Summary
Angiogenic factors have been demonstrated to play important roles in modulating angiogenesis of solid tumors. Recently, accumulating studies extensively indicated that some angiogenic factors widely exist in malignant cells of hematologic malignancy, which regulated the expression of a number of genes that were involved in abnormal proliferation, differentiation and apoptosis of these cells. With deep research of angiogenic factors, its expression, function and regulatory mechanism were gradually elucidated, and some of them were related to the development and prognosis of leukemia, or provide more possible strategies for treatment of patients with leukemia. Herein, we summarize the progress in study of some important angiogenic factors and hematological malignancies.
Keywords: Angiogenesis, angiogenic factors, leukemia
1. Introduction
Angiogenesis, new blood vessel formation, is fundamental to tumor progression and metastasis, which has been documented for solid tumors. In addition, it is also proved that the induction of new blood vessel formation is a dependent factor of hematological malignancies (1). For example, it was first reported by Perez-Atayde et al. in 1997 that the angiogenesis phenomenon existed in the bone marrow of childhood acute lymphoblastic leukemia (ALL) (2). Much subsequent research also discovered that a number of hematological malignancies were accompanied with angiogenesis which was related to the prognosis of childhood ALL or made a contribution to the development and progression of chronic lymphocytic leukemia (CLL) (3,4). Usually, angiogenesis is regulated by a balance of angiogenic and antiangiogenic cytokines, and angiogenesis can be induced by leukemia cells in the bone marrow, and leukemia may be more likely to be angiogenesis dependent, which raises the probability for antiangiogenic drugs in the treatment of leukemia. For example, some evidence indicated that several antiangiogenic drugs, such as targeting vascular endothelial growth factor (VEGF) or its receptors, are able to treat patients with cancer (5). Moreover inhibition of VEGF only is not as effective as first believed. Therefore, it is necessary to develop more effective targets for treatment of patients with leukemia (6). A lot of angiogenesis-inducing factors so far have been discovered, such as VEGF, fibroblast growth factor (FGF), angiogenin (Ang), hypoxia-inducible factor 1 (HIF-1), matrix metalloproteinase (MMP), c-myc gene, endothelin (ET), transforming growth factor (TGF), tumor necrosis factor (TNF), interleukin (IL), integrin, hepatocyte growth factor (HGF), placenta growth factor (PIGF) and so on. By way of combination with their ligand-receptors, these growth factors can promote the division of vascular endothelial cells and induce formation of new vessels, which will provide favorable conditions for the occurrence and progression of the tumors. In addition to the above-mentioned relationship between angiogenic factors and angiogenesis in patients with leukemia, their roles in modulating proliferation, differentiation and apoptosis were also gradually elucidated. This review will briefly introduce roles of some of these angiogenic factors in development of leukemias.
2. The role of VEGF involved in leukemia
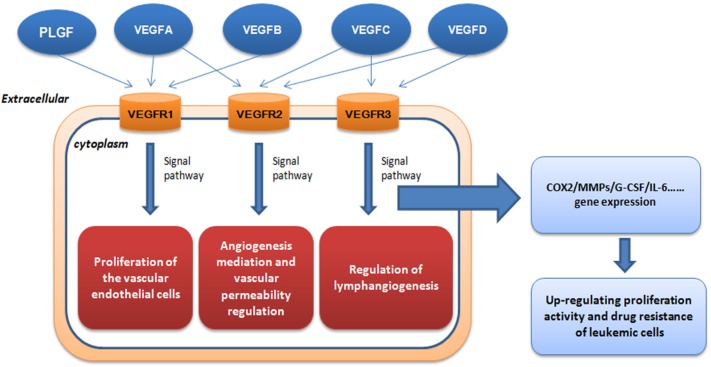
More than 40 molecules that play an important role in blood vessel recruitment have been identified, especially the roles of VEGF and its receptors (3). VEGF has at least six isoforms (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and PlGF) and all the isoforms are secreted as dimeric glycoproteins (7). First of all, VEGF becomes the most well characterized proangiogenic factor, and it was first purfied from the in vitro culture medium of bovine pituitary folliculo-stellate cells by Ferrara et al (8). Named after its mitogenic activity for vascular endothelial cells, VEGF is thought to be the highly specific co-mitogen for vascular endothelial cells and promoting factor of vascular permeability (9). As is shown in Figure 1, VEGF can act on vascular endothelial cells with high efficiency and specificity promote endangium regeneration and increase vascular permeability via three tyrosine kinase receptors (TKR): VEGFR-1 (also known as Flt-1), VEGFR-2 (also known as KDR/Flk-1) and VEGFR-3 (also known as Flt4) (10). It has been discovered that VEGF-2 plays a major role in VEGF-induced angiogenesis, and the binding between VEGF and receptor Flk-1 can activate the mitogen-activated protein kinase (MAPK) system via protein kinase C (PKC) or Ras protein to induce proliferation of vascular endothelial cells (11). It has been proved so far that VEGF is the only specific growth factor for angiogenesis, while other growth factors such as FGF and PDGF can act on various kinds of cells in addition to vascular endothelial cells with low specificity (12). VEGF can regulate development of hemopoietic stem cells, remodeling of extracellular matrix and regeneration of inflammatory cytokines. Furthermore, it seems that activation of VEGFR-2 plays a necessary and sufficient role mediating VEGF-dependent angiogenesis and induction of vascular permeability. In addition, VEGFR-1 and VEGFR-2 were predominantly expressed on vascular endothelial cells (13), and VEGFR-1 was expressed on other diverse cells, including hematopoietic stem cells (HSCs), vascular smooth muscle cells, monocytes and leukemia cells (14). However, VEGFR-2 is chiefly expressed on endothelial progenitor cells and megakaryocytes (15). Regulation of lymphangiogenesis was mainly dependent on binding of VEGF homologs VEGF-C, VEGF-D and VEGFR-3, which is largely restricted to lymphatic endothelial cells (6). In addition, expression of VEGF-D was found to be strongly expressed both in non-HL (NHL) and HL by HRS cells in line with a high number of tumor microvessels, suggesting a role for this cytokine in angiogenesis (16).
