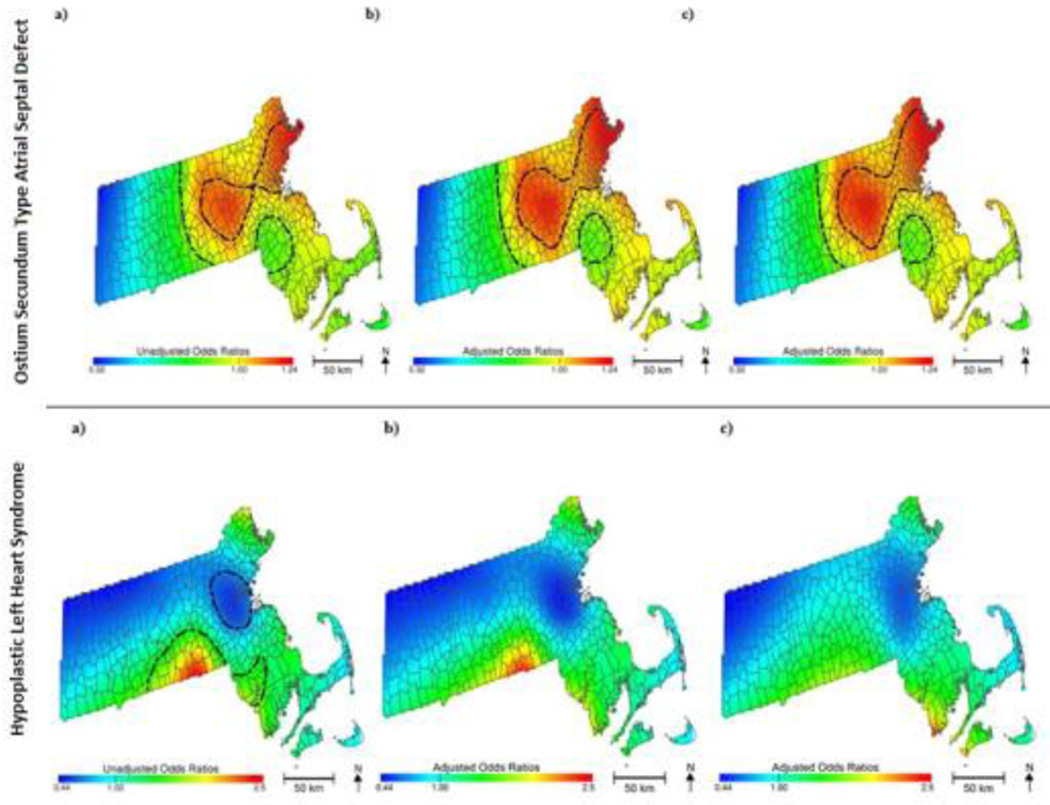
3.2 Geographic location
Spatial analyses using GAMs showed significant associations between geographic location and certain birth defects (eTable 6). Birth location remained statistically significant (P = 0.004) for ostium secundum atrial septal defects after adjusting for demographic, socioeconomic, behavioral risk factors, and PM2.5 exposure (Figure 2). The relationship between birth location and hypoplastic left heart syndrome was borderline statistically significant (P = 0.067) and was fully explained only after adding PM2.5 to the model (P = 0.144, Figure 2). To determine the influence of missing data on outcomes in the spatial analysis, five imputed data sets were generated to run the GAMs and permutation tests for hypoplastic left heart syndrome and ostium secundum atrial septal defect. Results were similar to those generated using the original dataset (data not shown).
Figure 2.
Geographic Patterns of Ostium Secundum Atrial Septal Defect and Hypoplastic Left Heart Syndrome Risk among infants conceived in Massachusetts, 2001–2008: (a) Unadjusted, (b) Adjusted without PM2.5 Exposure, and (c) Adjusted with PM2.5 Exposure. Statistically significant geographic areas of increased or decreased risk of birth defect are indicated using black contour lines. Maps adjusted for maternal education, race, smoking during pregnancy, alcohol consumption during pregnancy, prenatal care, infant sex, and season of conception.
