Abstract
Calcitonin gene-related peptide (CGRP) is a neuropeptide with well-established immunomodulatory functions. CGRP-containing nerves innervate dermal blood vessels and lymph nodes. We examined whether CGRP regulates the outcome of Ag presentation by Langerhans cells (LCs) to T cells through actions on microvascular endothelial cells (ECs). Exposure of primary murine dermal microvascular ECs (pDMECs) to CGRP followed by co-culture with LCs, responsive CD4+ T cells and Ag resulted in increased production of IL-6 and IL-17A accompanied by inhibition of IFN-γ, IL-4 and IL-22 compared to wells containing pDMECs treated with medium alone. Physical contact between ECs and LCs or T cells was not required for this effect and, except for IL-4, we demonstrated that IL-6 production by CGRP-treated pDMECs was involved in these effects. CD4+ cells expressing cytoplasmic IL-17A were increased while cells expressing cytoplasmic IFN-γ or IL-4 were decreased by the presence of CGRP-treated pDMECs and the level of retinoic acid receptor-related orphan receptor γt mRNA was significantly increased while T-bet and GATA3 expression was inhibited. Immunization at the site of intradermally administered CGRP led to a similar bias in CD4+ T cells from draining lymph node cells towards IL-17A and away from IFN-γ. Actions of nerve-derived CGRP on ECs may have important regulatory effects on the outcome of Ag presentation with consequences for the expression of inflammatory skin disorders involving Th17 cells.
Introduction
Neurologic status, including emotional state, influences immune function. The primary and secondary lymphoid organs including the spleen, thymus and lymph nodes are innervated and dendritic cells and lymphocytes express receptors for peptide and non-peptide products of nerves (1–5). Additionally, many studies have demonstrated that stress can have immunoregulatory effects both in humans and animals and these effects are mediated, at least in part, by neuroendocrine pathways (3–14). Also, there are reports that stress may exacerbate psoriasis and atopic dermatitis (6–8) and an atopic dermatitis-like rash in an animal model (11). The importance of the nervous system to inflammatory skin disease is highlighted by the findings that psoriasis clears in denervated skin (15, 16) and that some animal models of psoriasiform dermatitis depend on innervation for their expression (17, 18).
Endothelial cells (ECs)3, which line blood vessels within the dermis, contribute to cutaneous immunity and inflammation through many mechanisms. Amongst these is the ability to release cytokines and chemokines as well as expression of adhesion molecules involved in recruitment of inflammatory cells out of the vasculature and into the interstitium (19–23). In this regard, we have recently reported that the vasodilator and peptide neurotransmitter calcitonin gene-related peptide (CGRP) inhibits the stimulated expression of the chemokines CXCL8, CCL2 and CXCL1 by human dermal microvascular ECs (24). CGRP is a 37 amino acid neuropeptide generated by tissue-specific alternative processing of the calcitonin gene and is widely distributed in organs of the immune system as well as the central and peripheral nervous system (25). Of particular interest, in a murine model of psoriasiform dermatitis, in which the Tie2 receptor tyrosine kinase is over-expressed in keratinocytes, denervation of skin results in loss of the psoriasiform phenotype but administration of CGRP to the animal inhibits this loss (17), suggesting a key role for CGRP in the phenotype observed. In this regard, it has been reported that in lesions of psoriasis ECs have CGRP on their surface (26). Furthermore, both sympathetic and sensory nerves are associated with dermal vessels (27, 28) and also innervate lymph nodes (29). Moreover, recent evidence indicates that sympathetic neurotransmitters, including norepinephrine, regulate immune and inflammatory responses (30, 31).
Lymphocytes and APCs trafficking through the skin and exiting the vasculature to enter the interstitium of the dermis are closely associated with ECs during these processes. Furthermore, release of EC-derived factors on the abluminal side of vessels would be able to interact with immune cells in the interstitium, particularly those in a perivascular arrangement. Thus, we asked whether CGRP modulates the ability of ECs acting as bystanders, to regulate the outcome of Ag presentation by Langerhans cells (LCs) to CD4+ T cells. LCs are dendritic APCs that reside in the epidermis that, depending on circumstances, can present Ag for induction or regulation of arms of the immune response (32, 33). They were chosen as APCs for this study because their function has previously been shown to be directly regulated by neuropeptides (34–40) and, when stimulated by Ag, they traffic through EC-lined lymphatics to regional lymph nodes (41). Additionally, there is evidence that they can present Ag for generation of Th17 helper T cells (42, 43), believed to be important in the pathogenesis of certain inflammatory skin disorders including psoriasis (44, 45). In this regard, LCs are believed to play a role in some other inflammatory dermatoses (46).
We have now examined the effects of adding CGRP-treated or non-treated ECs to Ag presenting cultures of LCs and responding T cells, an environment perhaps similar to that in the dermis or regional lymph nodes during a local immune reaction, on the generation of T helper cell subtypes.
Materials and Methods
Mice
Six- to 12-wk-old female BALB/c (H-2d) and DO11.10 chicken OVA (cOVA) TCR transgenic (Tg) mice on a BALB/c background [C.Cg-Tg(DO11.10)10Dlo/J] mice were purchased from the Jackson Laboratory. The DO11.10 mice carry MHC class II-restricted, rearranged TCR α and β chain genes that encode a TCR that recognizes a fragment of cOVA (cOVA 323–339) presented by I-Ad (47, 48). All animal studies were approved by the Institutional Animal Care and Use Committee of the Weill Cornell Medical College.
Reagents
αCGRP was purchased from Bachem; a fragment of cOVA (cOVA323–339) was obtained from Peptides International; anti-mouse CD3 mAb along with isotype controls was obtained from R&D Systems and anti-mouse CD28 mAb from BD Biosciences. Mouse CGRP, CGRP8–37 and substance P (SP) were purchased from Bachem. Mouse adrenomedullin (1–50) (ADM) was purchased from Phoenix Pharmaceuticals. Mouse recombinant IL-6 was purchased from R&D system.
Media and cell lines
Complete medium (CM) consisted of RPMI 1640 (Mediatech), 10% FBS (American Type Culture Collection), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 0.1 mM essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, and 10 mM HEPES buffer (all from Mediatech).
Primary murine dermal microvascular ECs (pDMECs) from BALB/c mice were obtained from Cell Biologic and maintained in complete EC medium (consisting of Endothelial Basal Medium-2 supplemented with hydrocortisone, human fibroblast growth factor, human vascular endothelial growth factor, human epidermal growth factor, ascorbic acid, gentamicin and amphotericin, L-glutamine and 5% FBS (Endothelial Basal Medium-2 and supplement kits from Lonza). pDMEC were maintained in deplete EC medium (consisting of EBM-2 medium with 5% FBS and L-glutamine) overnight before experiment performed.
The bEnd.3 cell line (49) was obtained from the American Type Culture Collection (Manassas, VA). This cell line is an EC line established from the cerebral cortex of BALB/c mice and has many characteristics of freshly isolated ECs including expression of von Willebrand factor (50), ICAM-1 (51), and VCAM-1. bEnd.3 cells were cultured in DMEM (Mediatech) supplemented with 10% heat-inactivated FBS (Gemini Bio-Products, Sacramento, CA), 100 U/ml penicillin (Mediatech), 100 μg/ml streptomycin (Mediatech), and 2 mM L-glutamine (Mediatech).
Preparation of LCs
Epidermal cells were prepared using a modification of a standard protocol (37). Briefly, truncal skins of mice were shaved with electric clippers and chemically depilated. Subcutaneous fat and panniculus carnosus were removed by blunt dissection. Skin was floated dermis-side down for 45 min in Ca2+/Mg2+-free phosphate-buffered saline (PBS) containing 0.5 U of dispase/ml (BD Biosciences) and 0.38% trypsin (Mediatech). Epidermal sheets were collected by gentle scraping, washed, and dissociated by repetitive pipetting in HBSS (Mediatech) supplemented with 2% FBS. Epidermal cells were filtered through a 40 μm cell strainer (BD Biosciences) to yield ECs containing 2–3% LCs.
Epidermal cells were incubated with anti-I-Ad mAb (BD Biosciences) (5 μg/ml) for 30 min at 4°C. They were then incubated with goat anti-mouse IgG conjugated to magnetic microspheres (Dynabeads M-450; Invitrogen) for 10 min with continuous, gentle agitation. LCs were isolated by placing the tube in a magnetic particle concentrator (Invitrogen), discarding the supernatant and washing the bead-bound cells (up to five times) with HBSS containing 2% FBS. By FACS (using anti-I-Ad mAb), this procedure yields a cell population of ~95% LCs.
