Abstract
Malignant cells routinely violate cellular checkpoints that should initiate cell death in normal cells by triggering pro-apoptotic members of the BCL-2 family of proteins. To escape such death inducing signals, cancer cells often select for up regulation of anti-apoptotic BCL-2 family members including BCL-2, BCL-XL, BFL-1, BCL-W, and MCL-1. These family members prevent death by sequestering pro-apoptotic molecules. To counter this resistance mechanism, small molecule inhibitors of anti-apoptotic BCL-2 family members have been under development. These molecules have shown promise in pre-clinical and clinical testing to overcome apoptotic resistance, prompting cancer cells to undergo apoptosis. Alternatively, other strategies have taken advantage of the normal regulatory machinery controlling anti-apoptotic molecules and have used inhibitors of signaling pathways to down-modulate the expression of anti-apoptotic molecules thus tilting the balance in cancer cells to cell death. This review explores recent developments and strategies aimed at antagonizing anti-apoptotic BCL-2 family member action to promote the induction of cell death in cancer therapy.
Keywords: Apoptosis, BH3-mimetic, cancer, BCL-2 family, MCL-1, therapy
BCL-2 Family Basics
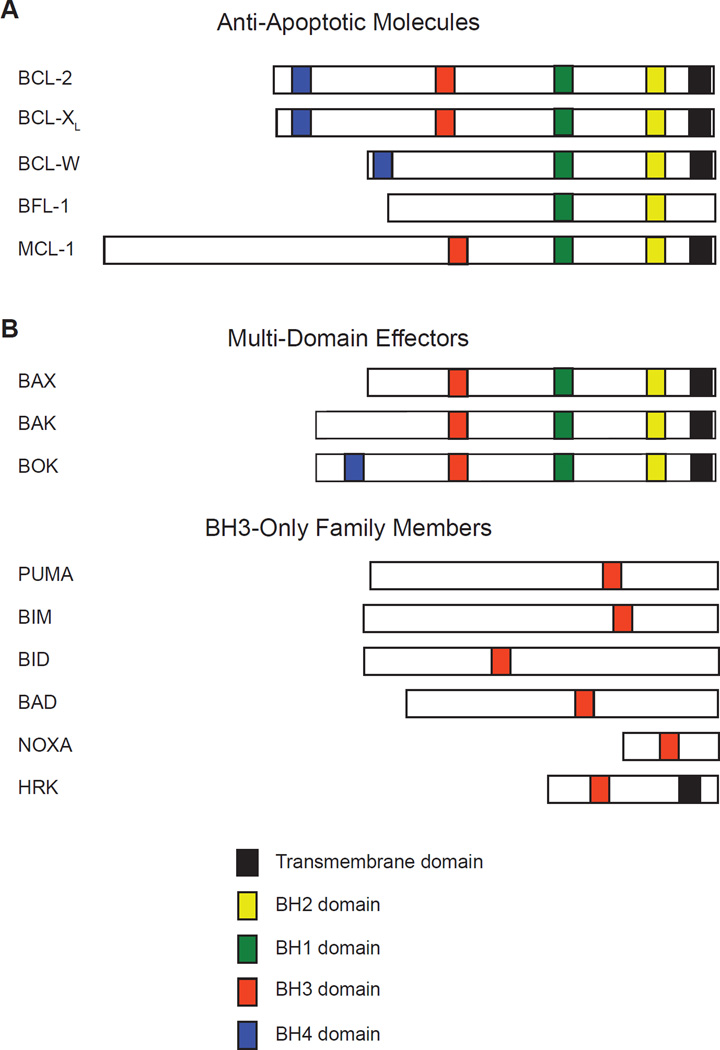
Apoptosis, or programmed cell death, is a genetic program regulating tissue homeostasis that was first identified in the nematode, C. elegans [1]. Members of the BCL-2 family, which regulate intrinsic cellular survival and death, share substantial evolutionary conservation with the primordial C. elegans molecules. The BCL-2 family is composed of both death inducing and pro-survival molecules (Figure 1). The anti-apoptotic molecules BCL-2, BCL-XL, BCL-W, BFL-1, and MCL-1 restrain the induction of cell death, thus promoting cellular survival. In opposition are pro-apoptotic BCL-2 family members, which actively participate in inducing cell death. Pro-apoptotic molecules can be sub-divided into the BH3-only family members (including BID, BAD, BIM, PUMA, NOXA, etc.) which respond to cellular signals that trigger cell death and the pro-apoptotic effectors (BAX and BAK) that integrate the cell death signals at the mitochondria [2]. The diverse collection of BH3-only family members act as cellular sentinels that, when activated by transcriptional and post-translational modifications, trigger the oligomerization of the pro-apoptotic effectors BAX and BAK on the mitochondrial outer membrane. The oligomers permeablize the mitochondrial outer membrane to release cytochrome c and other proteins. Released cytochrome c interacts with the initiator caspase-9 and APAF1, thus triggering caspase activation and the subsequent orderly destruction of the cell [3]. This process is critical to the maintenance of homeostasis and is responsible for eliminating damaged or obsolete cells not only during development, but also for the lifespan of the animal.
Figure 1. The BCL-2 Family of Apoptotic Regulators.
BCL-2 family members share a number of domains known as BCL-2 homology (BH) domains (indicated in colored segments). (A) Anti-apoptotic molecules, which antagonize the cell death process, contain multiple BH domains and often possess transmembrane (TM) domains that anchor these family members on cellular membranes including the mitochondrial outer membrane, nuclear membrane, and endoplasmic reticulum. (B) Pro-apoptotic molecules can be further sub-divided into two groups, the multi-domain effector molecules of BAX, BAK, and BOK that possess multiple BH-domains and TM domains that permit localization to the outer mitochondrial membrane and the BH3-only family members, which share only a minimal BH3-domain and are otherwise structurally quite dissimilar. The BH3-only family contains additional members not represented here. The BH and TM domains represented in this figure are those recognized by UniProt and the relative sizes of the family members are represented for comparison.
Specificity of Anti-Apoptotic BCL-2 Family Members
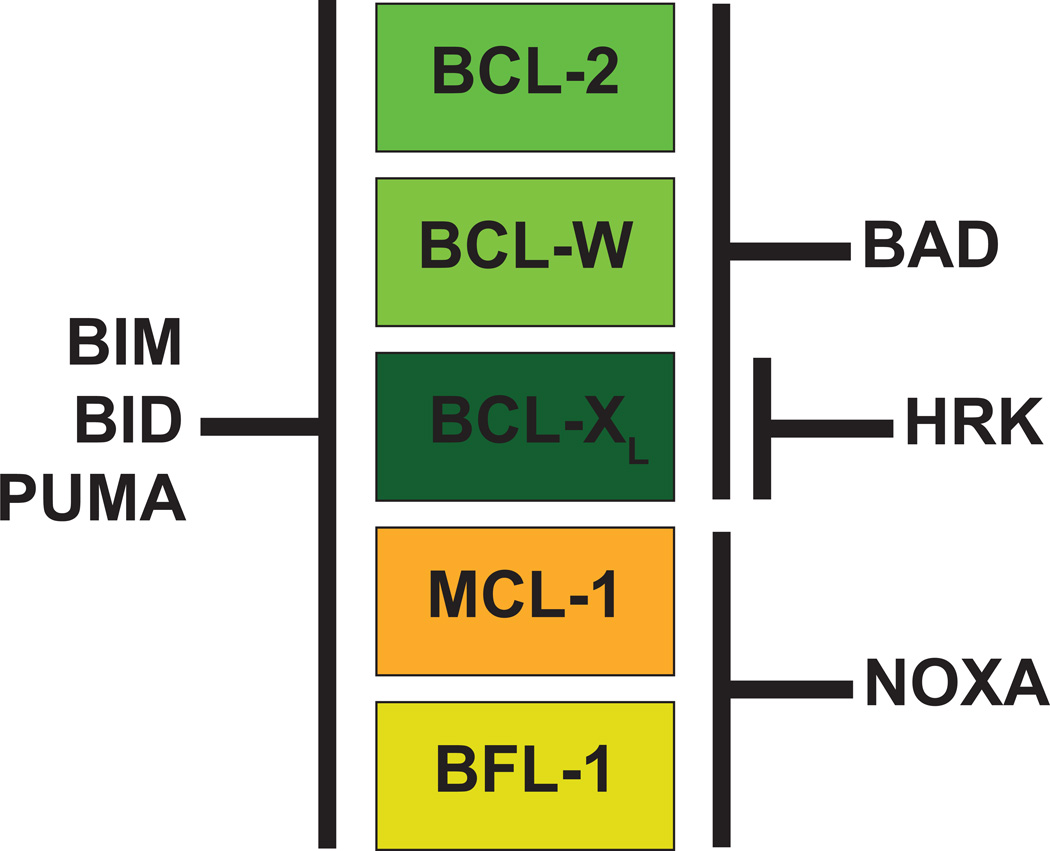
Anti-apoptotic BCL-2 family members antagonize cell death by directly binding BH3-only molecules as well as pro-apoptotic effectors; however, the ability of individual anti-apoptotic BCL-2 family members to antagonize pro-apoptotic molecules is not uniform [4]. The hydrophobic BH3-domain binding pockets of individual anti-apoptotic molecules dictate their ability to bind and antagonize the BH3-domains of the various pro-apoptotic molecules. Some BH3-only family members (e.g. BIM, BID, and PUMA) have the ability to bind all anti-apoptotic molecules with similar affinities (Figure 2). In contrast, other BH3-only family members have restricted abilities to interact with different anti-apoptotic BCL-2 family members. For example, anti-apoptotic BCL-2, BCL-XL, and BCL-W have similar capacities to bind the BH3-only family member BAD; however, neither MCL-1 nor BFL-1 can bind BAD [5, 6]. In contrast, only MCL-1 and BFL-1 are capable of binding the NOXA BH3-only family member, but none of the other anti-apoptotic molecules can bind NOXA (Figure 2). Another BH3-only, HRK is capable of binding BCL-XL, but does not interact with the other anti-apoptotics. The specificity for NOXA, BAD, and HRK can be used diagnostically to define the dependency of cells to individual anti-apoptotic molecules in a technique known as BH3-profiling [7].
