Abstract
Platelet aggregation is an essential response to tissue injury and is associated with activation of pro-oxidant enzymes, such as cyclooxygenase, and is also a highly energetic process. The two central energetic pathways in the cell, glycolysis and mitochondrial oxidative phosphorylation, are susceptible to damage by reactive lipid species. Interestingly, how platelet metabolism is affected by the oxidative stress associated with aggregation is largely unexplored. To address this issue, we examined the response of human platelets to 4-hydroxynonenal (4-HNE), a reactive lipid species which is generated during thrombus formation and during oxidative stress. Elevated plasma 4-HNE has been associated with renal failure, septic shock and cardiopulmonary bypass surgery. In this study, we found that 4-HNE decreased thrombin stimulated platelet aggregation by approximately 60%. The metabolomics analysis demonstrated that underlying our previous observation of a stimulation of platelet energetics by thrombin glycolysis and TCA (Tricarboxylic acid) metabolites were increased. Next, we assessed the effect of both 4-HNE and alkyne HNE (A-HNE) on bioenergetics and targeted metabolomics, and found a stimulatory effect on glycolysis, associated with inhibition of bioenergetic parameters. In the presence of HNE and thrombin glycolysis was further stimulated but the levels of the TCA metabolites were markedly suppressed. Identification of proteins modified by A-HNE followed by click chemistry and mass spectrometry revealed essential targets in platelet activation including proteins involved in metabolism, adhesion, cytoskeletal reorganization, aggregation, vesicular transport, protein folding, antioxidant proteins, and small GTPases. In summary, the biological effects of 4-HNE can be more effectively explained in platelets by the integrated effects of the modification of an electrophile responsive proteome rather than the isolated effects of candidate proteins.
Keywords: Platelet, Bioenergetics, 4-hydroxynonenal, Aggregation, click chemistry, metabolomics
INTRODUCTION
Lipid peroxidation can produce reactive electrophiles such as 4-hydroxy-2-nonenal (4-HNE), which in turn can alter cellular function by forming stable adducts with proteins[1–4]. 4-HNE has been identified in human atherosclerotic lesions, brain sections of patients with Alzheimer’s and Parkinson’s disease, kidney tissue of patients with renal cell carcinoma and diabetic nephropathy and placentas of women with preeclampsia [5–9]. Based on these studies, 4-HNE has been considered a negative prognostic marker and mediator of oxidative stress in a broad range of diseases. Conversely, some studies have reported that low concentrations of 4-HNE can have a protective role against oxidative stress through activation of Nrf2 and the induction of antioxidant enzymes such as heme oxygenase 1 (HO-1) and glutathione-S-transferase [10–12]. It has been shown that 4-HNE forms adducts with mitochondrial and glycolytic proteins, such as cytochrome c1 (complex III), cytochrome c, electron transfer flavoprotein alpha, glyceraldehyde-3-phosphate dehydrogenase, aldolase A, and inhibits the activity of these metabolic proteins [13–15].
The platelet offers an interesting and biologically relevant setting in which to assess the impact of 4-HNE, and how identification of protein adducts is linked to changes in platelet function to mechanism. This is important because previous studies have largely taken a candidate target approach to identifying mechanisms of 4-HNE dependent changes in function. It is now becoming clear that the biological effects of reactive electrophiles are mediated through the generation of an electrophile responsive proteome which has the potential to impact biological function through the cumulative effects at multiple targets [16, 17]. In the platelet, we can identify both effects on bioenergetics and platelet function and can link these to the protein targets so providing an ideal context to test these concepts.
Previous studies have shown that 4-HNE can affect platelet aggregation and possibly act as a negative feedback modulator of platelet function. However, the data presented in the literature are not consistent. For example, using platelet rich plasma (PRP) from healthy human volunteers it was reported that 4-HNE at high concentrations (0.5–2 mM) did not affect thrombin-induced (2 U/ml) aggregation [18]. However, the authors reported an approximately a 50% decrease in ADP stimulated aggregation of PRP when pre-treated with 4-HNE (330 µM) for 10 min [18]. Conversely, low concentrations of 4-HNE were more potent in inhibiting thrombin (0.5 U/ml) mediated aggregation in washed platelets [18]. In contrast, another study reported that incubation of healthy PRP with HNE for 1 min caused a significant potentiation of aggregation induced by thrombin (0.02 U/ml) and ADP, but not collagen when compared to control conditions [19]. The authors proposed that potentiation of aggregation was attributable to an increase in arachidonic acid release after 4-HNE and thrombin treatment, which would suggest an increase in levels of thromboxane A2 [19]. However, this would seem unlikely since most if not all of the exogenous HNE would be bound or react with the large amount of albumin present in PRP.
From previous studies, it is not clear if the changes seen in platelet aggregation were a direct or indirect result of HNE production. However, it is clear that 4-HNE has diverse effects on platelet aggregability, but the precise mechanisms underlying these effects are unclear. What is known is that 4-HNE reacts with nucleophilic amino acids such as cysteine, lysine, histidine, asparagine, and glutamine, and so modifies protein function [1–4, 17]. Here, we used protocols to assess bioenergetics, targeted metabolomics, platelet function and 4-HNE protein adduct formation in isolated human platelets. To determine the protein targets of 4-HNE in platelets, we used an analog of HNE, A-HNE to modify and enrich for HNE-adducted proteins. A-HNE contains a terminal alkyne group which can be conjugated to a biotin tag using click chemistry allowing for affinity enrichment using neutravidin resin and identification by mass spectrometry. Our studies revealed that 4-HNE decreased thrombin-dependent aggregation and caused a depression of mitochondrial respiration which was further blunted in the presence of thrombin. On the other hand, 4-HNE caused a compensatory increase in basal glycolysis. We found that A-HNE modified an electrophilic responsive proteome comprised of 72 proteins involved in key aspects of platelet function. Taken together these data highlight the pleiotropic nature of the interaction of 4-HNE with complex biological systems and the potentially interactive targets which could result in inhibition of platelet aggregation and metabolism.
METHODS
Platelet Isolation and Aggregation
For these experiments, platelets were isolated from platelet concentrates from ten donors between days 6–8 since collection. The platelet concentrates were obtained from the blood bank at the University of Alabama at Birmingham. Approval for collection and use of platelet concentrates was obtained from the University of Alabama at Birmingham Institutional Review Board (Protocol #X110718014). Platelets were isolated from the concentrates as described previously [20]. In brief, the concentrates were centrifuged at 1500 × g for 10 min and the pelleted platelets were washed with PBS containing prostaglandin I2 (1 µg/ml) prior to determination of platelet count by turbidimetry [20–22]. Aggregation was measured by monitoring change in light transmittance at 405 nm in a 96-well plate reader, after the addition of thrombin (0.5 U/ml) [23].
Measurement of Bioenergetics
Both oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured simultaneously in the extracellular flux (XF) analyzer as described previously [20, 24]. In brief, platelets were pre-treated with 4-HNE (0–30 µM), alkyne HNE (30 µM) or 1-nonanol and nonanal (30 µM) for 1h prior to performing the assay by sequential injection of oligomycin (1 µg/ml), FCCP (0.6 µM) and antimycin A (10 µM). To determine the effect of activation on bioenergetics, thrombin (0.5 U/ml) was injected prior to injection of the mitochondrial inhibitors.
Tagging Platelets with A-HNE
Platelets (200 × 106) were placed in suspension in 3.6 ml XF DMEM media and incubated with 30 µM Click Tag A-HNE (4-hydroxynon-2E-nonen-8-ynal) (Cayman, Ann Arbor, MI), or unmodified 4-HNE in an equal volume of vehicle (ethanol) for 1 h at 37°C in a non-CO2 incubator, and Click Chemistry was performed as previously described [25]. The platelets were washed 2× with PBS and lysed in 10mM Tris, 1% Triton X-100 containing protease inhibitor cocktail (Roche, Basel, Switzerland). The lysed samples were centrifuged at 20,000 × g for 10 min at 4°C and the supernatant was collected and the Lowry protein assay performed. Using equal amounts of cell lysate, NaBH4 (1 M) was added to the platelet lysate and incubated at room temperature (RT) for 1 h. Next, ascorbate (200 mM), cupric sulfate (100 mM) and azide-PEG3-biotin (0.5 mM, Axxora, Farmingdale, NY) were added and rotated end over end for 2h at RT. The protein was then precipitated by adding 2× volumes of ice cold methanol, and incubating on ice for 30 min. The samples were centrifuged at 20,000 × g for 10 min at 4°C and then the pellet was washed with ice cold methanol and re-suspended in 100 µl RIPA lysis buffer containing protease inhibitor cocktail (Roche, Basel, Switzerland). To assess the efficiency of the labeling, 10 µg of protein was subjected to SDS-PAGE gel electrophoresis and probed with streptavidin-horseradish peroxidase conjugate. For the pull down of biotinylated proteins, 75 µg of protein was incubated with 30 µl of NeutrAvidin Plus UltraLink resin (Thermo Scientific, Waltham, MA) slurry at room temperature for 1h on a shaker. The resin was washed 6X with RIPA buffer and then 15 µl of 2X sample loading buffer containing β-mercaptoethanol was added and heated at 80°C for 10 min. These samples were then separated on an SDS-PAGE gel and stained with Coomassie brilliant blue. To identify A-HNE modified proteins, the gel bands were excised and digested with trypsin (12.5 ng/µl) overnight, and an aliquot (5 µl) of the digest was first loaded onto a Nano cHiPLC 200 µm ID × 0.5 mm ChromXP C18-CL 3 µm 120 Å reverse-phase trap cartridge for desalting, then eluted onto a 200 µm ID × 15 cm ChromXP C18-CL 3 µm 120 Å reverse phase analytical column heated at 45°C using a 5–95% acetonitrile gradient over 42 min at a flow rate of 1000 nL/min (Eksigent, Dublin,CA). The SCIEX 5600 Triple-Tof mass spectrometer (SCIEX, Toronto, Canada) was used to analyze the separated peptides in positive ion mode. The IonSpray voltage was 2300 V and the declustering potential was 80 V. Ionspray and curtain gases were set at 10 psi and 25 psi, respectively. The interface heater temperature was 120°C.