Preparation of bone marrow-derived dendritic cells
Bone marrow derived cells (BMDCs) were prepared from BALB/c mouse femur bones using modifications of standard procedures (52). Briefly, mice were euthanized and leg areas were shaved and sprayed with 70% ethanol. Skin was removed from legs and surrounding area and muscle and tendons were cut/scraped from the femur bones which were subsequently cut from the body. Bones were washed in PBS in petri dishes and any remaining tissue was removed. Femur bones were placed in petri dishes containing 70% ETOH for 2–3 min, then rinsed 3 times with fresh PBS. To remove bone marrow, PBS was flushed through both ends of the bone with a syringe and needle into a 50 ml conical tube. Cells were spun down at 250 × g for 10 min., washed once with PBS and and once with medium and resuspended in complete medium (CM) (RPMI 1640, 10 % FBS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 25 mM HEPES, 1 mM essential amino acids, 0 .1 mM non-essential amino acids) containing 20–25 ng/ml GM-CSF (PeproTech). Cells were plated at 2.5–4 × 106 cells per 10 ml CM media in 10 cm petri dishes and incubated at 37°C, 5% CO2. Two days later 10 ml of fresh media was added to the plate. One to 2 days later days later 10 ml was removed from each plate, placed in 50 ml conical tubes and cells spun down [1200 rpm (300 × g) for 8 min]. Cells were resuspended in fresh media, returned to plates and additional fresh media was added to plates to bring total back to 10 ml. This feeding procedure was repeated every 2 days. BMDCs were harvested on day 8 or 9 and purified using binding to CD11c microbeads and MACS technology according to manufacturer’s instructions for resuspending and magnetic separation (Miltenyi Biotec). Non-adherent and loosely adherent proliferating DC aggregates were collected from plates, transferred to 50 ml conical tubes and spun at 250 × g, 8–10 min. Cells were washed once with MACS buffer (1x PBS, 1%FBS, 2mM EDTA), resuspended in 400 μl MACS buffer per 108 total cells and 100 μl CD11c microbeads were added per 108 total cells. The mixture was incubated for 15 min at 4–8°C, 10 ml MACs buffer was added, cells spun down as above and resuspended in 500 μl MACS buffer. Cells were loaded onto LS columns Miltenyi Biotec), washed and magnetically separated following manufacture’s procedures. Eluted cells were spun down, resuspended in 500 μl of MACS buffer and subjected to a second round of column purification. Eluted cells were spun down and washed once with PBS, twice with CM and counted for use in co-culture experiments.
Isolation of CD4+ T cells from DO11.10 Tg mice
DO11.10 Tg mouse spleens were mechanically disrupted to yield a single cell suspension and erythrocytes lysed. CD4+ cells were isolated by depletion of non-target cells. The non-target cells were indirectly magnetically labeled with a cocktail of biotin-conjugated monoclonal antibodies [CD8a, Cd11b, CD11c, Cd19, CD45R (B220), CD49b (DX5), CD105, anti-MHC class II, and Ter-119] as primary labeling reagent, and anti-biotin monoclonal antibodies conjugated to microbeads, as secondary labeling reagent. The magnetically labeled non-target cells were on a MACS Column in the magnetic field of a MACS separator, while the unlabeled CD4+ T cells passed through the column (Miltenyi Biotec).
RNA interference
Ten thousand pDMECs per well were plated in 96-well round-bottom plates (BD Falcon) in antibiotic-free medium and incubated at 37°C until adherence. The appropriate amount of ON-TARGET plus SMARTpool Mouse IL-6 short interfering RNA (siRNA) (Dharmacon) target sequences (5′-CCUAGUGCGUUAUGCCUAA-3′; 5′-UUACACAUGUUCUCUGGGA-3′; 5′-GGACCAAGACCAUCCAAUU-3′; and 5′-CUACCAAACUGGAUAUAAU-3′) or the corresponding ON-TARGET plus non-targeting pool were diluted in OptiMEM medium (Invitrogen) to obtain a final concentration of 100 nM. Lipofectamine 2000 (Invitrogen) was also diluted following the manufacturer’s instructions in OptiMEM. The diluted siRNA and Lipofectamine 2000 were mixed and incubated for 20 min at room temperature for complex formation. Twenty-five μl complexed siRNA was added to each well. After overnight incubation at 37°C, the medium was replaced with depleted EC medium for treatment with CGRP. Under these conditions, siRNA pretreatment resulted in ~67% reduction in release of IL-6 protein from muramyl dipeptide-stimulated bEnd.3 cells.
In vitro Ag presentation to DO11.10 T cells
Ten thousand pDMECs or bEnd.3 cells per well were plated in 96-well, round-bottom plates coated with 2% gelatin in depleted DMEM or depleted ECs medium respectively, then incubated overnight at 37°C. The following day, cells were treated with graded concentrations of CGRP or medium alone for 3 h at 37°C. Cells were then washed extensively, and 1 × 104 purified LCs (from BALB/c mice) and 2 × 105 purified CD4+ T cells (from DO11.10 Tg mice) were added to each well in a total of 250 μl of CM containing 10 μM cOVA323–339 (Peptides International). Supernatants were harvested 48 h later and analyzed by sandwich ELISA for cytokine content.
In some experiments, pDMECs were co-cultured in graded concentrations of CGRP8–37 during the period of culture in CGRP as above. In other experiments, no pDMECs or bEnd.3 cells were utilized; instead graded concentrations of IL-6 were added to antigen presenting cultures of LCs and CD4+ T cells.
For experiments utilizing Transwell plates, 1.25 × 104 ECs/well were plated in the lower chamber of 96-well Transwell plates (Corning Life Sciences) and 24-hours later treated for 3 hours with 100 nM CGRP or medium alone as above. Cells were then washed extensively, and 1.25 × 104 purified LCs (from BALB/c mice) and 2.5 × 105 purified CD4+ T cells (from DO11.10 Tg mice) in CM were added to the upper chamber in a total of 275 μl of medium containing 10 μM cOVA323–339 (Peptides International). Supernatants were harvested 48 h later and analyzed for cytokine content.
Cytokine determinations
Supernatant IL-17A, IL-6, IL-4, IFN-γ, and IL-22 levels were determined by sandwich ELISA following the manufacturer’s instructions. IL-22 ELISA kits were purchased from Antigenix America; IL-17A, IL-4 and IL-6 kits were from (R&D Systems) and IFN-γ kits were from BD Biosciences.
Real-time PCR
For gene expression analysis, CD4+ T cells from DO11.10 Tg and LCs from BALB/c mice were co-cultured with CGRP treated pDMECs in the presence of 10 μM OVA323–339 for 24 h. T cells were gently collected from mixed culture wells after 24 h incubation, LCs still bound to beads were removed by magnetic capture. By FACS analysis for CD3, the remaining cell populations were approximately 93% T cells. Total RNA was isolated from the remaining cells (primarily CD4+ T cells) using the RNeasy Plus Mini Kit (Qiagen); DNA eliminator columns were used to eliminate any contamination with genomic DNA. cDNA was synthesized using a high-capacity RNA-to-cDNA kit according to the manufacturer’s instructions (SuperScript® VILO™ cDNA Synthesis Kit, Invitrogen). Real-time PCR for murine IL-17A (5′-GAGCTTCCCAGATCACAGAG-3′ forward; 5′-AGACTACCTCAACCGTTCCA-3′ reverse), IL-6 (5′-CAAGTGCATCATCGTTGTTCA-3′ forward; 5′-GATACCACTCCCAACAGACC-3′ reverse), IFN-γ (5′-GAGCTCATTGAATGCTTGGC-3′ forward; 5′-CAGCAACAACATAAGCGTCAT-3′ reverse), retinoic acid receptor-related orphan receptor γt (RORγt) (5′-TCCCACATCTCCCACATTG-3′ forward; 5′-AATGTCTGCAAGTCCTTCCG-3′ reverse), T-bet (5′-CAAGACCACATCCACAAACATC-3′ forward; 5′-TTCAACCAGCACCAGACAG-3′ reverse), IL-4 (5′-TCTTTAGGCTTTCCAGGAAGTC-3′ forward; 5′-GAGCTGCAGAGACTCTTTCG-3′ reverse), IL-22 (5′-AATCGCCTTGATCTCTCCAC-3′ forward; 5′-GCTCAGCTCCTGTCACATC-3′ reverse) and GATA3 (5′-GTCCCCATTAGCGTTCCTC-3′ forward; 5′-CCTTATCAAGCCCAAGCGAA-3′ reverse) expression was performed using PrimeTime primers (Integrated DNA Technologies) as shown above, power SYBR Green PCR Master Mix (Invitrogen) and ABI 7900HT instrument (Invitrogen). Expression of each cytokine was normalized to glyceraldehyde 3-phosphate dehydrogenase (5′-GTGGAGTCATACTGGAACATGTAG-3′ forward; 5′-AATGGTGAAGGTCGGTGTG-3′ reverse).
CGRP receptor mRNA detection by RT-PCR
Total RNA was extracted from pDMECs of BALB/c mice using a total RNA extraction kit (Qiagen). A DNA elimination column (Qiagen) was used to eliminate any contamination with genomic DNA. Then 100 ng of RNA was reverse-transcribed into complementary DNA using SuperScript VILO cDNA Synthesis kit following the instructions of the manufacturer (Invitrogen). One-twentieth of the synthesized cDNA was amplified by PCR using gene-specific primers for RAMP1, RAMP2, RAMP3 and calcitonin receptor-like receptor (CRLR) and CGRP-receptor component protein (CRCP). Primer sequences were designed from GenBank sequences for the mRNA of mouse RAMP1 (5′-TGTGACTGGGGAAAGACCATACAG-3′ forward; 5′-ATGAGCAGCGTGACCGTAATG-3′ reverse); RAMP2 (5′-CCCAGAATCAATCTCATCCCAC-3′ forward and 5′-AGCAGTTCGCAAAGTGTATCAGG-3′reverse); RAMP3 (5′-GGTTCAGATTGTCCATACTTTGC-3′ forward and 5′-TCAAGAAGGAGGTTCACGCTCTAC-3′ reverse); CRLR (5′-CTACTATTTTCTGCTTCTTT-3′ forward and 5′-TTTGTGCTTATTTTCTTTCC-3′ reverse); CRCP, (5′-TGGCGGAATAGGAGATAAGA-3′ forward and 5′-AGACAGAAGGGACCGCATAA-3′ reverse). The PCR products were electrophoresed in 1.5% agarose gel, stained with ethidium bromide, and visualized with UV radiation.