Figure 2. Specificity of the Anti-Apoptotic BCL-2 Family Members for BH3-Only Members.
Some BH3-only proteins (BIM, BID, and PUMA) can interact with any of the five anti-apoptotic molecules (indicated in colored boxes). In contrast, other BH3-only molecules exhibit selectivity, only interacting with individual or sub-sets of anti-apoptotic molecules. The basis for this specificity is the binding interface of the anti-apoptotic molecule for the BH3-domains from the pro-apoptotic molecule. Two main groups have been defined largely on the ability to interact with BAD or NOXA. BCL-2, BCL-XL, and BCL-W (depicted in shades of green) all exhibit binding specificity to the BAD BH3-only molecule, but not to NOXA. In contrast, MCL-1 and BFL-1 (depicted in shades of orange) cannot interact with BAD, but possess specificity to interact with NOXA. The HRK BH3-only family member has remarkable specificity for BCL-XL.
BH3-Mimetic Small Molecules
The identification that pro-survival BCL-2 family members function by binding the BH3-domain of pro-apoptotic proteins was critical to the design of small molecular inhibitors of anti-apoptotic proteins, which are collectively known as BH3-mimetics. Their design is based on how the BH3-domain of BH3-only molecules fits into the hydrophobic cleft of the anti-apoptotic molecule. BH3-mimetics are typically designed to competitively bind to the BH3-binding groove of anti-apoptotic molecules to displace pro-apoptotic molecules. This ultimately leads to the activation of the multi-domain effector molecules BAX or BAK. This conceptual framework has served as the basis for the evolution of a number of small molecular inhibitors of pro-survival molecules.
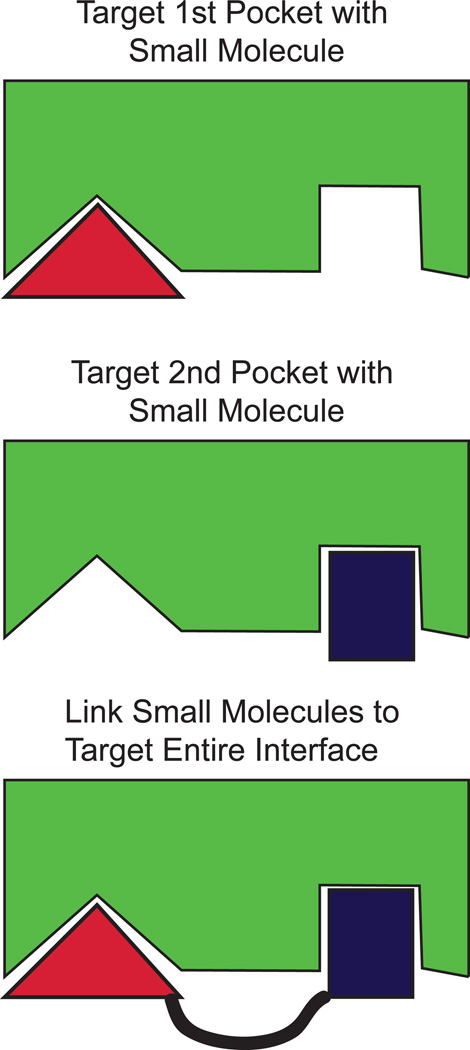
This approach has not been without challenges as it depends upon the design of small molecules capable of inhibiting large protein-protein interactions; often regarded as more challenging than the design of inhibitors of enzymatic activity. Pioneering work from scientists at Abbott Laboratories employed structure-activity relationships by nuclear magnetic resonance (so called SAR by NMR). This technique is based on sub-dividing the large protein-protein interacting face and individually targeting specific sub-domains by small molecules. In isolation, each small molecule may have a relatively poor affinity for the binding pocket, but after chemically linking the individual moieties the combined molecule can achieve a higher affinity than the individual small molecules (Figure 3). Using this technique and iterative optimization, scientists at Abbott Laboratories identified a small molecule inhibitor with sub-nanomolar binding affinity for BCL-XL, BCL-2, and BCL-W named ABT-737 [8]. This proof of principle development of a potent and effective inhibitor of members of the BCL-2 family was a landmark in apoptosis research and has been a harbinger of the design and development of a number of BH3-mimetic compounds with a broad scope of specificities.
Figure 3. Structure-Activity Relationships by Nuclear Magnetic Resonance (so called SAR by NMR).
Scientists at Abbott Laboratories pioneered targeting of anti-apoptotic BCL-2 family members based on structural studies of how BH3-only proteins interact with the BH3-binding pocket of the anti-apoptotic molecule. This protein-protein interaction face (indicated by green shape) is quite large when compared to the active site of an enzyme. Therefore, to target this interface, the sub-divided the interaction domain into separate “pockets” and used small molecule fragment screening (indicated by red and blue shapes) to interact with specific “pockets” separately. By using NMR to assess the binding of the small molecule to the pocket, a series of iterations were used to increase the affinity of the fragments to each pocket individually. Then, to increase affinity for interaction the small molecule fragments were chemically linked together (indicated by black line). Thus, the combined small molecule was able to bind both pockets and resulted in a dramatic increase in the affinity of interaction when compared to the separate fragments.
ABT-263 (navitoclax)
The initial success of ABT-737 in cell lines led to the development of an orally bioavailable derivative known as ABT-263 (navitoclax) [9]. Both molecules have sub-nanomolar affinity for BCL-2 and BCL-XL, exhibiting a similar pattern of specificity as the BH3-only molecule BAD, and induce apoptosis in a BAX and BAK-dependent manner (Figure 4). Like BAD, neither ABT-737 nor ABT-263 is capable of antagonizing the activity of anti-apoptotic MCL-1. Thus, the ability of these agents to show pro-apoptotic activity or anti-tumor activities depends on weak or absent MCL-1 expression [10]. Indeed, it was revealed that a common resistance mechanism in cancer cells to ABT-263 or ABT-737 is elevated MCL-1 expression [11, 12].