Eluted peptides were subjected to a time-of-flight survey scan from 400–1250 m/z to determine the top 20, most intense ions for MSMS analysis. Product ion time-of-flight scans (50 msec) were carried out to obtain the tandem mass spectra of the selected parent ions over the range from m/z 400–1500. Spectra are centroided and de-isotoped by Analyst software, version TF (Applied Biosystems). A β-galactosidase trypsin digest was used to establish and confirm the mass accuracy of the mass spectrometer.
The tandem mass spectrometry data were processed to provide protein identifications using Protein Pilot 4.5 search engine (SCIEX) using the Homo sapiens Sprot protein (Version 4/2014) and using a trypsin digestion and fixed iodoacetamide-modified cysteines parameters. To correct for false identifications, proteins also found in the control group and any proteins which had less than 2 peptides identified were excluded from further analysis.
Measurement of Platelet Integrity
The proportion of lactate dehydrogenase (LDH) released to the platelet medium relative to the lysed platelet was used to determine platelet integrity. Platelets were treated with either vehicle or 4-HNE (0–50 µM) for 1h, followed by the addition of either media or thrombin (0.5 U/ml) for 30 min. After this the media was collected and the platelets were lysed in PBS containing 0.1 % Triton X-100. Both the media and the lysates were centrifuged (10,000 × g, 10 min), and the supernatants were collected for analysis. LDH activity in both the media and lysates were measured by oxidation of NADH to NAD+ in the presence of pyruvate, by monitoring the disappearance of absorbance at 340 nm [26]. Intra-platelet LDH was calculated by dividing the rate of change of absorbance in the platelet lysates by the sum of the rate of change of absorbance in the media and lysate and expressed as a percentage.
Targeted Metabolomics Analysis
For targeted metabolomics analysis, suspended platelets (300 million) were treated with either vehicle, HNE (30 µM), thrombin (0.5 U/ml) or a combination of HNE/thrombin in XF media. After treatment, the platelets were sedimented by centrifugation and rinsed with cold PBS. Platelets were then metabolically clamped using methanol (cooled to -80°C), and centrifuged to remove precipitated protein. Methanol extracts were taken to dryness under a steam of nitrogen and reconstituted in 5% acetic acid prior to derivatization using Amplifex Reagent (AB Sciex, Concorde, Ontario, Canada). Derivatized samples and standards were analyzed by quantitative liquid chromatography-multiple reaction ion monitoring mass spectrometry analysis (LC-MRM-MS) as previously described [27]. Precursor ion product transitions for TCA cycle, glycolytic intermediates and glutaminolysis were: TCA cycle; citrate (m/z 191/87), cis-aconitate (m/z 173/85), α-ketoglutarate (m/z 261/118), succinate (m/z 117/73), fumarate (m/z 115/71), malate (m/z 133/115), oxaloacetate (m/z 247/118); glycolysis: glucose-6-phosphate (m/z 375/109), fructose-6-phosphate (m/z 375/74), pyruvate (m/z 204/144), lactate (m/z 89/43); glutaminolysis: glutamine (m/z 145/42), glutamate (m/z 143/102) and 2-hydroxy glutarate (m/z 147/129). The data are expressed as mmol metabolite per million platelets.
Statistics
All results are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA and the Tukey’s post hoc test. Values of p<0.05 were considered statistically significant.
RESULTS
Effect of 4-HNE on platelet aggregation and integrity
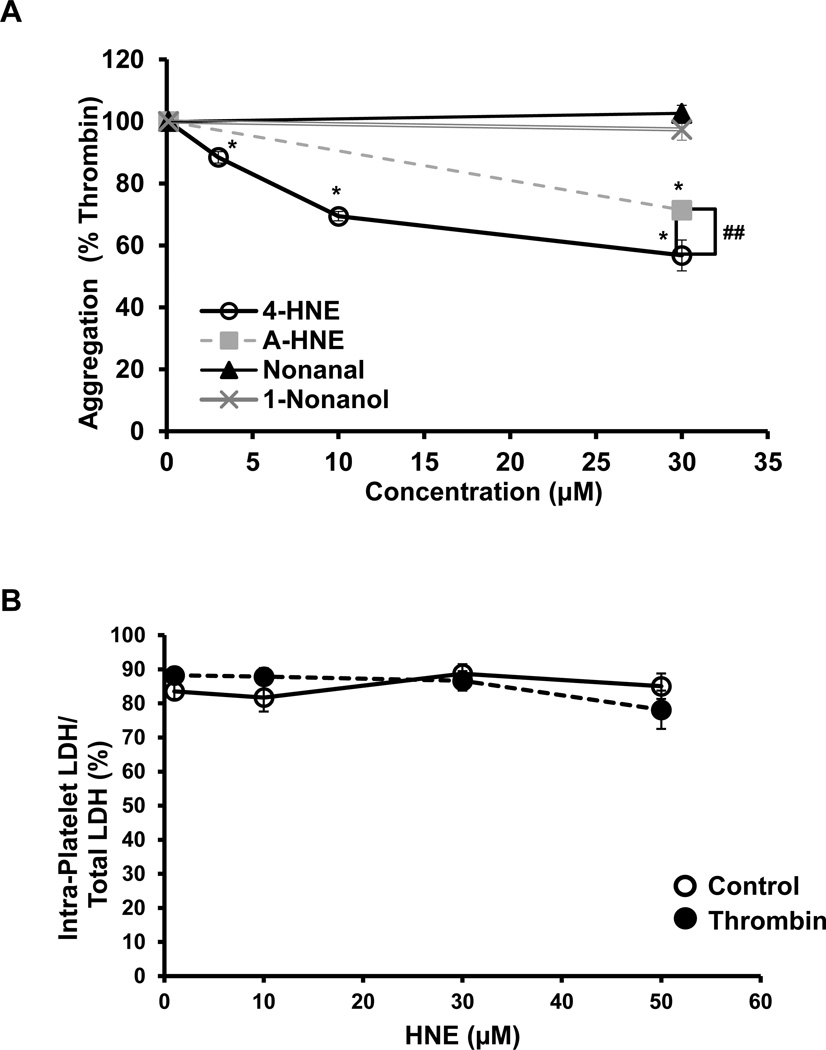
Platelets were incubated with either 4-HNE (0–30 µM), A-HNE (30 µM) or the non-electrophilic derivative of HNE, nonanal (30 µM) or the alcohol, 1-nonanol (30 µM) for 1h, after which a platelet aggregation assay was performed after the addition of thrombin (0.5 U/ml). As shown in Figure 1A, 4-HNE caused a concentration dependent decrease in platelet aggregation, which was approximately 60% at the highest concentration tested. To identify the proteins modified by HNE, an analog of HNE, with a terminal alkyne moiety was used. We confirmed that the effects of A-HNE (30 µM) and 4-HNE (30 µM) on platelet function were similar. We found that the A-HNE was capable of inhibiting platelet aggregation, to the same extent as 4-HNE. As expected the non-electrophilic derivatives of HNE, nonanal and 1-nonanol, had no effect on thrombin-mediated platelet aggregation.
Figure 1. Effect of 4-HNE on platelet aggregation and platelet rupture.
(A) Platelets were incubated with either HNE (0–30 µM), 4-HNE (30 µM), nonanal (30 µM), or 1-nonanol (30 µM) for 1h, followed by the addition of thrombin and monitoring of aggregation by light transmittance. Nonanal and 1-nonanol were used as non-electrophilic controls. (B) Platelets were treated with 4-HNE alone (0–50 µM) for 1h or in combination with thrombin (30 min), after which the samples were lysed and LDH activity was measured in the media and in the lysed platelets. Data expressed as mean ± SEM from one representative donor, n = 3 (Panel A) N = 5 donors and (B) N = 2 donors. *p<0.05, different from thrombin treatment, ## represents 4-HNE vs. A-HNE.