Sensitization of mice to dinitrofluorobenzene (DNFB)
BALB/c mice were divided into four groups of five. Mice were shaved on the dorsum with electric clippers, and injected intradermally with 100 μL of PBS containing 530 pmol CGRP, or PBS alone. Fifteen minutes after injection, the mice were painted with 10 μL of DNFB [1% in acetone and olive oil (4:1)] epicutanousely at the injection site.
Preparation of supernatants conditioned by CD4+ T cells stimulated with anti-CD3 and anti-CD28
Three days after immunization, mice were sacrificed and draining lymph nodes (axillary and inguinal) removed. Lymph nodes were mechanically disrupted and passed through a 70 μm nylon mesh to yield a single cell suspension. CD4+ T cells were isolated as described above. Ninety-six well flat-bottom plates were treated with 10 μg/ml of anti-mouse CD3 mAb in PBS overnight and washed. T cells were cultured (3 × 105 cells/well) in 250 μL of CM containing 2 μg/ml of anti-mouse CD28 mAb. Supernatants were collected 72 h after stimulation and cytokine contents were determined.
Flow cytometry
pDMECs were treated with 100 nM CGRP or medium alone for 3 h, washed 4 times, and then co-cultured with LCs and CD4+ T cells (from DO11.10 Tg mice) in the presence of 10 μM OVA323–339 for 48 h. For the last 5 h of co-culture, cells were stimulated with 50 ng/ml phorbol myristate acetate and 750 ng/ml ionomycin (Sigma-Aldrich). After 1 h, GolgiStop (BD Biosciences) was added to block cytokine secretion. LCs still bound to beads were then removed by magnetic capture. CD4+ T cells were surface stained for 20–30 minutes at 4°C with PerCP-Cy 5.5-labled anti-CD4 mAb (BD Biosciences) in PBS supplemented with 0.1% bovine serum albumin (BSA) and 0.1% sodium azide. After fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences), cells were stained with Alexa Fluor 647-labeled anti-IL-17 and fluorescein isothiocyanate (FITC) labled anti-IFN-γ (clone XMG1.2; BD Biosciences), phycoerythrin (PE) or Alexa Fluor 647-lableled anti-IL-17A (clone TC11–18H10; BD Biosciences), anti-IL-4 (clone 11B11, BD Biosciences) monoclonal antibodies. Analysis was performed on a FACSCalibur (BD Biosciences). Data analysis was conducted using CellQuest Pro software (BD Biosciences).
Biostatistics
Differences in average cytokine levels under different treatments at varying cOVA concentrations were analyzed using ANOVA. Data were log transformed prior to analysis to satisfy the underlying model assumptions. Average cytokine levels under each cOVA concentration were then compared between CGRP treatment and control groups. p-values were adjusted by controlling for the false discovery rate.
For assessment of mRNA levels, effects of intradermal administration of neuropeptides and effects of anti-IL-6 mAb on Ag presenting cultures, a linear mixed effects model was used to estimate the average level of the biomarkers under different treatments. This model takes into account variations for each treatment both within and between plates. Data were log transformed prior to analysis to satisfy the underlying model assumptions. Differences in the average level of the biomarker under pairs of experimental conditions of interest were evaluated using simultaneous tests for general linear hypotheses. p-values were again adjusted for multiple comparisons by controlling the false discovery rate.
Results
Pretreatment of primary dermal microvascular ECs (pDMECs) with CGRP biases Ag presentation towards enhanced IL-17A and IL-6 responses with reduced IFN-γ, IL-22 and IL-4 responses
Initial experiments examined the ability of ECs to present Ag to responsive T cells. ECs were co-cultured with CD4+ T cells from DO11.10 Tg mice in the presence of a fragment of chicken ovalbumin (cOVA323–339). Forty-eight hour supernatants were harvested and assessed for cytokine content by ELISA. No significant presentation was observed as assessed by production of IFN-γ, IL-6, IL-4 or IL-17A (data not shown). Since murine ECs do not express MHC class II molecules in the steady-state, this was not unexpected (53). We next performed experiments where ECs were added to Ag-presenting cultures containing LCs and T cells.
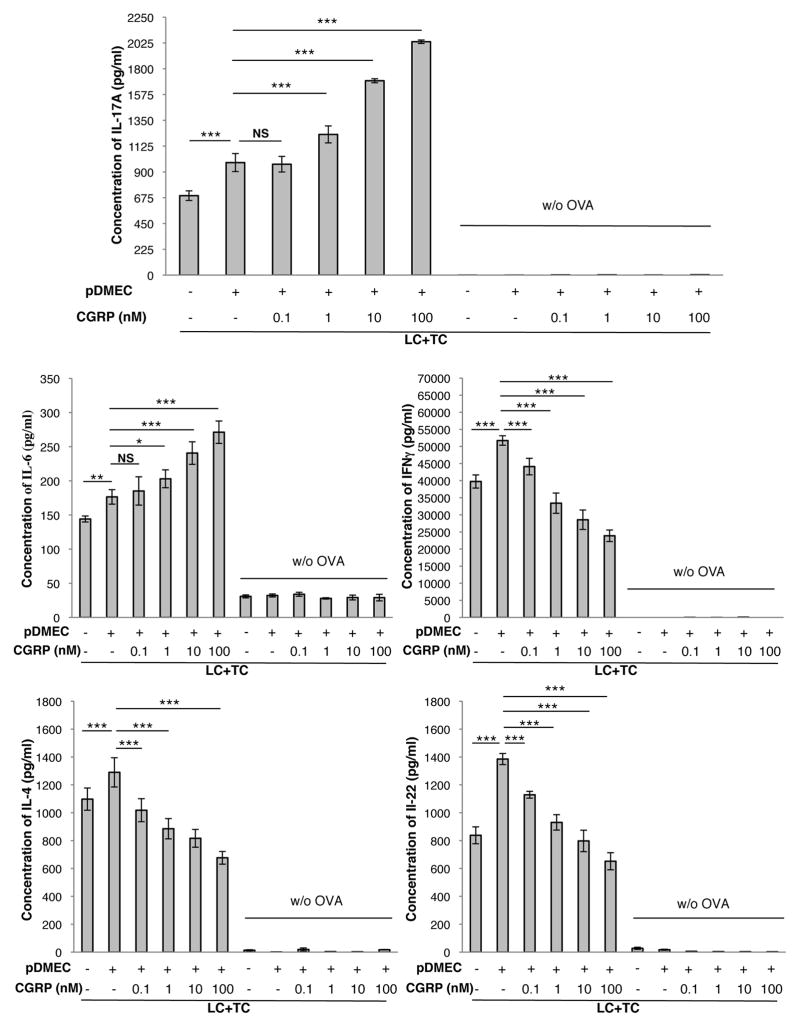
pDMECs were cultured in CGRP or medium alone for 3 h followed by extensive washing to remove CGRP. pDMECs were then co-cultured with LCs and CD4+ T cells from DO11.10 Tg mice in the presence of cOVA323–339. As shown in Figure 1 the presence of medium-treated pDMECs resulted in an Ag-dependent and significant increase in release of IL-17A, IL-6, IFN-γ and IL-22 with a small increase in IL-4. However, pretreatment of pDMECs with CGRP led to a dose-dependent and much greater increase in IL-17A and IL-6 production while the increase in IFN-γ, and IL-22 was completely reversed and production of IL-4 was significantly reduced. Similar results were obtained substituting cells of the BALB/c-derived EC line bEnd.3 for pDMECs in these experiments (Supplemental Figure 1).
FIGURE 1.
CGRP pretreatment of pDMECs biases LC Ag presentation to T cells towards an enhanced IL-17A and IL-6 response with a reduced IFNγ, IL-22 and IL-4 response. Medium- or CGRP-treated (0.1, 1, 10 and 100 nM concentrations) pDMECs were added to cultures of LCs, T cells (from DO11.10 Tg mice) and cOVA323–339. After 48 hours, supernatants were assessed for cytokine content. Addition of medium-treated pDMECs slightly but significantly enhanced IL-17A, IL-6 and IL-4 production alone with more substantial and significant increases in IFNγ and IL-22. Addition of CGRP-treated pDMECs led to a much larger increase in IL-17A and IL-6 production but largely eliminated the increased production of IFNγ and IL-22 with significant reductions in IL-4 production compared to that seen in wells with addition of medium-treated pDMECs. n=3 experiments, all groups. *p < 0.05, **p < 0.01, ***p < 0.001.