Figure 1.
The role of VEGF in leukemia progression. VEGF can act on vascular endothelial cells with high efficiency and specificity to promote endangium regeneration and increase vascular permeability via three tyrosine kinase receptors (TKR).
The induction of tumor angiogenesis by an angiogenic switch has become a characteristic of cancer (17). As the angiogenesis switch of tumors, VEGF can markedly increase vascular permeability. Many leukemic cell strains and primary cells can synthesize and secrete VEGF (18), which can modulate the malignant biological behavior of leukemic cells by two positive-feedback loops: paracrine and autocrine. VEGF secreted by leukemic cells interacts with the relevant receptors on the endothelial cell surface, and the endothelial cells produce growth factors such as G-CSF that acts on the leukemic cells in return to increase their proliferation activity and drug resistance, or directly acts on relevant receptors on the autologous cell surface to increase autologous proliferation activity and drug resistance (19). The functional interaction between VEGF-A and formyl peptide receptor-like 1 (FPRL1) mediated by secretion of connective tissue growth factor (GTGF) was demonstrated by some scientists (20). CTGF activates the downstream signals of FPRL1 by binding to FPRL1 directly, such as increase in intracellular Ca2+ concentration and extracellular signal-regulated kinase (ERK) phosphorylation. In recent studies, VEGF is thought to be closely related to MMPs (21), which showed the complexity of VEGF-mediated regulation of MMP-9 expression. In the other reports, it is demonstrated that repeated social defeat stress (RSDS) made a contribution to cancer angiogenesis and metastasis, which was partially associated with increased secretion of VEGF (22). The combination has the possibility to be used in clinical trials if it can be considered as a possible therapeutic option when conducting suitable further investigations.
Emerging evidence suggests that it is also involved in proliferation, abnormal differentiation, prognosis and treatment of acute myeloid leukemia (AML) (23,24). The upregulation of bone marrow microvessel density and the levels of plasma pro-angiogenic cytokines also support this above-mentioned theory (6). Simultaneously, the representative cytokines, VEGFs and their receptors are expressed on AML blasts in vascular and osteoblast niches in both the BM and the peripheral circulation (25). In the present review, we just focus on the discussion of VEGF as a therapy target for leukemia. Interestingly, as an anti-angiogenic therapeutic approach, it is becoming more and more effective and promising by using immunomodulatory drugs, for example, anti-VEGF monoclonal antibodies, VEGFR inhibitor and Histone deacetylase inhibitors (26,27).
Hence, anti-angiogenesis therapy based on the principle of inhibiting the physiological function of VEGF has become the hotspot of oncotherapies. For example, Ilorasertib (ABT-348) is a novel inhibitor of aurora kinase, and could inhibit biomarkers for aurora kinase and VEGF receptors (28). Heparan sulfate d-glucosaminyl 3-O-sulfotransferase-3B1 (HS3ST3B1) can promote angiogenesis and proliferation by induction of VEGF in AML cells. It was positively contributed to AML progression, and these activities were associated with an induction of proangiogenic factor VEGF expression and shedding (29). Ginsenoside Rg3 has been not only used in anti-angiogenic therapy of solid tumors, but also exhibits an anti-leukemia effect in part due to its anti-angiogenic activity via inhibiting PI3K/Akt and ERK1/2 pathways, which act to regulate expression of HIF-1α and VEGF (30). Histone deacetylase (HDAC) inhibitors have been reported to inhibit tumor angiogenesis via downregulation of angiogenic factors (31). The mechanism underlying VPA induced antiangiogenesis is associated with suppression of VEGF and its receptors (31). Furthermore, the combined use of histone deacetylase inhibitor VPA, all-trans retinoic acid (ATRA), and deoxyribonucleic acid polymerase-α inhibitor cytarabine (Ara-C) is now considered for disease-stabilizing treatment of AML, which was related to antiproliferative effects, and modulation of release of angiogenic mediator of endothelial cells respectively (32). Foretinib is a multiple kinase inhibitor undergoing clinical trials and it could suppress activity of VEGFR-2 (33). It could be able to inhibit VEGF-A, VEGF-C and Angiopoetin-2 stimulated tube formation by reducing VEGFR-2, VEGFR-3 and TIE-2 activation (34). However, although VEGF receptor inhibition is one potential mechanism by which AML could be treated, the receptor tyrosine kinase inhibitor AZD2171, against VEGF receptors KDR and FLT-1, could not get confirmed responses in other groups (35). Understanding the intricate cellular components of the bone marrow microenvironment can lead to discovery of novel extrinsic factors that are responsible for initiation and progression of leukemic disease. Activation of endothelial cells (ECs) by VEGF-A provides cues that enable leukemic cells to proliferate at higher rates and also increases adhesion of leukemia to ECs. Development of drugs that target the activation state of the vascular niche could prove to be an effective adjuvant therapy in combination with chemotherapeutic agents (36). On the other hand, high-level expression of VEGF-C is associated with chemoresistance and adverse prognosis in AML. VEGF-C induces COX-2-mediated resistance to chemotherapy through induction of ET-1 expression. Acting as a key regulator in the VEGF-C/COX-2 axis, ET-1 represents a potential target for ameliorating resistance to chemotherapy in AML patients (37). Lenalidomide is an IMID immunomodulatory agent clinically active in patients with CLL, the anti-CLL effect of lenalidomide is mediated through the alteration of microenvironmental elements, implying the modulation of several angiogenesis-related factors and disruption of CLL crosstalk with endothelial cells (38). Indeed, the leukemic environment is highly enriched with lymphangiogenic stimuli, and that inhibition of VEGFR-3 restored function of NK cells, providing therapeutic value of modulation of NK cells by blocking VEGFR-3 and provided a possibility of advanced therapeutic approaches using immune cells against myelogenous leukemia (39). Understanding the functional characterization of lymphangiogenic factors in the BM niche in AML will also be helpful in interrupting the engraftment of leukemic stem cells and enhancing immune cell function by modulating the tumor microenvironment (25).