In order to assess whether 4-HNE or thrombin caused platelet rupture and a decrease in integrity, the extent of LDH released to the media, after 4-HNE was determined. Platelets were pre-treated with 4-HNE (0–50 µM) for 1h, followed by addition of either media or thrombin (0.5 U/ml) for 30 min. There was no detectable change in percentage of intra-platelet LDH in proportion to LDH released to the media following 4-HNE treatment in the absence or presence of thrombin (Figure 1B). These data indicate that any changes observed in the bioenergetics were not due to platelet rupture. Total LDH levels did not change (data not shown).
Effect of 4-HNE on platelet bioenergetics
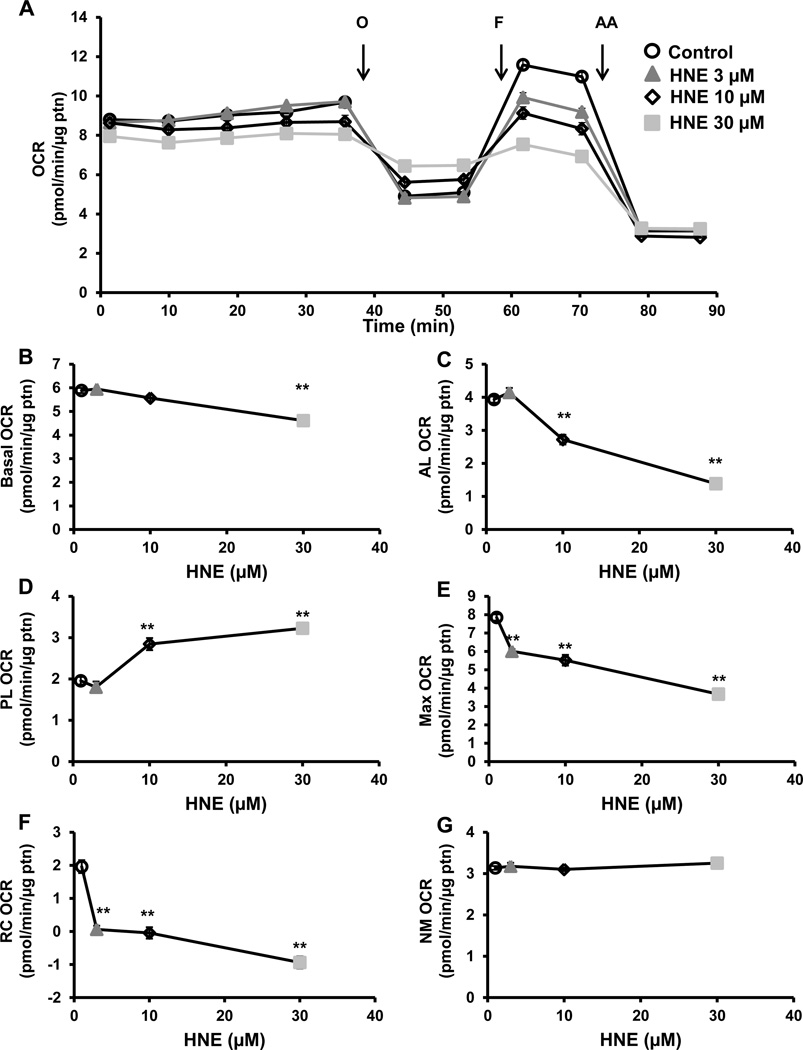
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured simultaneously in platelets pre-treated with 4-HNE (0–30 µM), and bioenergetics assessed using the mitochondrial stress test as we have described previously [24, 28, 29]. First, the basal rate of oxygen consumption was measured for 40 min, and showed a modest 4-HNE concentration dependent decrease in OCR at (Figure 2A and B). Next, oligomycin (1 µg/ml), a complex V inhibitor, was injected and as expected a decrease in OCR was observed. However, the magnitude of the ATP linked respiration decreased in a concentration dependent manner with 4-HNE treatment (Figure 2C). The remaining respiration after oligomycin, or proton leak, was consistently higher in the 4-HNE treated samples (Figure 2A and D). After 16 min, FCCP (0.6 µM), a proton ionophore was injected, leading to an increase in maximal respiration, which reached a lower value with the 4-HNE treated platelets depending on the concentration, and a corresponding decrease in reserve capacity (Figure 2A, E and F). Finally, antimycin A (10 µM) was injected, leading to inhibition of all non-mitochondrial electron transport-dependent oxygen consumption, and was subtracted from the basal and maximal bioenergetic parameters (Figure 2A). 4-HNE did not significantly change non-mitochondrial (NM) oxygen consuming processes (Figure 2A and G).
Figure 2. Effect of 4-HNE on mitochondrial respiration.
(A) Platelets were incubated with 4-HNE (HNE) (0–30 µM) for 1h after which a mitochondrial stress test was performed by first measuring basal OCR, followed by sequential injection of oligomycin (O) (1 µg/ml), FCCP (F) (0.6 µM), antimycin A (AA) (10 µM). Different indices of mitochondrial respiration (B) basal (basal OCR – AA OCR), (C) ATP-linked (AL) (basal OCR – oligomycin OCR), (D) proton leak (PL) (oligomycin OCR – AA OCR), (E) maximal (FCCP OCR – AA OCR), (F) reserve capacity (RC) (FCCP OCR – basal OCR) and (G) non-mitochondrial (NM) (AA OCR) were calculated. Data expressed as mean±SEM from one representative donor, n = 5–6 replicates. **p<0.01, different from thrombin.
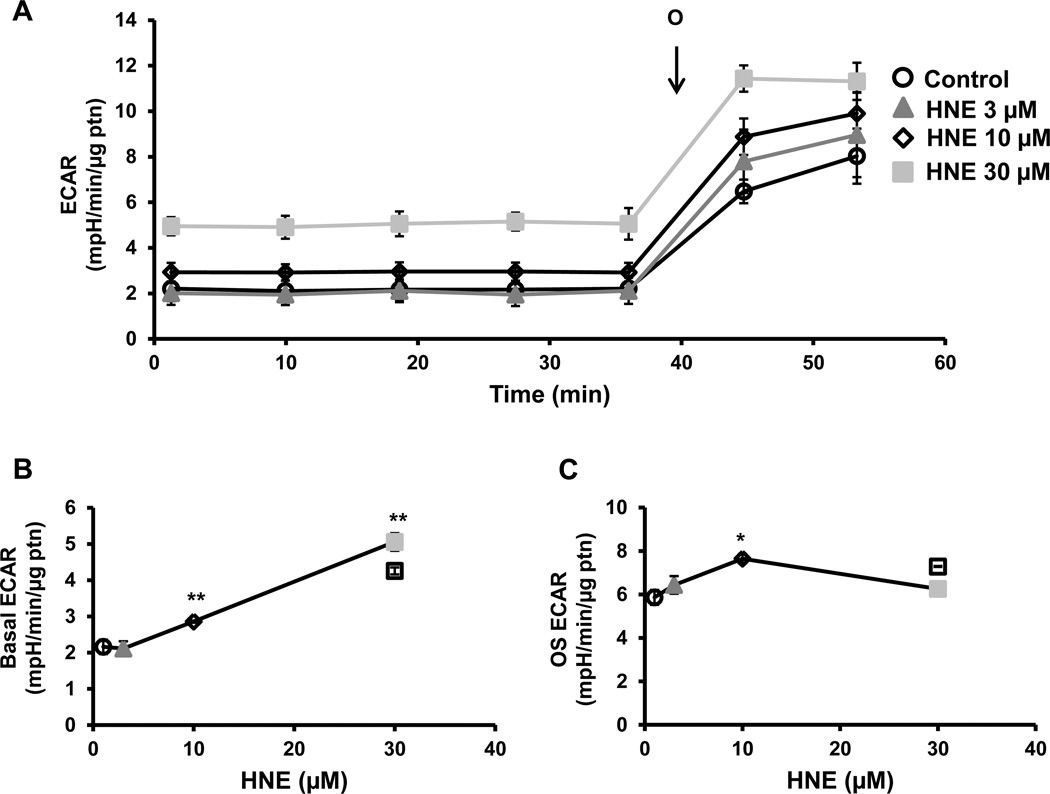
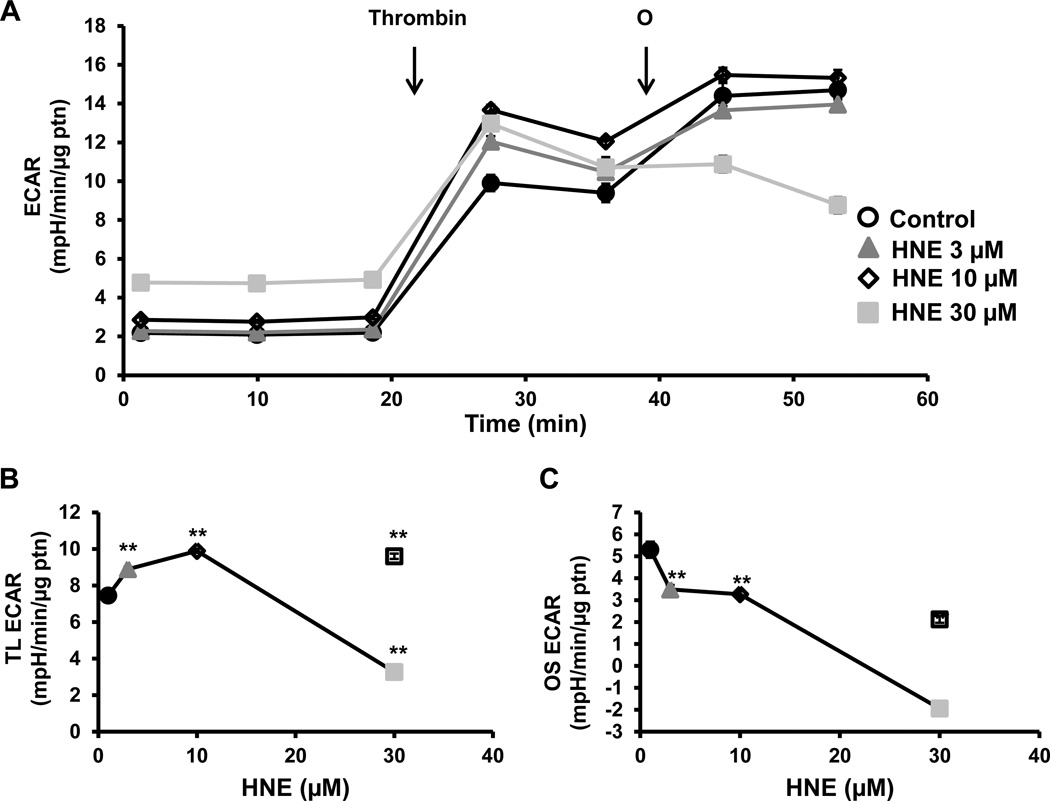