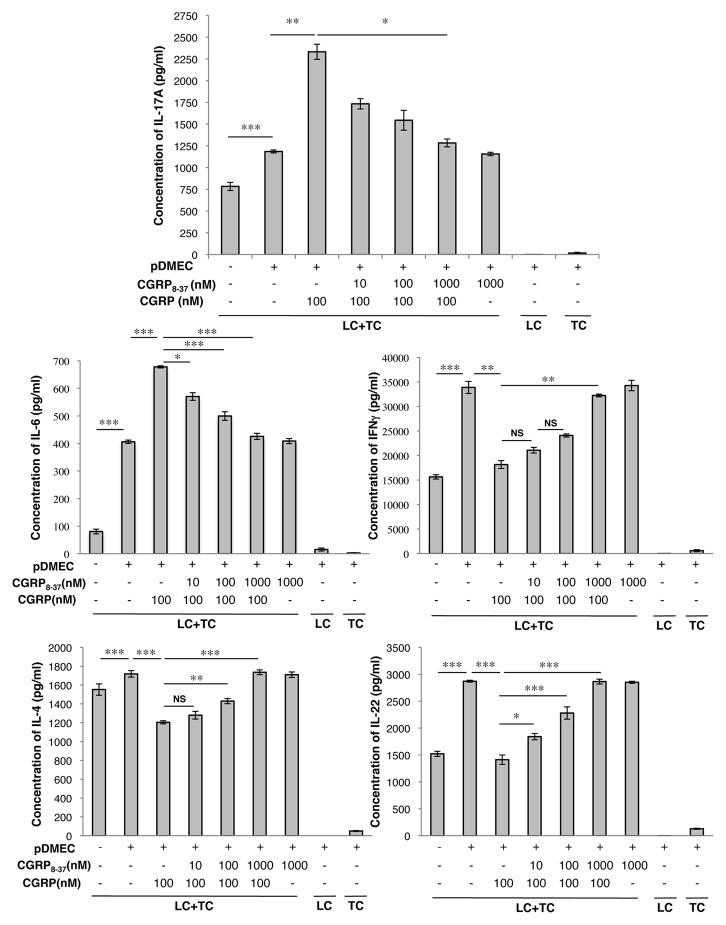
The specificity of the CGRP effect was shown by additional experiments comparing CGRP with SP (which co-localizes with CGRP in sensory nerves) and ADM (which binds to the CGRP receptor but with much lower avidity than to the ADM receptor). Although SP and ADM may have a very small amount of activity at inducing IL-17A bias, CGRP is dramatically more effective at biasing the outcome of antigen presentation towards IL-6 and IL-17A production and away from IFN-γ, IL-22 and IL-4 release (Supplemental Figure 2). Furthermore, addition of the CGRP receptor inhibitor CGRP8–37 blocked the effects of CGRP on the outcome of antigen presentation (Figure 2) and similar results were seen when bEnd.3 cells were substituted for pDMECs in these types of experiments (Supplemental Figure 3). Consistent with these findings, by RT-PCR, pDMECs were found to express the components of the CGRP receptor RAMP1 and CLR as well as RAMP2, RAMP3, calcitonin receptor-like receptor (CRLR) and calcitonin gene-related peptide-receptor component protein (CRCP) (Supplemental Figure 4).
FIGURE 2.
The effects of CGRP pretreatment of pDMECs on LC Ag presentation to T cells can be blocked with CGRP8–37. pDMECs were treated with graded concentrations of CGRP8–37 or medium alone during exposure to CGRP followed by washing and addition to cultures of LCs, T cells (from DO11.10 Tg mice) and cOVA323–339. After 48 hours, supernatants were assessed for cytokine content. The effects of CGRP on production of IL-17A, IL-6, IFNγ, IL-4 and IL-22 were all significantly inhibited by CGRP8–37 in a dose-dependent manner. n=3 experiments, all groups. *p < 0.05, **p < 0.01, ***p < 0.001.
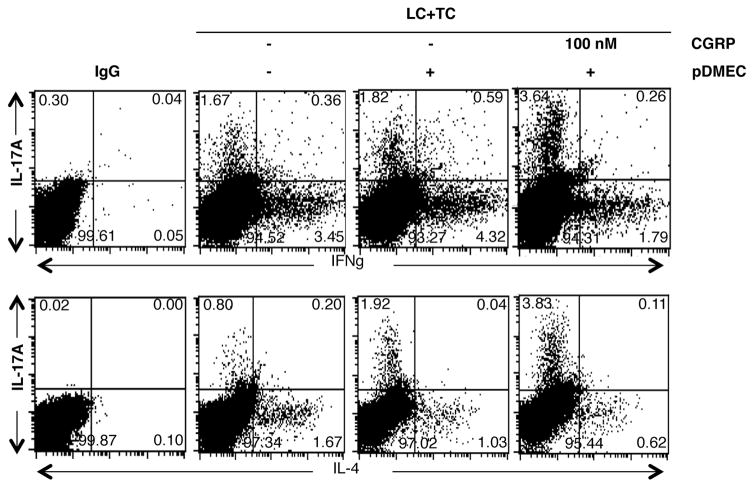
Pretreatment of pDMECs with CGRP enhances differentiation of T cells with intracellular IL-17A and reduces differentiation of T cells with intracellular IL-4 or IFN-γ
Additional experiments were set-up in the same manner with medium-pretreated or CGRP-pretreated pDMECs and isolated CD4+ T cells were studied by FACS analysis after 48 h of culture. Addition of medium-treated pDMECs to wells somewhat enhanced the proportion of cells expressing IL-17A or IFN-γ while slightly decreasing the numbers of cells expressing IL-4. However, addition of CGRP-treated pDMECs further enhanced the proportion of cells expressing intracellular IL-17A while the percentages of cells expressing intracellular IFN-γ or IL-4 were greatly reduced (Figure 3). Very few cells expressing intracellular IL-22 were observed, presumably because of the sensitivity of the assay (data not shown).
FIGURE 3.
CGRP pretreatment of pDMECs enhances differentiation of T cells containing intracellular IL-17A or IL-4 while decreasing expression of IFNγ. pDMECs pretreated with CGRP or medium alone were added to cultures of LCs, CD4+ T cells from DO11.10 Tg mice and cOVA323–339. Forty-eight hours later cells were harvested and CD4+ cells examined by FACS for intracellular IL-17A, IFNγ, or IL-4 content. Addition of medium-treated pDMECs somewhat enhanced the proportion of cells expressing IL-17A or IFNγ while slightly decreasing the numbers of cells expressing IL-4. Addition of CGRP-treated pDMECs further enhanced the proportion of cells expressing intracellular IL-17A while the proportions of cells expressing intracellular IFNγ or IL-4 were greatly reduced. Representative of 4 experiments.
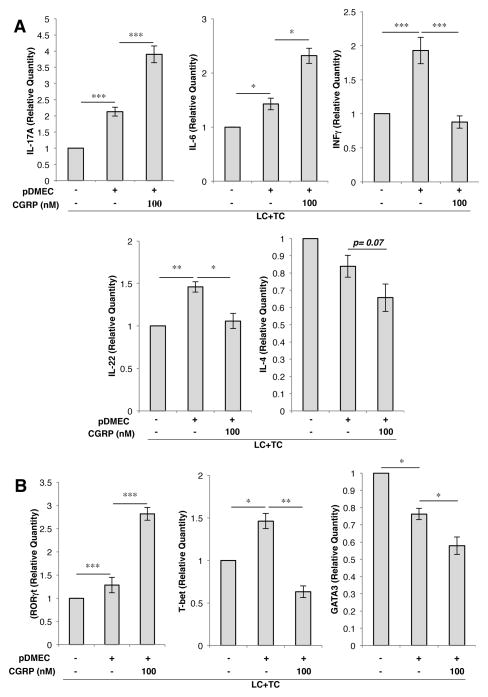
Pretreatment of pDMECs with CGRP yields T cells with increased levels of mRNA for IL-17A, IL-6 and RORγt accompanied by decreased levels of IFN-γ, IL-22, T-bet, and GATA3
Additional experiments were set-up as described above and CD4+ cells isolated by a magnetic antibody technique after 24 h of culture. Total RNA was extracted and real-time RT-PCR performed to assess IL-6, IFN-γ, IL-17A and IL-22 mRNA levels. The presence of medium-treated pDMECs during Ag presentation led to significantly enhanced levels of IL-17A, IL-6, IFN-γ and IL-22 mRNA while the IL-4 mRNA level was little changed (Figure 4A). The presence of CGRP-treated pDMECs during Ag presentation led to a much more substantial increase in IL-17A and IL-6 mRNA levels while the increase in IFN-γ and IL-22 mRNA levels was completely blocked (Figure 4A). The level of IL-4 mRNA showed a strong trend towards being decreased by the presence of CGRP pretreated pDMECs (p=0.07) (Figure 3A). Similarly, the level of mRNA for the transcription factor RORγt (Th17 cells) was slightly but significantly elevated by the presence of medium-treated pDMECs and much more substantially elevated by the presence of CGRP-treated pDMECs (Figure 4B).
FIGURE 4.
A, CGRP pretreatment of pDMECs induces T cells with increased levels of mRNA for IL-17A and IL-6 accompanied by decreased levels of mRNA for IFNγ, IL-22 and IL-4. pDMECs pretreated with CGRP or medium alone were added to cultures of LCs and CD4+ T cells from DO11.10 Tg mice. Twenty-four hours later, LCs still bound to beads were moved by magnetic capture and total RNA was isolated from the remaining cells. By RT-PCR cultures containing medium-treated pDMECs had significantly increased IL-17A, IL-6, IFNγ and IL-22 levels while a small and not significant decrease in the IL-4 mRNA level was observed. Addition of CGRP-treated pDMECs led to a substantial and significant further increase in IL-17A and IL-6 mRNA levels accompanied by loss of the increase in IFNγ and IL-22 mRNA seen with addition of medium-treated pDMECs. The level of IL-4 was further decreased compared to wells containing medium-treated pDMECs with a p value of 0.07. B, CGRP pretreated pDMECs lead to enhanced mRNA levels of RORγT accompanied by decreased levels of T-bet and GATA3. Addition of medium-treated pDMECs led to a small but significant decrease in GATA3 mRNA levels. Addition of CGRP-treated pDMECs resulted in a large increase in the level of RORγt mRNA, the loss of the increase in T-bet observed, and a further small but significant decrease in the level of GATA3 mRNA compared to wells containing medium-treated pDMECs. n=6 experiments for all except T-bet which was 7. *p < 0.05, **p < 0.01, *** p < 0.001.
