Abstract
Roux-en-Y gastric bypass (RYGB) surgery is a commonly performed and very effective method to achieve significant, long-term weight loss. Opioid analgesics are primarily used to manage postoperative pain as fewer alternative medication options are available for bariatric surgery patients than for the general population. Recent clinical studies support a greater risk for substance use following bariatric surgery, including an increased use of opioid medications. The present study is the first to study morphine self-administration in a rat model of RYGB. High fat diet-induced obese (HFD-DIO) rats underwent RYGB (n=14) or sham-surgery with ad libitum HFD (SHAM, n=14) or a restricted amount that resulted in weight matched to the RYGB cohort (SHAM-WM, n=8). An additional normal-diet (ND, n=7), intact (no surgery) group of rats was included. Two months after the surgeries, rats were fitted with jugular catheters and trained on a fixed ratio-2 lick task to obtain morphine intravenously. Both morphine-seeking (number of licks on an empty spout to obtain morphine infusion) and consumption (number of infusion) were significantly greater in RYGB than any control group beginning on Day 3 and reached a two-fold increase over a period of two weeks. These findings demonstrate that RYGB increases motivation for taking morphine and that this effect is independent of weight loss. Further research is warranted to reveal the underlying mechanisms and to determine whether increased morphine use represents a risk for opioid addiction following RYGB. Identifying risk factors preoperatively could help with personalized postoperative care to prevent opioid abuse and addiction.
Keywords: Addiction, Bariatric Surgery, Obesity, Prescription Opioids, Reward, Substance Use
1. Introduction
The growing epidemic of obesity and associated health consequences represent a major cause of preventable death. At present, Roux-en-Y gastric bypass (RYGB) is a highly effective surgical method to achieve significant, long-term weight loss (Marcus et al., 2009). In contrast to improved food preferences and reduced food cravings following RYGB (Brown et al., 1982; Halmi et al., 1981; Le Roux et al., 2011; Olbers et al., 2006), there appears to be a greater vulnerability for some patients to develop substance use disorder (SUD) after RYGB (Dutta et al., 2006). For example, recent clinical reports have revealed an increased risk for alcohol consumption (Ertelt et al., 2008; Hsu et al., 1998; King et al., 2012; Suzuki et al., 2012). Recently, our laboratory (Hajnal et al., 2012; Polston et al., 2013; Thanos et al., 2012) and others (Davis et al., 2013) reported increased alcohol self-administration in dietary obese (DIO) rats that underwent the RYGB operation, suggesting that a biological cause may drive the increase in substance use after the surgery.
The present study is the first to test whether RYGB also alters self-administration of morphine in an obese rat model of RYGB. The rationale of focusing on morphine was that most RYGB patients experience postoperative pain severe enough to require systemic opioid therapy (Cohen et al., 2013). Doctors have relatively few options for alternative pain relief in bariatric surgery patients. Non-steroidal anti-inflammatory agents should be avoided in this population (Sasse et al., 2008) and, while acetaminophen can be used, it is less efficacious in these patients (Sasse et al., 2008). Opioid use in patients with higher pain sensitivity, characteristic of obese patients even after weight loss (Cohen et al., 2013; Dodet et al., 2013), may lead to rapid development of tolerance, and escalating dosages can, themselves, increase pain sensitivity even when the initial cause has been resolved. In fact, an increased risk for chronic morphine use after bariatric surgery, RYGB in particular, is supported not only by anecdotal accounts in the popular media (Berthoud et al., 2012; Sóuter, 2007; Spencer, 2006), but also by clinical observations (Reslan et al., 2014; Saules et al., 2010; Wendling and Wudyka, 2011) including a recent large-sample, population-based, longitudinal study (Raebel et al., 2013) which confirmed a risk of increased chronic use of opioid drugs following RYGB. Among those who had no prior history, chronic opioid use also emerged after surgery (from 0 to ~5 mg daily morphine equivalents, escalating from years 2 and 3 after the surgery) such that 8% of the bariatric surgery population used opioids chronically, exceeding the 3% observed in the general population (Boudreau et al., 2009). In a recent study (Ivezaj et al., 2014), the majority of those who met criteria for substance use disorder (SUD) after surgery (68 %) did not have a pre-RYGB history of SUD. Therefore, with the increasing number of patients who have received or will receive the surgery, the problem of new onset SUD is expected to grow and the long-term adverse effects (i.e., health and social problems associated with addiction) to escalate. Thus, preclinical research using animal models to determine the impact of bariatric surgery on risk for opiate addiction is of high translational relevance.
2. Materials and methods
2. 1. Subjects and Diet
Forty-three Sprague-Dawley adult rats (Charles River, Wilmington, MA) were utilized during these studies. Rats at approximately 4 weeks of age, weighing between 200–225 g were housed in temperature and humidity controlled cages and maintained on a 12:12-hr light-dark cycle (lights on at 0700).
Rats were either maintained on normal diet or placed on a nutritionally complete high fat diet to induce obesity. The high fat diet (D12494, Research Diets Inc., New Brunswick, NJ) consists of 60% kcal fat, 20% kcal carbohydrates, and 20% kcal protein, providing a 5.24 kcal/gram diet. The rats were maintained on the high fat diet for 26–28 weeks prior to surgery and continued on the high fat diet throughout the experiment. Seven rats were maintained on normal chow (Harlan Teklad, Madison, WI), while thirty-six rats were placed on high fat chow. The high fat diet group was further separated into three surgical groups as follows: SHAM, n=14, SHAM WM (weight managed), n=8, and RYGB, n=14. Normal diet controls, RYGB, and SHAM rats were allowed ad lib access to food prior to and after surgery with water ad lib, except during training and drug schedule. SHAM WM rats were allowed ad lib access to food prior to surgery; however, after surgery these rats were given 5 HFD pellets daily as a weight managed group.