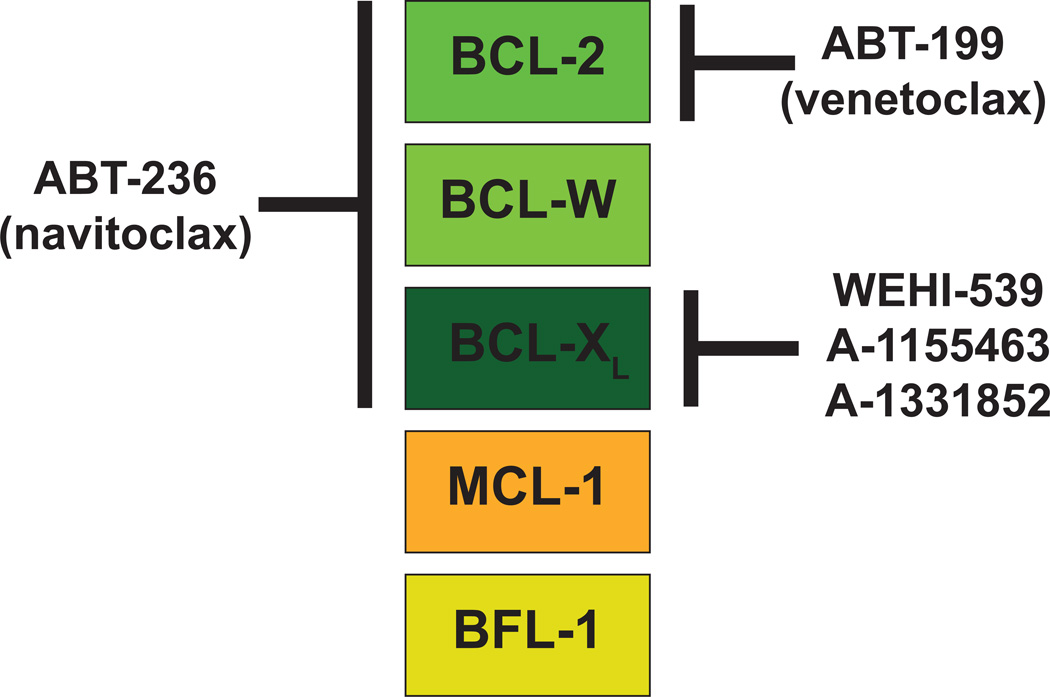
Figure 4. Specificity of the Anti-Apoptotic BCL-2 Family Members for BH3-Mimetic Molecules.
Like BH3-only molecules, the small molecule BH3-mimetics exhibit specificity for interacting with anti-apoptotic molecules (shown in colored boxes). The first in class molecule, ABT-737, and its orally-bioavailable derivative, ABT-263 (navitoclax), can interact with BCL-2, BCL-XL, and BCL-W much like the BAD BH3-only molecule. In contrast, the next generation inhibitor, ABT-199 (venetoclax), possesses specificity only for BCL-2 and does not inhibit the activity of BCL-XL or BCL-W and therefore does not induce the thrombocytopenia associated with ABT-263 administration. New BCL-XL inhibitors, WEHI-539, A-1155463, and A-1331852 were engineered to avoid interaction with BCL-2 and BCL-W, but have selectivity for BCL-XL. None of these compounds has appreciable affinity for MCL-1 or BFL-1. Thus, a common resistance mechanism to navitoclax and venetoclax has been elevated MCL-1 expression. The indicated BH3-mimetic small molecules are the furthest along in drug development and have well validated mechanisms of action.
Early clinical trials have demonstrated anti-tumor activity for ABT-263 in chronic lymphocytic leukemia (CLL) and small-cell lung cancer [13, 14]. Despite the promising results, phase I studies with ABT-263 revealed a dose-limiting, transient thrombocytopenia associated with treatment [13, 15]. Experimental examination of the basis for this toxicity revealed that mature platelets depend on BCL-XL expression to promote their survival; therefore, the death and clearance of aging platelets by ABT-263 is an “on-target” toxicity associated with its ability to target BCL-XL [16]. Despite the thrombocytopenia, additional clinical trials combining ABT-263 with other agents are still underway with the goal of fostering clinical responses while avoiding the toxicity.
ABT-199 (venetoclax)
ABT-199 represents a next generation BH3-mimetic in which its specificity has been tuned to specifically target BCL-2 (Figure 4). By targeting BCL-2, but not BCL-XL, ABT-199 does not induce the thrombocytopenia associated with BCL-XL inhibition [17]. Preclinical studies indicate potency for ABT-199 in killing cancer cells obtained from patients with acute myelogenous leukemia (AML), T-cell acute lymphoblastic leukemia (T-ALL), as well as CLL [18–20]. Clinical trials involving ABT-199 have reported efficacy in treating lymphoma and chronic lymphocytic leukemia (CLL). As evidence of its potency, in CLL patients single-agent ABT-199 treatment induced complete remissions in 25% of patients and some patients have experienced tumor lysis syndrome (TLS) due to rapid lymphoma killing [17, 21]. The potency of ABT-199 in CLL has led to its designation by the FDA as a “breakthrough therapy” for treating CLL and studies are underway to combine ABT-199 with other therapeutic agents. There is great anticipation that venetoclax will soon be approved by the FDA.
BCL-XL Inhibitors (WEHI-539, A-1155463, and A-1331852)
Despite the preclinical data from ABT-263 that demonstrated that BCL-XL is essential for the survival of mature platelets, there has remained interest in developing inhibitors with specificity for individual anti-apoptotic molecules to avoid toxicities associated with pan-BCL-2 inhibitors. To this aim, a number of groups have screened for selective BCL-XL inhibitors; a challenge made complicated by the similarities between the binding specificities of BCL-2 and BCL-XL. These efforts have resulted in the development of several BCL-XL selective inhibitors. The first, WEHI-539 was identified by disrupting the interaction between BCL-XL and BIM and was further optimized to increase the affinity of binding to the sub-nanomolar range with more than a 400-fold selectivity for BCL-XL over that of BCL-2, MCL-1, BCL-W, or A1 [22]. As evidence of its specificity, WEHI-539 induces apoptosis only in MCL-1-deficient cells and is ineffective in BCL-2 overexpressing cells. Furthermore, it rapidly induces the killing of isolated platelets in a caspase-dependent manner as anticipated. However, WEHI-539 is limited to use as an in vitro tool compound due to unfavorable chemical properties for in vivo use [23].
Scientist at AbbVie (formerly Abbott Laboratories) used fragment based NMR screening to identify two BCL-XL inhibitors suitable for in vivo use, A-1155463 and an orally-bioavailable version A-1331852 [23, 24]. When mice were treated with A-1155463 in vivo, a transient thrombocytopenia was observed within 6 hours, but the platelet numbers rebounded to normal by 72 hours after treatment [23]. As evidence of efficacy, A-1155463 administration produced a modest reduction in the tumor growth of a BCL-XL-dependent small cell lung cancer xenograft [23]. Furthermore, single-agent administration of A-1331852 delayed the progression of ALL xenografts and potentiated the effects of docetaxel in several xenograft models [24]. Therefore, A-1155463 and A-1331852 are capable of in vivo dosing for selective inhibition of BCL-XL.
“MCL-1 Selective” BH3-Mimetics in Development
The observation that elevated expression of anti-apoptotic MCL-1 results in resistance to ABT-263/ABT-737 and ABT-199 has resulted in a flurry of activity by many parties to develop MCL-1 selective inhibitors [11, 12, 25, 26]. Despite these efforts, MCL-1 has been a challenging target. To date, most MCL-1 inhibitors have been quite impotent and in many cases the ability of these compounds to directly inhibit MCL-1 has been questionable. Here is a summary of the current efforts to inhibit MCL-1 function therapeutically. While there are certainly additional inhibitors in development, including an orally active version produced by Astra-Zeneca presented at a meeting and other compounds that have been reported in patent literature, this review is focused on peer-reviewed findings for inhibitors that have been tested in biological systems.