The expression of mRNA for cytokines was also reflected in the expression of key transcription factors. T-bet mRNA was slightly but significantly elevated by the presence of medium-treated pDMECs and this elevation was completely abolished by the presence of CGRP-treated pDMECs (Figure 4B). A small but statistically significant decrease in the level of GATA3 mRNA was seen with addition of medium pretreated pDMECs while the addition of CGRP pretreated pDMECs led to a further and significant reduction in the mRNA level (Figure 4B).
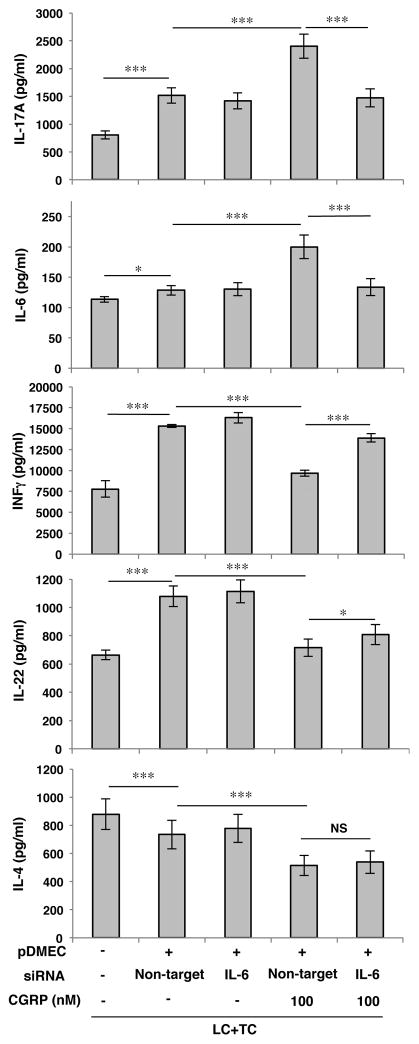
Pretreatment of pDMECs with siRNA to IL-6 prior to exposure to CGRP inhibited their ability to bias Ag presentation
To determine if IL-6 is involved in the process by which CGRP induces ECs to bias the outcome of Ag presentation, we treated pDMECs with IL-6 siRNA to knockdown IL-6 production prior to treatment with CGRP and co-culture with LCs and T cells. As shown in Figure 5, pretreatment of pDMECs with either siRNA to IL-6 or non-target siRNA without CGRP exposure appeared to enhance production of IL-17A, IL-6, IFN-γ and IL-22 while IL-4 release was slightly decreased. Pretreatment of pDMECs with IL-6 siRNA prior to CGRP exposure significantly inhibited IL-6 release and IL-17A production in these cultures while substantially and significantly reducing the inhibition of IFN-γ production (Figure 5). Only a slight, but significant, reversal of the effect of CGRP exposure by siRNA treatment of pDMECs was seen on the inhibition of of IL-22 mRNA and no effect was seen on suppression of IL-4 mRNA (Figure 5).
FIGURE 5.
Pretreatment of pDMEC with siRNA to IL-6 inhibited the ability of CGRP-treated pDMECs to bias Ag presentation. Treatment of pDMECs with non-target siRNA prior to culture of pDMECs in medium alone followed by addition to cultures of LCs, CD4+ T cells from DO11.10 Tg mice and cOVA323–339 led to small but significant increases in supernatant content of IL-17A and IL-6 and a substantial increase in IFNγ and IL-22 production along with a small but significant decrease in IL-4 production. Treatment of pDMECs with non-target siRNA prior to culture in CGRP and addition to cultures led to a significant and substantial increase in IL-17A and IL-6 production compared to wells containing non-target siRNA-treated pDMECs exposed to medium alone along with a significant decrease in IFNγ, IL-22 and IL-4 production. Addition of pDMECs treated with IL-6 siRNA prior to exposure to CGRP and addition to culture wells led to loss of the increase in IL-17A and IL-6 production observed with addition of non-target siRNA-treated pDMECs exposed to medium alone accompanied by a significant increase in IFNγ production, a small but significant increase in IL-22 production but no change in IL-4 production. n=3 experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
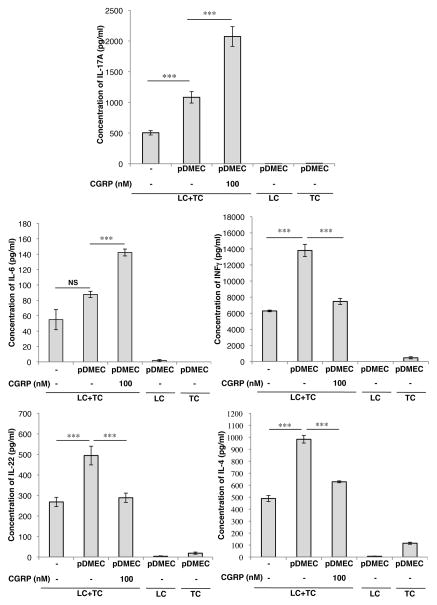
Regulatory effects of CGRP-treated pDMECs do not depend on cell-cell contact
To determine whether ECs must touch LCs and/or responding T cells, additional experiments were set-up in which Transwell inserts were used to spatially separate ECs from LCs and T cells and supernatant content of cytokines assessed. The changes seen in these experiments were similar, but not identical to the other experiments (as shown in Figure 1). As seen in the previous experiments, CGRP-treated pDMECs increased the expression of IL-6 and IL-17 and reduced the expression of IFN-γ and IL-22 (Figure 6). However, in the absence of pDMEC contact, medium-treated pDMECs greatly increased the expression of IL-4 and this effect was decreased when the pDMECs had been exposed to CGRP (Figure 6), suggesting that the regulation of IL-4 expression differs from the regulation of the other 4 cytokines we studied.
FIGURE 6.
Biasing of Ag presentation by CGRP-treated pDMECs does not require contact between pDMECs and s or CD4+ T cells. Medium- or CGRP-treated pDMECs were added to the lower chamber of wells in 96-well Transwell plates with LCs, and CD4+ T cells from DO11.10 Tg mice added to the upper chambers in the presence of cOVA323–339. The upper chamber of some control wells contained only LCs or only T cells. After 48 hours, supernatants were assessed for cytokine content. Addition of cells not exposed to CGRP significantly enhanced IL-17A, IFNγ, IL-22 and IL-4 concentrations. Pre-exposure of added pDMECs to CGRP led to a much larger increase in IL-17A and IL-6 production but largely eliminated the increased production of IFNγ, IL-22 and IL-4 seen in wells with addition of medium-treated pDMECs. n=3 experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
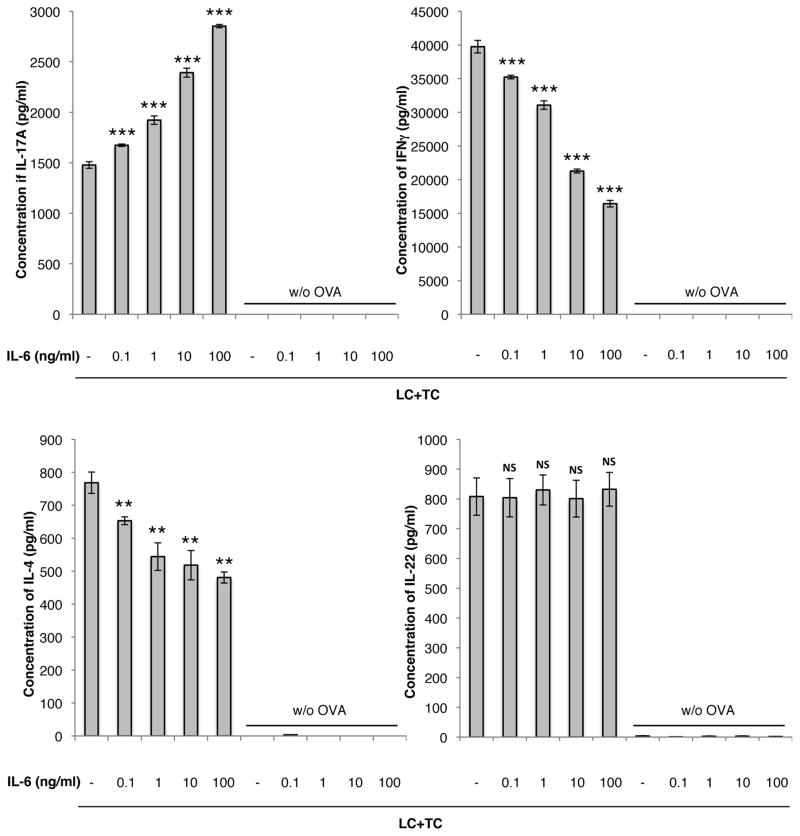
Given the evidence that (a) IL-6 expression by pDMECs is important for biasing of antigen presentation in this system and (b) that cell-cell contact is not needed, we performed experiments to determine if adding IL-6 to cultures of LCs, responding T cells and antigen in the absence of pDMECs could similarly bias the outcome of antigen presentation. As shown in Figure 7, addition of IL-6 to wells in the absence of pDMECs reproduced most of the findings of co-culture with CGRP-treated pDMECs including a significant increase in IL-17A production with a significant decrease in IFN-γ and IL-4 production. However, IL-22 release was unchanged by addition of IL-6.
FIGURE 7.
IL-6 biases LC Ag presentation to T cells towards an enhanced IL-17A response with a reduced IFNγ and IL-4 response. IL-6 was added to cultures of LCs, T cells (from DO11.10 Tg mice) and cOVA323–339. After 48 hours, supernatants were assessed for cytokine content. Addition of IL-6 significantly enhanced IL-17A production with a significant decrease in IL-4 production. IL-22 production was not affected by IL-6. n=2 experiments, all groups. *p < 0.05, **p < 0.01, ***p < 0.001 compared to no IL-6.