2.2. Drugs
Morphine sulfate (Sigma-Aldrich, St. Louis, MO) solution of 2.25 mg/mL (dissolved in sterile saline) was prepared for each self-administration chamber prior to the daily sessions. The rats were allowed to self-administer morphine in a dose of 0.225 mg per infusion.
2.3. Roux-en-Y Gastric Bypass and Sham Surgeries
After the animals were on high fat diet for 26 – 28 weeks, they underwent either RYGB or SHAM surgery. The techniques and perioperative care were previously described (Hajnal et al., 2012). Rats were fasted overnight and had water ad lib prior to surgery, then anesthetized (isoflurane: 3% for inductions, 1.5% for maintenance). All animals were pretreated with antibiotic (Gentamycin: 2.5 mg/kg, IM, APP Pharmaceuticals, LLC, Schamburg, IL and Ceftriaxone: 30 mg/kg, SC, Sandoz Inc., Princeton, NJ) and buprenorphine (Buprenex: 0.05 mg/kg, SC, Reckitt Benckiser Pharmaceuticals, Richmond, VA) for pain control. Utilizing a sterile procedure during the surgery, through a midline laparotomy, the stomach was separated in the RYGB procedure using a linear-cutting stapler (ETS-Flex Ethicon Endo surgery, 45mm blue load) to create a small gastric pouch isolated from the bypassed stomach. The jejunum was measured 15 cm from the ligament of Treitz and the distal segment was anastomosed end-to-side to form a pouch gastrojejunostomy. The proximal jejunum was anastomosed 15 cm along the distal limb end-to-side. Both anastomoses were created utilizing interrupted 5-0 monofilament suture material (Prolene) sutures. The muscle layer was closed using running 3-0 nylon suture and the skin layer was closed utilizing running 4-0 nylon suture. SHAM controls received a gastric manipulation to simulate the stapler insertion, then replaced to original position, followed by a transverse enterotomy 15 cm from the ligament of Treitz that was re-closed with 5-0 Prolene interrupted sutures. Postoperative care consisted of normal saline (10 mL bid, SC), ceftriaxone (30 mg/kg, SC), gentamycin (2.5 mg/kg, IM), and carprofen (5 mg/kg, SC) for 3 days. Animals received BOOST (Nestle Nutrition, Minneapolis, MN) 24 hr after surgery for 5 days with water ad lib, then return to their designated high fat diet.
2.4. Jugular Catheter Implantation
Approximately two months following RYGB or SHAM surgery, rats were anesthetized (isoflurane: 3% induction, 1.5% maintenance) and a catheter was surgically implanted into the right external jugular vein. The catheter design and surgical protocols are described elsewhere (Grigson and Twining, 2002). The catheter was routed subcutaneously to the back for access to be coupled to the self-administration apparatus. Catheters were flushed daily with 0.2 ml of heparinized saline to maintain patency, and verification with 0.2 ml of IV propofol (Diprivan 1%) as needed. Following catheterization, animals were allowed 5 days of recovery before behavioral training commenced.
2.5. Self-Administration Procedure
Training and testing of self-administration of morphine took place in six identical operant chambers (MED Associates, St. Albans, VT). After surgical recovery, animals were attached to a coupling assembly, where syringe pumps were connected to a swivel system in the test chambers, enabling the computer controlled IV infusion of morphine. Rats were overnight water deprived for continuous access training, where for 3 days rats received 30 min water access in the operant chambers and 3 hr of water access each afternoon in their home cages to maintain proper hydration. Following water training for 3 days, rats began a 16 hr overnight morphine access, where rats received access to all three spouts: spout 1 (left- “inactive” spout), spout 2 (center – “active” spout), and spout 3 (right – “water” spout), for 16 hours. Morphine delivery was triggered by a lickometer circuit, in that licks on an empty spout (the active spout) would trigger an infusion of 0.225 mg of morphine (100 µl in 2 s). After the 16 hr overnight access, the rats began daily morphine self-administration sessions of 1 hr duration. Rats were placed in the operant chambers with the three spouts that were empty. Upon program activation, spouts 1 and 2 were presented, with licks on the inactive spout producing no programmed consequences and licks on the active spout (triggering morphine infusions) counting towards the completion of the FR2 schedule of reinforcement. The FR2 schedule was performed for 10 consecutive days.
2.6. Statistical Analysis
All data were analyzed with Prism (Version 5, GraphPad, San Diego, CA) using Analysis of Variance (ANOVA). Body weight (g) was measured daily, presented as Mean ± SEM of Group (RYGB, SHAM, SHAM WM, Normal), and analyzed using two-way mixed factorial ANOVA with Group and Day (Pre-surgery and Pre-experiment) as independent factors and Bonferroni post-hoc tests. For the behavioral tests, the number of infusions and licks made on both empty spouts were measured and analyzed as dependent factors using two-way mixed factorial ANOVAs with Group and Day as independent factors. Significant findings were further analyzed using Bonferroni post-hoc tests. In addition, one-way ANOVAs with Tukey’s multiple comparison tests were used to compare effects (total empty spout licks and total infusions) across groups.