3. C-myc as an important tumor angiogenesis factor in leukemia
The c-myc gene is a member of the Myc proto-oncogene family, and it is a pleiotropic transcription regulator. It can induce the instability of the genome and affect the occurrence and progression of tumors directly or indirectly, so it plays an important role in cell proliferation and programmed cell death (40). Binding DNA in a sequence-specific manner and having a basic helix-loop-helix leucine zipper transcription factor can be attributed to c-myc (41). Heterodimerizing with corresponding protein partner, MAX, binding the enhancer box (E-box) sequence and stimulating transcription of some corresponding downstream genes provide support for the biological activities of c-myc (42). With E-box binding sites, these genes include ODC, ECA39, eIF4E, CDC25, CAD, CDK4, eIF4G1, hTert, and CCND1 which belong to the direct target genes of c-myc (43).
In recent years, it have been discovered that c-myc also acts as an important tumor angiogenesis factor (TAF) and participates in tumor angiogenesis, and c-myc was related to the expression of VEGF (44). There is a c-myc-binding region located at the 271bp of the VEGF promoter. This region can bind c-myc after the directed mutagenesis of the VEGF promoter and induce VEGF expression in hypoxic conditions to promote angiogenesis and tumorigenesis. On the other hand, some other reports also elucidated the role of c-myc in modulating angiogenesis. For example, tumor angiogenesis could be promoted by miRNA-induced c-myc activation (45), and IL-1β is the effector of the c-myc-induced angiogenesis initiation (46).
Moreover, c-myc is highly expressed in HL-60 leukemic cells and is related to proliferation and differentiation of the leukemic cells. The c-myc gene can be abnormally activated by amplification activation or gene rearrangement, and its expression level is related to the cell growth state and highly expressed in the undifferentiated stage. It is an interesting target for novel drug therapies because of its oncogenic activities and overexpression in a majority of human cancers (47). Binding inhibition of c-myc on gene promoters by some small molecule inhibitors has been tried (48,49), and found that some of them were effective in disrupting essential protein-DNA interactions, for example, Pyrrole-imidazole (PI) polyamides, specific sequence DNA-binding small molecules (50–52), and can inhibit a part of E-box-mediated c-myc downstream gene expression (47). EGFR variant III (EGFRvIII), which strongly induces neovascularization in tumors, could induce Angptl4 expression through the ERK/c-myc pathway. Therefore, ERK/c-myc, EGFRvIII or Angptl4 may be a possible therapeutic target (53). Tumor-associated macrophages (TAMs) can also express c-myc, which can regulate their phenotype and pro-tumor activities in vivo. Targeting c-myc function in TAMs may control tumor growth (54,55). As discovered in the above-mentioned reports, c-myc has become an ideal target of gene therapies for tumors or leukemias.
4. MMPs family in hematologic malignancies are gradually elucidated
MMPs are a series of zinc-finger-dependent proteinases with high homology (56). There are three common domains of the MMPs: pro-peptide, catalytic domain and hemopexin-like C-terminal domain. According to the distinct specificities of their amino acid sequences and substrates, they can be classified into five types: collagenases (MMP-1, MMP-8, MMP-13, and MMP-18), gelatinases (MMP-2, MMP-9), stromelysins (MMP-3, MMP-10 and MMP-11), matrilysins (MMP-7, MMP-26), and other types. MMPs can degrade the extracellular matrix which is thought to be the start signal and important pathway of angiogenesis, invasion and metastasis of tumors.
