Abstract
Angiogenesis is closely linked to and precedes eosinophilic infiltration in asthma. Eosinophils are recruited into the airway by chemoattractant eotaxins, which are expressed by endothelial cells, smooth muscles cells, epithelial cells, and hematopoietic cells. We hypothesized that bone marrow-derived proangiogenic progenitor cells that contain eotaxins contribute to the initiation of angiogenesis and inflammation in asthma. Whole lung allergen challenge of atopic asthma patients revealed vascular activation occurs within hours of challenge, and prior to airway inflammation. The eotaxin receptor CCR3 was expressed at high levels on submucosal endothelial cells in patients and murine model of asthma. Exvivo exposure of murine endothelial cells to eotaxins induced migration and angiogenesis. In mechanistic studies, wildtype mice transplanted with eotaxin-1/2 deficient bone marrow had markedly less angiogenesis and inflammation in an atopic asthma model, while adoptive transfer of proangiogenic progenitor cells from wildtype mice in an atopic asthma model into the eotaxin-1/2 deficient mice led to angiogenesis and airway inflammation. The findings indicate that TH2-promoting hematopoietic progenitor cells are rapidly recruited to the lung upon allergen exposure and release eotaxins that coordinately activate endothelial cells, angiogenesis, and airway inflammation.
Keywords: asthma, angiogenesis, proangiogenic hematopoietic progenitors, allergen challenge
Introduction
Angiogenic remodeling in asthma is one of the most consistent characteristics of airway remodeling, occurring in mild, moderate and severe asthmatic lungs(1-4). There is a strong correlation between the number of blood vessels in the bronchial wall and the severity of asthma in patients (2-4). Accumulating evidence from murine and patient studies suggests angiogenesis may be a critical component in the pathophysiology of asthma driven by proangiogenic progenitor cells recruited from the bone marrow (5-9). Eotaxins, a family of C-C chemokines including eotaxin-1 (eotaxin or CCL11), -2 (CCL24) and -3 in humans (10-12) and eotaxin-1 and -2 in mice (13, 14), are the prime eosinophil specific(15-21) chemoattractants in allergic airway inflammation as conclusively demonstrated by studies in mice genetically deficient for eotaxin-1/2 (22). Eotaxins act via a single chemokine receptor CCR3. In the mouse, CCR3 is predominantly expressed on eosinophils (22-24), while in humans CCR3 is also expressed on mast cells, basophils, and a subset of TH2 lymphocytes (25). Recent studies report that human and murine angiogenic endothelial cells express CCR3 (26, 27) and that binding of eotaxin on these cells induces proliferation and migration, suggesting eotaxins are angiogenic factors superior to vascular endothelial cell growth factor (VEGF) (26).
Studies in murine models showed that eotaxin-1 is expressed by vascular endothelial and smooth muscle cells and by airway smooth muscle and epithelial cells (14, 28), while eotaxin-2 is mainly expressed by airway lumen macrophages (14). Proangiogenic progenitor cells include a subset of hematopoietic stem cells and lineage progenitors with the capacity to promote neovascularization (reviewed in(29)). We recently reported that proangiogenic progenitor cells from asthmatic patients and ovalbumin (OVA) mouse model express high levels of eotaxin-1(30).
However, the contribution of bone marrow-derived eotaxins to airway inflammation and vascular cell activation and angiogenesis relative to eotaxins secreted by structural cells of the lungs remains unknown. Here we tested the hypothesis that bone marrow-derived proangiogenic progenitor cells that contain eotaxins contribute to the initiation of angiogenesis and inflammation in asthma.
Materials and Methods
Human Study Population
Asthmatics and healthy controls were enrolled in the study for bronchoscopy to collect bronchoalveolar lavage fluid or for whole lung allergen challenge, both performed as previously described (31). Endobronchial biopsies were obtained from some participants. Informed consent was obtained from all, and subjects had baseline characterization of lung functions and exhaled nitric oxide (NO) prior to bronchosocpy or whole lung allergen inhalation. Healthy donor lungs not used for transplantation were used as controls. Serum NO was determined by measure of total NO i.e., nitrite and nitrate as published previously (29, 32).
Animals
For all murine experiments six – eight-week-old female mice were used. Wildtype BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Eotaxin-1/2 double gene deletion mice previously generated by Dr. Rothenberg in the BALB/c background (22) were used. Bone marrow reconstitution was performed by sublethal whole body irradiation (total irradiation dose of 1,000 cGy) of the recipient mice to ablate the bone marrow, followed by tail vein injection of 5 × 106 bone marrow mononuclear cells obtained from donor mice. Bone marrow chimera mice were used for experiments four weeks after the bone marrow transfusion. Pilot experiments using eGFP bone marrow transplantation into wildtype mice showed > 95% eGFP cells in the recipient bone marrow using this protocol. TH2 airway inflammation was induced by intraperitoneal ovalbumin (10 μg OVA in 100 μL, Sigma-Aldrich, St. Louis, MO) or saline (vehicle) sensitization in 20% aluminum hydroxide (alum) as adjuvant and challenge as described previously (30). Mice were challenged for 45 minutes with aerosolized 1% OVA (wt/vol in sterile PBS) or PBS alone for one week using an ultrasonic 2000 nebulizer (Nouvag, Lake Huges, Ca). Saline-alum sensitized and PBS challenged groups served as controls. Twenty-four hours after the final challenge, animals were euthanized and samples collected for analysis. In experiments involving adoptive transfer of proangiogenic cells, animals received a daily tail vein injection of ∼ 0.5 × 106 cells immediately after allergen challenge. In control experiments mice were injected with bone marrow mononuclear cells after magnetic activated cell sorting depletion of SCA-1+ cells using anti-Sca-1 Microbead Kit (Miltenyni Biotec, San Diego, Ca). Immortomouse was purchased from Charles River (Burlington, MA). All animal experiments were approved by the local Institutional Animal Care and Use Committee (IACUC).
Isolation of bone marrow proangiogenic hematopoietic progenitor cells
Bone marrow-derived proangiogenic progenitor cells were isolated as reported (30, 33) by culture of bone marrow mononuclear cells on fibronectin-coated plates in angiogenic medium (EBM-2 medium supplemented with 20% FBS and 20 ng/mL VEGF) for 7 – 12 days. Non-adherent cells were washed away and adherent colonies of proliferating cells were harvested for experiments by trypsinization. Cells were stimulated with IL-25 (R&D systems, Minneapolis, MN) or IL-33 (R&D) for some experiments. These cells were c-Kit+ SCA-1+ VEGFR2+ as previously reported (33).
Quantification of microvessel density and airway inflammation
Microvessel density of the lungs was quantified by staining tissue sections with polyclonal rabbit anti-Von Willebrand Factor antibodies (Dako Cytomation, Glostrup, Denmark) as described previously (7, 33). CCR3 staining was performed on a Ventana Benchmark XT automated immunostainer (Roche Diagnostics Corporation, Indianapolis, IN) utilizing a Ventana iVIEW DAB Detection kit with mild CC2 (citrate) retrieval for 36 minutes and without the provided secondary. Instead, Vector goat anti-rabbit biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) was used at 1:200 for 8 minutes. Primary antibody, monoclonal rabbit anti-CCR3 (Abcam, Cambridge, MA) was diluted 1:1200 and incubated for 32 minutes with heat. Slides were counterstained with Hematoxylin II, dehydrated, cleared and permanently mounted for viewing. Airway inflammation was assessed by quantification of inflammatory cells in bronchoalveolar lavage fluid (BALF) as described (7, 33).