Extracellular acidification rate (ECAR) which is a measure of glycolysis in platelets and can be derived from proton production during glycolysis or mitochondrial respiration derived CO2, was measured simultaneously in these experiments. We have shown previously that in platelets approximately 30% of the basal ECAR and more than 90% of the thrombin-stimulated ECAR can be ascribed to glycolysis [24]. Furthermore, basal ECAR increased concentration-dependently with 4-HNE and, since this increase is associated with a decrease in basal OCR, it is unlikely to be due to increased proton production from the TCA cycle (Figure 3A and B). Oligomycin-sensitive ECAR is a measure of the upregulation of glycolysis after mitochondrial ATP synthase is inhibited. Oligomycin-sensitive ECAR was calculated by subtracting the rate before oligomycin addition from the rate after oligomycin addition. Low concentrations of 4-HNE had no effect on oligomycin-dependent ECAR, but at a higher concentration (10 µM), caused a significant increase in ECAR after oligomycin injection compared to control (Figure 3C). However, with the highest concentration of 4-HNE (30 µM), the increase in oligomycin sensitive ECAR was attenuated (Figure 3C).
Figure 3. Effect of 4-HNE glycolysis.
(A) Platelets were incubated with 4-HNE (HNE) (0–30 µM) for 1h after which basal ECAR was measured, followed by injection of oligomycin (O) (1 µg/ml). Different indices of glycolytic function (B) basal (basal ECAR) and (C) oligomycin sensitive (OS) (oligomycin ECAR – basal ECAR) were calculated. Data expressed as mean±SEM from one representative donor, n = 5–6 replicates. *p<0.05,**p<0.01, different from thrombin.
Effect of 4-HNE on platelet bioenergetics following thrombin
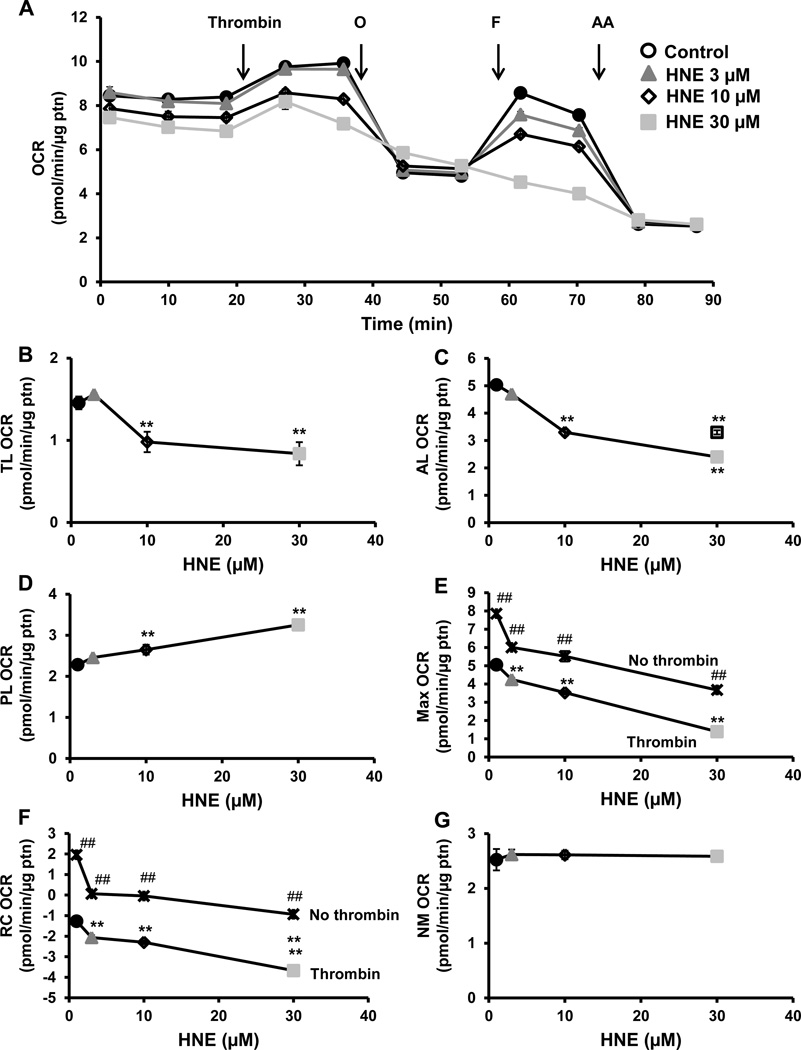
The next experiment was performed as described in Figure 2, but with the injection of thrombin (0.5 U/ml), followed by oligomycin (1 µg/ml), FCCP (0.6 µM), and antimycin A (10 µM). As we have shown previously [24], thrombin addition stimulated OCR, but this was inhibited in the 4-HNE treated groups in a concentration dependent manner (Figure 4A). The other bioenergetic parameters, ATP-linked, maximal OCR and reserve capacity showed a similar decrease in response to 4-HNE in the presence of thrombin (Figure 4C, E and F). Proton leak increased in a 4-HNE concentration dependent manner in the presence of thrombin (Figure 4D). We compared the concentration dependent effects of 4-HNE with and without thrombin and found a significant difference with maximal respiration and reserve capacity with the combined treatment of 4-HNE and thrombin (Figure 4E and 4F). The maximal OCR was further decreased in the presence of thrombin, perhaps due to depletion of the reserve capacity due to the increased energy demand of thrombin activation.
Figure 4. Effect of 4-HNE on mitochondrial respiration in the presence of thrombin.
(A) Platelets were incubated with 4-HNE (HNE) (0–30 µM) for 1h after which a mitochondrial stress test was performed by first measuring basal OCR, followed by sequential injection of thrombin (0.5 U/ml) oligomycin (O) (1 µg/ml), FCCP (F) (0.6 µM), antimycin A (AA) (10 µM). Indices of mitochondrial respiration: (B) thrombin linked (TL) (thrombin OCR – basal OCR), (C) ATP-linked (AL) (thrombin OCR – oligomycin OCR), (D) proton leak (PL) (oligomycin OCR – AA OCR), (E) maximal (FCCP OCR – AA OCR), (F) reserve capacity (RC) (FCCP OCR – thrombin OCR) and (G) non-mitochondrial (NM) (AA OCR) were calculated. The no thrombin HNE treatment data were added to the maximal OCR and reserve capacity plots. Data expressed as mean±SEM from one representative donor, n = 5–6 replicates. **p<0.01, different from thrombin. ##p<0.01, different from the thrombin and 4-HNE treatments.  No thrombin traces.
No thrombin traces.