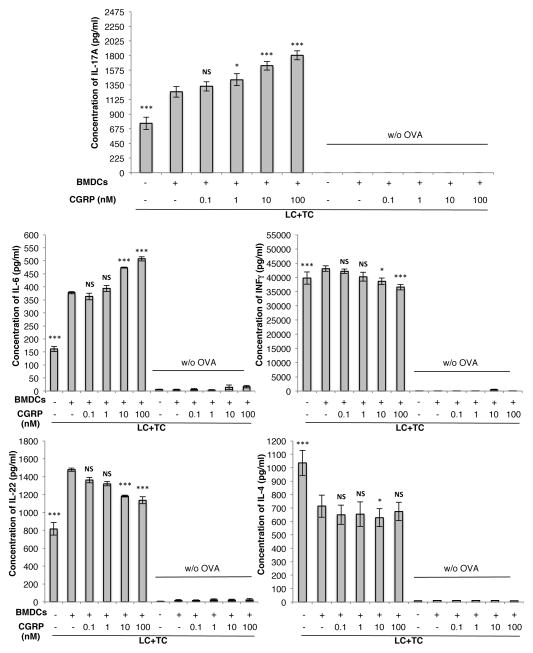
Bone marrow-derived dendritic cells also respond to CGRP-treated pDMECs but with a lesser magnitude
pDMECs were cultured in CGRP or medium alone for 3 h followed by extensive washing to remove CGRP. pDMECs were then co-cultured with BMDCs and CD4+ T cells from DO11.10 Tg mice in the presence of cOVA323–339. The presence of medium-treated pDMECs and Ag resulted in a dose-dependent and significant increase in release of IL-17A, IL-6, IFN-γ and IL-22 with a significant decrease in IL-4 (Figure 8). However, pretreatment of pDMECs with CGRP led to a greater increase in IL-17A and IL-6 production while the increase in IFN-γ, and IL-22 was reversed and production of IL-4 was perhaps somewhat reduced (a significant reduction was only seen with the 10 nM CGRP concentration).
FIGURE 8.
CGRP pretreatment of pDMECs biases BMDC Ag presentation to T cells towards an enhanced IL-17A and IL-6 response with a reduced IFNγ and IL-22 response. Medium- or CGRP-treated pDMECs were added to cultures of BMDCs, T cells (from DO11.10 Tg mice) and cOVA323–339. After 48 hours, supernatants were assessed for cytokine content. Addition of medium-treated pDMECs slightly but significantly enhanced IL-17A and IFNγ (very slightly) production alone with more substantial and significant increases in IL-6, and IL-22. A significant decrease in IL-4 production occurred. Addition of CGRP-treated pDMECs led to a significant increase in IL-17A and IL-6 production, a small but significant decreased production of IFNγ and IL-22 with little or no reduction in IL-4 production compared to that seen in wells with addition of medium-treated pDMECs. n=3 experiments, all groups. *p < 0.05, **p < 0.01, ***p < 0.001.
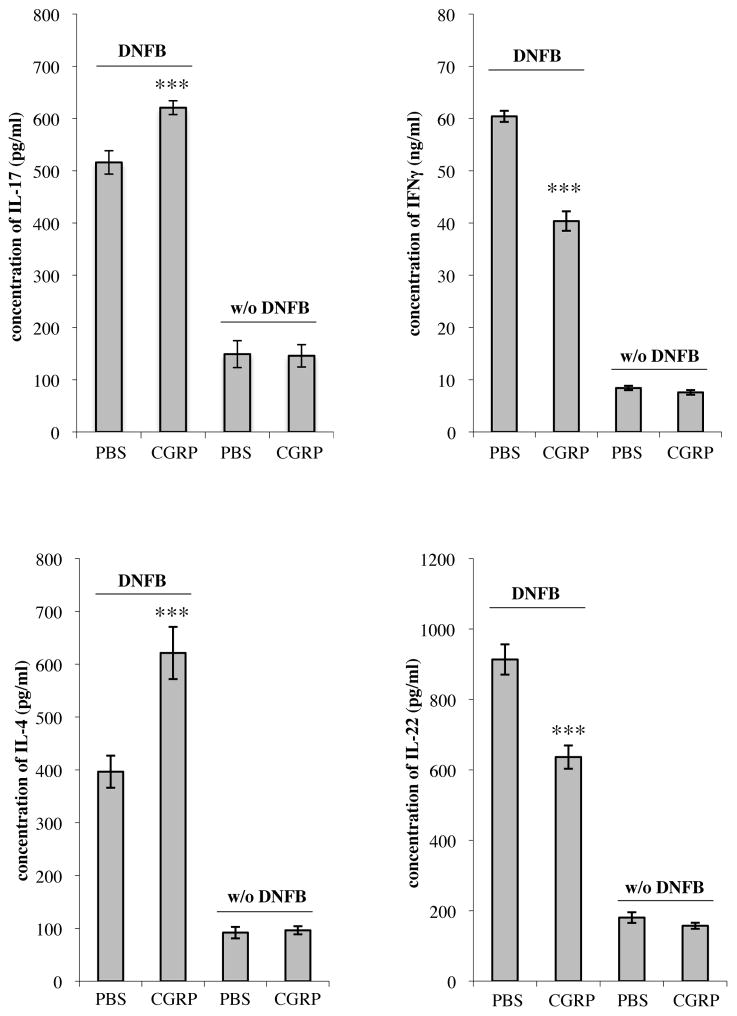
Intradermal CGRP biases the CD4+ lymph node cell response to epicutaneous immunization towards an IL-17A and IL-4 response while inhibiting IL-22 and IFN-γ responses
To determine whether CGRP can modulate the immune response in vivo, groups of BALB/c mice were injected intradermally with CGRP or medium alone. Fifteen minutes later, mice were immunized by topical application of DNFB at sites of injection. Three days later, draining lymph nodes were harvested and a single cell suspension of lymphocytes was stimulated in culture with anti-CD3 and anti-CD28. After 72 h, supernatants were assayed for cytokine content. Lymphocytes from mice treated with CGRP produced significantly more IL-17A and IL-4 but significantly less IL-22 and IFN-γ compared with cells from control mice (Figure 9).
FIGURE 9.
CGRP administered intradermally can modulate the immune response in vivo. Groups of BALB/c mice were injected intradermally with medium alone or CGRP. They were then immunized by application of DNFB at sites of injection. Three days later draining lymph nodes were harvested, a single-cell suspension of CD4+ lymphocytes were stimulated in culture with anti-CD3 and anti-CD28 monoclonal antibodies. After 72 hours, supernatants were harvested and cytokine content assessed by ELISA. Production of IL-17A and IL-4 were significantly increased in lymphocytes obtained from mice immunized at CGRP-treated sites compared to those at medium-treated sites while production of IFNγ and IL-22 was significantly decreased. n=10 mice per group for all groups except IL-17A, for which n=15 mice. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
ECs are uniquely positioned to allow for products of nerves to influence immune reactions within the skin. First, blood vessels and, probably, lymphatics within the skin are surrounded by both sensory and sympathetic nerves (27, 28, 54). Secondly, immune cells, both APCs and lymphocytes, are closely associated with ECs as they exit vessels at sites of immunologic reactions or, conversely, leave the skin to enter lymphatic or blood vessels. Many inflammatory and immunologic disorders of the skin are characterized by perivascular T cells along with APCs including, for example, in the case of psoriasis, LCs (45, 46). Thus, there is an anatomic basis by which nerves may release transmitters, such as CGRP, which then bind to receptors on ECs that are then able to bias the outcome of Ag presentation towards generation of IL-17A producing cells and away from IFN-γ and IL-4 producing cells.
T cells that predominantly produce IL-17A and other members of the IL-17 family of cytokines have been termed Th17 cells (55). These cells play a physiologic role in protection against extracellular organisms (55). They also appear to be involved in the pathophysiology of a number of skin disorders marked by abnormal immune reactivity, notably psoriasis (56). In the mouse these cells also produce IL-22 and some do in humans (57). However, there are also cells that produce IL-22 but not IL-17 (56). Interestingly, our work indicates that exposure of ECs to CGRP reduces IL-22 expression.
Our results demonstrate that CGRP can regulate the outcome of Ag presentation through effects on ECs acting as bystanders. As shown above, CGRP-exposed pDMECs lead to markedly enhanced IL-17A and IL-6 production with decreased release of IFN-γ, IL-22 and IL-4 accompanied by enhanced generation of Th17 cells and the CGRP effect could be blocked by treating ECs in the presence of the CGRP receptor blocker CGRP 8–37. Interestingly, blocking of IL-6 expression by pDMECs through the use of siRNA for IL-6 inhibited the effect on all cytokines except IL-4, although the effect on suppression of IL-22 was very small. Experiments substituting addition of IL-6 to antigen presenting culture for addition of CGRP-treated pDMECs gave similar results, skewing of the results of antigen presentation with increased IL-17A accompanied by decreased IFN-γ and IL-4 production but no effect was observed on IL-22 release. Thus, the effects of CGRP-treated endothelial cells on IL-22 production in antigen presenting cultures appear to be mediated by factors other than IL-6 alone. Although addition of IL-6 to antigen presenting cell cultures in the absence of ECs did result in decreased IL-4 production, treatment of pDMECs with siRNA for IL-6 did not inhibit the effect of CGRP-treated pDMECs on IL-4 production in our assay. Furthermore, the effects of CGRP-treated pDMECs in the system did not depend on cell-cell contact with the interesting exception that the presence of medium-treated pDMECs greatly enhanced IL-4 release in the Ag presenting cell cultures when cell-cell contact did not take place while this was not seen when cell-cell contact was possible. Thus, the role of IL-6 in the effect we have observed for IL-4 production with CGRP-treated pDMECs in our assay requires further study. Also, in contrast to the effect seen with addition of IL-6 to Ag presentation cultures, contact between pDMECs and Langerhans cells and/or responding T cells appears to be important for the alteration of the IL-4 response in Ag presenting cultures seen in the presence of CGRP-treated ECs.