Some studies have reported that MMPs expression was related to metastatic potential of various human tumors, and plays an essential part during the development of leuko-diapedesis (57) and disseminated intravascular coagulation (DIC) (58). As to the modulation of MMPs, there are some important transcription factors, such as activator protein-1 (AP-1), Ets transcription factors PEA3, nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) and signal transducer and activator of transcription (STAT) families, which are involved in the regulation of MMPs expression, and many growth factors enhance the levels of transcriptional regulation of VEGF (59). The enhancement of the invasive properties of human tumor cells was also related to the low expression of tissue inhibitors of matrix metalloproteinases (TIMP) (60), including TIMP-1,-2, -3 and -4. In addition, the expression of TIMP levels could directly affect the activity level of MMPs (61). Constitutive release of several MMPs and TIMPs can be secreted by primary human AML cells and affect the behavior of leukemia cells. As for clinical use of MMPs and TIMPs, this must be better clarified before we use such therapeutic agents in the treatment of human AML (62). The amount of ECM molecules including type IV, V and XI collagens, laminin and aggrecan core protein will also be digested by MMPs and was related to the angiogenesis and metastasis of tumors (63), for example, MMP-1 as a negative regulatory factor in angiogenesis, growth and metastasis of tumors (64), MMP-13 promoting secretion of VEGF and inducing tumor angiogenesis in vivo (65), and important roles of stromelysin members MMP-3, MMP-10 and MMP-11 in activation of proMMPs or activation of tumor cells (66). Due to lack of a hemopexin domain, matrilysins (MMP-7 and -26) can be considered as MMPs, and it has been discovered that MMP-7 not only plays an important role in the degradation of the extracellular matrix proteins, but also plays an equally important role in protein activation, degradation, abscission and other biochemical processes of non-extracellular matrix proteins, which is essential for tumor growth and tumor angiogenesis (67). The role of MMP-26 is different from most other MMPs and needs further investigation (68). Meanwhile, MMPs are usually overly expressed in tumors, and overexpression of the gelatinases including MMP-2 and MMP-9 is always accompanied by growth, metastasis and angiogenesis of the tumors, with different roles concerning collagenases, for example MMP-2 targeting collagens I,II and III, but not MMP-9 (63).
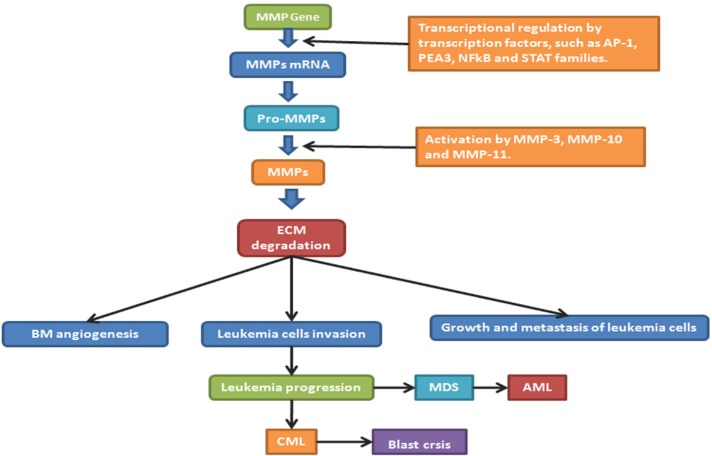
Reports about the functions of MMP-2 and MMP-9 in hematologic malignancies are gradually increasing, and it is thought they are closely related to VEGF (69). Serum MMP-9 not only can be likely to predict clinical outcome of patients with early CLL but also can help to refine the prognosis of CLL (70). In MDS, MMPs expression may produce a useful tool for diagnosis, prognosis and a possible target for clinical treatment. Similar to chemokines, primary human AML cells usually show constitutive release of several MMPs (71), such as matrix MMP-2 and MMP-9, TIMP1 and VEGF (26). In addition, the proteolytic release of VEGF from tumor matrix by MMPs, predominantly by MMP-9, which was delivered into the tumor microenvironment by tumor-infiltrating leukocytes, plays an important role in modulating proliferation of leukemia cells (72). Moreover, as for a mutually coordinated manner on a transcriptional level, it can regulate expression and secretion of VEGF and MMPs. According to a common master regulator, a hypoxia-inducible factor HIF-1 was closely related to expression of both VEGF and MMP-9 (73), and VEGF could significantly reduce MMP- 9 production in B-cell leukemia cells (21). MMPs blockade can completely inhibit VEGF production and significantly reduce the volume of angiogenic vasculature (22). It has been confirmed in some research that activation of MMP-2 is dependent on metallopeptidase inhibitor 2 (TIMP-2) and membrane type-1 matrix metalloproteinase (MT1-MMP). TIMP-2 is the activity inhibitor of MMP-2, whereas, when the concentration of TIMP-2 increases to a certain level, it will combine with MT1-MMP and activate MMP-2, which will give rise to a succession of tumor activation (66). Figure 2 shows the role of MMPs and its inhibitors in the progression of hematological malignancies such as MDS, AML and chronic myeloid leukemia (CML) (74). Because of the role of MMP in development of leukemias, specific MMP inhibitors are currently not only being developed but also being considered for cancer therapy. However, there is nothing harvested in passing clinical trials so far (75). More attention should be paid on targeting of MMPs as an antileukemic strategy due to the importantance in future clinical development of this therapeutic strategy (71). Therefore, in multiple ways, MMPs directly and indirectly influence the VEGF-mediated development of an angiogenic vasculature.
Figure 2.
The regulation of MMPs and their roles in leukemia progression. AP-1: activator protein-1; NFkB: nuclear factor kappa-light-chain-enhancer of activated B cells; STAT: signal transducer and activator of transcription; BM: bone marrow; ECM: extra cellular matrix; GF: growth factor; EGFR: epidermal growth factor; MDS: myelodysplastic syndrome; AML: acute myeloid leukemia; CML: chronic myeloid leukemia.