3. Results
3.1. Body Weight and Food Intake
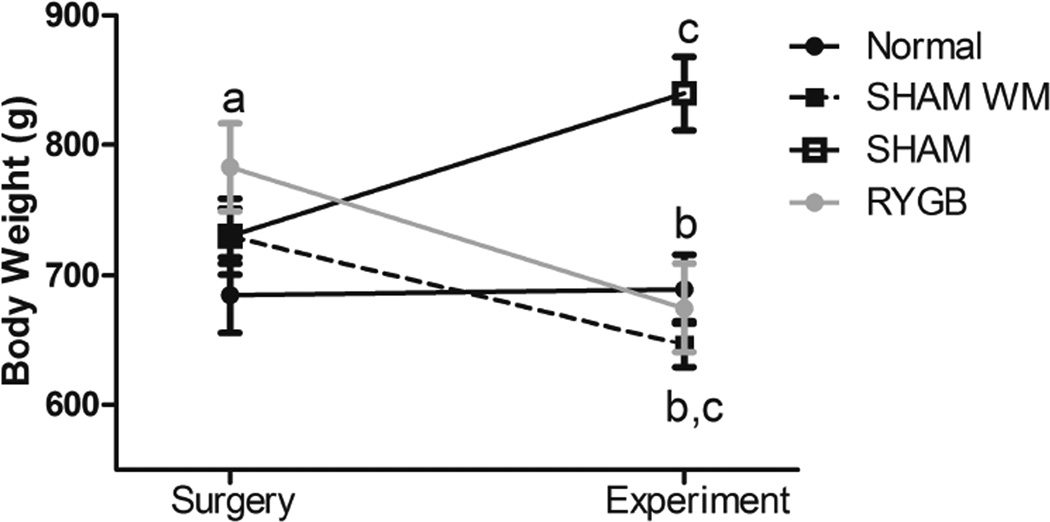
The rats’ weights were measured prior to the surgical procedure day and at the beginning of the self-drug administration experiment (Fig. 1). ANOVA revealed a significant effect for Group by Day interaction (F(3,68) = 6.16, p<0.001). Posthoc tests showed that the RYGB rats were significantly heavier compared to the normal group of rats on the day of their surgery (p<0.05), but not statistically different from the other groups that received HFD. After surgery, the RYGB rats lost a significant (p<0.05) amount of weight by the time of self-administration testing (782.75 ± 33.66 g vs. 656.71 ± 33.97g, ~16%). At the beginning of the behavioral tests, RYGB rats weighed significantly less that SHAM rats (p<0.001) but were not statistically different from SHAM WM and Normal diet cohorts. In fact, SHAMWM rats that were allowed ad lib access to HFD prior to surgery and thereafter were given a restricted daily allotment of HFD achieved a significant (p<0.05) weight loss compared to SHAM controls but showed no significant weight difference compared to the RYGB group.
Figure 1.
Diet induced obese rats’ body weight at time of Surgery and at time of the Experiment. a, p<0.05 Comparing RYGB day of surgery to normal diet controls. b, p<0.001 Comparing SHAM on the day of the experiment to RYGB, SHAM WM, and normal diet controls. c, p<0.05 Comparing day of surgery to experiment day in all but normal diet.
3.2. Operant Performance and Morphine Intake
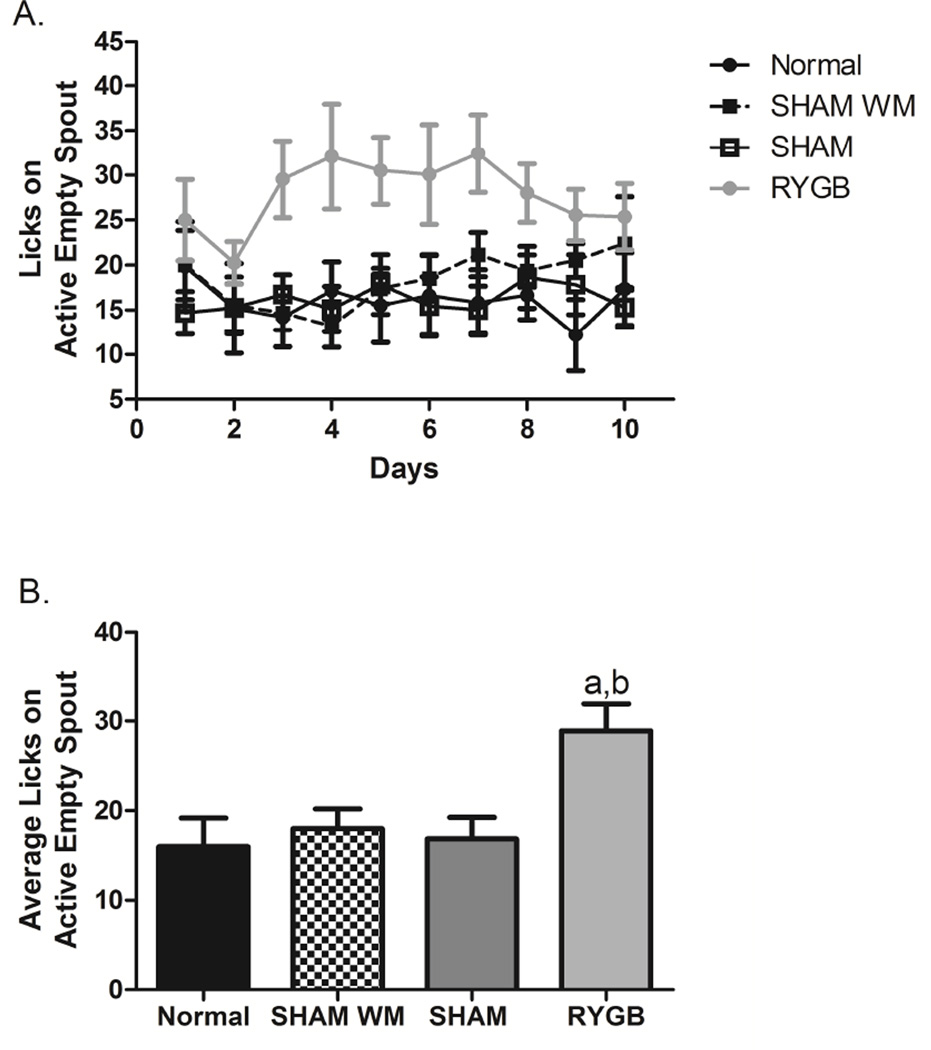
Morphine delivery was triggered by a lickometer circuit and licks on the empty active spout as well as licks on the inactive spouts were recorded throughout the FR2 schedule for each group of rats for 10 days. ANOVA revealed a significant effect for group × trials (F(3,306)=26.47, p<0.0001) for the empty active spout licks, i.e., morphine seeking (Fig 2A) but not for inactive spout licks (not shown). Throughout the 10 days of the FR2 schedule, post-hoc tests showed that the RYGB group of animals compared to the SHAM, SHAM WM, and the Normal groups made more licks on the active empty spout (p<0.05), particularly across days 3 to 8 (Fig. 2A). The range of licks by the RYGB rats was between 20 and 30 licks, where the SHAM, SHAM WM, and normal group of rats produced around 15 licks throughout the FR2 schedule days. When observing the average number of licks exhibited on the empty active spout during the entire experiment (Fig. 2B), ANOVA showed again a significant group effect (F(3,35)=5.11, p<0.01) with the RYGB group licking significantly more compared to the normal and SHAM WM groups (p<0.05), as well as the SHAM group (p<0.005).
Figure 2.
A: Time line of the 10 FR2 schedule days observing licks on the active empty spout. B: Average number of licks observed on the active empty spout for the FR2 schedule days. a, p<0.05 Comparing RYGB to SHAM WM and normal diet controls. b, p<0.005 Comparing RYGB to SHAM.
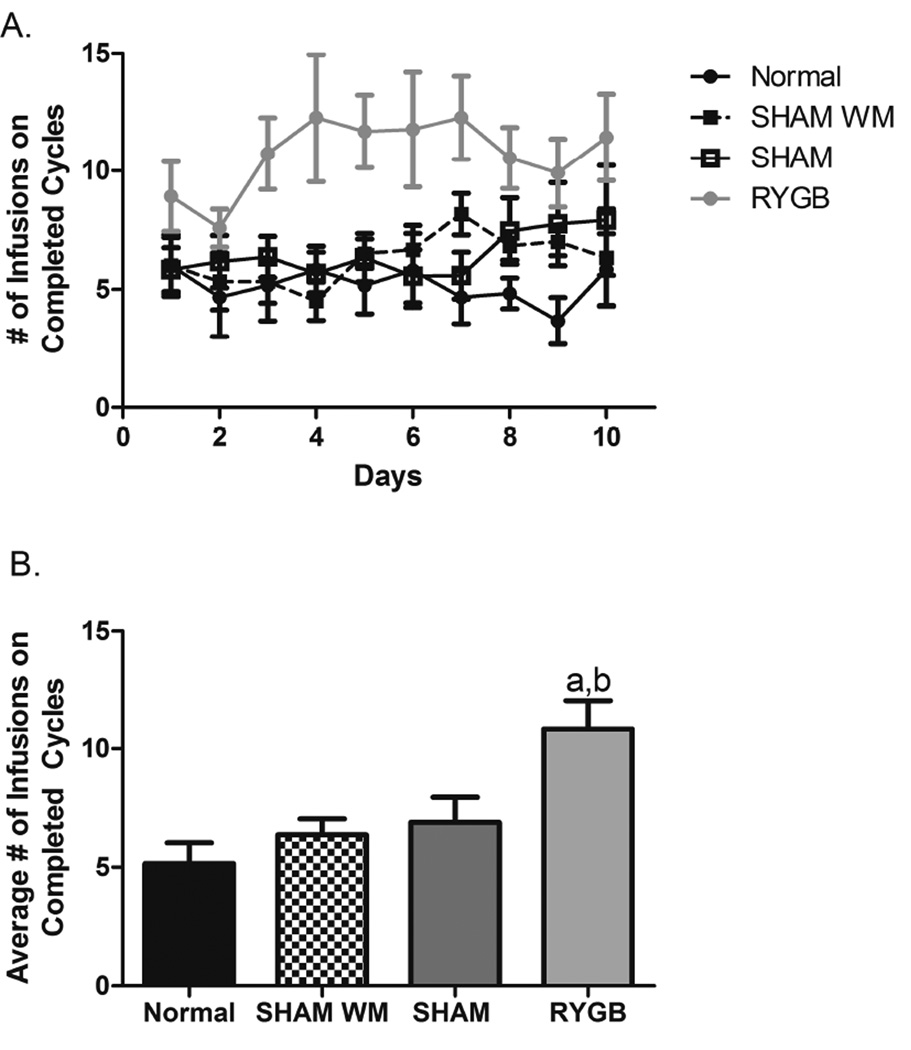
The infusion of morphine was dependent upon the completion of the FR2 schedule of reinforcement or two licks on the active empty spout operant. In general, the infusion data mirrored the response data such that the RYGB group self-administered more infusions/trials than did the SHAM, SHAM WM, and Normal groups (see Figure 3A). Support for this conclusions was provided by a significant group × trials ANOVA (F(3,310)=25.88, p<0.0001). Again, post hoc tests revealed that rats in the RYGB group took more infusions of morphine than did rats in each of the three control conditions, p < .05. Post-hoc tests showed that compared to controls the RYGB rats received more infusions from day 3 to day 8 (Fig. 3A). The number of infusions RYGB group received during the FR2 sessions ranged between 8 and 13 infusions. The SHAM, SHAM WM, and Normal groups self-administered infusions ranging between 3 and 7 per session. When the average number of infusions received over the entire 10 days of the testing was analyzed by one-way ANOVA and Tukey’s posthoc tests, we found the number of infusions was significantly greater for RYGB (Fig. 3B). RYGB infused significantly more morphine than SHAM and SHAM WM (p<0.05), as well as Normal diet group (p<0.01).
Figure 3.
A: Time line of the number of infusions on completed cycles each group received during the 10 FR2 test days. B: Average number of infusions on completed cycles observed for the FR2 test days. a, p<0.01 Comparing RYGB to normal diet controls.
b, p<0.05 Comparing RYGB to SHAM and SHAM WM.
4. Discussion
Here we show that in HF-DIO rats RYGB increases morphine self-administration. Furthermore, we demonstrate that this effect was due to factors other than weight loss, as the amount of morphine the RYGB rats self-administered was significantly greater than that of the HF-DIO surgical control rats that were subject to chronic caloric restriction to achieve a weight loss similar to the RYGB rats. This latter finding is potentially important as it demonstrates changes in responsiveness to reward following RYGB directly affecting the opioid system in addition to mechanisms that may change reward threshold by affecting the dopamine system as has been shown in the case of caloric deprivation (Carr, 2002). An additional finding supporting the notion of a potential risk of addiction was an escalation observed in the amount of morphine that the RYGB rats self-administered in our study. Such a phenomenon has been seen with drugs of abuse and is considered a hallmark of persistent addiction liability (Edwards and Koob, 2013). It has to be noted, however, that the present study used a ‘low demand’ FR-2 schedule of reinforcement task and relatively short sessions with the primary goal of investigating differences in drug intake between RYGB and control animals over a short period of time. Future studies, therefore, using a progressive ratio schedule of reinforcement task and extended access paradigms with assessment of extinction/reinstatement are warranted to determine whether the increased morphine self-administration after RYGB leads to addiction.
Whereas development of substance use in bariatric patients is likely influenced by numerous psychosocial and demographic factors, the findings from the present study using animals strongly suggest that a biological cause may drive the increase in morphine self-administration. Multiple biological factors likely contribute to increasing chronic opioid use after RYGB. For example, obese individuals demonstrate more pain sensitivity and lower pain detection thresholds than those who are not obese and altered pain processing persists after bariatric surgery (Dodet et al., 2013). Furthermore, since opioids are absorbed in the gastrointestinal tract, typically in the duodenum and proximal jejunum (Lotsch et al., 1999; Tan et al., 1989), changes in pharmacokinetics due to altered anatomy, or reduced body adiposity following bariatric surgery may raise the potential for opioid addiction (Lloret-Linares et al., 2014). Future studies in this high fat diet-induced obese rat model, are warranted to investigate changes in pain threshold and pharmacokinetics of opioid analgesics. In addition, there is high comorbidity between depression and addiction (Koob, 2008; Kosten et al., 1998; Markou et al., 1998) and there is a higher prevalence of depression in the bariatric surgery population (Bocchieri et al., 2002; Burgmer et al., 2007). A recent study (Tabibian et al., 2015) examined bariatric surgery patients’ response to a chronic pain rehabilitation program compared to non-bariatric patients with chronic pain. At discharge from the pain management program, bariatric patients demonstrated significantly higher scores for emotional concerns and were taking more morphine on an equivalent dose basis (+44%) as well as benzodiazepines and were less likely to complete treatment compared to controls. These findings suggest an association between increased prescription opioid use and altered pain and mood regulation following bariatric surgery, and calls for routine pain assessment of the patients pre- and post-surgery. Nevertheless, a recent study (Raebel et al., 2013) failed to find causality between prior history of chronic pain or depression and the increase in postsurgical opioid use, pointing to post-operative changes as causal factors.
Another plausible factor driving increased morphine use after bariatric surgery is weight loss. There is significant evidence that drugs of abuse hijack the neurocircuitry that mediates appetitive motivation and reward (Cardinal and Everitt, 2004; Corwin and Hajnal, 2005; Di Chiara, 2005; Grigson, 2002; Kelley and Berridge, 2002; Volkow and Wise, 2005). There is extensive research providing support for the regulation of drug effects by mechanisms of energy balance and body weight regulation (Daws et al., 2011; DiLeone, 2009; Marinelli et al., 1996). Studies in rats have shown that food restriction increases sensitivity to the rewarding properties of drugs of abuse (Berthoud and Zheng, 2012; Cabeza de Vaca and Carr, 1998; Carr et al., 2010; Carr et al., 2000). Therefore, it is plausible that excessive weight loss after RYGB exacerbates sensitivity to drug reward. In contrast, a recent study (Raebel et al., 2013) found that opioid use was independent of weight loss achieved by the surgery. In accordance, the present data showed that RYGB rats displayed significantly greater morphine self-administration compared to calorically restricted, weight-matched surgical controls. Thus, weight loss alone is unlikely to explain the increased use of opioid drugs following RYGB.
Although it was not directly investigated in the present study (i.e., lean rats, were not included because bariatric surgery is not performed in lean individuals), it is possible that change in diet may contribute to increased substance use. In fact, rats with free access to HFD display impaired acquisition of cocaine self-administration (Wellman et al., 2007) and amphetamine-conditioned place preference (Davis et al., 2008). Thus, one may suppose that the reduced consumption of fatty foods following the surgery (Behary and Miras, 2015) may reverse blunted reward and drug seeking. Recent brain imaging studies suggest that following RYGB, food-cues may elicit reduced activation in brain (Ochner et al., 2011; Ochner et al., 2012a; Ochner et al., 2012b; Scholtz et al., 2014). Perhaps, more surprisingly, RYGB appears also to result in blunted activation in the prefrontal cortex to food cues (Ochner et al., 2012a; Ochner et al., 2012b), an area thought to be involved in inhibition of impulsive behaviors. In a recent imaging study, we found that a sub-set of high fat dietary obese rats, those that maintained weight loss at higher level following RYGB, showed an increased activation of brain reward areas in response to cues associated with highly stimulating treats (Thanos et al., 2015). These, and other data, collectively suggest a greater risk for some patients to engage in alternative excessive behavior (Goldstein et al., 2007; Kalivas and Volkow, 2005; Volkow and Baler, 2013; Wang et al., 2004a), and they fit well the clinical observations of increased risk among RYGB patients for substituting food with alcohol (Ertelt et al., 2008; Hsu et al., 1998; King et al., 2012; Suzuki et al., 2012) or use of other substances (Conason et al., 2013; Dutta et al., 2006). The ‘symptom substitution’ theory (Kazdin, 1982) posits that the successful elimination of a particular symptom without treating the underlying cause will result in the appearance of a substitute symptom. Thus, an increase in substance use following RYGB might occur because the surgery largely eliminates excessive eating (Niego et al., 2007) and results in remission of food addiction (Pepino et al., 2014) without altering individual predispositions to addictive behaviors.
A common denominator for food and drug reward is the dopamine system with similar abnormalities in obesity and addiction (Blum et al., 2014; Tomasi and Volkow, 2013; Volkow et al., 2013). Prior literature suggests that both weight-reduced and obese animals have similar deficits in dopamine neurotransmission (Geiger et al., 2009; Pothos et al., 1995a; Pothos et al., 1995b) also termed as ‘reward deficiency syndrome’ (Blum et al., 1996) which may explain their altered propensity to self-administer either palatable food or drugs. In fact, brain imaging studies have demonstrated reductions in dopamine D2 receptor in the striatum of both obese humans (Wang et al., 2001) and in rats (Thanos et al., 2008; Wang et al., 2004b) similar to those seen in drug of abuse (Volkow et al., 2013). Interestingly, RYGB in humans has been shown to alter the expression of D2Rs in the striatum, although the results are controversial (Dunn et al., 2010; Steele et al., 2010). Thus, future research should seek to identify the underlying neural correlates.
5. Conclusions
To the best of our knowledge, this is the first study to demonstrate increased morphine self-administration in an animal model of RYGB with no prior history of drug exposure. This finding is consistent with clinical observations of an increased risk of new onset substance use in some patients who undergo RYGB, and in turn, suggests a biological mechanism. Further research is warranted to reveal the underlying mechanisms and determine whether increased morphine use represents a risk for opioid addiction following RYGB. Identifying risk factors preoperatively could help with personalized postoperative care to prevent opioid use and addiction.
Highlights.
-
-
RYGB in high fat dietary rats increased self-administration of morphine
-
-
The effects were independent of weight loss: did not occur in weight-matched rats
-
-
The findings are consistent with clinical data and suggest a biological mechanism
Acknowledgements
This research was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant DK080899 (to A.H.). The authors thank Dr. James E. Polston for performing a pilot study testing the hypothesis and methods and Dr. Patricia Sue Grigson for her comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors declare that there are no conflicts of interest.
Authors Contribution
JMB conducted the experiments and analyzed the data. CSF programmed the self-administration cages and helped with data analysis. NH and AMR performed the surgeries and NH provided perioperative care. AH designed and supervised research. AH and AMR contributed to the interpretation of data. JMB and AH drafted/wrote the paper. All authors have critically reviewed content and approved the final version submitted for publication.
REFERENCES
- Behary P, Miras AD. Food preferences and underlying mechanisms after bariatric surgery. The Proceedings of the Nutrition Society. 2015:1–7. doi: 10.1017/S0029665115002074. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Zheng H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiology & behavior. 2012;107:527–532. doi: 10.1016/j.physbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Zheng H, Shin AC. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Annals of the New York Academy of Sciences. 2012;1264:36–48. doi: 10.1111/j.1749-6632.2012.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Frontiers in psychology. 2014;5:919. doi: 10.3389/fpsyg.2014.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchieri LE, Meana M, Fisher BL. A review of psychosocial outcomes of surgery for morbid obesity. J Psychosom Res. 2002;52:155–165. doi: 10.1016/s0022-3999(01)00241-0. [DOI] [PubMed] [Google Scholar]
- Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Campbell CI, Merrill JO, Silverberg MJ, Banta-Green C, Weisner C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EK, Settle EA, Van Rij AM. Food intake patterns of gastric bypass patients. J Am Diet Assoc. 1982;80:437–443. [PubMed] [Google Scholar]
- Burgmer R, Petersen I, Burgmer M, de Zwaan M, Wolf AM, Herpertz S. Psychological outcome two years after restrictive bariatric surgery. Obes Surg. 2007;17:785–791. doi: 10.1007/s11695-007-9144-9. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neuroscience. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Chronic food restriction in rats augments the central rewarding effect of cocaine and the delta1 opioid agonist, DPDPE, but not the delta2 agonist, deltorphin-II. Psychopharmacology (Berl) 2000;152:200–207. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Clark SM, Saules KK. Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eating behaviors. 2013;14:216–219. doi: 10.1016/j.eatbeh.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Smith AN, Henriksen BS. Postoperative Opioid Requirements Following Roux-en-Y Gastric Bypass in Patients Receiving Continuous Bupivacaine Through a Pump System: A Retrospective Review. Hosp Pharm. 2013;48:479–483. doi: 10.1310/hpj4806-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles SL, Dixon JB, O'Brien PE. Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity (Silver Spring) 2008;16:608–614. doi: 10.1038/oby.2007.99. [DOI] [PubMed] [Google Scholar]
- Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148:145–150. doi: 10.1001/2013.jamasurg.265. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Hajnal A. Too much of a good thing: neurobiology of non-homeostatic eating and drug abuse. Physiol Behav. 2005;86:5–8. doi: 10.1016/j.physbeh.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Magrisso IJ, Grayson BE, Seeley RJ, Benoit SC. Roux en Y gastric bypass increases ethanol intake in the rat. Obes Surg. 2013;23:920–930. doi: 10.1007/s11695-013-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61:1123–1128. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: a case of homology? Physiol Behav. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ. The influence of leptin on the dopamine system and implications for ingestive behavior. Int J Obes (Lond) 2009;33(Suppl 2):S25–S29. doi: 10.1038/ijo.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodet P, Perrot S, Auvergne L, Hajj A, Simoneau G, Decleves X, Poitou C, Oppert JM, Peoc'h K, Mouly S, Bergmann JF, Lloret-Linares C. Sensory impairment in obese patients? Sensitivity and pain detection thresholds for electrical stimulation after surgery-induced weight loss, and comparison with a nonobese population. Clin J Pain. 2013;29:43–49. doi: 10.1097/AJP.0b013e31824786ad. [DOI] [PubMed] [Google Scholar]
- Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, Kessler RM, Abumrad NN. Decreased dopamine type 2 receptor availability after bariatric surgery: Preliminary findings. Brain Res. 2010;1350:123–130. doi: 10.1016/j.brainres.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Morton J, Shepard E, Peebles R, Farrales-Nguyen S, Hammer L, Albanese C. Methamphetamine Use Following Bariatric Surgery in an Adolescent. Obesity Surgery. 2006;16:780–782. doi: 10.1381/096089206777346646. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behavioural pharmacology. 2013;24:356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg Obes Relat Dis. 2008;4:647–650. doi: 10.1016/j.soard.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop MR, Eysenck SB. A further investigation into the personality of drug addicts in treatment. Br J Addict. 1980;75:305–311. doi: 10.1111/j.1360-0443.1980.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Behav. 2002;76:389–395. doi: 10.1016/s0031-9384(02)00758-8. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND, Thanos PK. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One. 2012;7:e49121. doi: 10.1371/journal.pone.0049121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5:457–464. [PubMed] [Google Scholar]
- Hsu LK, Benotti PN, Dwyer J, Roberts SB, Saltzman E, Shikora S, Rolls BJ, Rand W. Nonsurgical factors that influence the outcome of bariatric surgery: a review. Psychosomatic medicine. 1998;60:338–346. doi: 10.1097/00006842-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Ivezaj V, Saules KK, Schuh LM. New-Onset Substance Use Disorder After Gastric Bypass Surgery: Rates and Associated Characteristics. Obes Surg. 2014 doi: 10.1007/s11695-014-1317-8. [DOI] [PubMed] [Google Scholar]
- Kalarchian MA, Marcus MD, Levine MD, Courcoulas AP, Pilkonis PA, Ringham RM, Soulakova JN, Weissfeld LA, Rofey DL. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164:328–334. doi: 10.1176/ajp.2007.164.2.328. quiz 374. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Wagner A, Bischoff-Grethe A. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol Psychiatry. 2013;73:836–842. doi: 10.1016/j.biopsych.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Symptom substitution, generalization, and response covariation: implications for psychotherapy outcome. Psychol Bull. 1982;91:349–365. [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas AP, Pories WJ, Yanovski SZ. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–2525. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Hedonic Homeostatic Dysregulation as a Driver of Drug-Seeking Behavior. Drug Discov Today Dis Models. 