After basal rate of ECAR was established, thrombin (0.5 U/ml) was injected and ECAR was measured for 16 min (Figure 5A). There was an increase in the thrombin linked ECAR with the lower concentrations of 4-HNE whereas the 30µM concentration resulted in a decrease (Figure 5B). There was a concentration dependent decrease in oligomycin sensitive ECAR which was most pronounced with the 30µM 4-HNE treated platelets (Figure 5C).
Figure 5. Effect of 4-HNE on glycolysis in the presence of thrombin.
(A) Platelets were incubated with 4-HNE (HNE) (0–30 µM) for 1h after which basal ECAR was measured, followed by sequential injections of thrombin (0.5 U/ml) and oligomycin (O) (1 µg/ml). Indices of glycolytic function (B) thrombin-linked (TL) (thrombin ECAR) and (C) oligomycin sensitive (OS) (oligomycin ECAR – thrombin ECAR) were calculated. Data expressed as mean±SEM from one representative donor, n = 5–6 replicates. **p<0.01, different from thrombin.
Effect of HNE on platelet metabolism
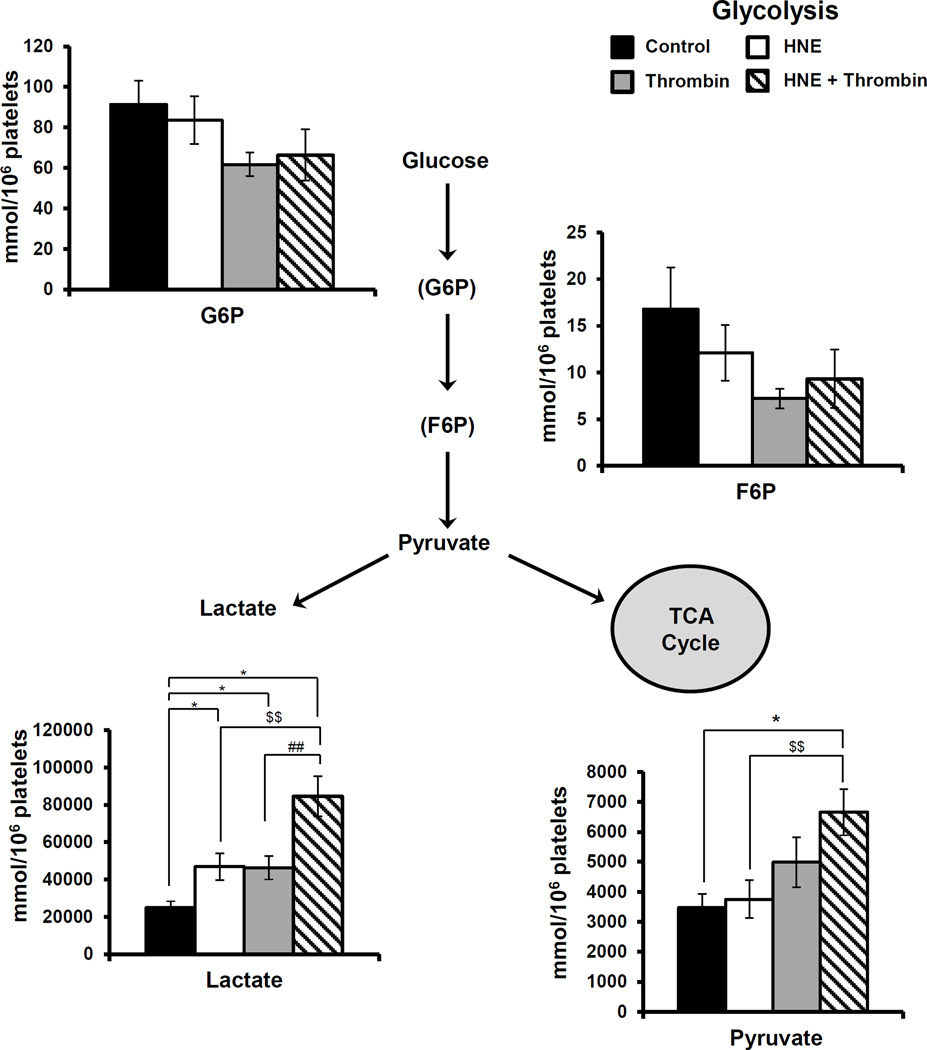
Using a targeted metabolomics approach we measured intermediates of glycolysis such as glucose-6-phosphate (G6P), fructose-6-phosphate (F6P), lactate and pyruvate (Figure 6). The intermediates in the initial phases of glycolysis (F6P and G6P) did not show any significant changes with any of the conditions tested. Consistent with the changes in ECAR lactate was increased significantly with both HNE (30 µM) and thrombin treatment. Interestingly, when the platelets were pre-treated with HNE prior to activation with thrombin a further increase in lactate production was observed coupled with an increase in pyruvate.
Figure 6. Targeted Glycolytic Metabolomics.
Platelets were incubated with either media, HNE (30 µM), thrombin (0.5 U/ml) or treated with HNE prior to thrombin exposure as described under Methods. Data represent mean ± SEM, N=5 donors; p<0.05 was taken as statistically significant.
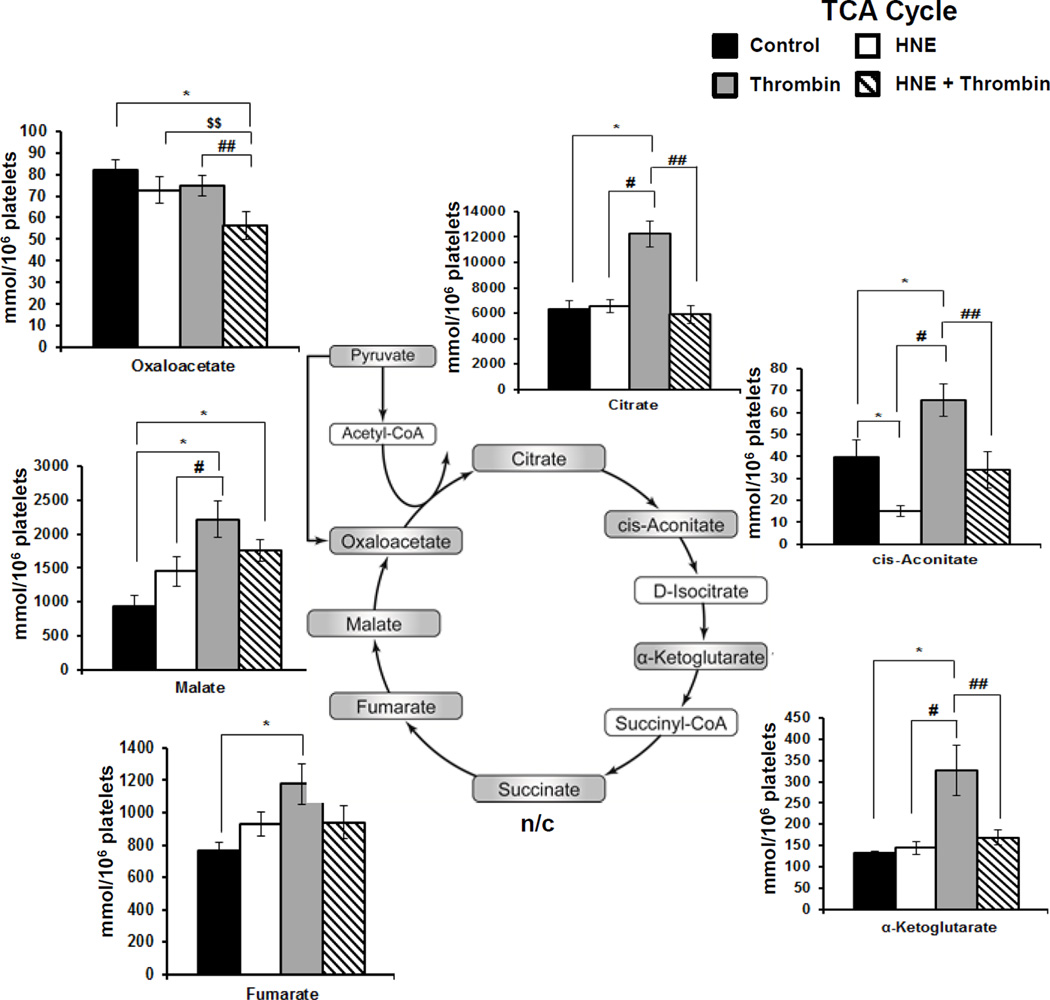
We next focused on intermediates of the TCA cycle (Figure 7). When platelets were exposed to HNE (30 µM) alone no major changes in the levels of metabolites were observed except for a significant decrease in cis-aconitate which would be consistent with the modification of aconitase. Interestingly, thrombin activation of platelets resulted in significant increases in citrate, cis-aconitate, α-ketoglutarate, fumarate and malate. In marked contrast when platelets were first pre-treated with HNE (30 µM) prior to activation with thrombin this prevented the thrombin-dependent decrease in citrate, cis-aconitate and α-ketoglutarate and accumulation of pyruvate levels suggesting inhibition of substrate oxidation in the mitochondria.