Obatoclax (GX15-070) was one of the first BH3-mimetic compounds reported to inhibit MCL-1, albeit with low affinity. In vitro and in cultured cells, obatoclax inhibited the interaction between MCL-1 and pro-apoptotic BAK to induce cell death, even in ABT-737-resistant cells [27]. Obatoclax targets MCL-1 along with other anti-apoptotic BCL-2 family members all with relatively low affinity, so it represents a so called “pan-BCL-2” inhibitor. Importantly, several studies have reported that obatoclax can induce killing in a BCL-2 family protein independent manner, indicating that its action may not be purely as a BH3-mimetic [28]. Obatoclax has completed a number of Phase I and II clinical trials for a number of malignancies including non-small cell lung cancer (NSCLC), CLL, and acute myelogenous leukemia (AML) with modest efficacy [29]. In NSCLC, combining obatoclax with topotecan or docetaxel in relapsed patients resulted in minimal responses [30, 31]. Common toxicities were neutropenia and dose-limiting induction of drowsiness, euphoria, and disorientation during intravenous infusion [31, 32]. At this time, no additional obatoclax clinical trials are ongoing according to the NIH clinical trials website.
The Gossypol Family of inhibitors are plant-derived “pan-BCL-2” inhibitors capable of inhibiting BCL-2, BCL-XL, and MCL-1 [33]. The affinities for early Gossypol-derivatives for anti-apoptotic molecules were relatively modest and there have been reports that variants can induce death even in cells deficient in intrinsic apoptosis, indicative of off-target effects beyond BCL-2 family member inhibition [34, 35]. Despite the short-comings of the initial derivatives, a number of variants of this family are still being developed including AT-101 and BI97C1 (sabutoclax) [36]. These agents appear to have improved potency, but still exhibit off-target effects [37, 38]. Phase I clinical trials for AT-101 indicate that it is well tolerated with treatable neutropenia as a common toxicity [39]. At this time, clinical trials have not revealed efficacy in metastatic prostate cancer or recurrent lung cancer either as single-agent or when combined with standard therapies [40–42]. However, additional clinical trials are ongoing for AT-101, often in combination with other agents, in relapsed CLL, subsets of NSCLC, and advanced laryngeal cancer.
TW-37 is another gossypol-derivative “pan-BCL-2” BH3-mimetic compound capable of inhibiting MCL-1, BCL-2, and BCL-XL [43]. Like obatoclax, TW-37 shows some toxicity even to cells in which the intrinsic apoptotic machinery has been compromised indicating that it is capable “off-target” cell death [35]. Despite this, in pre-clinical data TW-37 showed potency as a single agent against diffuse large cell lymphoma (DLCL) cell lines and synergized with other chemotherapeutics in xenografted mice [43]. TW-37 has also been reported to induce the induction of the BH3-only family member NOXA [35]; potentially representing an indirect repression MCL-1 expression as NOXA induction has been reported to lead to the degradation of MCL-1 [44].
S1 is another “pan-BCL-2 inhibitor” BH3-mimetic small molecule that was characterized by its ability to displace a BID BH3-peptide from BCL-2 [45]. At higher concentrations, S1 can also disrupt the interaction between MCL-1 and the pro-apoptotic effector BAK in cell lines. However, S1’s ability to directly inhibit MCL-1 has been questioned, as treatment of cells with S1 also induces the expression of NOXA that could be responsible for antagonizing MCL-1 function [34, 38]. In either case, S1 induced killing is BAX and/or BAK dependent [45]. Preclinical evidence indicates that S1 treatment can kill human primary ALL samples in culture and appears to delay tumor growth in hepatocarcinoma xenograft models [45].
Maritoclax is a BH3-mimetic identified due to its ability to displace the BIM BH3-peptide from MCL-1, but it is unable to displace the BIM BH3-peptide from BCL-XL indicating selectivity for MCL-1 [46]. Maritoclax rapidly induces the proteasome-dependent degradation of MCL-1 [46]. Therefore, maritoclax may have dual function in not only displacing MCL-1 from pro-apoptotic molecules, but also causing the elimination of MCL-1 via proteolysis. In either case, maritoclax has demonstrated efficacy in killing acute myeloid leukemia (AML) cell lines both in vitro and in vivo [47]. Lastly, maritoclax treated cells are more sensitive to other BH3-mimetic compounds such as ABT-737 raising the possibility that it would be a good combinatorial candidate [46].
EU-5346 (compound 9) is a small molecule inhibitor identified by Eutropics Pharmaceuticals by screening a small molecule library for displacement of a BIM BH3-peptide from recombinant MCL-1 [48]. To attenuate the toxic effects of BCL-XL inhibition, a counter screen for molecules that exhibited a preference for MCL-1 above recombinant BCL-XL was also implemented. Modified derivatives of the initial screening hit were designed to generate EU-5346, a small molecule that possesses an IC50=310 nM for MCL-1 and 40 µM for BCL-XL [48]. In cultured human cancer cell lines, EU-5346 kills cell lines defined as MCL-1-dependent by inducing mitochondrial permeabilization [48]. EU-5346 is still in preclinical developmental and is considered a tool compound.
MIM1 is a small molecule inhibitor identified by a small molecule screen to displace a stapled alpha-helical BH3-peptide from MCL-1 itself from recombinant MCL-1 [49]. MIM1 is a prototype inhibitor with selectivity for MCL-1, but not anti-apoptotic BCL-XL in model leukemic cell lines [49]. However, MIM1 appears to lack the potency necessary to move forward with clinical evaluation. This may reflect the small size of MIM1 and the fact that it only targets a portion of MCL-1’s BH3-binding pocket [49]. Importantly, MIM1 does appear to be on-pathway as cells lacking intrinsic apoptotic machinery are resistant [35]. It is anticipated that additional medicinal chemistry of MIM1 will improve the potency.
UMI-77 is an analog of UMI-59, a small molecule that was identified as an MCL-1 inhibitor by a high-throughput screening approach in which molecules were tested to disrupt the interaction between MCL-1 and a BID BH3-peptide [50, 51]. The improved UMI-77 selectively binds MCL-1 (Ki=0.49 µM) and to a lesser extent A1/BFL-1 (Ki=5.33 µM) and BCL-W (Ki=8.19 µM)[50]. In contrast UMI-77 poorly binds to BCL-2 and BCL-XL making it an MCL-1 “selective inhibitor”. Mechanistically, UMI-77 induces killing of pancreatic cancer cells that correlates with the expression levels of MCL-1 and BAK and induces caspase-dependent intrinsic apoptosis [50]. As further evidence of the on-pathway efficacy, UMI-77 only poorly induces the death of BAX and BAK doubly-deficient MEF lines and requires the expression of MCL-1 for activity in cell lines [50]. Lastly, UMI-77 can slow the growth of pancreatic cancer cell line xenografts when treated in vivo highlighting its potential in pre-clinical models [50].
MCL-1 inhibitor A-1210477 is the culmination of efforts by AbbVie to design an MCL-1 selective inhibitor by high-throughput screening. Using extensive iterative chemical modification and structural evaluation, scientists at AbbVie were able to generate A-1210477 with picomolar binding to MCL-1 (Ki=0.43 nM) while exhibiting much poorer binding to BCL-2, BCL-XL, BCL-W, and BFL-1 (Ki>0.66 µM) [52, 53]. In cell based assays, A-1210477 is able to disrupt the interaction between MCL-1 and pro-apoptotic BIM and can induce death of multiple myeloma and non-small cell lung cancer cell lines in vitro even as a single agent [53]. Strikingly, A-1210477 appears to function similar to BIM as treatment with the agent induces the stabilization of MCL-1 in cell lines serving as a bio-marker for activity [53]. Importantly, by combining A-1210477 with navitoclax a potent synergy of interaction was observed in cell lines. Unfortunately, A-1210477 does not have favorable pharmacokinetics for in vivo use and is therefore limited to utility as a tool compound for in vitro and cell based studies. However, the anticipation is that additional development based on the efficacy of A-1210477 will lead to the development of a suitable in vivo inhibitor.