5. VEGF-related HIF-1 involved in hematological malignancy
HIF-1 was first discovered as a hypoxia-inducible nuclear factor connected to the hypoxia response element (HRE) of the erythropoietin (EPO) gene. It is an oxygen-sensitive transcription activator that can activate the expression of many hypoxia response genes. In hypoxia HIF-1α becomes stable and can interact with the auxiliary co-stimulatory factor CBP/P300 to regulate activity of oncogenes (76). Nuclear translocation of HIF-1a is in need of the participation of its innate PAS domain and C-terminal nuclear transport signal, but how these sequences interact with oxygen-sensitive signals in detail still needs further clarification. HIF-1 could be negatively regulated by inhibitory PAS domain protein (IPAS), of which the pro-apoptotic activity is by way of binding to pro-survival Bcl-2 family proteins (77). To date it has been confirmed that in aerobic conditions the conserved proline residue in HIF-1α can be hydroxylated so that it can be recognized by the E3 ubiquitin ligase complex containing the tumor suppressor gene product VHL protein and undergo further proteasomal degradation (78). HIF-1α not only stimulates Ang production but also regulates the expression level of the Ang receptor; In addition, HIF-1α also plays an important role in the metabolism of the extracellular matrix. HIF-α has various other effects after activation such as causing adaptive changes in cell metabolism, shifting aerobic metabolism to glycolysis-centered anaerobic metabolism and meanwhile stimulating renal cells to produce EPO which will act as the main regulator inducing angiogenesis in malignant tumors (79). HIF-1α can also affect angiogenesis and promote the growth and development of tumor cells by enhancing the expression of downstream target genes including VEGF (80).
It has been discovered in some research that with accumulation of HIF-1α in myeloid leukemic cells and normal hemotologic stem cells the simulacrum of hypoxia or anoxia will lead to cell differentiation (81). The Guoqiang Chen research group has discovered that hypoxia can lead to differentiation of leukemic cells accompanied by accumulation of the hypoxia inducible factor HIF-1α, and thereafter, that hypoxia can induce differentiation and maturation of leukemic cells into neutrophils (82). In addition, HIF-1α protein promotes tumor growth, angiogenesis, and metastasis (83), and it could be increased by hypoxia in primary human AML cells. Similarly, low HIF-1α expression and also release of several proangiogenic cytokines by leukemia cells can be induced by low oxygen concentration (84). It is reported in some research that HIF-1α protein can motivate differentiation of AML cells through an independent transcription mechanism, inhibiting AML progress (85). Studies showed that HIF-1a suppresses the expression of miR-17 and miR-20a, two miRNAs that can alleviate HIF-1α-induced differentiation and hypoxia of AML cells, by downregulating c-Myc expression (86). Moreover, miR-17 and miR-20a immediately inhibit p21 and STAT3 expression (86). Some observations indicated that HIF-1α, which plays an important role in the survival of ALL blasts, may be conductive to chemoresistance. It is proposed that targeting HIF-1α itself (87), its upstream mediators such as mTOR, or the glycolytic pathways will improve therapeutic efficacy in ALL (88).
6. Angiogenin (Ang) family related to leukemogenesis
Ang is the secreted type of the angiogenic factors and the angiogenin family includes Ang-1, Ang-2, Ang-3, Ang-4 and the Ang-like proteins. These family members have similar structures including secreted protein signal sequence, coiled-coil domain and fibrinogen-like domain. The angiogenin promoter contains the binding site for TCF/LEF, and TCF/LEF is a transctription factor with bilateral regulatory effects which acts as the intermediate medium of the Wnt/β-catenin signaling pathway (89). Ang-1 can support remodeling and maturation of vascular endothelial cells, and promote establishment of local collateral circulation. Furthermore, depending on an attributed anti-inflammatory role, Ang1 not only can promote pericyte-dependent vessel integrity but also can support a Tie2-constitutively activated state. As a result of this, Ang-1 is able to modulate the quiescent vascular endothelium (90). Acting in a mutually complementary manner in angiogenesis, VEGF mainly functions in early stages and Ang-1 mainly functions in later stages. Although Ang-1 doesn't take part in initiation of angiogenesis, it can maintain stability of the vessels and promote maturation of the newly formed vessels. Ang1 and Ang-2 are widely different in their biological roles although they are homologous (91). As the antagonist of Ang-1, on the one hand, during vasculogenesis and inflammation Ang-2 can be strongly released. On the other hand, Ang-2 can be considered as an angiogenic inhibitor that is involved in destabilization and vascular remodelling (90,92). Ang-2 is related to angiogenesis initiation and extention and affects cell growth and metastasis. With high Ang-2 levels, there is an associated upregulation of VEGF (93). Ang-2 doesn't directly stimulate angiogenesis, but in the presence of VEGF, Ang-2 can inhibit the stabilizing effects of Ang-1 on the vessels, eliminate restriction on angiogenesis caused by the basal membrane and peripheral stromal cells and enhance sensitivity of endothelial cells to growth factors, so that angiogenesis in tumors is promoted (94).
This is an important reason that tumor initiation and development contribute to the increasing levels of Ang in malignancies (95). Ang upregulation was investigated in AML, myelodysplastic syndrome (MS), and a variety of other malignancies (96). CLL patients may have a negative impact on their disease course with a notable increase of Ang-2 ligand expression (97), which is accompanied with an aberrant vascularization in (bone marrow) BM sections (98). Recent epigenetic evidence showed that Ang-2 displays lower DNA methylation in CLL and correlates with poor prognosis and shorter survival (97). Additionally, Binet staging can be linked to elevated levels of Ang-2 (93). It was shown in a series of experiments that Ang-2 regulates the pathophysiology of CLL cells through the Ang-Tie signalling pathways (5). The theory of suggesting that Ang is a possible prognostic marker and potential target of anti-angiogenic therapy was proposed by Pavlov et al., previously (99). Ang-1 expression in BM sections, which is an independent prognostic factor towards overall survival of MDS patients, has potential to be a new biomarker for predicting clinical treatment (100). In human acute myelogenous leukemia (AML) the balance between Ang-1 and the Ang-2 plays an important role for both in leukemogenesis. The release from AML cells, is a major source of Ang-1 in leukemic BM. On the contrary, local Ang-2 is released less commonly (101). Further investigation will make a contribution to act on the angiopoietin system as a possible therapeutic target in AML patients.