Although unadjusted models indicated significant spatial variation across Massachusetts for other defects, adjusted models suggested that the patterns were due to socioeconomic, demographic, and behavioral risk factors, and including PM2.5 did not alter the geographic pattern.
4. Discussion
We examined the spatial relationship between PM2.5 and other traffic-related measures using anatomical groupings of cardiac, neural tube and orofacial birth defects. There is evidence to support the hypothesis that exposure to PM2.5 and traffic-related air pollution increases risk of patent foramen ovale and patent ductus arteriosus, as these defects displayed a positive but non-significant association with PM2.5. Patent foramen ovale displayed positive significant associations with both distance to major roadways and traffic density.
We assessed traffic-related air pollution at various spatial scales, as our PM2.5 measures represent larger scale pollution whereas traffic density and distance to major roads represent more local measures of pollution. Perimembranous ventricular septal defects demonstrated a positive but non-significant association with PM2.5 exposure but not with local measures of pollution. Ostium secundum atrial septal defects and insufficiency of the aortic valve defects were significantly associated with local measures of air pollution, but not PM2.5, indicating that there may be a specific local pollutant affecting the development of these defects.
There was a noticeable rise in risk for ostium secundum type atrial septal defect, patent ductus arteriosus, patent foramen ovale, insufficiency of the aortic valve, and cleft lip with or without palate among infants with birth addresses around 1000–1500 meters away from a major roadway (Figure 1). It is not clear that the rise and decline in risk around 1000–1500 meters is statistically significant as it is contained with the CI at shorter distances for most outcomes. It is therefore difficult to tell if the higher risk at that distance is meaningful or an effect of random variation for most birth defects. However, the CIs are narrower for insufficiency of the aortic valve, suggesting that the pattern is meaningful for that outcome. Moreover, the shared pattern of increased risk estimates around 1000–1500 meters suggests that some unmeasured risk factor for birth defects may be more common in that range. We examined the confounders of infants whose birth address was between 1000–1500 meters away from the nearest major roadway and found they were similar to births residing at other distances. Further investigation is needed to better understand the shared pattern of increased risk estimates around 1000–1500 meters.
Interestingly, we observed inverse associations between PM2.5 and traffic density and cleft lip with or without palate, although the association with PM2.5 was not significant. Other studies have found no association between cleft lip with or without palate and PM2.5 (16, 18), yielding null inverse associations. One study also found a consistent, but non-significant inverse association with PM2.5 and cleft lip with or without palate across all PM2.5 quartiles (17).
In our secondary analysis of effect modification, we found that women with the lowest education level (less than high school) had the highest effect estimate compared to women with at least high school or college education for a majority of birth defects. Although there is some evidence for effect modification, this stratified analysis contains small numbers of cases, especially in the less than high school category, and therefore the effect estimates have wide confidence intervals.
The mapped results of our crude GAMs indicated there was a statistically significant association between geographic location and certain birth defects. Residential location remained significantly associated with ostium secundum atrial septal defects in the fully adjusted spatial model, suggesting that the persistent areas of increased risk are not fully explained by individual risk factors included in the model. These spatial patterns may be due to unidentified environmental or social determinants. Adjusting for PM2.5 exposure influenced the spatial patterns for hypoplastic left heart syndrome only, suggesting that spatial patterns of increased risk for all other defects are not strongly associated with PM2.5. Furthermore, despite analyzing eight years of births for an entire state, rare birth defects are prone to small case numbers, limiting the power to detect significant spatial associations.
We investigated three groups of atrial septal defects; ostium secundum atrial septal defects, atrial septal defects, NOS, and patent foramen ovale. To our knowledge no other studies have examined the influence of PM2.5 exposure on risk of ostium secundum atrial septal defects. We did not find any studies that investigated the role of patent foramen ovale and air pollutants. We found an overall positive significant association between atrial septal defects, NOS and residential distance to major roadways (p= 0.030) and increased risk with PM2.5 (OR= 1.23, 95% CI: 0.78, 1.90). Gilboa et al. also found a positive significant association between PM10 and atrial septal defects in Texas (5). We found a non-significant positive association between patent ductus arteriosus and PM2.5 while Agay-Shay et al. found a significant inverse association with isolated patent ductus arteriosus and PM2.5 (11). Another study (43) found significant increased odds of patent ductus arteriosus and PM10 while utilizing further restrictive criteria for this outcome group based on active surveillance records, but we were unable to replicate this method because of limited information from the Massachusetts birth defects registry. Although there are differences in the composition of PM10 and PM2.5, this may indicate that general particulate matter exposure may be associated with risk of patent ductus arteriosus. We did not find strong evidence supporting an association between neural tube defects and traffic-related measures of air pollution. Similarly, Padula et al. found no association with PM2.5 or traffic density and neural tube defects (17).