Figure 7. Targeted Metabolomics of TCA Cycle Intermediates.
Platelets were incubated with either media, HNE (30 µM), thrombin (0.5 U/ml) or treated with HNE prior to thrombin exposure as described under Methods. Data represent mean ± SEM, N=5 donors; p<0.05 was taken as statistically significant.
Effect of A-HNE on platelet aggregation and bioenergetics
To identify the proteins modified by HNE, an alkyne derivative of HNE was used. After the reaction of platelets with A-HNE, it was then conjugated to biotin. First, we confirmed that the effects of A-HNE (30 µM) and 4-HNE (30 µM) on platelet function were similar, as shown in (Figure 6). We found that A-HNE (30 µM) was capable of inhibiting aggregation although it was slightly less potent than 4-HNE (30 µM) (Figure 1A). Although, some minor differences in the absolute magnitude of the effects of A-HNE compared to HNE in the bioenergetic responses were observed these were essentially similar (Supplementary Figure 1,2).
Non-electrophilic analogues of HNE do not affect platelet bioenergetics
Due to the electrophilic nature of HNE and its capacity to reactive with nucleophilic residues on proteins, which have been shown to be critical in the aggregation cascade, we used two analogs of HNE which are not electrophilic in nature, nonanal and 1-nonanol. Nonanal lacks the carbon-carbon double bond and the electron withdrawing hydroxyl group and 1-nonanol, a straight chain fatty alcohol. As shown in Supplementary Figure 5, neither nonanal nor 1-nonanol had a significant effect on OCR, thrombin-linked OCR/ECAR. There was a small effect of on the oligomycin dependent ECAR by 1-nonanol the mechanism of which is unclear.
Identification of A-HNE modified proteins in platelets
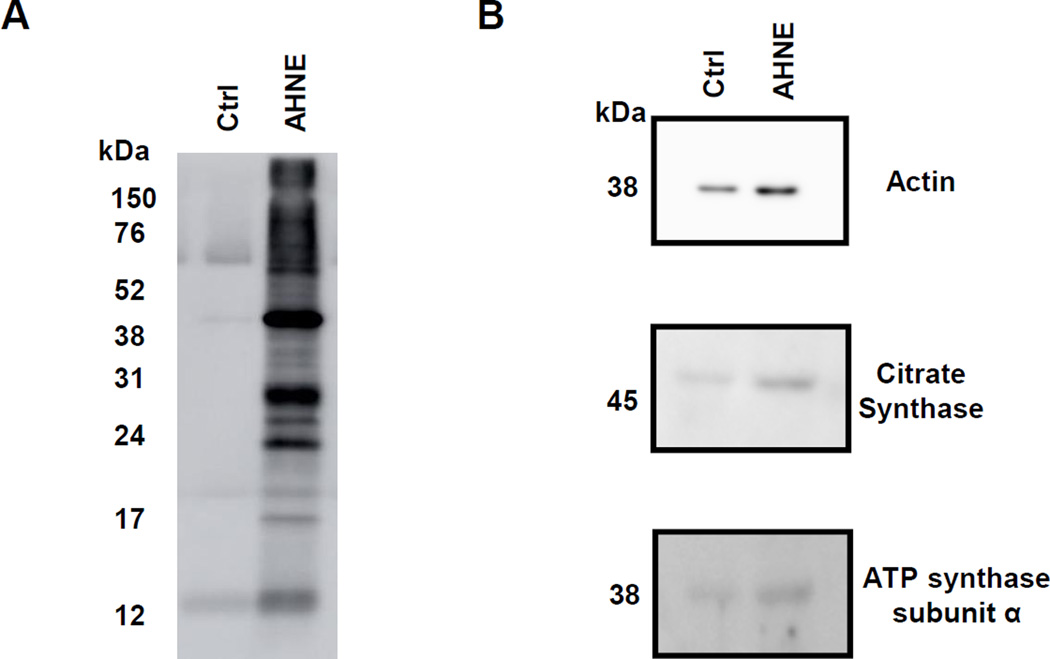
To determine the protein targets of A-HNE, platelets were treated with either media alone or A-HNE (30 µM) for 1hr. After the incubation, the samples were subjected to Click Chemistry and conjugated with biotin, followed by protein enrichment using a pull-down with NeutrAvidin. The lysates of the post-pulldown reaction (5 µl) were separated on a 15% SDS page gel, and enrichment of the biotinylated proteins in the A-HNE treated platelets was evident (Figure 8A). Using a candidate protein approach, western blot analyses were performed on the post pulldown lysates (15 µl). The lysates were probed for the presence of actin, citrate synthase, ATP synthase subunit α, and the signal was enriched in the A-HNE group, consistent with these proteins reacting with A-HNE (Figure 8B).
Figure 8. Modification of platelet proteins by A-HNE.
Platelets were incubated with either media or AHNE (30 µM) for 1h, after which Click Chemistry was performed and the biotinylated proteins were pulled down with NeutrAvidin beads. The lysates were separated using SDS-PAGE gel and (B) western blot for streptavidin and (B) western blot for actin, citrate synthase and ATP synthase subunit α were performed. Data from an individual platelet donor.
Next the proteins in the pull-down with and without A-HNE treatment were identified using nanoLC-tandem mass spectrometry of their tryptic peptides. Only proteins identified on the basis of two or more peptides were included in the analysis. This resulted in a total of 72 proteins that were identified to be potentially modified by AHNE in platelets (Supplementary Table 1 – 9). The proteins identified could be broadly divided into functional classes, including those involved in adhesion (total 3 proteins), cytoskeletal/shape change (20 proteins), protein folding (6 proteins), small GTPases (11 proteins), metabolism (10 proteins), aggregation (3 proteins), antioxidant (5 proteins), vesicle transport (3 proteins) and other signaling related proteins (11 proteins) (Supplementary Table 1 – 9).
DISCUSSION
The process of platelet aggregation involves the liberation of plasma membrane arachidonic acid which generates both enzymatic and non-enzymatic lipid peroxidation products [30–32]. The non-enzymatic lipid peroxidation product 4-HNE is a marker of oxidative stress, and has been associated with a number of different pathologies [5–9]. Platelets are frequently activated under pro-inflammatory and pro-oxidant conditions resulting in exposure to exogenous 4-HNE. Understanding how 4-HNE affects platelet aggregability is then important in targeting diseases associated with platelet dysfunction and oxidative stress. There are conflicting reports of the effects of 4-HNE on platelets, with some studies reporting no effect on thrombin-mediated platelet aggregation, while others showing inhibition of aggregation and others in contrast potentiation [18, 19, 33]. In this study we integrated the HNE-electrophile responsive proteome with the functional responses of platelets. The concentrations of 4-HNE used here are consistent with levels reached in pathological conditions, where concentrations of 10 µM to greater than 100 µM have been reported in the plasma of patients during liver failure and localized plasma membranes of diseases with high oxidative stress [34, 35].
Incubation of washed platelets with 4-HNE (0–30 µM), caused a concentration dependent decrease in thrombin-stimulated aggregation which was similar to A-HNE (Figure 1A). It is not always appreciated that the terminal azide tag, used for performing Click Chemistry can modify the reactivity or location relative to the parent molecule. This is a potential limitation of the present study, nevertheless, A-HNE (30 µM) also caused a decrease in aggregation and bioenergetics, comparable to 4-HNE, making it possible to attempt to relate the proteome modified to the changes in function we have observed.
The HNE-dependent decrease in aggregation observed could be due to the potential modifications of proteins involved in signaling, metabolism or the aggregation process itself. The first step in the platelet activation cascade is the adhesion of the platelet to the subendothelium through receptors present on the surface, and A-HNE potentially modified 3 proteins involved in adhesion (Supplementary Table 1) [36, 37]. These include platelet glycoprotein IX, integrin alpha-6 and multimerin-1. Since mutations of glycoprotein IX have been shown to disrupt formation of the glycoprotein Ib-IX-V complex which binds von Willebrand factor (vWF), then modification of this protein could affect adhesion, but this is not a feature of the in-vitro thrombin-dependent aggregation model we have used here [38, 39].
The next step during activation involves a change in platelet morphology, accompanied by the rearrangement of cytoskeletal components to aid in the spreading of the platelet, which are features of the thrombin-dependent aggregation used in the present study [40]. It has been shown that shape change is required for ADP-induced aggregation and for irreversibility of the bridges formed between platelets through the alpha IIb beta 3 receptor following thrombin induced aggregation [41, 42]. Actin-related protein 2/3, adenylyl cyclase-associated protein 1, myosin regulatory light chain 12A and B, myosin regulatory light polypeptide 9 and myosin, proteins that regulate actin polymerization, were potentially modified by A-HNE, and if their functions were altered, could affect cytoskeletal reorganization and aggregation (Supplementary Table 2) [43–48].