It should also be mentioned that in control wells containing non-CGRP-treated pDMECs, IL-4 expression was increased in some experiments but not in others. Indeed, in one set of experiments GATA3 mRNA levels showed a small reduction in responding T cells from wells containing non-CGRP-treated pDMECs (Figure 4B). The reasons for these discrepancies are not clear but the various experiments performed differed in experimental protocol and/or endpoints measured and these differences may account for some of the variations seen. These findings might also relate to the apparently more complex nature of regulation of T cell IL-4 production in our system, compared to the other cytokines examined, as described in the paragraph above. However, in all experiments utilizing LC antigen presentation to T cells, when pDMECs were pretreated with CGRP, IL-4 expression was decreased.
The peptides SP and ADM did not replicate the effects of CGRP in this system demonstrating the specificity of the CGRP effects, although some effects were seen on some cytokine responses. The failure of ADM to have a large effect strongly indicates that CGRP is working through actions at the CGRP receptor rather than the ADM receptor. The role of these other peptides in modifying the outcome of antigen presentation remains to be fully elucidated.
One caveat to these results is that the population of pDMECs may have a small contaminating population of other cell-types. To control for the possibility that the effects we have observed relate to a contaminating cell-type, experiments were also performed with the clonal bEnd.3 cell line substituted for pDMECs. Very similar results were observed (Supplemental Figures 1 and 3), minimizing the possibility that CGRP was acting through a contaminating population in our pDMECs preparations.
The effects of CGRP-treated pDMECs in Ag presenting cultures were very similar when BMDCs were employed as APCs in place of LCs. Whether this is a general phenomenon with regard to a large, diverse range of APCs is an important question that should be examined subsequently.
These findings may have important implications for regulation of immune processes within the skin, both physiologically and pathophysiologically, by the nervous system. Psoriasis is a disorder in which Th17 cells and IL-17A are believed to have key roles (58). Of particular interest, it has long been known that denervation of skin bearing psoriasis leads to an improvement or clearing of the psoriasis (15,16). Thus, products of nerves would appear to play an important role in the pathogenesis of this disorder. The key role of IL-17A in the pathophysiology of psoriasis is highlighted by the recent reports that an antibody against IL-17A and its receptor is highly efficacious in treating psoriasis (59). A limitation to this hypothesis, however, is that there is considerable evidence that IL-22 is also involved in the pathophysiology of psoriasis (60). It induces acanthosis in several models and enhanced levels of IL-22 are found in psoriatic skin (60–62). IL-22 was originally described as a significant product of Th17 cells. However, more recent work has shown that IL-22 in the skin is also derived from CD8+ T cells, RORγt+ innate lymphocytes, dermal γδ T cells (which also produce IL-17A) and CD4+ T cells that produce IL-22 but not IL-17 (Th22 cells) (57, 63, 64). Indeed, there is some evidence in animal models that γδ T cells are necessary for psoriatic plaque formation (65). Thus, it may be that IL-22 in psoriasis is not necessarily derived from CD4+ IL-17A producing cells. Thus, CGRP may play a role in the pathogenesis of psoriasis via effects on ECs despite inducing decreased IL-22 production from CD4+ T cells. Of interest, development of an antibody against IL-22 for treatment of psoriasis was discontinued in 2011 after a phase 2 study; there are no publications resulting from these trials and the results of the psoriasis trial were reportedly not promising (66, 67).
Experiments examining the ability of intradermally administered CGRP to alter the outcome of the T cell response to immunization with a hapten strongly suggest that our in vitro observations have in vivo relevance. Administered CGRP resulted in changes in the generation of T helper cell subtypes similar to that seen in the in vitro experiments in which CGRP exposed ECs were added to Ag presenting cultures. The direction of change in cytokine expression from CD4+ T cells obtained from lymph nodes draining the sites of immunization pretreated with CGRP were the same in direction as seen in the in vitro experiments with the exception of IL-4. In this regard, we have previously reported that exposure of LCs to CGRP enhances their Ag presenting ability to stimulate IL-4 responses (37). Thus, in our in vivo experiments, multiple cell types, including both EC and LCs, are likely impacted. Thus, a direct effect of CGRP on LCs, inducing them to present Ag for an IL-4 response, might account for the disparate effect seen on production of this cytokine in the in vivo experiments. An effect of CGRP on biasing of the immune response from effects on dermal ECs is consistent with our IL-17A, IFN-γ and IL-22 results. Nonetheless, other putative targets cannot be excluded from this type of experiment and the actual loci of action of the CGRP (including possible cell-types and signals involved) cannot be stated with certainty.
The in vivo effect of CGRP on biasing the response toward the Th17 pole was relatively modest. However, these results may understate the actual physiologic effects of this pathway in vivo. CGRP was administered only once, at a single concentration. The half-life of CGRP in the circulation is only 7–10 minutes (68) and it is unlikely to be much longer in the skin. Presumably, a putative physiologic effect of this pathway would depend on the local concentration at ECs in dermal vessels and it is likely that under some conditions CGRP is released chronically or repetitively by nerves in close proximity to ECs. Definitive experiments to determine the in vivo relevance of CGRP signaling at the level of the EC, although beyond the scope of this report, can be pursued in the future through the use of inducible, conditional knockout animals lacking CGRP receptors in ECs.
The observation that CGRP-treated ECs exert their effects in the absence of contact with T cells excludes the possibility that under the influence of factors in the Ag-presentation milieu they become APCs themselves that contribute to the outcomes observed. This is a significant observation as human ECs have been shown to present Ag, especially after stimulation with T cell products, most notably IFN-γ (69, 70). Thus, it appears that the effect is mediated by production of soluble mediators including, at least in part, IL-6. In this regard, it should be mentioned that CGRP has previously been reported to induce AP-1 activity in pre-B cells (71) and c-fos and IL-6 mRNA transcription in bone marrow-derived macrophages (72). These pathways may account for the induction of IL-6 production by pDMECs exposed to CGRP in our experimental system.
Interestingly, there may be other pathways by which nerves may influence the expression of psoriasis. We have previously shown that exposure of LCs to vasoactive intestinal polypeptide or pituitary adenylate-activating peptide biases LC Ag presentation towards generation of Th17 cells in vitro (73). Thus, if this occurs in vivo and Th17 cells indeed play a role in the pathophysiology of psoriasis, these neuropeptides may have an important role. Similarly, application of imiquimod to murine skin induces a psoriasis-like inflammation. A subset of sensory neurons expressing the ion channels TRPV1 and Nav1.8 has been shown to be required for this inflammatory response and imaging indicated that a large fraction of dermal dendritic cells are in close contact with these nociceptors (74). Evidence was presented suggesting the nerves influence dermal dendritic cells to release IL-23, that then induce gamma-delta T cells to produce IL-17, driving the inflammation observed. If this mechanism is operant in psoriasis, this pathway too may be important in the pathogenesis of the disorder.
Overall, the results of our investigations strongly suggest that a novel pathway exists by which the nervous system can regulate immunity through actions on ECs. Future work will determine the physiologic, pathophysiologic and therapeutic importance of these findings.
Supplementary Material
Footnotes
This work was supported by NIH Grant 1 R21 AR064907 (RDG and JAW), the Jacob L. and Lillian Holtzmann Foundation (RDG), the Edith C. Blum Foundation (RDG), the Carl and Fay Simons Family Trust (RDG), the Seth Sprague Educational and Charitable Foundation (RDG), the Lewis B. and Dorothy Cullman Foundation (RDG), the Filomen M. D’Agostino Foundation, contributions from Tully Plesser (RDG) and NIH Clinical and Translational Science Award RR024996 (XKZ).
Abbreviations used in this article: CGRP, calcitonin gene-related peptide; CM, complete medium; cOVA, chicken OVA; DNFB, dinitrofluorobenzene; EC, endothelial cell; LC, Langerhans cell; pDMEC, primary murine dermal microvascular EC; RORγt, retinoic acid receptor-related orphan receptor γt; siRNA, short interfering RNA; Tg, transgenic.