7. The relationship between FGF and leukemias
FGF is a kind of mitogen-activated angiogenic factor, which plays an important role in many tumors. FGF is a protein family composed of at least 23 members: FGF1 to FGF23. Acidic FGF (aFGF or FGF1) and basic FGF (bFGF or FGF2) were discovered earliest and also are the most widely studied ones with the most important functions so far. With their respective acidic isoelectric point and alkaline isoelectric point, FGF1 and FGF2 are called acidic fibroblast growth factor and alkaline fibroblast growth factor respectively. They are transported with the same mechanisms that can be enhanced in the presence of heparin. They act on the same receptor but the affinity of aFGF is 30 to 100 times stronger than that of bFGF. It was first reported by Gospodarowicz that the alkaline fibroblast growth factor was extracted and purified from the cerebrum and pituitary with physical and chemical methods (102). During the 80's, the amino acid sequence of bFGF was clarified, and during the 90's, recombinant bFGF was produced by genetic engineering abroad and home successively, which forcefully propelled research concerning bFGF.
Various studies have shown that bFGF can stimulate and regulate proliferation and differentiation of various types of cells stemming from mesoderm and neuroectoderm such as vascular endothelial cells, epithelial cells, myoblasts, osteoblasts and neurogliocytes which play an important role in embryogenesis and tissue healing, and additionally upregulate VEGF expression (103). As a strong mitogenic factor and chemokine of vascular endothelial cells, bFGF is produced by paracrine and autocrine cells, and can combine with different receptors on the surface of vascular endothelial cells including TKR, heparan sulfate proteoglycan and cell adhesion molecules (CAMs) to activate their vasogenic characteristics (104,105). bFGF can also activate the P13K signaling pathway to inhibit apoptosis of vascular endothelial cells and promote angiogenesis (106). As a chemokine, bFGF can attract many kinds of vascular intimal cells by chemotaxis and induce them to produce proteolytic enzymes and collagenase which can promote the proliferation and migration of vascular endothelial cells as well as degrade extracellular matrix proteins to induce angiogenesis (107).
It is reported that upregulation of bFGF can be detected in acute myelocytic leukemia (AML), chronic myelomonocytic leukemia (CML), and CLL, and is correlated with poorer prognosis (108). Through the FGF receptor 3/RAS/c-RAF/mitogen-activated protein kinase pathway, it was indicated that FGF2 is able to promote the growth of both short- and long-term assays (109). At the same time, FGF2 in the marrow was decreased relating to ponatinib, which furthermore suggests that FGF receptor inhibition can interrupt FGF2-mediated resistance (110). In addition, developing combinations of kinase inhibitors that circumvent resistance can provide a potential chance to identify critical ligand-RTK pathways of resistance (110).
8. Perspectives
As angiogenic factors and their functions are more and more deeply understood, targeted therapy aiming at angiogenesis in hematologic malignancies is attached to more and more importance. In targeted therapy based on antibodies against the vessels of hematologic malignancies, it is crucial to detect new specific target molecules of tumor vessels. Targeting to each segment of tumor angiogenesis and relevant regulatory factors, developing angiogenesis inhibitors to restrict tumor growth and metastasis has become a new approach and effective means in oncotherapies. In recent years, combination therapy with multiple targets has provided a brand-new research direction for anti-angiogenesis. Although this research is still confined to basic experimentation and clinical trials without reflection of its value in clinical practice, with the development of basic research and anti-angiogenic agents, the deepening of tumor vascular targeting therapy will open up a new area for treatment of hematologic malignancies.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81101605, 81172792, 81573467), the ‘Twelfth Five-Year’ National Science and Technology Support Program (2013BAI07B02), the Natural Science Foundation of Shandong Province of China (ZR2011HL045, ZR2015YL028, 2015ZRC03102) and the Project for Laureate of Taishan Scholar (NO.ts201511075).