To better understand the association of PM2.5 and traffic-related exposure on birth defects, our study includes improved exposure assessment methods, standardized definitions of cases, and important confounders. We believe we have captured several important SES measures, but we are less confident about our ability to capture alcohol and smoking behaviors from self-reported measures. We used satellite data to obtain fine spatial distribution estimates of PM2.5 during pregnancy for all of Massachusetts. The use of measured PM2.5 for each 4 km square grid cell allows for more fine-scale measures of exposure compared to previous studies that have relied on measures from stationary monitoring stations. We believe that using this finer spatial resolution of PM2.5 reduced residential exposure misclassification compared to using monitoring stations alone. By including distance to major roadways and traffic density, we were able to assess the influence of local road networks on risk of birth defects.
Although we were able to control for important covariates, we were unable to directly control for folic acid supplementation, a major contributor of neural tube defects (44). As a proxy for folic acid supplementation we used the adequacy of prenatal care index. This index measures prenatal care based on initiation and adequacy of received services. It has been found that adequacy of prenatal care is a useful proxy for folic acid supplementation during pregnancy (45).
We utilized critical windows of exposure specific to birth defect anatomical location. Previous studies have used average exposures over the first trimester of pregnancy or gestational weeks 3–8. Organ development is time specific, therefore the use of very specific exposure windows can strongly influence the effect estimates and allow for evaluation of exposureresponse relationships to be more readily observed (46, 47). We noticed variability in effect estimates when assessing exposure during the first trimester of pregnancy compared to narrow critical windows of exposure for transposition of the great vessel, tetralogy of fallot, endocardial cushion defect, patent ductus arteriosus, coarctation of the aorta, pulmonary artery anomalies, atrial septal defects, NOS, and spina bifida (eTable 3).
This analysis includes birth defect diagnoses among still births and diagnoses made up to one year of life. However, we are unable to more fully investigate the effects of fetal toxicity as we do not have records of fetal deaths. Fetal deaths may be more sensitive to the effects of air pollution than those who survive to birth with birth defects. Therefore, we may not have captured the conceptions most at risk. We hypothesize that the detected association would be weaker than the true association had all at risk subjects been included in this analysis.
This study only assessed the relationship between birth defects and traffic-related exposures using residential location of mother at time of delivery. Pregnant women are a mobile population and many women may not have been living in the same home during the early prenatal period as they were at the time of birth of the infant. We believe that this may lead to exposure misclassification but it is likely to be non-differential among cases and controls (48). Residential mobility was estimated to be between 3% and 14% in recent American studies based on birth cohorts (48, 49) and mobility rates did not differ among mothers of infants born with birth defects and mothers of infants born without birth defects (48). Additionally, maternal time-activity patterns were not accounted for in our investigation. This may result in exposure misclassification, although it is also likely to be non-differential among cases and controls. Recent studies have found that outdoor residential levels of exposure act as a good surrogate for personal exposure (50). Both sources of exposure misclassification may increase type 2 error so our null results should be interpreted with caution.
Given the multiple comparisons involved in testing a large range of birth defects with three different metrics for traffic-related air pollution, this analysis may result in significant associations due to chance (e.g., type 1 errors). Therefore we have emphasized associations that are observed for more than one measure of traffic-related air pollution. Furthermore, we acknowledge that PM2.5 is a complex mixture of particles with varying toxicity and we do not have measures of composition.
5. Conclusions
In summary, we found evidence to suggest that PM2.5 exposure during pregnancy may be associated with risk of patent foramen ovale, patent ductus arteriosus, and perimembranous ventricular septal defects. Our findings also support a possible relationship between ostium secundum atrial septal defects and insufficiency of the aortic valve with local traffic-related air pollutants. Our spatial analyses show that there were geographic regions with increased risk of ostium secundum atrial septal defects in Massachusetts, even after accounting for PM2.5 exposure. There is limited evidence to suggest that cleft lip with or without palate may have an inverse association with PM2.5 exposure and traffic-related air pollution in general. To fully understand the influence of PM2.5 on birth defects, larger studies of these specific birth defect groups using valid PM2.5 estimates are needed.
Supplementary Material
Highlights.
Improved PM2.5 satellite measures enable full advantage of a statewide birth cohort.
Some evidence of association of patent foramen ovale and patent ductus arteriosus with traffic related air pollution.
Limited evidence of association of neural tube defects with traffic related air pollution.
Limited evidence of association of orofacial defects with traffic related air pollution.
Atrial septal defects display significant spatial variation.
Acknowledgments
Grant support: This work was supported by grant numbers R01ES019897 and P42ES007381 from the National Institute of Environmental Health (NIEHS). Its contents are solely the responsibility of the authors and do not necessarily represent the views of NIH. The work of Y.L. and X. H. was partially supported by NASA Applied Sciences Program (grant no. NNX11AI53G).
Abbreviations
- AADT
annual average daily traffic
- AOD
aerosol optical depth
- CI
confidence interval
- GAMs
generalized additive models
- GOES
Geostationary Operational Environmental Satellite
- ICD-9-CM
International Classification of Diseases, Clinical Modification, Ninth Revision
- NOS
not otherwise specified
- NOAA
National Oceanic and Atmospheric Administration
- OR
odds ratio
- PM2.5
particulate matter with diameter of 2.5 µm or less
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared.
The Institutional Review Boards of the University of California at Irvine and the MA Department of Public Health approved this research.
REFERENCES
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael, et al. The National Birth Defects Prevention Study. Public Health Reports. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dadvand P, Rankin J, Rushton S, Pless-Mulloli T. Ambient air pollution and congenital heart disease: A register-based study. Environ Res J. 2011;111:435–441. doi: 10.1016/j.envres.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Dolk H, Armstrong B, Lachowycz K, Vrijheid M, Rankin J, Abramsky L, et al. Ambient air pollution and risk of congenital anomalies in England, 1991–1999. Occup Environ Med. 2010;67:223–227. doi: 10.1136/oem.2009.045997. [DOI] [PubMed] [Google Scholar]
- 5.Gilboa SM, Mendola P, Olshan AF, Langlois PH, Savitz DA, Loomis D, et al. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol. 2005;162:238–252. doi: 10.1093/aje/kwi189. [DOI] [PubMed] [Google Scholar]
- 6.Padula AM, Tager IB, Carmichael SL, Hammond SK, Yang W, Lurmann F, et al. Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin Valley of California. Paediatr Perinat Epidemiol. 2013;27:329–339. doi: 10.1111/ppe.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schembari A, Nieuwenhuijsen MJ, Salvador J, de Nazelle A, Cirach M, Dadvand P, et al. Traffic-related air pollution and congenital anomalies in Barcelona. Environ Health Perspect. 2014;122:317–323. doi: 10.1289/ehp.1306802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrijheid M, Martinez D, Manzanares S, Dadvand P, Schembari A, Rankin J, et al. Ambient air pollution and risk of congenital anomalies: A systematic review and meta-analysis. Environ Health Perspect. 2011;119:598–606. doi: 10.1289/ehp.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116:680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Expo Sci Environ Epidemiol. 2007;17:426–432. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- 11.Agay-Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C. Air pollution and congenital heart defects. Environ Res. 2013;124:28–34. doi: 10.1016/j.envres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Hansen CA, Barnett AG, Jalaludin BB, Morgan GG. Ambient air pollution and birth defects in Brisbane, Australia. PloS one. 2009;4:e5408. doi: 10.1371/journal.pone.0005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim OJ, Ha EH, Kim BM, Seo JH, Park HS, Jung WJ, et al. PM10 and pregnancy outcomes: A hospital-based cohort study of pregnant women in Seoul. J Occup Environ Med. 2007;49:1394–1402. doi: 10.1097/JOM.0b013e3181594859. [DOI] [PubMed] [Google Scholar]
- 14.Stingone JA, Luben TJ, Daniels JL, Fuentes M, Richardson DB, Aylsworth AS, et al. Maternal exposure to criteria air pollutants and congenital heart defects in offspring: Results from the national birth defects prevention study. Environ Health Perspect. 2014;122:863–872. doi: 10.1289/ehp.1307289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen EK, Zmirou-Navier D, Padilla C, Deguen S. Effects of air pollution on the risk of congenital anomalies: A systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11:7642–7668. doi: 10.3390/ijerph110807642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall EG, Harris G, Wartenberg D. Oral cleft defects and maternal exposure to ambient air pollutants in New Jersey. Birth Defects Res A Clin Mol Teratol. 2010;88:205–215. doi: 10.1002/bdra.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padula AM, Tager IB, Carmichael SL, Hammond SK, Lurmann F, Shaw GM. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am J Epidemiol. 2013;177:1074–1085. doi: 10.1093/aje/kws367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinkikoor-Imler LCDJ, Meyer RE, Luben TJ. Early prenatal exposure to air pollutoin and its association with birth defects in a state wide birth cohort from North Carolina. Birth Defects. Res A Clin Mol Teratol. 2013;97:696–701. doi: 10.1002/bdra.23159. [DOI] [PubMed] [Google Scholar]
- 19.Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102:182–190. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh JK, Wilhelm M, Su J, Goldberg D, Cockburn M, Jerrett M, et al. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am J Epidemiol. 2012;175:1262–1274. doi: 10.1093/aje/kwr469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Christopher SA. Intercomparison between satellite-derived aerosol optical thickness and PM2.5 mass: Implications for air quality studies. Geophys Res Lett. 2003;30(21):2095. [Google Scholar]
- 22.Liu Y, Sarnat JA, Kilaru A, Jacob DJ, Koutrakis P. Estimating ground-level PM2.5 in the eastern United States using satellite remote sensing. Environ Sci Technol. 2005;39(9):3269–3278. doi: 10.1021/es049352m. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Franklin M, Kahn R, Koutrakis P. Using aerosol optical thickness to predict ground-level PM2.5 concentrations in the St. Louis area: A comparison between MISR and MODIS. Remote Sens Environ. 2007;107(1–2):33–44. [Google Scholar]
- 24.Liu Y, Paciorek CJ, Koutrakis P. Estimating regional spatial and temporal variability of PM2.5 concentrations using satellite data, meteorology, and land use information. Environ Health Perspect. 2009;117:886–892. doi: 10.1289/ehp.0800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Waller LA, Al-Hamdan MZ, Crosson WL, Estes MG, Jr, Estes SM, et al. Estimating ground-level PM2.5 concentrations in the southeastern U.S. using geographically weighted regression. Environmental Research. 2013;121(0):1–10. doi: 10.1016/j.envres.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Waller LA, Lyapustin A, Wang Y, Al-Hamdan MZ, Crosson WL, et al. Estimating ground-level PM2.5 concentrations in the southeastern United States using MAIAC AOD retrievals and a two-stage model. Remote Sens Environ. 2014a;140(0):220–232. [Google Scholar]
- 27.Hu X, Waller LA, Lyapustin A, Wang Y, Liu Y. 10-year spatial and temporal trends of PM2.5 concentrations in the southeastern US estimated using high-resolution satellite data. Atmos Chem Phys. 2014b;14(12):6301–6314. doi: 10.5194/acp-14-6301-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X, Waller LA, Lyapustin A, Wang Y, Liu Y. Improving satellite-driven PM2.5 models with Moderate Resolution Imaging Spectroradiometer fire counts in the southeastern U.S. Journal of Geophysical Research: Atmospheres. 2014c;119(19) doi: 10.1002/2014JD021920. 2014JD021920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmospheric Environment. 2011;45(35):6267–6275. [Google Scholar]
- 30.Kahn R, Banerjee P, McDonald D, Diner DJ. Sensitivity of multiangle imaging to aerosol optical depth and to pure-particle size distribution and composition over ocean. J Geophys Res-Atmos. 1998;103(D24):32195–32213. [Google Scholar]
- 31.van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, et al. Global Estimates of Ambient Fine Particulate Matter Concentrations from Satellite-Based Aerosol Optical Depth: Development and Application. Environ Health Perspect. 2010;118(6):847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Liu Y, Coull BA, Schwartz J, Koutrakis P. A novel calibration approach of modis aod data to predict PM2.5 concentrations. Atmos Chem Phys. 2011;11:7991–8002. [Google Scholar]
- 33.Opitz JM, Clark EB. Heart development: An introduction. Am J Med Genet A. 2000;97:238–247. [PubMed] [Google Scholar]
- 34.Sadler TW. Mechanisms of neural tube closure and defects. Ment. Retard. Dev. Disabil. Res. Rev. 1998;4:247–253. [Google Scholar]
- 35.Trines J, Hornberger LK. Evolution of heart disease in utero. Pediatr Cardiol. 2004;25:287–298. doi: 10.1007/s00246-003-0592-2. [DOI] [PubMed] [Google Scholar]
- 36.Adar SD, Kaufman JD. Cardiovascular disease and air pollutants: evaluating and improving epidemiological data implicating traffic exposure. Inhal Toxicol. 2007;19(suppl 1):135–149. doi: 10.1080/08958370701496012. [DOI] [PubMed] [Google Scholar]
- 37.Lipfert FW, Wyzga RE. On exposure and response relationships for health effects associated with exposure to vehicular traffic. J Expo Sci Environ Epidemiol. 2008;18:588–599. doi: 10.1038/jes.2008.4. [DOI] [PubMed] [Google Scholar]
- 38.Medina-Ramon M, Goldberg R, Melly S, Mittleman MA, Schwartz J. Residential exposure to traffic-related air pollution and survival after heart failure. Environ Health Perspect. 2008;116:481–485. doi: 10.1289/ehp.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 40.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira V, Webster T, Weinberg J, Aschengrau A, Ozonoff D. Spatial analysis of lung, colorectal, and breast cancer on Cape Cod: An application of generalized additive models to case-control data. Environ Health: A Global Access Science Source. 2005;4:11. doi: 10.1186/1476-069X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster T, Vieira V, Weinberg J, Aschengrau A. Method for mapping population-based case-control studies: An application using generalized additive models. Int J Health Geogr. 2006;5:26. doi: 10.1186/1476-072X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strickland MJ, Klein M, Correa A, Reller MD, Mahle WT, Riehle-Colarusso TJ, et al. Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am J Epidemiol. 2009;169:1004–1014. doi: 10.1093/aje/kwp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald N, Sneddon J. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 45.Lunet N, Rodrigues T, Correia S, Barros H. Adequacy of prenatal care as a major determinant of folic acid, iron, and vitamin intake during pregnancy. Cada SaudePublica. 2008;24:1151.43–1157.43. doi: 10.1590/s0102-311x2008000500022. [DOI] [PubMed] [Google Scholar]
- 46.Wilson JG. Embryological considerations in teratology. Ann N Y Acad Sci. 1965;123:219–227. doi: 10.1111/j.1749-6632.1965.tb12260.x. [DOI] [PubMed] [Google Scholar]
- 47.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108:451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J Expo Sci Environ Epidemiol. 2006;16:538–543. doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- 49.Shaw GM, Malcoe LH. Residential mobility during pregnancy for mothers of infants with or without congenital cardiac anomalies: A reprint. Arch Environ Health. 1992;47:236–238. doi: 10.1080/00039896.1992.9938355. [DOI] [PubMed] [Google Scholar]
- 50.Nethery E, Leckie SE, Teschke K, Brauer M. From measures to models: An evaluation of air pollution exposure assessment for epidemiological studies of pregnant women. Occup Environ Med. 2008;65:579–586. doi: 10.1136/oem.2007.035337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.