2008;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Markou A, Koob GF. Depression and stimulant dependence: neurobiology and pharmacotherapy. J Nerv Ment Dis. 1998;186:737–745. doi: 10.1097/00005053-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011 doi: 10.1152/ajpregu.00139.2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent MR, Swencionis C. Addictive personality and maladaptive eating behaviors in adults seeking bariatric surgery. Eating behaviors. 2012;13:67–70. doi: 10.1016/j.eatbeh.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Lloret-Linares C, Hirt D, Bardin C, Bouillot JL, Oppert JM, Poitou C, Chast F, Mouly S, Scherrmann JM, Bergmann JF, Decleves X. Effect of a Roux-en-Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clinical pharmacokinetics. 2014;53:919–930. doi: 10.1007/s40262-014-0163-0. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Weiss M, Ahne G, Kobal G, Geisslinger G. Pharmacokinetic modeling of M6G formation after oral administration of morphine in healthy volunteers. Anesthesiology. 1999;90:1026–1038. doi: 10.1097/00000542-199904000-00016. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Kalarchian MA, Courcoulas AP. Psychiatric Evaluation and Follow-Up of Bariatric Surgery Patients. Am J Psychiatry. 2009;166:285–291. doi: 10.1176/appi.ajp.2008.08091327. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res. 1996;724:251–255. doi: 10.1016/0006-8993(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Niego SH, Kofman MD, Weiss JJ, Geliebter A. Binge eating in the bariatric surgery population: a review of the literature. Int J Eat Disord. 2007;40:349–359. doi: 10.1002/eat.20376. [DOI] [PubMed] [Google Scholar]
- Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, Teixeira J, Hirsch J, Geliebter A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Annals of surgery. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Laferrere B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci Res. 2012a;74:138–143. doi: 10.1016/j.neures.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J, Teixeira J. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience. 2012b;209:128–135. doi: 10.1016/j.neuroscience.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, Lonroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Stein RI, Eagon JC, Klein S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity. 2014;22:1792–1798. doi: 10.1002/oby.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, Volkow ND, Hajnal A. Roux-en-Y Gastric Bypass Increases Intravenous Ethanol Self-Administration in Dietary Obese Rats. PLoS One. 2013;8:e83741. doi: 10.1371/journal.pone.0083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995a;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obesity research. 1995b;3(Suppl 4):525S–529S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Raebel MA, Newcomer SR, Reifler LM, Boudreau D, Elliott TE, DeBar L, Ahmed A, Pawloski PA, Fisher D, Donahoo WT, Bayliss EA. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310:1369–1376. doi: 10.1001/jama.2013.278344. [DOI] [PubMed] [Google Scholar]
- Reslan S, Saules KK, Greenwald MK, Schuh LM. Substance misuse following Roux-en-Y gastric bypass surgery. Substance use & misuse. 2014;49:405–417. doi: 10.3109/10826084.2013.841249. [DOI] [PubMed] [Google Scholar]
- Sasse KC, Ganser J, Kozar M, Watson RW, McGinley L, Lim D, Weede M, Smith CJ, Bovee V. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18:530–534. doi: 10.1007/s11695-007-9335-4. [DOI] [PubMed] [Google Scholar]
- Saules KK, Wiedemann A, Ivezaj V, Hopper JA, Foster-Hartsfield J, Schwarz D. Bariatric surgery history among substance abuse treatment patients: prevalence and associated features. Surg Obes Relat Dis. 2010;6:615–621. doi: 10.1016/j.soard.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW, Goldstone AP. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891–902. doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sóuter Trading one addiction for another. People. 2007 [Google Scholar]
- Spencer J. The new science of addiction. Wall Street Journal. 2006 [Google Scholar]
- Steele K, Prokopowicz G, Schweitzer M, Magunsuon T, Lidor A, Kuwabawa H, Kumar A, Brasic J, Wong D. Alterations of Central Dopamine Receptors Before and After Gastric Bypass Surgery. Obesity Surgery. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes Surg. 2012;22:201–207. doi: 10.1007/s11695-010-0346-1. [DOI] [PubMed] [Google Scholar]
- Tabibian A, Grothe KB, Mundi MS, Kellogg TA, Clark MM, Townsend CO. Bariatric Surgery Patients' Response to a Chronic Pain Rehabilitation Program. Obes Surg. 2015 doi: 10.1007/s11695-015-1634-6. [DOI] [PubMed] [Google Scholar]
- Tan T, Kuramoto M, Takahashi T, Nakamura H, Nakanishi Y, Imasato Y, Yoshimura H. Characteristics of the gastrointestinal absorption of morphine in rats. Chemical & pharmaceutical bulletin. 1989;37:168–173. doi: 10.1248/cpb.37.168. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang G-J, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo µPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Subrize M, Miller ML, Bellezza R, Cooney RN, Leggio L, Wang GJ, Rogers AM, Volkow ND, Hajnal A. Roux-en-Y Gastric Bypass Alters Brain Activity in Regions that Underlie Reward and Taste Perception. PLoS One. 2015;10:e0125570. doi: 10.1371/journal.pone.0125570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, Wang GJ, Volkow ND, Hajnal A. Gastric bypass increases ethanol and water consumption in diet-induced obese rats. Obes Surg. 2012;22:1884–1892. doi: 10.1007/s11695-012-0749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Critical reviews in biochemistry and molecular biology. 2013;48:1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry. 2013;70:661–663. doi: 10.1001/jamapsychiatry.2013.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Sarwer DB, Fabricatore AN, Jones L, Stack R, Williams NS. Psychosocial and behavioral status of patients undergoing bariatric surgery: what to expect before and after surgery. Med Clin North Am. 2007;91:451–469. xi–xii. doi: 10.1016/j.mcna.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004a;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004b;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu ZL, Chen B. Association study of dopamine D2, D3 receptor gene polymorphisms with motor fluctuations in PD. Neurology. 2001;56:1757–1759. doi: 10.1212/wnl.56.12.1757. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling A, Wudyka A. Narcotic addiction following gastric bypass surgery--a case study. Obesity surgery. 2011;21:680–683. doi: 10.1007/s11695-010-0177-0. [DOI] [PubMed] [Google Scholar]