References
- 1. Medinger M, Passweg J. Angiogenesis in myeloproliferative neoplasms, new markers and future directions. Memo. 2014; 7:206-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997; 150:815-821. [PMC free article] [PubMed] [Google Scholar]
- 3. Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014; 26:605-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piechnik A, Dmoszynska A, Omiotek M, Mlak R, Kowal M, Stilgenbauer S, Bullinger L, Giannopoulos K. The VEGF receptor, neuropilin-1, represents a promising novel target for chronic lymphocytic leukemia patients. Int J Cancer. 2013; 133:1489-1496. [DOI] [PubMed] [Google Scholar]
- 5. Aguirre Palma LM, Gehrke I, Kreuzer KA. Angiogenic factors in chronic lymphocytic leukaemia (CLL): Where do we stand? Crit Rev Oncol Hematol. 2015; 93:225-236. [DOI] [PubMed] [Google Scholar]
- 6. Medinger M, Passweg J. Role of tumour angiogenesis in haematological malignancies. Swiss Med Wkly. 2014; 144:w14050. [DOI] [PubMed] [Google Scholar]
- 7. Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001; 280:C1367-1374. [DOI] [PubMed] [Google Scholar]
- 8. Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989; 161:851-858. [DOI] [PubMed] [Google Scholar]
- 9. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219:983-985. [DOI] [PubMed] [Google Scholar]
- 10. de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992; 255:989-991. [DOI] [PubMed] [Google Scholar]
- 11. Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000; 60:5117-5124. [PubMed] [Google Scholar]
- 12. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;4 07:242-248. [DOI] [PubMed] [Google Scholar]
- 13. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9:669-676. [DOI] [PubMed] [Google Scholar]
- 14. Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001; 193:1005-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casella I, Feccia T, Chelucci C, Samoggia P, Castelli G, Guerriero R, Parolini I, Petrucci E, Pelosi E, Morsilli O, Gabbianelli M, Testa U, Peschle C. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood. 2003; 101:1316-1323. [DOI] [PubMed] [Google Scholar]
- 16. Bardelli M, Leucci E, Schürfeld K, Bellan C, Passiatore G, Rocchigiani M, Bartolommei S, Orlandini M, Zagursky J, Lazzi S, De Falco G, Tosi P, Oliviero S, Leoncini L. VEGF-D is expressed in activated lymphoid cells and in tumors of hematopoietic and lymphoid tissues. Leuk Lymphoma. 2007; 48:2014-2021. [DOI] [PubMed] [Google Scholar]
- 17. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100:57-70. [DOI] [PubMed] [Google Scholar]
- 18. Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ, Cairnduff F, Selby PJ, Perren TJ, Lansdown M, Banks RE. Vascular endothelial growth factor (VEGF) in breast cancer: Comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000; 60:2898-2905. [PubMed] [Google Scholar]
- 19. Dias S, Hattori K, Heissig B, Zhu Z, Wu Y, Witte L, Hicklin DJ, Tateno M, Bohlen P, Moore MA, Rafii S. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A. 2001; 98:10857-10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee MS, Ghim J, Kim SJ, Yun YS, Yoo SA, Suh PG, Kim WU, Ryu SH. Functional interaction between CTGF and FPRL1 regulates VEGF-A-induced angiogenesis. Cell Signal. 2015; 27:1439-1448. [DOI] [PubMed] [Google Scholar]
- 21. Woenne EC, Lederle W, Zwick S, Palmowski M, Krell H, Semmler W, Mueller MM, Kiessling F. MMP inhibition blocks fibroblast-dependent skin cancer invasion, reduces vascularization and alters VEGF-A and PDGF-BB expression. Anticancer Res. 2010; 30:703-711. [PubMed] [Google Scholar]
- 22. Wu X, Liu BJ, Ji S, Wu JF, Xu CQ, Du YJ, You XF, Li B, Le JJ, Xu HL, Duan XH, Dong JC. Social defeat stress promotes tumor growth and angiogenesis by upregulating vascular endothelial growth factor/extracellular signal-regulated kinase/matrix metalloproteinase signaling in a mouse model of lung carcinoma. Mol Med Rep. 2015; 12:1405-1412. [DOI] [PubMed] [Google Scholar]
- 23. Haouas H. Angiogenesis and acute myeloid leukemia. Hematology. 2014; 19:311-323. [DOI] [PubMed] [Google Scholar]
- 24. Song G, Li Y, Jiang G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets (Review). Oncol Rep. 2012; 28:1935-1944. [DOI] [PubMed] [Google Scholar]
- 25. Lee JY, Kim HJ. (Lymph)angiogenic influences on hematopoietic cells in acute myeloid leukemia. Exp Mol Med. 2014; 46:e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marinaccio C, Nico B, Maiorano E, Specchia G, Ribatti D. Insights in Hodgkin Lymphoma angiogenesis. Leuk Res. 2014; 38:857-861. [DOI] [PubMed] [Google Scholar]
- 27. Kon Kim T, Gore SD, Zeidan AM. Epigenetic Therapy in Acute Myeloid Leukemia: Current and Future Directions. Semin Hematol. 2015; 52:172-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia-Manero G, Tibes R, Kadia T, et al. Phase 1 dose escalation trial of ilorasertib, a dual Aurora/VEGF receptor kinase inhibitor, in patients with hematologic malignancies. Invest New Drugs. 2015; 33:870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L, Song K, Zhou L, Xie Z, Zhou P, Zhao Y, Han Y, Xu X, Li P. Heparan sulfate d-glucosaminyl 3-O-sulfotransferase-3B1 (HS3ST3B1) promotes angiogenesis and proliferation by induction of VEGF in acute myeloid leukemia cells. Journal of cellular biochemistry. 2015; 116:1101-1112. [DOI] [PubMed] [Google Scholar]