Quantification of eotaxins
Eotaxin-1 and eotaxin-2 levels in BALF and plasma were quantified by using Quantikine ELISA Kits (R&D Systems Inc, Minneapolis, MN). Expression of eotaxins by proangiogenic hematopoietic progenitor cells was quantified using immunofluorescence as described in details elsewhere (30). Rabbit anti-mouse eotaxin-1 (1/50, Santa Cruz) and goat anti-mouse eotaxin-2 (1/50, Santa Cruz) polyclonal antibodies were used. Biotinylated donkey anti-rabbit (1/1,000 Jackson ImmunoResearch Laboratories, West Grove, PA) and streptavidin Alexa Fluor 568 (1/500, Life Technologies. Carlsbad, CA) were used as secondary and tertiary reagents, respectively, for eotaxin-1 detection. Donkey anti-goat Alexa Fluor 488 (1/500, Life Technologies) was used as secondary antibody for eotaxin-2. Values from different experiments were normalized against OVA/OVA group.
Isolation of Endothelial Cells
Wildtype mouse lung endothelial cells are difficult to propagate, but lung endothelial cells are easily cultured from the Immortomouse (34). The transgenic mice carry a thermolabile SV40 large antigen. At 33°C, the SV40 oncogene product is stable, allowing easy propagation of endothelial cells. At higher temperatures, the oncogene is rapidly degraded, allowing for the large-scale generation of endothelial cells for in vitro functional studies (35). Lungs from these mice were digested into single cell suspension using collagenase and dispase. Hematopoietic cells were depleted by a negative sorting for CD45. The CD45-negative cell population was stained for CD31 to sort endothelial cells as described (33). Cells were expanded at 33°C. For experiments, the cells were cultured at 37°C in MCDB-131 complete medium (VEC Technologies Inc. Rensselaer, NY) and used between passage 5 – 7.
Migration Assay
Modified Boyden chamber migration assay was performed as published (36). In short, lower chambers were loaded with different concentrations of eotaxin-1 or eotaxin-2, and a collagen type I-coated 8.0 μm pore PVPF polycarbonate membrane was placed on top. A quantity of 10 × 103 endothelial cells in 50 μL serum free EBM-2 medium was added to the upper chamber. The chamber was incubated at 37°C in a 5% CO2 humidified incubator for 4 hours to allow migration of the cells. After incubation, cells remaining on the top of the membrane were removed. Migrated cells on the bottom of the filter were fixed in 10% formalin, stained with hematoxylin, and the number of migrated cells in each well was counted using light microscopy.
Angiogenesis Assay
In vitro Angiogenesis Assay Kit (EMD Millipore Temecula, CA) was used according to manufacturer's instructions. A quantity of 300 × 103 cells in 500 μL MCDB-131 complete medium were seeded on angiogenesis gel in 12-well. Eotaxin-1 or eotaxin-2 was added at a final concentration of 10 ng/mL. Cells were incubated at 37°C in a 5% CO2 humidified incubator for 4 hours. Per well, three random images were captured at 5× magnification using an inverted microscope (Leica DM IRB). Network analysis of tube-forming endothelial cells was performed in an automated fashion using customized visual basic macros developed within Image-Pro Plus (v7.0, Media Cybernetics, Silver Spring, MD). Briefly, phase-contrast images were imported into Image-Pro in batch mode. Each phase-contrast image was then “flattened” to normalize uneven background illumination, spectrally enhanced, and then morphologically “opened” to equalize intensity of the tube network. These steps enabled application of a fixed threshold to segment the cell network (will be referred to as the “tube mask”) that was subsequently “skeletonized' to create continuous single pixel-width medial lines along the entire tube network. To eliminate spurious skeletal branches, a “pruning” filter was applied to remove branches of a predefined length connected to a single branch node. The final total length of the skeleton and segmented tube network area were subsequently exported to Excel.
Flow Cytometry
Proangiogenic cell colonies were harvested and dispersed into single cell suspension using trypsin. Bone marrow cells were isolated from hind legs followed by lysis of red blood cells using ammonium chloride. Non-specific Fc binding sites were blocked by pre-incubation of cells in Fc-block (Affymetrix eBiosciences, San Diego, CA). Maximum 1 × 106 cells/test were stained using the following cell surface antibodies (CD33-PE (1/50, Santa Cruz, all other antibodies Affymetrix eBiosciences); TER-119-PE (1/25); CD14-PE (1/50); CD19-PE (1/200); CD3-APC (1/125), all antibodies were used at 10 μL/test. CD40-FITC (1/200); CD86 (B7-2)-FITC (1/800); CD80 (B7-1)-FITC (1/400), and MHC Class II-FITC (1/3200), (all antibodies from Affymetrix eBiosciences) were used as cell surface markers for antigen presenting cell. These antibodies were used at 100 μL/test. All antibody dilutions were determined by titration for optimal concentration. Isotype controls were used to assess non-specific binding.
Each incubation was performed for 30 min on ice, and cells were washed twice after each incubation with 1% BSA, 0.02% NaN3 in PBS.
Whole bone marrow was used to analyze eotaxin-2 expression by flow cytometry. Bone marrow from long bones was flushed out in PBS, and red blood cells were lysed using ammonium chloride. Cells were stained with SCA1-APC (1/200, BD); c-Kit-PE (1/200, BD); VEGFR2-APC-Cy7 (1/50, BD) or lineage-specific antibodies as described above, followed by fixation with 4% paraformaldehyde (10 min, room temperature). SCA1, c-Kit and VEGFR-2 antibodies were used at 100 μL/test. After a washing step in PBS, permeabilization was performed using 2% FBS, 0.02% Tween-20 in PBS (30 min, room temperature). Permeabilized cells were stained with goat anti-mouse eotaxin-2 (1/50, Santa Cruz) for 30 min at room temperature, followed by donkey anti-goat Alexa Fluor 488 (1/500, Life Technologies) as second step. Normal goat IgG was used as negative control for eotaxin-2 staining. Background fluorescence was subtracted for the quantification of eotaxin fluorescence intensity levels. For the quantification of eotaxin-2 in SCA1+c-Kit+VEGFR2+ progenitors, mononuclear cells were gated on FSC/SSC plots after dead cell exclusion, followed by gating of SCA1+c-Kit+ cells. Eotaxin-2 expression was analyzed on eotaxin-2/VEGFR-2 dots on the gated SCA1+c-Kit+ cells.
ST2 (IL-33R)-PerCP-eFluor710 (1/80, eBioscience); CD90-FITC (1/40, SouthernBiotech, Birmingham, AL); IL7R alpha (CD127)-eFluor 450 (1/80, eBioscience) antibodies were used to stain IL-33 stimulated proangiogenic progenitors for ILC2 cell surface antigens, followed intracellular staining for cytokines using IL-5-APC (1/40, Biolegend, San Diego, Ca) and IL-13-PE (1/200, eBioscience). GolgiStop (BD) was added to the cell culture 6 hours prior to harvest. Cell surface and intracellular cytokine stains were performed as described above for lineage markers and intracellular eotaxin-2 staining. Live/Dead Blue dye (ThermoFisher Scientific) was used to exclude dead cells. All ILC2 antibodies were titrated to determine optimal dilutions using OVA/OVA splenocytes.
Expression of CCR3 on lung endothelial cells was analyzed in lung single cell suspension obtained by whole organ digestion (33). Lung single cell suspensions were pre-treated with Fc-block, and aliquots of 1 × 106 cells/tube were stained with CD31-PE-Cy7 (1/130, Affymetrix eBiosciences), CD45-APC (1/50, Affymetrix eBiosciences) and CCR3-PE (1/2.5, R&D). All antibodies were used at 10 μL/test. Cells stained with CD31-PE-Cy7 and CD45-APC in combination with CCR3 isotype-PE matched control were used as gating control for CCR3+ endothelial cells. Each incubation was performed for 30 min on ice, and cells were washed twice after each incubation with 1% BSA, 0.02% NaN3 in PBS. After the final wash, cells were resuspended in 7-AAD (BD Biosciences, San Jose, CA) to discriminate dead cells.