Following shape change, alpha and dense granules release their contents, including calcium, ADP and vWF that potentiate aggregation [49]. Defects in granule release are associated with bleeding disorders [50]. Fusion of the granules with the plasma membranes enables this release reaction, and syntaxin binding protein-2 and the small GTPases Rab 6, 11 and 27 are responsible for regulation of the fusion machinery [51]. Potential A-HNE modification of Rab GTPases and syntaxin binding protein-2, may hinder granule release and may contribute to decreased aggregation (Supplementary Table 4 and 8).
More directly, 3 proteins involved in the regulation of aggregation were potentially modified by A-HNE, which could have an inhibitory effect on aggregability if their functions were hampered (Supplementary Table 6). Specifically, the integrin beta 3, of the alpha IIb beta 3 complex was identified as a target for A-HNE. The alpha IIb beta 3 receptor binds fibrinogen and vWF, forming bridges between platelets so enabling aggregation [52]. Furthermore, the signaling protein disulfide isomerase (PDI) required for activation of integrin alpha IIb beta 3 by thiol reduction, was also potentially modified by A-HNE (Supplementary Table 8) [53]. A previous study has shown that 4-HNE modification of PDI inhibits its activity in human endothelial cells and macrophages [54]. Consequently, PDI modifications in the platelet could inhibit its activity and play a role in decreasing aggregation.
We have previously reported that platelet aggregation with thrombin requires both a contribution from mitochondrial oxidative phosphorylation and glycolysis [24]. Therefore, we examined whether the inhibition of thrombin-dependent aggregation was associated with modification of metabolic proteins. The overall characteristics of the 4-HNE or A-HNE-dependent inhibition of respiration are a decreased maximal respiration and ATP linked respiration and increased proton leak (Figure 2 and Supplementary Figure 1). The candidate proteins identified using A-HNE (30 µM) were consistent with these observations. The modification of ATP synthase subunits, could lead to decreased ATP linked respiration (Supplementary Table 5). Increased, proton leak has previously been reported in rat cardiomyocytes, neurons and chicken embryo fibroblasts on exposure to 4-HNE, which can be related to its effects on uncoupling proteins [15, 55–57]. From the candidate proteins identified by A-HNE, one possibility is that the loss of MnSOD increases the steady state level of superoxide, which has previously been reported to have an uncoupling effect (Supplementary Table 7) [58]. Several of the metabolic proteins modified, if inhibited by A-HNE, would decrease substrate flux and so limit the extent of maximal respiration. The metabolomic analysis suggests that inhibition of glycolysis at the level of hexokinase-1 and triosephosphate isomerase does not have a major impact. The increased levels of TCA cycle intermediates on the addition of thrombin is consistent with our previous data showing that thrombin-induced platelet aggregation resulted in a stimulation of respiration [24]. We noted that thrombin stimulated respiration either approaches or is less than maximal respiration (Figure 4) suggesting that the oxidation of TCA cycle metabolites by the electron transport chain has now become limiting leading to their accumulation. The metabolomic data are consistent with HNE-dependent inhibition of malate and isocitrate dehydrogenases, which would decrease metabolic flux through the TCA cycle (Supplementary Table 5). Additionally, there is evidence for the inhibition of the enzymatic activities of ATP synthase in rat liver mitochondria and isocitrate dehydrogenase in rat heart mitochondria by 4-HNE modifications [59, 60].
In summary, we have shown that thrombin-mediated aggregation of washed platelets is inhibited by 4-HNE. Inhibition of aggregation by HNE was accompanied by a decrease in mitochondrial oxidative phosphorylation, which was further exacerbated by thrombin. However, there was a compensatory increase in glycolysis on addition of thrombin and HNE indicating that although glycolytic proteins were modified by HNE they were not affecting glycolytic flux to a significant extent. In contrast a profound inhibition of substrate oxidation by the TCA cycle with the combined treatment of thrombin and HNE was noted. We found that the HNE-electrophile responsive proteome encompasses proteins required for regulation of platelet metabolism and aggregation, suggesting that the inhibition of aggregation caused by 4-HNE is a result of the integrated effects of HNE on multiple pathways rather than on any one target. These data have important implications for the interpretation of modification of biological processes mediated by the post-translational modification of proteins by electrophiles and other reactive species.
Supplementary Material
HIGHLIGHTS.
The effect of 4-HNE on platelet aggregation and metabolism were determined.
4-HNE inhibited thrombin induced platelet aggregation.
4-HNE decreased mitochondrial respiration in the resting and thrombin activated state.
A-HNE modifies proteins regulating platelet activation and metabolism.
ACKNOWLEDGEMENTS
This work was supported by American Heart Association 13PRE16390001 (SR), and the O’Brien Center P30 DK079337 (VDU,SB). The mass spectrometer used in these studies was obtained from funds provided by a NIH Shared Instrumentation Grant (S10 RR027822 to SB).
Abbreviations
- 4-HNE
4-hydroxynonenal
- AA
antimycin A
- A-HNE
alkyne HNE
- ECAR
extracellular acidification rate
- LDH
lactate dehydrogenase
- MnSOD
manganese superoxide dismutase
- OCR
oxygen consumption rate
- PRP
platelet rich plasma
- vWF
von Willebrand Factor
- XF
Extracellular Flux
- TCA
tricarboxylic acid cycle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURE
Victor Darley-Usmar receives some project support from Seahorse Bioscience but it is not related to the data included in this manuscript.
REFERENCES
- 1.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free radical biology & medicine. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto M, Sibata T, Wasada H, Toyokuni S, Uchida K. Structural basis of protein-bound endogenous aldehydes. Chemical and immunochemical characterizations of configurational isomers of a 4-hydroxy-2-nonenal-histidine adduct. The Journal of biological chemistry. 2003;278:5044–5051. doi: 10.1074/jbc.M210129200. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Chen J, Zhu H, Xiong YL. Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J Agric Food Chem. 2012;60:9727–9736. doi: 10.1021/jf3026277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Minkler PE, Sayre LM. Mass spectroscopic characterization of protein modification by 4-hydroxy-2-(E)-nonenal and 4-oxo-2-(E)-nonenal. Chem Res Toxicol. 2003;16:901–911. doi: 10.1021/tx0300030. [DOI] [PubMed] [Google Scholar]
- 5.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando Y, Brannstrom T, Uchida K, Nyhlin N, Nasman B, Suhr O, Yamashita T, Olsson T, El Salhy M, Uchino M, Ando M. Histochemical detection of 4-hydroxynonenal protein in Alzheimer amyloid. J Neurol Sci. 1998;156:172–176. doi: 10.1016/s0022-510x(98)00042-2. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto K, Toyokuni S, Uchida K, Ogawa O, Takenewa J, Kakehi Y, Kinoshita H, Hattori-Nakakuki Y, Hiai H, Yoshida O. Formation of 8-hydroxy-2'-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer. 1994;58:825–829. doi: 10.1002/ijc.2910580613. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki D, Miyata T, Saotome N, Horie K, Inagi R, Yasuda Y, Uchida K, Izuhara Y, Yagame M, Sakai H, Kurokawa K. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol. 1999;10:822–832. doi: 10.1681/ASN.V104822. [DOI] [PubMed] [Google Scholar]
- 9.Morikawa S, Kurauchi O, Tanaka M, Yoneda M, Uchida K, Itakura A, Furugori K, Mizutani S, Tomoda Y. Increased mitochondrial damage by lipid peroxidation in trophoblast cells of preeclamptic placentas. Biochemistry and molecular biology international. 1997;41:767–775. doi: 10.1080/15216549700201801. [DOI] [PubMed] [Google Scholar]
- 10.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circulation research. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda A, Nakamura Y, Ohigashi H, Osawa T, Uchida K. Cellular response to the redox active lipid peroxidation products: induction of glutathione S-transferase P by 4-hydroxy-2-nonenal. Biochemical and biophysical research communications. 1997;236:505–509. doi: 10.1006/bbrc.1997.6585. [DOI] [PubMed] [Google Scholar]