- 30. Zeng D, Wang J, Kong P, Chang C, Li J, Li J. Ginsenoside Rg3 inhibits HIF-1alpha and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. J Cell Biochem. 2014; 7:2172-2178. [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang ZH, Hao CL, Liu P, Tian X, Wang LH, Zhao L, Zhu CM. Valproic acid inhibits tumor angiogenesis in mice transplanted with Kasumi1 leukemia cells. Mol Med Rep. 2014; 9:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kvestad H, Evensen L, Lorens JB, Bruserud O, Hatfield KJ. In Vitro Characterization of Valproic Acid, ATRA, and Cytarabine Used for Disease-Stabilization in Human Acute Myeloid Leukemia: Antiproliferative Effects of Drugs on Endothelial and Osteoblastic Cells and Altered Release of Angioregulatory Mediators by Endothelial Cells. Leuk Res Treatment. 2014; 2014:143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huynh H, Ong R, Soo KC. Foretinib demonstrates anti-tumor activity and improves overall survival in preclinical models of hepatocellular carcinoma. Angiogenesis. 2012; 15:59-70. [DOI] [PubMed] [Google Scholar]
- 34. Chen HM, Tsai CH, Hung WC. Foretinib inhibits angiogenesis, lymphangiogenesis and tumor growth of pancreatic cancer in vivo by decreasing VEGFR-2/3 and TIE-2 signaling. Oncotarget. 2015; 6:14940-14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mattison R, Jumonville A, Flynn PJ, Moreno-Aspitia A, Erlichman C, LaPlant B, Juckett MB. A phase II study of AZD2171 (cediranib) in the treatment of patients with acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia & lymphoma. 2015; 56:2061-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poulos MG, Gars EJ, Gutkin MC, Kloss CC, Ginsberg M, Scandura JM, Rafii S, Butler JM. Activation of the vascular niche supports leukemic progression and resistance to chemotherapy. Exp Hematol. 2014; 42:976-986 e971-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hua KT, Lee WJ, Yang SF, Chen CK, Hsiao M, Ku CC, Wei LH, Kuo ML, Chien MH. Vascular endothelial growth factor-C modulates proliferation and chemoresistance in acute myeloid leukemic cells through an endothelin-1-dependent induction of cyclooxygenase-2. Biochim Biophys Acta. 2014; 1843:387-397. [DOI] [PubMed] [Google Scholar]
- 38. Maffei R, Fiorcari S, Bulgarelli J, et al. Endothelium-mediated survival of leukemic cells and angiogenesis-related factors are affected by lenalidomide treatment in chronic lymphocytic leukemia. Exp Hematol. 2014; 42:126-36.e1. [DOI] [PubMed] [Google Scholar]
- 39. Lee JY, Park S, Min WS, Kim HJ. Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett. 2014; 354:281-289. [DOI] [PubMed] [Google Scholar]
- 40. Wang F, Chan LW, Cho WC, Tang P, Yu J, Shyu CR, Tsui NB, Wong SC, Siu PM, Yip SP, Yung BY. Novel approach for coexpression analysis of E2F1-3 and MYC target genes in chronic myelogenous leukemia. Biomed Res Int. 2014; 2014:439840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blackwood EM, Eisenman RN. Max: A helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991; 251:1211-1217. [DOI] [PubMed] [Google Scholar]
- 42. Liu YC, Li F, Handler J, Huang CR, Xiang Y, Neretti N, Sedivy JM, Zeller KI, Dang CV. Global regulation of nucleotide biosynthetic genes by c-Myc. PLoS One. 2008; 3:e2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res. 2008; 68:5326-5334. [DOI] [PubMed] [Google Scholar]
- 44. Mizukami Y, Fujiki K, Duerr EM, Gala M, Jo WS, Zhang X, Chung DC. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3-kinase/Rho/ROCK and c-Myc. J Biol Chem. 2006; 281:13957-13963. [DOI] [PubMed] [Google Scholar]
- 45. Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006; 38:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006; 20:2527-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mishra R, Watanabe T, Kimura MT, et al. Identification of a novel E-box binding pyrrole-imidazole polyamide inhibiting MYC-driven cell proliferation. Cancer Sci. 2015; 106:421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang H, Chauhan J, Hu A, Pendleton K, Yap JL, Sabato PE, Jones JW, Perri M, Yu J, Cione E, Kane MA, Fletcher S, Prochownik EV. Disruption of Myc-Max heterodimerization with improved cell-penetrating analogs of the small molecule 10074-G5. Oncotarget. 2013; 4:936-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011; 146:904-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nickols NG, Szablowski JO, Hargrove AE, Li BC, Raskatov JA, Dervan PB. Activity of a Py-Im polyamide targeted to the estrogen response element. Mol Cancer Ther. 2013; 12:675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang F, Nickols NG, Li BC, Marinov GK, Said JW, Dervan PB. Antitumor activity of a pyrrole-imidazole polyamide. Proc Natl Acad Sci U S A. 2013; 110:1863-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raskatov JA, Nickols NG, Hargrove AE, Marinov GK, Wold B, Dervan PB. Gene expression changes in a tumor xenograft by a pyrrole-imidazole polyamide. Proc Natl Acad Sci U S A. 2012; 109:16041-16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Katanasaka Y, Kodera Y, Kitamura Y, Morimoto T, Tamura T, Koizumi F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer. 2013; 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pello OM, Andres V. Role of c-MYC in tumor-associated macrophages and cancer progression. Oncoimmunology. 2013; 2:e22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A, Doni A, Nebuloni M, Swigart LB, Evan GI, Mantovani A, Locati M. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012; 119:411-421. [DOI] [PubMed] [Google Scholar]
- 56. Eisen AZ, Jeffrey JJ, Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968; 151:637-645. [DOI] [PubMed] [Google Scholar]
- 57. Tschesche H. Leukodiapedesis, function, and physiological role of leucocyte matrix metalloproteinases. Adv Exp Med Biol. 1997; 421:285-301. [DOI] [PubMed] [Google Scholar]
- 58. Ries C, Loher F, Zang C, Ismair MG, Petrides PE. