Summary
Apixaban, a non-vitamin K antagonist oral anticoagulants, is a Factor Xa inhibitor that is prescribed for the treatment of non valvular atrial fibrillation. Rectus sheath hematoma is a rare but significant complication of oral anticoagulant treatment. The important causes of rectus sheath hematoma include treatment with anticoagulants, hematologic diseases, trauma, intense physical activity, coughing, sneezing and pregnancy. In this report, we describe case of a 71-year-old woman undergoing apixaban treatment for non valvular atrial fibrillation who presented with spontaneous rectus sheath hematoma.
Keywords: Apixaban, oral anticoagulant, rectus sheath hematoma
1. Introduction
In recent years, new oral anticoagulants are used as the alternatives of warfarin in non-valvular atrial fibrillation (1). Apixaban is a directly absorbed, competitive Factor Xa inhibitor with an extremely fast-onset action profile. An additional advantage to the use of apixaban is that it is not reported to adversely interact with any known drug. When compared to warfarin, therapeutic intervention with apixaban (2 × 5 mg dose) has been found to be significantly more efficacious in the treatment of non valvular atrial fibrillation; use of apixaban is strongly associated with a marked decrease in the incidence of stroke, formation of systemic emboli, major bleeding, intracranial bleeding, death and common end (2). Unfortunately, unlike warfarin, apixaban has no known antidote available for therapeutic use hence bleeding, following apixaban treatment, is considered as a major complication and is often difficult to manage. Formation of rectus sheath hematoma is one such severe complication that requires a close follow up as it is associated with a high risk of mortality.
In this communication, we have presented a unique case regarding spontaneous development of rectus sheath hematoma following apixaban treatment. To our knowledge such an occurrence has not been previously reported in literature.
2. Case report
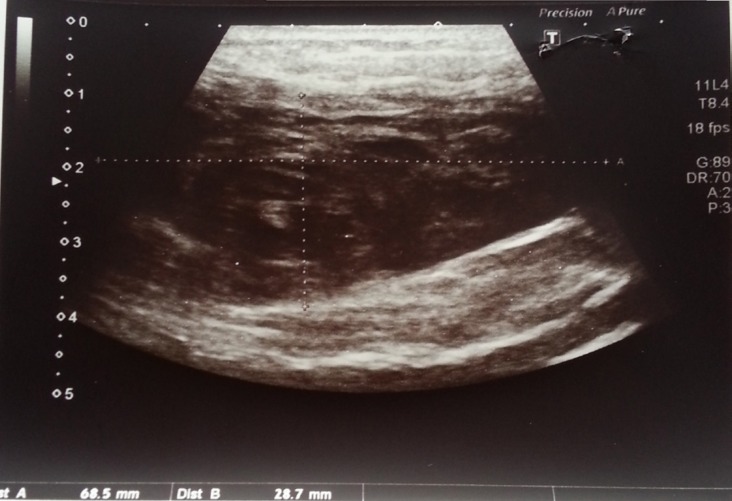
A 71-year-old female patient with a history of atrial fibrillation and coronary artery disease was referred to the cardiology clinic following complaints of abdominal pain and dyspnea for the last 24 hours. It was also noted that she had been suffering from a cough for the past one week as a result of an upper respiratory tract infection. The patient's blood pressure was recorded as 100/60 mmHg, heart rate was arrhythmic at 94 beatsmin. The patient's body weight is 70 kg. On physical examination, it was noticed that she had a tender, hard, colorless soft tissue swelling located on the left side of the umbilicus. Laboratory evaluation revealed mild anemia (Hb: 11.5 mg/dL) as well as renal function impairment [blood urea: 79.6 mg/dL, creatinine: 1.83 mg/dL, predicted Glomerular Filtration Rate (GFR): 33 mL/min]. Results of other laboratory tests were within normal acceptable limits. As a result of the patient's impaired renal function, computed tomography with contrast was ruled out as a diagnostic option and it was decided to employ emergent abdominal ultrasonography (USG) instead. USG revealed the presence of a left sided rectus sheath hematoma measuring 11 × 7.5 × 3 cm in size (Figure 1).
Figure 1.
Left sided rectus sheath hematoma on abdominal USG.
As part of a treatment module for her non valvular atrial fibrillation, the patient had been using apixaban at a dose of 10 mg/day (2 × 5 mg) for the past three months. She had no history of trauma, had not undergone any surgical procedure and had not been prescribed any other drug that could account for the bleeding and hematoma formation. For relieving symptoms of upper respiratory tract infection the patient was taking suitable antibiotics and drugs. Her daily drug schedule was as follows: diltiazem 120 mg b.i.d., digoxin 0.125 mg/day and furosemide 40 mg/day. Her CHADS-VASc score was 3 (Age one point, Coronary arter disease one point and Female one point) with a HASBLED score of 2.
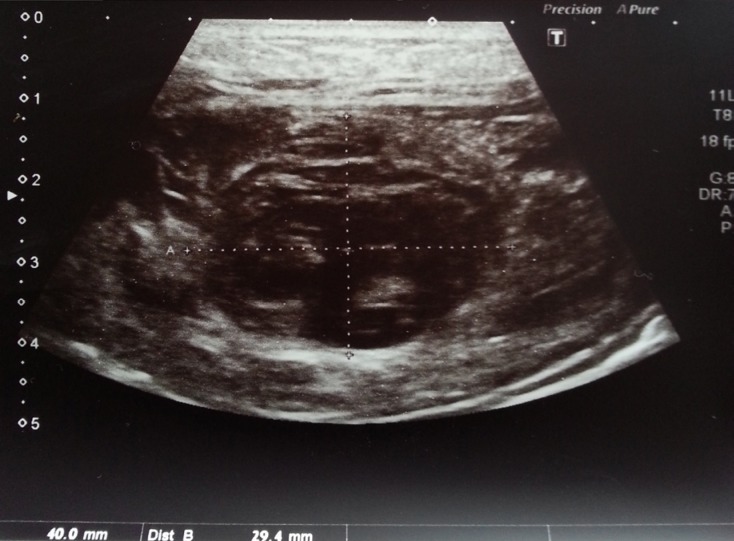
The patient was moved to intensive care unit (ICU) and apixaban treatment was instantly stopped. Intravenous fluid resuscitation was commenced and vital signs such as blood pressure, heart rate, respiratory rate and pulse oxygen saturation were constantly monitored. Hemoglobin values were regularly controlled and showed no significant decrease during the course of follow up analysis. Two days following intervention, the abdominal USG revealed regression of the rectus sheath hematoma (size: 4 × 3 cm) (Figure 2). Apixaban treatment was reinstated 5 days later with a reduced dose of 5 mg/day (2 × 2.5 mg). The patient was discharged from the hospital and asked to revisit for follow-up after a period of one week. A week later she underwent routine examination at the clinic but no medical complaint, symptom of hematoma or any other complication was detected.
Figure 2.
Regression of size of the hematoma on abdominal USG in the follow-up.
3. Discussion
Unlike warfarin, treatment with apixaban does not necessitate an INR follow up. This is one of the primary reasons for the increased use of apixaban and other novel oral anticoagulants in the treatment of non valvular atrial fibrillation. A severe drawback associated with this issue is the increased incidence of bleeding related complications. The situation is further complicated by the absence of specific antidotes to these oral anticoagulants; this makes management of above mentioned bleeding complications extremely difficult because only follow up supportive treatment can be offered to the patient (3). The half life of apixaban is 12 hours and about 25% of the drug is renally excreted (4). For this reason supportive treatment and follow up of apixaban treated patients is very crucial. In our case, the patient had been receiving apixaban (daily dose: 2 × 5 mg) for treatment of non valvular AF for a period of 3 months.
Rectus sheath hematoma is a rare but significant complication of oral anticoagulant treatment. The important etiological causes of rectus sheath hematoma include anticoagulant treatment, hematologic diseases, trauma, intense physical activity, coughing, sneezing and pregnancy (5). Older patients, because of their deteriorating physical status, are especially vulnerable to rectus sheath weakness. Under such conditions, it is imperative to diagnose early, immediately evaluate and begin treatment so as to prevent the occurrence of severe complications including hemodynamic instability, abdominal compartment syndrome, multi-organ dysfunction and even death. The main strategy for treatment is discontinuation of the responsible drug, regular hemoglobin follow up, fluid replacement and blood transfusion as and when required. Our patient was predisposed to the formation of rectus sheath hematoma as a result of her advanced age and her week-long cough attack. Upon diagnosis we immediately terminated apixaban treatment and commenced fluid replacement. In the ICU, hemoglobin controls were obtained and vital signs were rigorously monitored. In our case no blood transfusion was required as the hemoglobin values were stable. Control USG revealed that the hematoma was decreasing in size as a result of the steps outlined above.
Factors that increase the tendency to bleed while undergoing apixaban treatment are well documented in literature. Some of the most important risk factors include advanced age, a history of bleeding, stroke or transient ischemic attack, diabetes, decreased creatinine clearance and drug use (6). Although it has been previously reported that with conventional oral anticoagulant use, there is a decreased incidence of bleeding among patients with mild renal impairment (50–80 mL/min), this is not applicable for patients suffering from moderate and severe renal impairment (GFR < 50 mL/min) (7). In our case the patient suffered from renal function impairment (GFR: 33 mL/min) and this, in our opinion, might have increased the risk of bleeding. Reduced apixaban doses (2 × 2.5 mg/day) are suggested in patients with two or more of the following criteria: The age being ≥ 80 years, the body weight being ≤ 60 kg, or the serum creatinine level being ≥ 1.5 mg/dL (2). In our case the patient had one criteria (the serum creatinine level was ≥ 1.5 mg/dL). However, it is well known that the risk of hemorrhage increases in patients with renal dysfunction (6,7). In addition, the concomitant use of apixaban and strong dual inhibitors of P-gp and CYP3A4 are not recommended, or the dose must be reduced (8). Diltiazem is a moderate inhibitor of dual P-gp and CYP3A4, and increases plasma concentration of apixaban (9). In our case the patient had renal dysfunction, major/mortal bleeding like rectus sheath hematoma, and was receiving concomitant diltiazem therapy, therefore we reduced the apixaban dosage (2 × 2.5 mg/day).
Drug interaction with apixaban treatment is a rare phenomenon but a recent study has reported that ketoconazole and diltiazem are observed to increase plasma concentration of apixaban (9). In direct relation to the aforementioned findings, our patient was undergoing diltiazem therapy along with apixaban and this drug interaction might have impacted the tendency to bleed.
In literature there are documented cases that report the formation of muscle hematomas following anticoagulant or anti-platelet therapy (10). To our knowledge, only two reports that associated the formation of rectus sheath hematoma with use of the new oral anticoagulant rivaroxaban are known (3,11). Our case report is the first of its kind to highlight the association between apixaban treatment and formation of rectus sheath hematoma.
In conclusion, patients on apixaban therapy who complain of abdominal pain must always be evaluated for formation of rectus sheath hematoma. Upon diagnosis appropriate treatment modalities must be immediately planned and executed.
References
- 1. Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013; 15:625-651. [DOI] [PubMed] [Google Scholar]
- 2. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981-992. [DOI] [PubMed] [Google Scholar]
- 3. Kocayigit I, Can Y, Sahinkus S, Aydin E, Vatan MB, Kilic H, Gunduz H. Spontaneous rectus sheath hematoma during rivaroxaban therapy. Indian J Pharmacol. 2014; 46:339-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009; 37:74-81. [DOI] [PubMed] [Google Scholar]
- 5. Cherry WB, Mueller PS. Rectus sheath hematoma: Review of 126 cases at a single institution. Medicine (Baltimore). 2006; 85:105-110. [DOI] [PubMed] [Google Scholar]
- 6. Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, Huber K, Jansky P, Steg PG, Hanna M, Thomas L, Wallentin L, Granger CB. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol. 2014; 63:2141-2147. [DOI] [PubMed] [Google Scholar]
- 7. Pathak R, Pandit A, Karmacharya P, Aryal MR, Ghimire S, Poudel DR, Shamoun FE. Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol. 2015; 115:323-327. [DOI] [PubMed] [Google Scholar]
- 8. Hellwig T, Gulseth M. Pharmacokinetic and pharmacodynamic drug interactions with new oral anticoagulants: What do they mean for patients with atrial fibrillation? Ann Pharmacother. 2013; 47:1478-1487. [DOI] [PubMed] [Google Scholar]
- 9. Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA, Zhang D, Yu Z, Dias C, Shenker A, LaCreta F. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015; 79:838-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cakar MA, Kocayigit I, Aydin E, Demirci H, Gunduz H. Clopidogrel-induced spontaneous pectoral hematoma. Indian J Pharmacol. 2012; 44:526-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tas Tuna A, Palabiyik O, Beyaz SG. Retracted: Spontaneous rectus sheath haematoma associated with rivaroxaban treatment. J Clin Pharm Ther. 2015; 40:486-488. [DOI] [PubMed] [Google Scholar]