All samples were analyzed on a LSRII flow cytometer equipped with 5 lasers (488 nm, 639 nm, 532 nm, 355 nm and 405 nm) with standard configurations. For ILC2 antigen analysis and eotaxin-2 expression in SCA-1+c-Kit+VEGFR2+ progenitors, samples were run on a LSRFortessa flow cytometer equipped with 5 lasers (488 nm, 641 nm, 561 nm, 355 nm and 407 nm) with standard configurations. A minimum of 10,000. 50,000 or 200,000 events were acquired for the analysis of proangiogenic cells, bone marrow and lung single cell suspensions, respectively. Data were stored as listmode files and analyzed using Flowjo 9.4.6 or Flowjo V10 software (Treestar Ashland, Or).
Statistical analysis
Statistical analysis was performed using JMP 5.1 software program. ANOVA or Student's t-test were used for comparisons of parametric data, and Wilcoxon test was used for comparison of nonparametric data, as appropriate. p-values <0.05 were considered as significant. Mean ± SE value for each group is shown.
Results
Endothelial Activation is an Early Response to Inhaled Allergens in Human Asthma
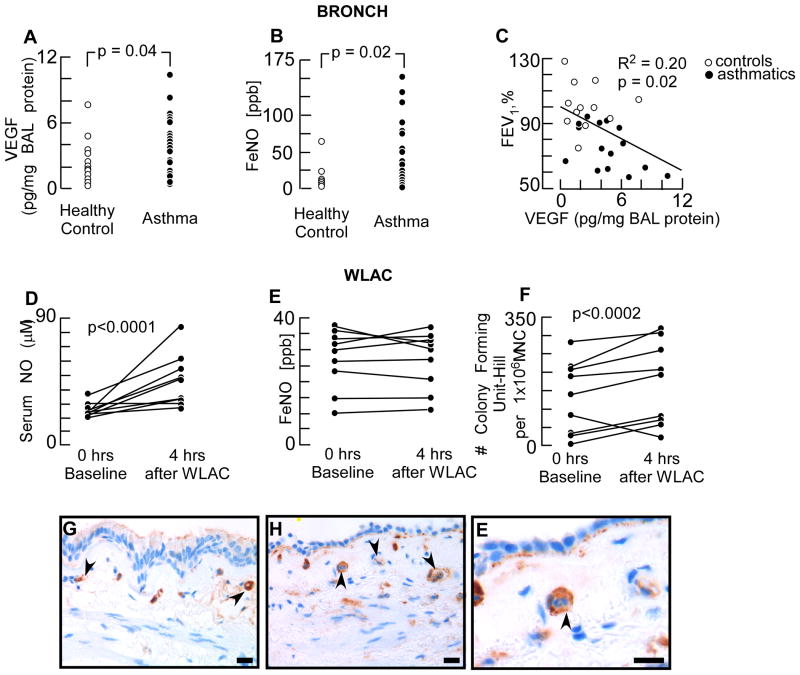
Demographics and clinical characteristics of participants are summarized in table I. The airways of asthma patients have a proangiogenic milieu as shown by increased VEGF levels in BAL fluid (Fig 1A), analogous to the higher levels of exhaled NO (Fig 1B). There was a significant correlation between airflow limitation as measured by %FEV1 and VEGF levels (Fig 1C). Exposure of patients to whole lung allergen to initiate an experimental asthma exacerbation resulted in a rapid vascular response. Within 4 hours after allergen exposure serum NO levels increased in all asthmatics (Fig 1D) (serum NO: before challenge 25.6 ± 1.7 μM; after challenge 46.6 ± 6.0 μM, p = 0.008). The exhaled NO, an indirect measure for airway inflammation (37), at 4 hours after allergen was similar to levels at baseline (Fig. 1 E). There was no correlation between serum and exhaled NO levels. In strong support of the early vascular response to allergen, proangiogenic progenitors were rapidly mobilized from the bone marrow into the circulation, as indicated by increased colony forming units of cells derived from blood (Fig 1F). Diluent control challenge was not part of our study. Others have shown that control challenge of asthma patients doesn't induce progenitor cell mobilization (9). The findings of the rapid vascular responses to allergen prompted us to analyze angiogenic markers on the airway endothelium. Asthmatic and healthy lungs were evaluated for CCR3 by immunohistochemistry. Bronchial capillary blood vessels in both healthy control subjects and asthma patients were positive for CCR3 (Fig 1 G-E), indicating that the bronchial endothelium has the potential to become angiogenic upon binding of eotaxins (26, 27). Altogether these findings show that proangiogenic progenitors are rapidly mobilized and vascular endothelium rapidly activated in asthmatics undergoing an experimental asthma attack.
Table I. Characteristics of the study subjects.
| Healthy controls | Asthma | |
|---|---|---|
| Number | 12 | 31 |
| Age (years) | 35 ± 3 | 38 ± 2 |
| Methacholine PC20 (μg/mL) | ND | 3.4 ± 1.4 |
| Predicted FEV1 (%) | 104.4 ± 4.0 | 81.0 ± 2.9 |
| Predicted FVC (%) | 102.8 ± 4.0 | 89.0 ± 3.7 |
| FEV1/FVC (%) | 80.6 ± 1.3 | 72.6 ± 1.8 |
| Sex (male/female) | 6/6 | 10/21 |
| Race (AA/C/H/O) | 4/5/0/3 | 10/18/0/3 |
Values are presented as mean ± SE. AA= African American, C= Caucasian, H= Hispanic, O = Other, M = Male, F = Female, ND= not done.
Fig 1. Early Endothelial Activation after Allergen Exposure in Human Asthma.
[A] VEGF levels in BAL fluid measured by ELISA. [B] Fraction of exhaled NO (FeNO) in asthma and healthy control subjects. Each dot represents data from one subject. [C] Correlation between %FEV1 and BAL VEGF levels. Open dots are values from healthy controls and filled dots from asthma patients. [D – F] Serum and exhaled NO levels, and circulating proangiogenic hematopoietic progenitors before and after whole lung allergen challenge (WLAC). [G-E] Immunohistochemistry of CCR3 expression in submucosal endothelium. Healthy control [G] and asthmatic [H-E] human bronchi showed CCR3 expression on endothelial cells in the bronchial capillaries. High power image [E] showed CCR3 positive capillary endothelium with an inflammatory cell in the lumen. Scale bar = 200 μm
Up-regulation of Eotaxin Receptor CCR3 on Lung Endothelial Cells Contributes to Pathological Angiogenesis in OVA Model of Asthma
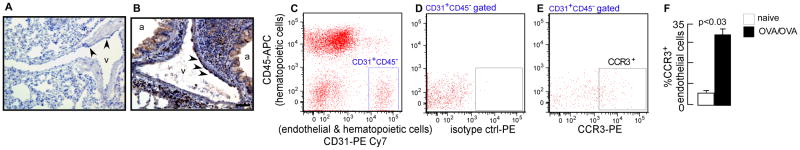
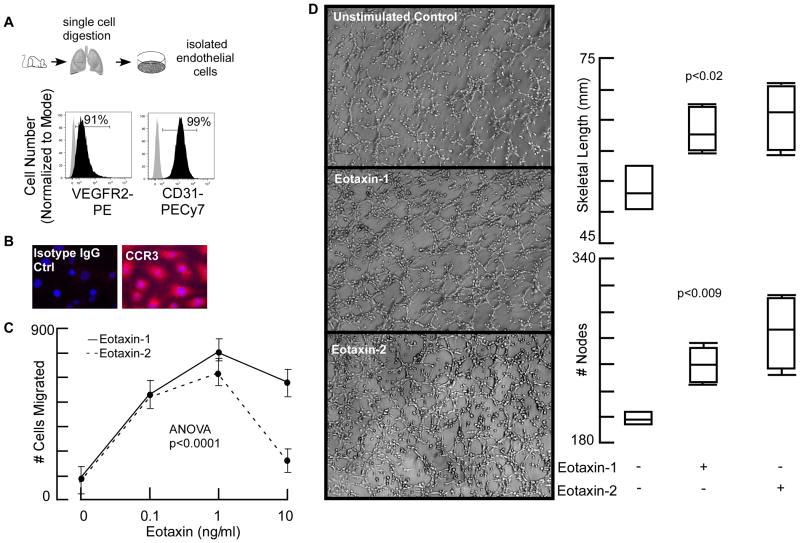
To quantify the expression of CCR3 in asthma, we turned to a mouse model of antigen-induced airway inflammation. Immunohistochemistry showed that naïve mouse lung endothelium has CCR3 expression at low levels (Fig 2A) compared to asthmatic mice, where it is strongly positive (Fig 2B). Flow cytometric analysis and quantification of the receptor showed strong upregulation of CCR3 on endothelial cells from OVA/OVA mice lungs (Fig 2C-F). The results demonstrate that eotaxin receptor CCR3, a marker for pathological angiogenesis (26, 27), is up-regulated on endothelial cells in the murine asthma model. The expression of CCR3 by lung endothelial cells raised the question of whether eotaxin could drive angiogenesis in asthma. To test this, endothelial cells were evaluated exvivo (Fig 3A). Endothelial expression of CCR3 in cultures could be detected by fluorescence staining of cells (Fig 3B). Boyden-chamber migration assay demonstrated a dose-dependent migration of the endothelial cells towards eotaxin-1 and eotaxin-2 (Fig 3C). Matrigel tube formation assay showed that eotaxins induced robust angiogenesis (Fig 3D). Total angiogenic skeletal length and number of branching points (nodes) were significantly higher in the presence of eotaxins (Fig 3D). These data identify that eotaxin promotes angiogenesis, providing a mechanism for the TH2-induced pathologic angiogenesis in asthma.
Fig 2. Increased expression of eotaxin receptor CCR3 on lung endothelial cells in murine asthma model.
CCR3 expression was analyzed by immunohistochemistry on paraffin embedded lung sections and flow cytometry of lung single cell suspensions. [A] Naïve mouse lung endothelium showed expression of CCR3 at low levels. [B] Allergen exposed mouse lung had segments of the endothelium expressing CCR3. [C] Flow cytometric gating of lung endothelial cells (CD31+CD45-). [D & E] Analysis of CCR3 expression on the endothelial cells. [F] Open bar naïve mouse lung; closed bar OVA sensitized and day 6 ova challenged lung (n=3). Scale bar = 200 μm
Fig 3. Eotaxins Induced Lung Endothelial Cell Migration and Angiogenesis.
[A] Purity of isolated lung endothelial cells assessed by flow cytometry. [B] Immortomouse lung endothelial cells express eotaxin receptor CCR3. [C] Migration of lung endothelial cells toward eotaxins was analyzed using a Boyden chamber assay. There was a dose-dependent increase in endothelial migration toward both eotoxins. As expected, at a higher concentration the migration was inhibited. [D] In vitro Matrigel assays were performed to analyze the effects on angiogenic tube formation. Cells were seeded on Matrigel in the presence or absence of eotaxin. After four hours, phase-contrast images were captured for automated quantification. Eotaxins increased total skeletal length and number of branching points. Mean ± SE values of 4 mice per group are shown.
TH2-Polarization Regulates Eotaxin Expression by Murine Proangiogenic Progenitor Cells
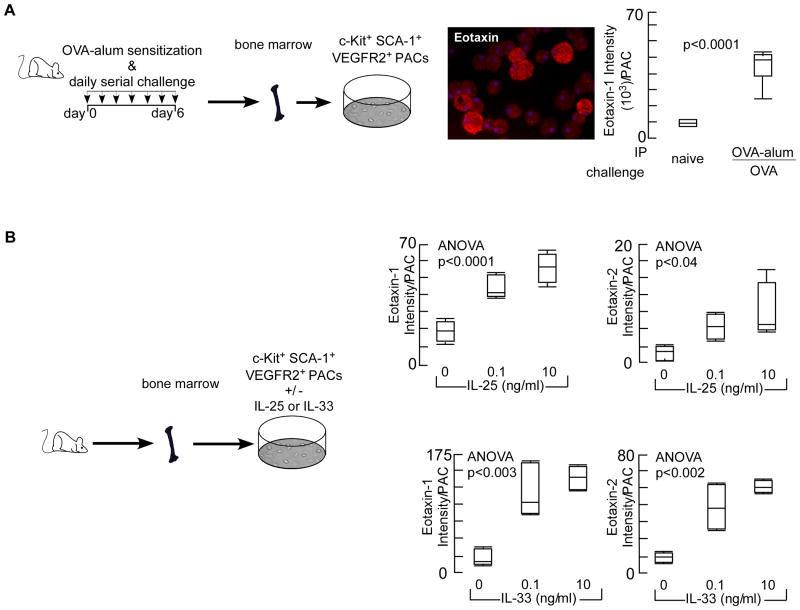
Proangiogenic progenitor cells carry high levels of eotaxins in asthmatics, making them an immediate source for genesis of atopic inflammation and angiogenesis (30). Because allergic asthma is characterized by TH2 cytokine inflammation, we analyzed whether the induction of eotaxins in proangiogenic progenitor cells is controlled by TH2 immune response. Bone marrow-derived proangiogenic cells from TH2 polarized mice (OVA-alum/OVA) had greater eotaxin expression than cells from naïve animals (Fig 4A). IL-25 (38-42) and IL-33 (35, 43) are central upstream initiators of TH2 response and in vitro stimulation of proangiogenic progenitor cells obtained from bone marrows of naïve wildtype mice showed a dose-dependent increase in the expression of eotaxins (Fig 4B). The data reveal that the high level expression of eotaxin in the bone marrow proangiogenic progenitor cells is a fundamental change in asthma, i.e. a TH2-promoting proangiogenic progenitor endotype.
Fig 4. Eotaxin Expression by Proangiogenic Progenitors is TH2-Dependent.
[A] TH2 response was induced in mice by sensitizing animals with OVA-aluminum hydroxide (OVA-alum) followed by OVA challenge. Proangiogenic hematopoietic progenitors (PACs) were isolated and expression of eotaxins was analyzed. Cytospins of proangiogenic progenitors were made, and immunofluoresence quantification for eotaxin was performed. Quantification of eotaxin content/cell. Box plot graphs of 6 mice in each group are shown. Eotaxin-2 was not quantified in this experiment [B] Proangiogenic hematopoietic progenitors were isolated from naÏve mice and ex vivo stimulated with TH2-regulators IL-25 or IL-33. Eotaxin was quantified on cytospins as described under [A] or Methods section. Both eotaxin 1&2 were upregulated by IL-25 or IL-33 in a dose dependent-manner. Box plot graphs of 4 mice/group are shown.
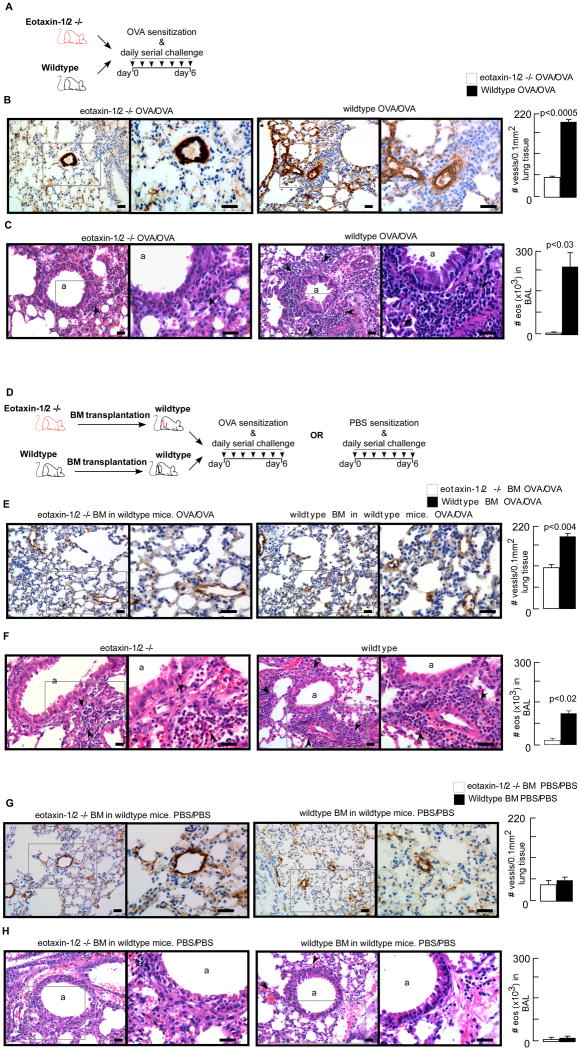
Reduced Angiogenesis and Airway Inflammation in Wildtype Mice Reconstituted with Eotaxin-1/2 Deficient Bone Marrow
Wildtype OVA/OVA mice exhibited increased airway inflammation and angiogenesis in contrast to eotaxin-1/2 deficient mice (Fig 5 A – C). To mechanistically evaluate the role of the proangiogenic cells in asthma pathogenesis, wildtype mice were engrafted with bone marrow cells isolated from wildtype or eotaxin-1/2 knockout mice in the OVA model (Fig 5D). Microvessel density of the lungs in recipients of eotaxin-1/2 KO bone marrow was markedly decreased as compared to those mice receiving wildtype bone marrow (Fig 5E). Inflammation of the airways and influx of eosinophils in peribronchial areas and in the BAL fluid were also blunted in wildtype mice engrafted with eotaxin-1/2 bone marrow (Fig 5F). Negative control groups, including mice undergoing bone marrow transplantation but PBS sensitized and PBS challenged (PBS/PBS) had no increase in angiogenesis or airway inflammation (Fig 5 G – H). The data show that bone marrow eotaxins control angiogenesis and inflammation, identifying bone marrow-derived eotaxin-rich cells as critical initiators of allergic airway disease.
Fig 5. Eotaxin-1/2 deficient bone marrow transplantation into wildtype mice reduced pathological angiogenesis and eosinophilic airway inflammation.
[A] Wildtype or eotaxin-1/2 deficient mice were sensitized and challenged with OVA allergen. Twenty-four hours after the final allergen exposure, animals were euthanized for analysis. [B] Lung angiogenesis was quantified on paraffin embedded tissue sections stained for endothelial cell marker von Willebrand Factor (vWF). OVA/OVA wildtype mice had a higher microvessel density compared to OVA/OVA eotaxin-1/2 deficient mice. [C] Airway inflammation was analyzed by staining lung sections for hematoxylin & eosin. Eosinophils in the bronchoalveolar lavage (BAL) were quantified by differential staining of BAL cytospins with Diff-Quick. Airway inflammation was blunted in OVA/OVA eotaxin-1/2 deficient compared to OVA/OVA wildtype mice. Black arrows indicate inflammatory cells. [D] Bone marrow mononuclear cells isolated from wildtype or eotaxin-1/2 deficient mice were engrafted into sublethally irradiated wildtype mice. Four weeks after the bone marrow transplantation animals were sensitized and challenged with OVA allergen or PBS. Twenty-four hours after the final allergen exposure, animals were euthanized for analysis. [E] Lung angiogenesis was quantified on paraffin embedded tissue sections stained for endothelial cell marker von Willebrand Factor (vWF). Compared to recipients of wildtype bone marrow, animals receiving eotaxin-1/2 deficient bone marrow exhibited significantly decreased microvessel density in the OVA/OVA group. [F] Airway inflammation was analyzed by staining lung sections for hematoxylin & eosin. Eosinophils in the bronchoalveolar lavage (BAL) were quantified by differential staining of BAL cytospins with Diff-Quick. Airway inflammation was significantly reduced in OVA/OVA wildtype mice reconstituted with eotaxin-1/2 deficient bone marrow. Black arrows indicate inflammatory cells. [G – H] Recipients of wildtype or eotaxin-1/2 deficient bone marrow in the PBS/PBS groups showed no increase in lung microvessel density or airway inflammation. Mean ± SE values of 4 mice/group are shown. Low power (200×) and high power (400×) images are shown. Scale bar in each image represents 100 μm.
Adoptive Transfer of Wildtype Proangiogenic Progenitor Cells in Eotaxin-1/2 Deficient Mice Confers Airway Inflammation and Angiogenesis
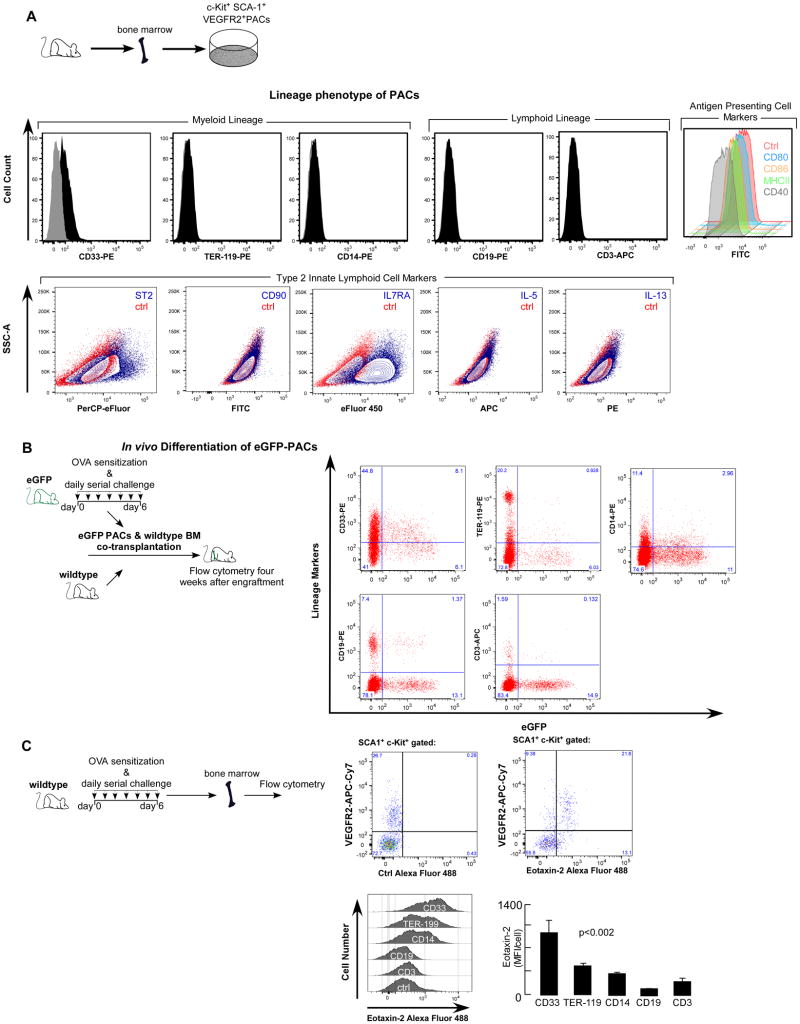
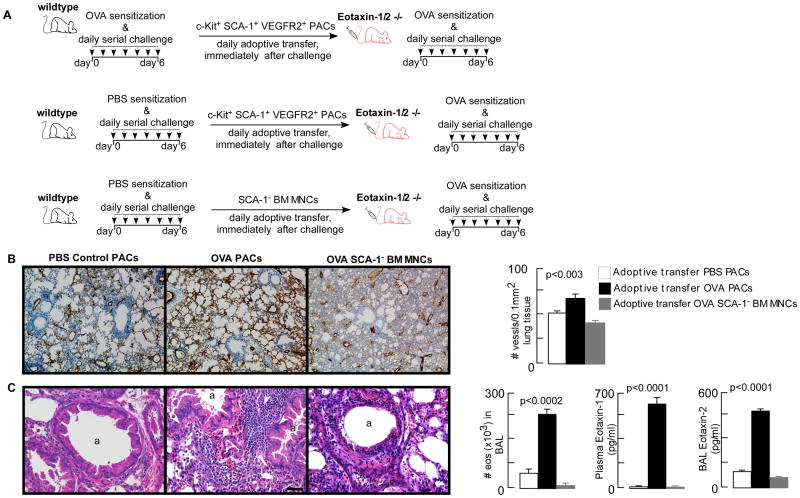
A variety of bone marrow-derived cells, including mature hematopoietic cells and hematopoietic progenitor cells, can induce angiogenic activity (29). Among these cells, proangiogenic progenitor cells are the major contributors to angiogenesis (29). Further, we and others have shown that bone marrow-derived proangiogenic progenitor cells are linked to angiogenesis and eosinophilic airway inflammation (5, 7-9, 30, 33). Proangiogenic progenitor cells from asthmatic patients and murine models of asthma express hematopoietic stem/progenitor cell surface antigens (7, 30, 33), but it is unknown from which exact hematopoietic compartment the proangiogenic progenitor cells are derived. To analyze this, bone marrow proangiogenic progenitor cells were isolated from OVA/OVA eGFP mice and co-engrafted with wildtype bone marrow into sublethally irradiated recipients. OVA/OVA proangiogenic progenitor cells expressed hematopoietic progenitor cell and pan-myeloid marker CD33, but were negative for erythroid (Ter-119), monocyte (CD14), B-cell (CD19) and T-cell (CD3) markers (Fig 6A). Proangiogenic progenitor cells were also negative for antigen presenting cell antigens including MHC II and co-stimulatory molecules CD40, CD80 (B7-1), and CD87 (B7-2) (Fig 6A). The co-expression of SCA1 and c-Kit by the proangiogenic progenitors and their response to IL-25 and IL-33, characteristics of type 2 innate lymphoid cells (ILC2) antigens (44), lead us to characterize IL-33 stimulated proangiogenic progenitors for ILC2 signature antigens ST2, IL17R, CD90, IL-5 and IL-13 (44). Proangiogenic progenitors expressed ST2, IL7R alpha, but are negative for CD90, IL-5 and IL-13 (Fig 6A), demonstrating that these cells are not ILC2. After engraftment into wildtype mice, eGFP proangiogenic progenitor cells were able to differentiate into both myeloid and lymphoid lineages, confirming that these cells are hematopoietic progenitors (Fig 6B). Compared to wildtype hematopoietic stem/progenitor cells, OVA/OVA proangiogenic progenitor cells exhibited greater monocyte, but less erythroid and T-cell, differentiation (Table II). Eotaxin-2 antibodies were suitable for flow cytometry, and data shown in Fig 6C demonstrates eotaxin-2 expression by bone marrow SCA1+c-Kit+VEGFR2+ progenitors and cells in the various hematopoietic lineages in OVA/OVA mice. Majority of the SCA-1+c-Kit+VEGFR2+ progenitors (66.1 ± 7.8%, mean ± SE values, n=4) were positive for eotaxin-2. Consistent with CD33 expression by PACs, CD33+ bone marrow cells exhibited highest eotaxin-2 expression followed by TER-119 (erythroid) and CD14 (monocytic) cells. B-cells (CD19) had the lowest levels, while T-cells (CD3) were also positive for eotaxin-2, similar to prior report (45). PBS/PBS or naïve mice bone marrow cells exhibited low level of eotaxin-2 expression, similar to eotaxin-1 as reported previously (30). We determined whether the proangiogenic progenitor cells are the significant source of the bone marrow eotaxin-rich cells driving angiogenesis and eosinophilia. Proangiogenic progenitor cells were isolated from bone marrow of OVA sensitized and airway OVA exposed (OVA/OVA) mice. Animals sensitized with saline and challenged with PBS (control) were used as a source for non-asthmatic control proangiogenic progenitor cells. Eotaxin-1/2 KO mice were injected intravenously with proangiogenic progenitor cells immediately after each allergen challenge (Fig 7A). Adoptive transfer of proangiogenic progenitor cells from wildtype OVA/OVA mice, but not proangiogenic progenitor cells from wildtype control mice, led to robust angiogenesis and airway inflammation in the eotaxin-1/2 KO mice (Fig 7 B – C). Data presented here and previously published (33) show that proangiogenic progenitors co-express SCA-1, c-Kit, VEGFR2, and CD33. Adoptive transfer of SCA-1-depleted bone marrow mononuclear cells failed to induce angiogenesis or airway inflammation, demonstrating that progenitor cells, but not their progeny, are a significant source for eotaxin-induced airway inflammation and angiogenesis. Eotaxin-2 levels were increased BAL fluid, but eotaxin-1 levels were increased in plasma only, in mice receiving wildtype OVA/OVA proangiogenic progenitor cells (Fig 7C). This compartmentalized expression of eotaxin-1 and eotaxin-2 plays a functional role in the recruitment of eosinophils from the peripheral blood into the airways, as reported previously (46). The findings demonstrate that proangiogenic progenitor cells are hematopoietic progenitors and serve as a primary source of the eotaxins, which lead to angiogenesis and eosinophilic airway inflammation.
Fig 6. Proangiogenic cells were able to engraft bone marrow and differentiated into hematopoietic lineages.
[A] Proangiogenic hematopoietic progenitors (PACs) were isolated from OVA/OVA eGFP mice. We reported previously that these are CD45+SCA-1+c-Kit+VEGFR2+. Here we show that the cells express pan-myeloid marker CD33, but are negative for erythroid (TER-119) or monocyte (CD14) antigens, and don't express B-cell (CD19) and T-cell (CD3) markers. PACs were also negative for antigen presenting cell antigens including MHC II and co-stimulatory molecules CD40, CD80 (B7-1), and CD87 (B7-2). IL-33 stimulated PACs were analyzed for type 2 innate lymphoid cell markers and were positive for ST2 and IL7RA, but negative for CD90 and intracellular IL-5 and IL-13. [B] To analyze the hematopoietic differentiation potential of these cells, mice were sublethally irradiated and engrafted with ∼0.7×106 eGFP proangiogenic cells and equal number of wildtype bone marrow cells. After four weeks bone marrow cells were isolated and analyzed by flow cytometry. GFP+ and GFP- cells were gated for lineage antigen expression. Proangiogenic hematopoietic progenitor cell population was able to differentiate into both myeloid and lymphoid lineages, demonstrating that these cells are hematopoietic progenitors. Mean ± SE values of 4 mice/group are shown. [C] Bone marrow cells were isolated from OVA/OVA mice and the expression of eotaxin-2 was analyzed by flow cytometry. To analyze the expression of eotaxin-2 in SCA1+c-Kit+VEGFR2+ cells, gated SCA1+c-Kit+ cells plotted on eotaxin-2/VEGFR-2 plots. Background boundary for VEGFR2 channel was set on cells stained for SCA1 and c-Kit only. Cut off for eotaxin-2 expression was determined by replacing eotaxin-2 antibodies by isotype control IgG. Overlay histograms show expression level by the various hematopoietic lineages. Bar graphs show median fluorescence intensity (MFI) per cell. Mean ± SE values of 4 mice/group are shown.
Table II. eGFP OVA/OVA proangiogenic progenitors co-injected with wildtype bone marrow were able to engraft bone marrow and differentiated into hematopoietic lineages.
| WT population | eGFP+ population | p-value | |
|---|---|---|---|
| Lymphoid | |||
| CD19 | 8.88 ± 1.56 | 11.38 ± 3.88 | NS |
| CD3 | 1.76 ± 0.18 | 0.76 ± 0.20 | 0.01 |
| Myeloid | |||
| CD14 | 12.95 ± 0.40 | 18.42 ± 0.95 | 0.006 |
| CD33 | 50.28 ± 1.89 | 55.42 ± 3.40 | NS |
| Erythroid | |||
| TER-119 | 24.09 ± 1.05 | 17.65 ± 1.63 | 0.02 |
Bone marrow cells were RBC-lysed, stained for lineage antigens and analyzed on a LSRII flow cytometer. Distribution of lineage antigens were quantified on gated eGFP+ or eGFP- (WT) cells. Mean ± SE values of 4 mice/group are shown.
Fig 7. Adoptive transfer of wildtype proangiogenic cells into eotaxin-1/2 deficient mice induced pathological angiogenesis and eosinophilic airway inflammation.
[A] Proangiogenic hematopoietic progenitors were isolated from OVA/OVA or PBS/PBS wildtype mice and intravenously injected (∼0.5×106 cells/mouse) into eotaxin-1/2 knockout mice after each allergen challenge. Adoptive transfer of SCA-1 depleted bone marrow mononuclear cells was used as additional control. Twenty-four hours after the final allergen inhalation, animals were analyzed. [B] Lung angiogenesis was quantified on paraffin embedded tissue sections stained for endothelial cell marker von Willebrand Factor (vWF). Compared to recipients of PBS/PBS progenitors or OVA/OVA SCA-1 depleted bone marrow mononuclear cells, animals receiving OVA/OVA progenitors showed significantly increased microvessel density. [C] Airway inflammation, analyzed by eosinophils in the bronchoalveolar lavage (BAL), showed increased eosinophilia in eotaxin-1/2 deficient mice injected with OVA/OVA progenitors. White arrows indicate inflammatory cells on hematoxylin & eosin staining. Eotaxin concentration was measured in plasma by ELISA. Compared to animals injected with PBS/PBS wildtype progenitors or OVA/OVA SCA-1 depleted bone marrow mononuclear cells, animals receiving OVAOVA progenitors had significantly increased eotaxin-1 in the plasma and eotaxin-2 in BAL. Mean ± SE values of 3-4 mice/group are shown. Scale bar = 100 μm.
Discussion
Here, we show that activation of the endothelium is one of the earliest responses to inhaled allergens as evidenced by vascular release of NO and mobilization of bone marrow-derived proangiogenic progenitors. Eotaxin-rich TH2 promoting proangiogenic progenitor cells interact with the lung vascular endothelium to initiate angiogenesis and consequently eosinophilic airway inflammation. The proangiogenic cells have bone marrow engraftment capacity and are able to differentiate into in all hematopoietic lineages, but predominantly in the myeloid monocytic series. CCR3, the receptor for eotaxins and a marker of angiogenic endothelium, was present at high levels on lung endothelium in asthmatics and mice exposed to allergen. Both eotaxin-1 and eotaxin-2 could induce migration and angiogenic tube formation by CCR3+ lung endothelial cells. Altogether, the findings reveal that early activation of the endothelium and mobilization of TH2-promoting eotaxin-rich proangiogenic progenitor cells are requisite for the pathologic angiogenesis and eosinophilic airway inflammation in allergen-induced asthma.
Recruitment of bone marrow proangiogenic cells into allergen exposed lungs is one of the earliest events in animal models (7) and in patients (9). Onset of angiogenesis is initiated shortly after the lung homing of proangiogenic cells, followed by increased influx of eosinophils and worsening of lung function (7, 9). Inhibition of angiogenesis blunts the pathological TH2-response (33). Here, eotaxins released by proangiogenic cells were a critical contributor of this process. Proangiogenic cells secrete a plethora of angiogenic factors (47) to maintain endothelium integrity and promote healthy vessels. Little is known about angiogenic factors secreted by these cells that specifically contribute to the pathological angiogenesis. Our data show that eotaxins are a family of such cytokines contributing to abnormal neovascularization in inflamed airways. The expression of eotaxins by the proangiogenic cells and the up-regulation of its receptor CCR3 on the endothelium are both tightly regulated by TH2 pathways and allergen exposure. Although structural cells in the lung also express eotaxins, the data suggest that this is not sufficient to induce full-blown airway disease. In line with these findings, others have shown that eotaxin expression by resident tissue cells is not sufficient to induce eosinophilia in macular degeneration (26). The lung endothelium in asthma may be the important gatekeeper for the influx of eosinophils by regulating eotaxin release by the circulating proangiogenic cells (30). Overall, these findings indicate that bone marrow-derived eotaxins are critically important in the initiation of eosinophilic inflammation in response to atopic events. Airway hyperreactivity was not studied in this work. While airway inflammation and airway hyperreactivity are both critical aspects of asthma, eosinophilic airway inflammation and airway hyperreactivity may occur independently from each other (48-50).
Notably in this report, the bone marrow hematopoietic stem/progenitor cell compartment was TH2-promoting without direct bone marrow allergen exposure, suggesting that signals from the allergen exposed airway epithelium or other lung cells induced a TH2 response in the bone marrow. In keeping with this observation, we have reported previously that allergen sensitization alone is sufficient to induce eotaxin in the bone marrow (30). The data indicate that IL-25 and IL-33 are likely potent factors in this change in the myeloid compartment, as eotaxin expression could be induced in the proangiogenic cells by IL-25 or IL-33 in vitro. IL-25 (IL-17E) is a relatively recently described upstream master regulator of TH2-responses (38-42). Mice treated with IL-25 have greater eotaxin expression in many organs (42). IL-25 is expressed by several cell types such as airway epithelial cells (38), activated mast cells (51), T-cells (42), eosinophils (52) and endothelial cells (53-55), but the precise cellular targets of IL-25 remain elusive (39, 56). IL-25 can act on non-B/non-T/non-mast cell bone marrow-derived myeloid progenitor (39), T-cells (40) (57), and IL17RB+ granulocytes (58). Many cell types express IL-33, including epithelial cells, fibroblasts, smooth muscle cells, and hematopoietic cells such as macrophages and dendritic cells (59). IL-33 induces the release of TH2 cytokines such as IL-4, IL-13 and IL-5 by acting on a variety of immune cells and IL-25 receptor positive type 2 innate immune cells (35, 42, 60). In a recent study, IL-33-stimulated human hematopoietic progenitor cells exhibited secretion of IL-5 and IL-13 (43). Here, IL-25 and IL-33 are also observed to induce eotaxin expression by bone marrow proangiogenic hematopoietic progenitors. Immunophenotyping of the proangiogenic progenitors cells showed that while these cells do not express markers characteristic for type 2 innate lymphoid cells, they do differentiate into various hematopoietic lineages after bone marrow restitution. However, there may still be the possibility that in vivo differentiation of progenitors into T-cells or type 2 innate lymphoid cells may contribute to the observed phenotype. Further studies are needed to dissect the potential overlap between the differentiation pathways of type 2 innate lymphoid cells cells and proangiogenic progenitors.