References
- 1.Bulloch K. Neuroanatomy of lymphoid tissue: A review. In: Guillermin R, Cohn M, Melnechuk T, editors. Neural Modulation of Immunity. Raven Press; New York, NY: 1985. pp. 111–141. [Google Scholar]
- 2.Felton DL, Ackerman KD, Wiegand SJ, Felton SY. Nonadrenergic sympathetic innervation of the spleen: I. Nerve fibers associated with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 3.Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Walker RF, Codd EE. Neuroimmunomodulatory interactions of norepinephrine and serotonin. J Neuroimmunol. 1985;10:41–58. doi: 10.1016/0165-5728(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 5.Lorton D, Bellinger DL, Felten SY, Felten DL. Substance P innervation of spleen in rats: nerve fibers associate with lymphocytes and macrophages in specific compartments of the spleen. Brain Behav Immunol. 1991;5:29–40. doi: 10.1016/0889-1591(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 6.Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Misery L. Atopic dermatitis and the nervous system. Clin Rev Allergy Immunol. 2011;41:259–266. doi: 10.1007/s12016-010-8225-z. [DOI] [PubMed] [Google Scholar]
- 8.Khansari DN, Murgo AJ, Faith RE. Effects of stress on the immune system. Immunol Today. 1990;11:170–175. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 9.Sirinek LP, O’Dorisio MS. Modulation of immune function by intestinal neuropeptides. Acta Oncol. 1991;30:509–517. doi: 10.3109/02841869109092410. [DOI] [PubMed] [Google Scholar]
- 10.Dhabhar FS, Saul AN, Holmes TH, Daugherty C, Neri E, Tillie JM, Kusewitt D, Oberyszyn TM. High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS One. 2012;7(4):e33069. doi: 10.1371/journal.pone.0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharm. 2008;33:566–573. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- 12.Flint MS, Depree KM, Rich BA, Tinkle SS. Differential regulation of sensitizer-induced inflammation and immunity by acute restraint stress in allergic contact dermatitis. J Neuroimmunol. 2003;140:28–40. doi: 10.1016/s0165-5728(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 13.Flint MS, Morgan JB, Shreve SN, Tinkle SS. Restraint stress and corticotropin releasing hormone modulation of murine cutaneous POMC mRNA. Stress. 2003;6:59–62. doi: 10.1080/1025389031000088426. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–6109. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- 15.Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220–221. [PubMed] [Google Scholar]
- 16.Raychaudhuri SP, Farber EM. Are sensory nerves essential for the development of psoriatic lesions? J Am Acad Dermatol. 1993;28:488–489. doi: 10.1016/s0190-9622(08)81760-4. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530–1538. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 19.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 20.Swerlick RA, Lawley TJ. Role of microvascular endothelial cells in inflammation. J Invest Dermatol. 1993;100:111S–115S. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10:370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- 22.Cid MC. Endothelial cell biology, perivascular inflammation, and vasculitis. Cleveland Clinic J Med. 2002;69:45–49. doi: 10.3949/ccjm.69.suppl_2.sii45. [DOI] [PubMed] [Google Scholar]
- 23.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Stohl LL, Zhou X, Ding W, Granstein RD. Calcitonon gene-related peptide inhibitis chemokine production by human dermal microvascular endothelial cells. Brain Behav Immun. 2011;25:787–799. doi: 10.1016/j.bbi.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arulmani U, Maassenvandenbrink A, Villalón CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 26.He Y, Ding G, Wang X, Zhu T, Fan S. Calcitonin gene-related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl) 2000;113:747–751. [PubMed] [Google Scholar]
- 27.Dalsgaard CJ, Jonsson CE, Hökfelt T, Cuello AC. Localization of substance P-immunoreactive nerve fibers in the human digital skin. Experientia. 1983;39:1018–1020. doi: 10.1007/BF01989781. [DOI] [PubMed] [Google Scholar]
- 28.Dalsgaard CJ, Björklund H, Jonsson CE, Hermansson A, Dahl D. Distribution of neurofilament-immunoreactive nerve fibers in human skin. Histochem. 1984;81:111–114. doi: 10.1007/BF00490102. [DOI] [PubMed] [Google Scholar]
- 29.Bellinger DL, Lorton D, Felten SY, Felten DL. Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol. 1992;14:329–344. doi: 10.1016/0192-0561(92)90162-e. [DOI] [PubMed] [Google Scholar]
- 30.Seiffert K, Hosoi J, Torii H, Ozawa H, Ding W, Campton K, Wagner JA, Granstein RD. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans cells. J Immunol. 2002;168:6128–6135. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 31.Manni M, Maestroni GJ. Sympathetic nervous modulation of the skin innate and adaptive immune response to peptidoglycan but not lipopolysaccharide: involvement of beta-adrenoceptors and relevance in inflammatory diseases. Brain Behav Immun. 2008;22:80–88. doi: 10.1016/j.bbi.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Streilen JW, Bergstresser PR. LC:APC of the epidermis. Immunobiology. 1984;168:285–300. doi: 10.1016/S0171-2985(84)80117-5. [DOI] [PubMed] [Google Scholar]
- 33.Stingl G, Katz SI, Clement L, Green I, Shevach EM. Immunologic functions of Ia bearing epidermal LC. J Immunol. 1978;121:2005–2013. [PubMed] [Google Scholar]
- 34.Hosoi J, Egan CL, Lerner EA, Murphy GF, Grabbe S, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 35.Asahina A, Moro O, Hosoi J, Lerner EA, Xu S, Takashima A, Granstein RD. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci USA. 1995;92:8323–8327. doi: 10.1073/pnas.92.18.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asahina A, Hosoi J, Beissert S, Stratigos A, Granstein RD. Inhibition of the induction of delayed-type and contact hypersensitivity by calcitonin gene-related peptide. J Immunol. 1995;154:3056–3061. [PubMed] [Google Scholar]
- 37.Ding W, Stohl L, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells towards Th2-type immunity. J Immunol. 2008;181:6020–1606. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding W, Wagner JA, Granstein RD. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NFκB activation. J Invest Dermatol. 2007;127:2357–2367. doi: 10.1038/sj.jid.5700858. [DOI] [PubMed] [Google Scholar]
- 39.Kodali S, Friedman I, Ding W, Seiffert K, Wagner JA, Granstein RD. Pituitary adenylate cyclase-activating polypeptide inhibits cutaneous immune function. Eur J Immunol. 2003;33:3070–3079. doi: 10.1002/eji.200324085. [DOI] [PubMed] [Google Scholar]
- 40.Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol. 2004;173:6082–6088. doi: 10.4049/jimmunol.173.10.6082. [DOI] [PubMed] [Google Scholar]
- 41.Jakob T, Ring J, Udey MC. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol. 2001;108:688–696. doi: 10.1067/mai.2001.118797. [DOI] [PubMed] [Google Scholar]
- 42.Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Review. Arthritis Res Therapy. 2009;11:257. doi: 10.1186/ar2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jabbari A, Johnson-Huang LM, Krueger JG. Role of the immune system and immunological circuits in psoriasis. G Ital Dermatol Venereol. 2011;146:17–30. [PubMed] [Google Scholar]
- 46.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci USA. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montesano R, Pepper MS, Möhle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- 50.Azuma M, Tamatani T, Fukui K, Bando T, Sato M. Enhanced proteolytic activity is responsible for the aberrant morphogenetic development of SV40-immortalized normal human salivary gland cells grown on basement membrane components. Lab Invest. 1994;70:217–227. [PubMed] [Google Scholar]
- 51.Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- 52.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 53.Mestas J, Hughes CCW. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–238. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 54.Boggon RP, Palfrey AJ. The microscopic anatomy of human lymphatic trunks. J Anat. 1973;114:389–405. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 57.Adami S, Cavani A, Rossi F, Girolomoni G. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487–97. doi: 10.1007/s40259-014-0098-x. [DOI] [PubMed] [Google Scholar]
- 58.Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141–150. doi: 10.1016/j.jaad.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 59.Brown G, Malakouti M, Wang E, Koo JY, Levin E. Anti-IL-17 phase II data for psoriasis: A review. J Dermatolog Treat. 2015;26:32–36. doi: 10.3109/09546634.2013.878448. [DOI] [PubMed] [Google Scholar]
- 60.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Ann Rev Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 62.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. Mol Med (Berl) 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 63.Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu YL, Yang B, Ma J, Wang H, Huang F, Zhang J, Chen H, Wu C. Interleukin-21 induces the differentiation of human Tc22 cells via phosphorylation of signal transducers and activators of transcription. Immunology. 2011;132:540–548. doi: 10.1111/j.1365-2567.2010.03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray EE, Ramírez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, Cyster JG. Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol. 2013;14:584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levy LL, Solomon SM, Emer JJ. Biologics in the treatment of psoriasis and emerging new therapies in the pipeline. Psoriasis: Targets and Therapy. 2012;2:29–43. [Google Scholar]
- 67.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9:302–309. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kraenzlin ME, Ch’Ng JL, Mulderry PK, Ghatei MA, Bloom SR. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul Pept. 1985;10:189–197. doi: 10.1016/0167-0115(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 69.Collins T, Korman AJ, Wake CT, Boss JM, Kappes DJ, Fiers W, Ault KA, Gimbrone MA, Jr, Strominger JL, Pober JS. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci USA. 1984;81:4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pober JS, Gimbrone MA, Jr, Collins T, Cotran RS, Ault KA, Fiers W, Krensky AM, Clayberger C, Reiss CS, Burakoff SJ. Interactions of T lymphocytes with human vascular endothelial cells: role of endothelial cells surface antigens. Immunobiology. 1984;168:483–494. doi: 10.1016/s0171-2985(84)80132-1. [DOI] [PubMed] [Google Scholar]
- 71.McGillis JP, Miller CN, Schneider DB, Fernandez S, Knopf M. Calcitonin gene-related peptide induces AP-1 activity by a PKA and c-fos-dependent mechanism in pre-B cells. J Neuroimmunol. 2002;123:83–90. doi: 10.1016/s0165-5728(01)00484-2. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez S1, Knopf MA, Bjork SK, McGillis JP. Bone marrow-derived macrophages express functional CGRP receptors and respond to CGRP by increasing transcription of c-fos and IL-6 mRNA. Cell Immunol. 2001;209:140–148. doi: 10.1006/cimm.2001.1795. [DOI] [PubMed] [Google Scholar]
- 73.Ding W, Manni M, Stohl LL, Zhou XK, Wagner JA, Granstein RD. Pituitary adenylate cyclase-activating peptide and vasoactive intestinal polypeptide bias Langerhans cell Ag presentation toward Th17 cells. Eur J Immunol. 2012;42:901–911. doi: 10.1002/eji.201141958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.