“Direct Activators” Bax Agonists
While the majority of anti-cancer therapies involving the BCL-2 family have been focused on releasing the inhibitory effects of anti-apoptotic molecules, others have approached the problem by investigating mechanisms to directly activate the multi-domain pro-apoptotic effectors. The benefit of this approach is that it can bypass the selectivity of the anti-apoptotic molecules, thus making a “one-size-fits-all” strategy to induce apoptosis in tumor cells. To date the majority of these approaches have targeted the BAX molecule and screened for small molecules that can drive its activation and subsequent oligomerization. Several strategies for identifying BAX agonists have been utilized including directly targeting BAX’s hydrophobic binding pocket, activating the so called “BH3-trigger site” formed by alpha-helices 1 and 6, and targeting the phosphorylation of serine 184 of BAX [54–56]. These approaches have all identified small molecules that could trigger BAX-dependent, but BAK-independent killing of model cell lines and tumor cell lines. The biggest concern about this direct-activation strategy is the possibility of triggering toxicity to normal tissues. Preliminary reports indicate that at least in a xenograft model of lung cancer, small-molecule BAX agonists triggered tumor killing without inducing appreciable toxicity highlighting the potential for this strategy [54]. Further research will be necessary to identify whether there is a therapeutic window for use of direct multi-domain effector agonists without triggering toxic effects in humans. It is anticipated that similar strategies will also lead to BAK agonist small molecules, thus adding another weapon to the arsenal of cancer therapy.
Roles for Anti-Apoptotic BCL-2 Family in Normal Biology
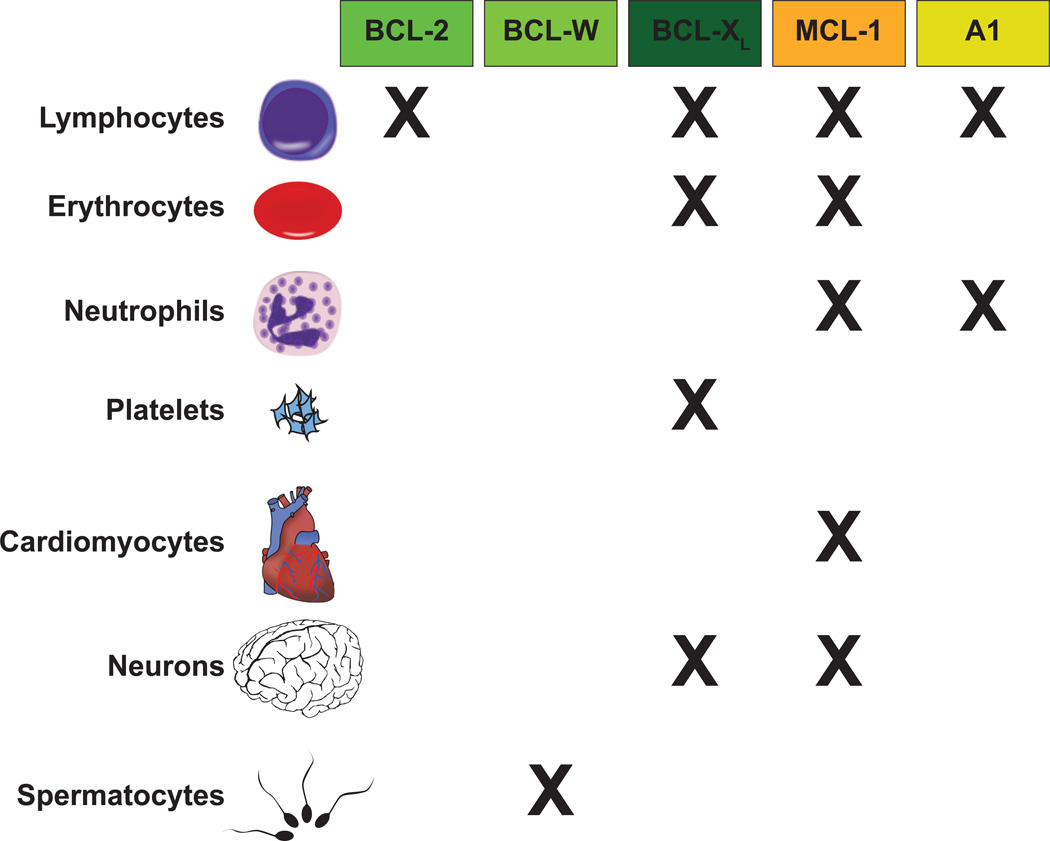
Evidence from mouse genetic ablation experiments has revealed selective roles for most anti-apoptotic members of the BCL-2 family in promoting survival during the development and homeostasis of many normal animal tissues (Figure 5). For example, genetic ablation of anti-apoptotic BCL-2 results in viable mice, but a majority of animals die at young ages due to polycystic kidney disease and fulminant apoptosis of mature lymphocytes [57–59]. Mice lacking BCL-XL die at embryonic day 13 exhibiting massive apoptosis throughout the developing brain and death of erythrocyte precursors [60, 61]. Mice lacking BCL-W have few developmental abnormalities, but male mice are sterile due to failed spermatogenesis [62, 63]. Silencing of isoforms of A1, the mouse homologs of human BFL-1, resulted in impaired thymocyte development, B lymphocyte homeostasis and proliferation as well as sensitizing granulocytes to spontaneous cell death [64].
Figure 5. Anti-Apoptotic BCL-2 Family Members Possess Selective Functions in Promoting the Survival of Normal Cellular Lineages.
Genetic ablation studies have revealed that while anti-apoptotic molecules share the ability to interact with some BH3-only molecules, they exhibit remarkable selectivity in promoting the survival of normal cellular lineages. Mice deficient in BCL-2, BCL-W, and A1 (mouse homolog of BFL-1) anti-apoptotic molecules often have quite specific deficiencies in normal cell survival. In contrast, mice lacking BCL-XL and MCL-1 are embryonic lethal. Conditional knockout approaches have revealed that BCL-XL is required for a number of cellular lineages. In contrast, MCL-1 appears to be important for a wide-array of normal tissues. The “X” represents a role in the survival of the indicated lineage; however, the relative contribution of individual anti-apoptotic molecules is varied. Please consult the text for a more complete explanation of the contribution of individual pro-survival molecules in specific cellular lineages.
In contrast to the relatively restricted roles for other anti-apoptotic BCL-2 family members, anti-apoptotic MCL-1 appears to have more generalizable roles being responsible for promoting cellular survival both during development and in differentiated lineages. During hematopoiesis, MCL-1 is essential to promote the survival of all the progenitor populations including the hematopoietic stem cells [65]. It is required to promote survival during all stages of both T and B lymphoid development as well as for naïve, effector, regulatory, and memory lymphocyte populations [5, 66–69]. During myelopoiesis, lacking MCL-1 prevents the survival of mature granulocytes, but surprisingly deletion of MCL-1 in monocytes and macrophages is tolerated, but renders the cells more sensitive to stress induced cell death [70, 71]. In unpublished work, MCL-1 is also important for early red blood cell progenitor survival. Genetic ablation of MCL-1 in developing neurons induces widespread apoptosis in neuronal progenitors and deletion of MCL-1 in mature neurons renders them highly sensitive to cell death stimuli [72, 73]. In mature cardiomyocytes, deletion of MCL-1 results in a fatal, dilated cardiomyopathy accompanied by loss of contractility, death of myofibrils and appearance of resultant fibrosis [74, 75]. Therefore, MCL-1 is a critical modulator of cellular survival to a variety of cellular lineages.
These observations, all made using permanent genetic ablation, indicate a general dependence for MCL-1 expression in many critical cell types. Therefore, it is possible that efficient inhibition of MCL-1 function by BH3-mimetics could be associated with toxicities to a myriad of normal cell types. Alternatively, it is possible that pharmacological inhibition of MCL-1 might be transient and incomplete, thus providing a therapeutic window in which malignant cells can be triggered to die without compromising normal cells. Only in vivo testing of MCL-1 inhibitors will be able to arbitrate this outcome.
In addition to MCL-1’s well-recognized function on the outer mitochondrial membrane where it antagonizes cell death, a truncated species of MCL-1 is imported into the mitochondrial matrix where it promotes normal mitochondrial energy production [76]. As genetic deletion abrogates both MCL-1’s ability to inhibit pro-apoptotic molecules as well as its function in maintaining mitochondrial energy production, it has been thus far challenging to assess the relative contributions of these functions on cell lineage survival [77]. Furthermore, it is unclear whether both of these diverse functions require MCL-1’s BH3-binding pocket and would be similarly affected by MCL-1-selective inhibitors. Further exploration of the contribution of MCL-1 functions will be essential to our understanding the potential for toxicity resulting from MCL-1 inhibition.
Potential for Indirect Inhibition of MCL-1 to Promote Sensitivity to BH3-mimetics
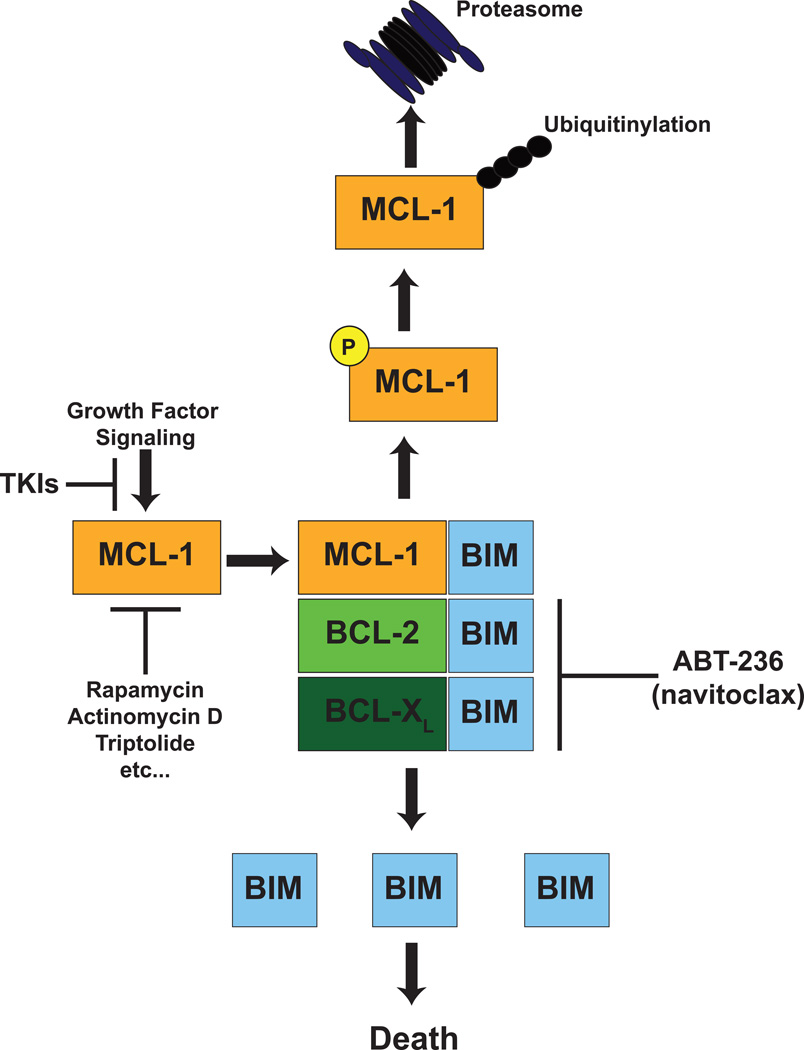
Due to the current lack of a potent and selective MCL-1 inhibitor and the possibility that efficient MCL-1 inhibition may have undesired toxicities, a number of groups have taken an alternative approach to utilize combination therapies that can attenuate MCL-1 expression to sensitize otherwise ABT-263-resistant tumors. MCL-1 is well-documented to be a labile anti-apoptotic molecule, but its turnover is regulated by many cellular signaling pathways [78, 79]. It is the target of phosphorylation events that foster its ubiquitinylation by a number of E3 ligases including MULE, β-TrCP, and FBW7 [80–83]. Furthermore, deubiquitinating enzymes such as USP9x can remove poly-ubiquitin chains, thus stabilizing MCL-1 expression [84]. MCL-1 elimination by the proteasome can also occur in an ubiquitin-independent manner highlighting the complex control on this anti-apoptotic BCL-2 family member [85]. Since MCL-1 has such a rapid turnover, a number of strategies to block new protein synthesis result in the elimination of MCL-1 expression (Figure 6). For example, general transcriptional inhibitors can rapidly induce the loss of MCL-1 expression in cancer cell lines including actinomycin D and triptolide [86]. Similarly, the CDK9 kinase inhibitor flavopiridol causes the transcriptional repression of MCL-1 leading to its elimination [87]. At the translational level, drugs like rapamycin and other inhibitors of the mammalian target of rapamycin (mTOR) like AZD8055 that have been shown to repress MCL-1 expression in a number of tumor types rendering the cells sensitive to ABT-737 [88–91].
Figure 6. Strategies to Attenuate MCL-1 Expression in Malignancy to Render Cells Susceptible to BH3-Mimetics.
Currently, MCL-1 expression renders cancer cells resistant to the best developed BH3-mimetic small molecules such as venetoclax and navitoclax. To restore susceptibility of cancer cells to these agents, strategies to decrease MCL-1 expression have been explored in pre-clinical models. A variety of cellular signaling pathways promote MCL-1 expression. Since MCL-1 is an extremely labile anti-apoptotic molecule, a number of agents that block MCL-1 mRNA synthesis or protein translation (e.g. rapamycin, actinomycin D, triptolide, CDK inhibitors, tyrosine kinase inhibitors etc.) have shown efficacy in repressing MCL-1 expression in cancer cells. Furthermore, MCL-1 turnover by the proteasome also is regulated at a number of levels. First, MCL-1 ubiquitinylation by E3 ligases (e.g. MULE, βTrCP, and FBW7) can be facilitated by phosphorylation of MCL-1 by a number of signaling pathways including GSK3β, JNK, and ERK. Thus, activation of these signaling pathways or inhibition of repressive signaling (e.g. AKT, etc.) can foster the elimination of MCL-1 by the proteasome. In contrast, removal of these phosphorylation events by phosphatases like PP2A or removal of polyubiquitin chains by deubiquitinases like USP9x can promote MCL-1 stability by blocking degradation. Lastly, a number of cellular stresses can lead to the induction of NOXA, an MCL-1-selective BH3-only molecule, which has been shown to promote MCL-1 degradation. In summary, there are a number of methods by which MCL-1 protein can be actively degraded in cells, thus representing potential mechanisms by which non-MCL-1 targeting BH3-mimetic agents (e.g. venetoclax and navitoclax) can be combined to drive cancer cell apoptosis.
MCL-1 stability is modulated by a number of signaling pathways, therefore by using inhibitors of these signaling pathways MCL-1 expression can be reduced in tumor cell lines [79]. One of the first examples of this was the identification of sorafenib, a kinase inhibitor developed to inhibit C-Ras and B-Raf that also inhibits a number of other kinases including VEGFRs, PDGFRs, Flt3, and c-Kit. Sorafenib has been shown to repress MCL-1 protein expression in a number of different malignancies including leukemia, lymphoma, breast cancer, multiple myeloma, hepatocellular carcinoma without affecting mRNA expression [92–94]. In another study, the PI3K inhibitor (GDC-0941) was shown to synergize with ABT-263 to abolish the ability of glioma-stem cells to form neurospheres in culture by reducing MCL-1 expression [95]. MCL-1 protein stability is regulated by the action of PI3K-Akt, which acts in part by inactivating GSK3β, a kinase that marks MCL-1 for ubiquitin-mediated degradation [83]. Not surprisingly, many Akt inhibitors act to down regulate MCL-1 expression and can render cells more sensitive to BH3-mimetics.
In ABT-263-resistant mouse B-ALL cell lines, the BCR-ABL oncofusion, which activates many cellular signaling pathways, acts in part to maintain expression of MCL-1 [96]. Therefore, BCR-ABL-inhibiting tyrosine kinase inhibitors (TKIs) dasatinib or imatinib repressed MCL-1 expression and potently synergized with ABT-263 to induce leukemia killing [96]. These studies demonstrate that while MCL-1 expression is a common resistance mechanism against BH3-mimetics like navitoclax and venetoclax, it is possible to use combinatorial drug treatment to repress MCL-1 expression and re-sensitize cells.
Concluding Remarks
It has been almost 30 years since it was appreciated that anti-apoptotic members of the BCL-2 family contribute a survival advantage to cancer cells. Now, we stand on the verge of having effective therapeutic agents to antagonize some of these survival pathways and improve the cancer therapy. This is truly the beginning to a new chapter on the BCL-2 family in cancer biology.
Acknowledgments
This review benefited from helpful discussions with Brian Koss and Dr. Amit Budhraja of the Opferman laboratory. The review was supported by R01HL102175 from the National Institute of Heart, Lung and Blood (J.T.O); the American Cancer Society 119130-RSG-10-255-01-LIB (J.T.O); a Cancer Center Support Grant P30CA021765; and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 2.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 4.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 6.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Ryan J, Letai A. BH3 profiling in whole cells by fluorimeter or FACS. Methods. 2013;61:156–164. doi: 10.1016/j.ymeth.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 9.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 10.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, Nolan C, Chiu YL, Busman T, Xiong H, Krivoshik AP, Humerickhouse R, Shapiro GI, Rudin CM. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, Cui Y, Busman TA, McKeegan EM, Krivoshik AP, Enschede SH, Humerickhouse R. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, Cui Y, Busman T, Elmore SW, Rosenberg SH, Krivoshik AP, Enschede SH, Humerickhouse RA. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. The lancet oncology. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, Josefsson EC, Alwis I, Ono A, Willcox A, Andrews RK, Mason KD, Salem HH, Huang DC, Kile BT, Roberts AW, Jackson SP. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 17.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 18.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, Dohner H, Gaidzik VI, Galinsky I, Golfman LS, Haferlach T, Harutyunyan KG, Hu J, Leverson JD, Marcucci G, Muschen M, Newman R, Park E, Ruvolo PP, Ruvolo V, Ryan J, Schindela S, Zweidler-McKay P, Stone RM, Kantarjian H, Andreeff M, Konopleva M, Letai AG. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer discovery. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, Degryse S, Cante-Barrett K, Briot D, Clappier E, Lammens T, De Moerloose B, Benoit Y, Poppe B, Meijerink JP, Cools J, Soulier J, Rabbitts TH, Taghon T, Speleman F, Van Vlierberghe P. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124:3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 20.Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, Loh ML, Hunger SP, Wood B, DeAngelo DJ, Stone R, Harris M, Gutierrez A, Kelliher MA, Letai A. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer discovery. 2014;4:1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ABT-199 Shows Effectiveness in CLL. Cancer discovery. 2014;4:OF7. doi: 10.1158/2159-8290.CD-NB2014-102. [DOI] [PubMed] [Google Scholar]
- 22.Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, Baell JB, Colman PM, Deshayes K, Fairbrother WJ, Flygare JA, Gibbons P, Kersten WJ, Kulasegaram S, Moss RM, Parisot JP, Smith BJ, Street IP, Yang H, Huang DC, Watson KG. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9:390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 23.Tao ZF, Hasvold L, Wang L, Wang X, Petros AM, Park CH, Boghaert ER, Catron ND, Chen J, Colman PM, Czabotar PE, Deshayes K, Fairbrother WJ, Flygare JA, Hymowitz SG, Jin S, Judge RA, Koehler MF, Kovar PJ, Lessene G, Mitten MJ, Ndubaku CO, Nimmer P, Purkey HE, Oleksijew A, Phillips DC, Sleebs BE, Smith BJ, Smith ML, Tahir SK, Watson KG, Xiao Y, Xue J, Zhang H, Zobel K, Rosenberg SH, Tse C, Leverson JD, Elmore SW, Souers AJ. Discovery of a Potent and Selective BCL-XL Inhibitor with in Vivo Activity. ACS medicinal chemistry letters. 2014;5:1088–1093. doi: 10.1021/ml5001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, Belmont LD, Nimmer P, Xiao Y, Ma XM, Lowes KN, Kovar P, Chen J, Jin S, Smith M, Xue J, Zhang H, Oleksijew A, Magoc TJ, Vaidya KS, Albert DH, Tarrant JM, La N, Wang L, Tao ZF, Wendt MD, Sampath D, Rosenberg SH, Tse C, Huang DC, Fairbrother WJ, Elmore SW, Souers AJ. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Science translational medicine. 2015;7:279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 25.Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, Hsi ED, Almasan A. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell death & disease. 2015;6:e1593. doi: 10.1038/cddis.2014.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiron D, Dousset C, Brosseau C, Touzeau C, Maiga S, Moreau P, Pellat-Deceunynck C, Le Gouill S, Amiot M. Biological rational for sequential targeting of Bruton tyrosine kinase and Bcl-2 to overcome CD40-induced ABT-199 resistance in mantle cell lymphoma. Oncotarget. 2015;6:8750–8759. doi: 10.18632/oncotarget.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Belec L, Billot X, Acoca S, Purisima E, Wiegmans A, Cluse L, Johnstone RW, Beauparlant P, Shore GC. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, Bornmann W, Kantarjian H, Viallet J, Samudio I, Andreeff M. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, Viallet J, Cheson BD. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, Travis W, James L, Ginsberg MS, Juergens R, Markus S, Tyson L, Subzwari S, Kris MG, Krug LM. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung cancer. 2011;74:481–485. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiappori A, Williams C, Northfelt DW, Adams JW, Malik S, Edelman MJ, Rosen P, Van Echo DA, Berger MS, Haura EB. Obatoclax mesylate, a pan-bcl-2 inhibitor, in combination with docetaxel in a phase 1/2 trial in relapsed non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:121–125. doi: 10.1097/JTO.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiappori AA, Schreeder MT, Moezi MM, Stephenson JJ, Blakely J, Salgia R, Chu QS, Ross HJ, Subramaniam DS, Schnyder J, Berger MS. A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer. Br J Cancer. 2012;106:839–845. doi: 10.1038/bjc.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. Journal of medicinal chemistry. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 34.Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, Eastman A. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–24895. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013;20:1475–1484. doi: 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, Wu B, Cellitti J, Zhai D, Yang L, Dahl R, Fisher PB, Reed JC, Pellecchia M. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. Journal of medicinal chemistry. 2010;53:4166–4176. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varadarajan S, Butterworth M, Wei J, Pellecchia M, Dinsdale D, Cohen GM. Sabutoclax (BI97C1) and BI112D1, putative inhibitors of MCL-1, induce mitochondrial fragmentation either upstream of or independent of apoptosis. Neoplasia. 2013;15:568–578. doi: 10.1593/neo.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soderquist R, Pletnev AA, Danilov AV, Eastman A. The putative BH3 mimetic S1 sensitizes leukemia to ABT-737 by increasing reactive oxygen species, inducing endoplasmic reticulum stress, and upregulating the BH3-only protein NOXA. Apoptosis. 2014;19:201–209. doi: 10.1007/s10495-013-0910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schelman WR, Mohammed TA, Traynor AM, Kolesar JM, Marnocha RM, Eickhoff J, Keppen M, Alberti DB, Wilding G, Takebe N, Liu G. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Investigational new drugs. 2014;32:295–302. doi: 10.1007/s10637-013-9999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan R. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:1757–1760. doi: 10.1097/JTO.0b013e31822e2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, Wood BA, Leopold L. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:781–785. doi: 10.1097/JTO.0b013e31820a0ea6. [DOI] [PubMed] [Google Scholar]
- 42.Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, Galsky MD, Berry WR, Karlov P, Holmlund JT, Wood BA, Brookes M, Leopold L. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2012;23:1803–1808. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]
- 43.Mohammad RM, Goustin AS, Aboukameel A, Chen B, Banerjee S, Wang G, Nikolovska-Coleska Z, Wang S, Al-Katib A. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 44.Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, Fairlie WD, Hinds MG, Colman PM. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Song T, Zhang T, Gao J, Wu G, An L, Du G. A novel BH3 mimetic S1 potently induces Bax/Bak-dependent apoptosis by targeting both Bcl-2 and Mcl-1. International journal of cancer Journal international du cancer. 2011;128:1724–1735. doi: 10.1002/ijc.25484. [DOI] [PubMed] [Google Scholar]
- 46.Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, Krishnegowda G, Awwad A, Dewey A, Liu X, Amin S, Cheng C, Qin Y, Schonbrunn E, Daughdrill G, Loughran TP, Jr, Sebti S, Wang HG. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012;287:10224–10235. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doi K, Liu Q, Gowda K, Barth BM, Claxton D, Amin S, Loughran TP, Jr, Wang HG. Maritoclax induces apoptosis in acute myeloid leukemia cells with elevated Mcl-1 expression. Cancer biology & therapy. 2014;15:1077–1086. doi: 10.4161/cbt.29186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard DJ, Lena R, Bannister T, Blake N, Pierceall WE, Carlson NE, Keller CE, Koenig M, He Y, Minond D, Mishra J, Cameron M, Spicer T, Hodder P, Cardone MH. Hydroxyquinoline-derived compounds and analoguing of selective Mcl-1 inhibitors using a functional biomarker. Bioorganic & medicinal chemistry. 2013 doi: 10.1016/j.bmc.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen NA, Stewart ML, Gavathiotis E, Tepper JL, Bruekner SR, Koss B, Opferman JT, Walensky LD. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chemistry & biology. 2012;19:1175–1186. doi: 10.1016/j.chembiol.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abulwerdi F, Liao C, Liu M, Azmi AS, Aboukameel A, Mady AS, Gulappa T, Cierpicki T, Owens S, Zhang T, Sun D, Stuckey JA, Mohammad RM, Nikolovska-Coleska Z. A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Molecular cancer therapeutics. 2014;13:565–575. doi: 10.1158/1535-7163.MCT-12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abulwerdi FA, Liao C, Mady AS, Gavin J, Shen C, Cierpicki T, Stuckey JA, Showalter HD, Nikolovska-Coleska Z. 3-Substituted-N-(4-hydroxynaphthalen-1-yl)arylsulfonamides as a novel class of selective Mcl-1 inhibitors: structure-based design, synthesis, SAR, biological evaluation. Journal of medicinal chemistry. 2014;57:4111–4133. doi: 10.1021/jm500010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruncko M, Wang L, Sheppard GS, Phillips DC, Tahir SK, Xue J, Erickson S, Fidanze S, Fry E, Hasvold L, Jenkins GJ, Jin S, Judge RA, Kovar PJ, Madar D, Nimmer P, Park C, Petros AM, Rosenberg SH, Smith ML, Song X, Sun C, Tao ZF, Wang X, Xiao Y, Zhang H, Tse C, Leverson JD, Elmore SW, Souers AJ. Structure-guided design of a series of MCL-1 inhibitors with high affinity and selectivity. Journal of medicinal chemistry. 2015;58:2180–2194. doi: 10.1021/jm501258m. [DOI] [PubMed] [Google Scholar]
- 53.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, Kovar P, Tanaka A, Bruncko M, Sheppard GS, Wang L, Gierke S, Kategaya L, Anderson DJ, Wong C, Eastham-Anderson J, Ludlam MJ, Sampath D, Fairbrother WJ, Wertz I, Rosenberg SH, Tse C, Elmore SW, Souers AJ. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell death & disease. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin M, Li R, Xie M, Park D, Owonikoko TK, Sica GL, Corsino PE, Zhou J, Ding C, White MA, Magis AT, Ramalingam SS, Curran WJ, Khuri FR, Deng X. Small-molecule Bax agonists for cancer therapy. Nature communications. 2014;5:4935. doi: 10.1038/ncomms5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao G, Zhu Y, Eno CO, Liu Y, Deleeuw L, Burlison JA, Chaires JB, Trent JO, Li C. Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol Cell Biol. 2014;34:1198–1207. doi: 10.1128/MCB.00996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol. 2012;8:639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 58.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 59.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci U S A. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motoyama N, Kimura T, Takahashi T, Watanabe T, Nakano T. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J Exp Med. 1999;189:1691–1698. doi: 10.1084/jem.189.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x- deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 62.Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Kontgen F, Adams JM, Cory S. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci U S A. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 64.Ottina E, Grespi F, Tischner D, Soratroi C, Geley S, Ploner A, Reichardt HM, Villunger A, Herold MJ. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood. 2012;119:6032–6042. doi: 10.1182/blood-2011-12-399089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 66.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tripathi P, Koss B, Opferman JT, Hildeman DA. Mcl-1 antagonizes Bax/Bak to promote effector CD4(+) and CD8(+) T-cell responses. Cell Death Differ. 2013;20:998–1007. doi: 10.1038/cdd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DH, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM, Erickson LD, Fairfax K, Mackay F, Strasser A, Nutt SL, Tarlinton DM. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013;14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, Kelly MA, MacKenzie AE, Park DS, Opferman JT, Slack RS. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malone CD, Hasan SM, Roome RB, Xiong J, Furlong M, Opferman JT, Vanderluit JL. Mcl-1 regulates the survival of adult neural precursor cells. Molecular and cellular neurosciences. 2012;49:439–447. doi: 10.1016/j.mcn.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase S, Schuetz JD, Rehg JE, Opferman JT. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev. 2013;27:1351–1364. doi: 10.1101/gad.215855.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, Fischer KM, Sussman MA, Miyamoto S, Gustafsson AB. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013;27:1365–1377. doi: 10.1101/gad.215871.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, Youle RJ, Green DR, Opferman JT. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perciavalle RM, Opferman JT. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Opferman JT. Unraveling MCL-1 degradation. Cell Death Differ. 2006;13:1260–1262. doi: 10.1038/sj.cdd.4401978. [DOI] [PubMed] [Google Scholar]
- 80.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 81.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O'Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ, Seshagiri S, Ludlam MJ, Leong KG, Dueber EC, Maecker H, Huang DC, Dixit VM. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 82.Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, Lee DF, Liu JC, Zhong Q, Wang X, Hung MC. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. Deubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 85.Stewart DP, Koss B, Bathina M, Perciavalle RM, Bisanz K, Opferman JT. Ubiquitin-independent degradation of antiapoptotic MCL-1. Mol Cell Biol. 2010;30:3099–3110. doi: 10.1128/MCB.01266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei G, Margolin AA, Haery L, Brown E, Cucolo L, Julian B, Shehata S, Kung AL, Beroukhim R, Golub TR. Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell. 2012;21:547–562. doi: 10.1016/j.ccr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma Y, Cress WD, Haura EB. Flavopiridol-induced apoptosis is mediated through up-regulation of E2F1 and repression of Mcl-1. Molecular cancer therapeutics. 2003;2:73–81. [PubMed] [Google Scholar]
- 88.Preuss E, Hugle M, Reimann R, Schlecht M, Fulda S. Pan-mammalian target of rapamycin (mTOR) inhibitor AZD8055 primes rhabdomyosarcoma cells for ABT-737-induced apoptosis by down-regulating Mcl-1 protein. J Biol Chem. 2013;288:35287–35296. doi: 10.1074/jbc.M113.495986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Faber AC, Coffee EM, Costa C, Dastur A, Ebi H, Hata AN, Yeo AT, Edelman EJ, Song Y, Tam AT, Boisvert JL, Milano RJ, Roper J, Kodack DP, Jain RK, Corcoran RB, Rivera MN, Ramaswamy S, Hung KE, Benes CH, Engelman JA. mTOR inhibition specifically sensitizes colorectal cancers with KRAS or BRAF mutations to BCL-2/BCL-XL inhibition by suppressing MCL-1. Cancer discovery. 2014;4:42–52. doi: 10.1158/2159-8290.CD-13-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, Golub TR, Armstrong SA. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, Trojahn U, Wendel HG, Charest A, Bronson RT, Kogan SC, Nadon R, Housman DE, Lowe SW, Pelletier J. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahmani M, Nguyen TK, Dent P, Grant S. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mol Pharmacol. 2007;72:788–795. doi: 10.1124/mol.106.033308. [DOI] [PubMed] [Google Scholar]
- 93.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 94.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, Adjei AA. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 95.Pareja F, Macleod D, Shu C, Crary JF, Canoll PD, Ross AH, Siegelin MD. PI3K and Bcl-2 inhibition primes glioblastoma cells to apoptosis through downregulation of Mcl-1 and Phospho-BAD. Molecular cancer research : MCR. 2014;12:987–1001. doi: 10.1158/1541-7786.MCR-13-0650. [DOI] [PubMed] [Google Scholar]
- 96.Koss B, Morrison J, Perciavalle RM, Singh H, Rehg JE, Williams RT, Opferman JT. Requirement for antiapoptotic MCL-1 in the survival of BCR-ABL B-lineage acute lymphoblastic leukemia. Blood. 2013;122:1587–1598. doi: 10.1182/blood-2012-06-440230. [DOI] [PMC free article] [PubMed] [Google Scholar]