- 12.Levonen AL, Hill BG, Kansanen E, Zhang J, Darley-Usmar VM. Redox regulation of antioxidants, autophagy, and the response to stress: Implications for electrophile therapeutics. Free radical biology & medicine. 2014;71C:196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andringa KK, Udoh US, Landar A, Bailey SM. Proteomic analysis of 4-hydroxynonenal (4-HNE) modified proteins in liver mitochondria from chronic ethanol-fed rats. Redox Biol. 2014;2C:1038–1047. doi: 10.1016/j.redox.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlisser AE, Yan J, Hales BF. Teratogen-induced oxidative stress targets glyceraldehyde-3-phosphate dehydrogenase in the organogenesis stage mouse embryo. Toxicological sciences : an official journal of the Society of Toxicology. 2010;118:686–695. doi: 10.1093/toxsci/kfq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. The Biochemical journal. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higdon AN, Landar A, Barnes S, Darley-Usmar VM. The Electrophile Responsive Proteome: Integrating Proteomics and Lipidomics with Cellular Function. Antioxidants & redox signaling. 2012 doi: 10.1089/ars.2012.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. The Biochemical journal. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurst JS, Slater TF, Lang J, Juergens G, Zollner H, Esterbauer H. Effects of the lipid peroxidation product 4-hydroxynonenal on the aggregation of human platelets. Chemico-biological interactions. 1987;61:109–124. doi: 10.1016/0009-2797(87)90033-0. [DOI] [PubMed] [Google Scholar]
- 19.Selley ML, McGuiness JA, Jenkin LA, Bartlett MR, Ardlie NG. Effect of 4-hydroxy-2,3-trans-nonenal on platelet function. Thrombosis and haemostasis. 1988;59:143–146. [PubMed] [Google Scholar]
- 20.Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Laboratory investigation; a journal of technical methods and pathology. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walkowiak B, Kesy A, Michalec L. Microplate reader--a convenient tool in studies of blood coagulation. Thrombosis research. 1997;87:95–103. doi: 10.1016/s0049-3848(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 23.Bednar B, Condra C, Gould RJ, Connolly TM. Platelet aggregation monitored in a 96 well microplate reader is useful for evaluation of platelet agonists and antagonists. Thrombosis research. 1995;77:453–463. doi: 10.1016/0049-3848(95)93881-y. [DOI] [PubMed] [Google Scholar]
- 24.Ravi S, Chacko B, Sawada H, Kramer PA, Johnson MS, Benavides GA, O'Donnell V, Marques MB, Darley-Usmar VM. Metabolic plasticity in resting and thrombin activated platelets. PloS one. 2015;10:e0123597. doi: 10.1371/journal.pone.0123597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem Res Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HU B. Methoden der enzymatischen Analyse. Berlin: Akademie Verlag; 1970. [Google Scholar]
- 27.Bolisetty S, Traylor A, Zarjou A, Johnson MS, Benavides GA, Ricart K, Boddu R, Moore RD, Landar A, Barnes S, Darley-Usmar V, Agarwal A. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. American journal of physiology. Renal physiology. 2013;305:F255–F264. doi: 10.1152/ajprenal.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell'Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biological chemistry. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou LY, Chatham JC, Hill BG, Zhang JH, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radical Biology and Medicine. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazzana N, Ganci A, Cefalu AB, Lattanzio S, Noto D, Santoro N, Saggini R, Puccetti L, Averna M, Davi G. Enhanced lipid peroxidation and platelet activation as potential contributors to increased cardiovascular risk in the low-HDL phenotype. Journal of the American Heart Association. 2013;2:e000063. doi: 10.1161/JAHA.113.000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seghieri G, Martinoli L, di Felice M, Anichini R, Fazzini A, Ciuti M, Miceli M, Gaspa L, Franconi F. Plasma and platelet ascorbate pools and lipid peroxidation in insulin-dependent diabetes mellitus. European journal of clinical investigation. 1998;28:659–663. doi: 10.1046/j.1365-2362.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- 32.Marcus AJ. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. Journal of lipid research. 1978;19:793–826. [PubMed] [Google Scholar]
- 33.Malle E, Ibovnik A, Leis HJ, Kostner GM, Verhallen PF, Sattler W. Lysine modification of LDL or lipoprotein(a) by 4-hydroxynonenal or malondialdehyde decreases platelet serotonin secretion without affecting platelet aggregability and eicosanoid formation. Arteriosclerosis, thrombosis, and vascular biology. 1995;15:377–384. doi: 10.1161/01.atv.15.3.377. [DOI] [PubMed] [Google Scholar]
- 34.Smathers RL, Fritz KS, Galligan JJ, Shearn CT, Reigan P, Marks MJ, Petersen DR. Characterization of 4-HNE modified L-FABP reveals alterations in structural and functional dynamics. PloS one. 2012;7:e38459. doi: 10.1371/journal.pone.0038459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 36.Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Current pharmaceutical design. 2009;15:1358–1372. doi: 10.2174/138161209787846702. [DOI] [PubMed] [Google Scholar]
- 37.Lopez JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. Journal of thrombosis and haemostasis : JTH. 2005;3:1737–1744. doi: 10.1111/j.1538-7836.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 38.Wright SD, Michaelides K, Johnson DJ, West NC, Tuddenham EG. Double heterozygosity for mutations in the platelet glycoprotein IX gene in three siblings with Bernard-Soulier syndrome. Blood. 1993;81:2339–2347. [PubMed] [Google Scholar]
- 39.Clemetson JM, Kyrle PA, Brenner B, Clemetson KJ. Variant Bernard-Soulier syndrome associated with a homozygous mutation in the leucine-rich domain of glycoprotein IX. Blood. 1994;84:1124–1131. [PubMed] [Google Scholar]
- 40.Loftus JC, Choate J, Albrecht RM. Platelet activation and cytoskeletal reorganization: high voltage electron microscopic examination of intact and Triton-extracted whole mounts. The Journal of cell biology. 1984;98:2019–2025. doi: 10.1083/jcb.98.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torti M, Festetics ET, Bertoni A, Sinigaglia F, Balduini C. Agonist-induced actin polymerization is required for the irreversibility of platelet aggregation. Thrombosis and haemostasis. 1996;76:444–449. [PubMed] [Google Scholar]
- 42.Milton JG, Frojmovic MM. Adrenaline and adenosine diphosphate-induced platelet aggregation require shape change. Importance of pseudopods. The Journal of laboratory and clinical medicine. 1984;104:805–815. [PubMed] [Google Scholar]
- 43.Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 44.Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, Chen L, Varella-Garcia M, Sacks PG, Frederick B, Raben D, Weed SA. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–8025. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Ghai P, Wu H, Wang C, Field J, Zhou GL. Mammalian adenylyl cyclase-associated protein 1 (CAP1) regulates cofilin function, the actin cytoskeleton, and cell adhesion. The Journal of biological chemistry. 2013;288:20966–20977. doi: 10.1074/jbc.M113.484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adelstein RS, Conti MA, Anderson W., Jr Phosphorylation of human platelet myosin. Proceedings of the National Academy of Sciences of the United States of America. 1973;70:3115–3119. doi: 10.1073/pnas.70.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higashihara M, Takahata K, Kurokawa K. Effect of phosphorylation of myosin light chain by myosin light chain kinase and protein kinase C on conformational change and ATPase activities of human platelet myosin. Blood. 1991;78:3224–3231. [PubMed] [Google Scholar]
- 48.Zucker-Franklin D, Grusky G. The actin and myosin filaments of human and bovine blood platelets. The Journal of clinical investigation. 1972;51:419–430. doi: 10.1172/JCI106828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelson AD. Platelets. London ; Waltham, MA: Academic Press; 2013. [Google Scholar]
- 50.White JG. Platelet granule disorders. Critical reviews in oncology/hematology. 1986;4:337–377. doi: 10.1016/s1040-8428(86)80027-0. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 52.Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms. Journal of thrombosis and haemostasis : JTH. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 53.Manickam N, Sun X, Li M, Gazitt Y, Essex DW. Protein disulphide isomerase in platelet function. British journal of haematology. 2008;140:223–229. doi: 10.1111/j.1365-2141.2007.06898.x. [DOI] [PubMed] [Google Scholar]
- 54.Muller C, Bandemer J, Vindis C, Camare C, Mucher E, Gueraud F, Larroque-Cardoso P, Bernis C, Auge N, Salvayre R, Negre-Salvayre A. Protein disulfide isomerase modification and inhibition contribute to ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal. 2013;18:731–742. doi: 10.1089/ars.2012.4577. [DOI] [PubMed] [Google Scholar]
- 55.Schneider L, Giordano S, Zelickson BR, M SJ, G AB, Ouyang X, Fineberg N, Darley-Usmar VM, Zhang J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free radical biology & medicine. 2011;51:2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassiter K, Dridi S, Piekarski A, Greene E, Hargis B, Kong BW, Bottje W. Bioenergetics in chicken embryo fibroblast cells: evidence of lower proton leak in spontaneously immortalized chicken embryo fibroblasts compared to young and senescent primary chicken embryo fibroblast cells. Comp Biochem Physiol A Mol Integr Physiol. 2014;175:115–123. doi: 10.1016/j.cbpa.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. Embo J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo J, Prokai-Tatrai K, Nguyen V, Rauniyar N, Ughy B, Prokai L. Protein targets for carbonylation by 4-hydroxy-2-nonenal in rat liver mitochondria. Journal of proteomics. 2011;74:2370–2379. doi: 10.1016/j.jprot.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. The Journal of biological chemistry. 2003;278:45154–45159. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.