Altogether, the early response of CCR3 positive vascular endothelium in the lung and eotaxin-rich proangiogenic progenitor cells in the bone marrow reveal a vascular origin of atopic inflammation of asthma, which offers possible new targets to prevent allergen-induced exacerbations.
Acknowledgments
The authors thank the Lerner Research Institute Imaging and Flow Cytometry Core staff and Jodi Hanson, Benjamin Savasky, and Colin Venner for excellent technical assistance. We thank Dr. Booki Min from the Department of Immunology for helpful discussion. Kewal Asosingh is a Scholar of the International Society for Advancement of Cytometry.
Grant Support: Supported by the American Heart Association grant 11SDG4990003, and NIH grants HL103453, HL081064, and HL109250, and the Alfred Lerner Memorial Chair in Innovative Biomedical Research at the Cleveland Clinic.
References
- 1.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 2.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–906. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrugt B, Wilson S, Bron A, Holgate ST, Djukanovic R, Aalbers R. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J. 2000;15:1014–1021. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 4.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116:477–486. doi: 10.1016/j.jaci.2005.07.011. quiz 487. [DOI] [PubMed] [Google Scholar]
- 5.Asosingh K, Erzurum SC. Angioplasticity in asthma. Biochem Soc Trans. 2009;37:805–810. doi: 10.1042/BST0370805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med. 2004;170:683–690. doi: 10.1164/rccm.200311-1539WS. [DOI] [PubMed] [Google Scholar]
- 7.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178:6482–6494. doi: 10.4049/jimmunol.178.10.6482. [DOI] [PubMed] [Google Scholar]
- 8.Doyle TM, Ellis R, Park HJ, Inman MD, Sehmi R. Modulating progenitor accumulation attenuates lung angiogenesis in a mouse model of asthma. Eur Respir J. 2011;38:679–687. doi: 10.1183/09031936.00133210. [DOI] [PubMed] [Google Scholar]
- 9.Imaoka H, Punia N, Irshad A, Ying S, Corrigan CJ, Howie K, O'Byrne PM, Gauvreau GM, Sehmi R. Lung homing of endothelial progenitor cells in humans with asthma after allergen challenge. Am J Respir Crit Care Med. 2011;184:771–778. doi: 10.1164/rccm.201102-0272OC. [DOI] [PubMed] [Google Scholar]
- 10.Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 11.Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, Sano Y, Hanai N, Furuya A, Ohta K, Matsushima K, Yoshie O, Yamamoto K, Hirai K. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothenberg ME. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- 14.Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 16.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao JL, Sen AI, Kitaura M, Yoshie O, Rothenberg ME, Murphy PM, Luster AD. Identification of a mouse eosinophil receptor for the CC chemokine eotaxin. Biochem Biophys Res Commun. 1996;223:679–684. doi: 10.1006/bbrc.1996.0955. [DOI] [PubMed] [Google Scholar]
- 18.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combadiere C, Ahuja SK, Murphy PM. Cloning and functional expression of a human eosinophil CC chemokine receptor. J Biol Chem. 1996;271:11034. [PubMed] [Google Scholar]
- 21.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. [Google Scholar]
- 22.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HH, Ochkur SI, McGarry MP, Lee NA, Lee JJ. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003;284:L169–178. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 24.Grimaldi JC, Yu NX, Grunig G, Seymour BW, Cottrez F, Robinson DS, Hosken N, Ferlin WG, Wu X, Soto H, O'Garra A, Howard MC, Coffman RL. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J Leukoc Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- 25.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, Oppenheim JJ. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 28.Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol. 2006;533:277–288. doi: 10.1016/j.ejphar.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 29.Rose JA, Erzurum S, Asosingh K. Biology and flow cytometry of proangiogenic hematopoietic progenitors cells. Cytometry A. 2015;87:5–19. doi: 10.1002/cyto.a.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asosingh K, Hanson JD, Cheng G, Aronica MA, Erzurum SC. Allergen-induced, eotaxin-rich, proangiogenic bone marrow progenitors: a blood-borne cellular envoy for lung eosinophilia. J Allergy Clin Immunol. 2010;125:918–925. doi: 10.1016/j.jaci.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol (1985) 2003;95:436–440. doi: 10.1152/japplphysiol.01127.2002. discussion 435. [DOI] [PubMed] [Google Scholar]
- 32.Naples R, Laskowski D, McCarthy K, Mattox E, Comhair SA, Erzurum S. in press. Carboxyhemoglobin and methemoglobin in asthma. Lung. doi: 10.1007/s00408-015-9686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asosingh K, Cheng G, Xu W, Savasky BM, Aronica MA, Li X, Erzurum SC. Nascent endothelium initiates Th2 polarization of asthma. J Immunol. 2013;190:3458–3465. doi: 10.4049/jimmunol.1202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oboki K, Nakae S, Matsumoto K, Saito H. IL-33 and Airway Inflammation. Allergy, asthma & immunology research. 2011;3:81–88. doi: 10.4168/aair.2011.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 37.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR A. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith SG, Gugilla A, Mukherjee M, Merim K, Irshad A, Tang W, Kinoshita T, Watson B, Oliveria JP, Comeau M, O'Byrne PM, Gauvreau GM, Sehmi R. Thymic stromal lymphopoietin and IL-33 modulate migration of hematopoietic progenitor cells in patients with allergic asthma. J Allergy Clin Immunol. 2015;135:1594–1602. doi: 10.1016/j.jaci.2014.12.1918. [DOI] [PubMed] [Google Scholar]
- 44.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 45.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- 46.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 47.Duong HT, Erzurum SC, Asosingh K. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis. 2011;14:411–422. doi: 10.1007/s10456-011-9228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 49.Makela MJ, Kanehiro A, Borish L, Dakhama A, Loader J, Joetham A, Xing Z, Jordana M, Larsen GL, Gelfand EW. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci U S A. 2000;97:6007–6012. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Sanctis GT, MacLean JA, Hamada K, Mehta S, Scott JA, Jiao A, Yandava CN, Kobzik L, Wolyniec WW, Fabian AJ, Venugopal CS, Grasemann H, Huang PL, Drazen JM. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189:1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 52.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183:5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 53.Sonobe Y, Takeuchi H, Kataoka K, Li H, Jin S, Mimuro M, Hashizume Y, Sano Y, Kanda T, Mizuno T, Suzumura A. Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cepsilon-dependent manner. J Biol Chem. 2009;284:31834–31842. doi: 10.1074/jbc.M109.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2009;220:499–508. doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]
- 55.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, Schow P, Gurney AL. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 56.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 57.Wong CK, Li PW, Lam CW. Intracellular JNK, p38 MAPK and NF-kappaB regulate IL-25 induced release of cytokines and chemokines from costimulated T helper lymphocytes. Immunol Lett. 2007;112:82–91. doi: 10.1016/j.imlet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012;18:751–758. doi: 10.1038/nm.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]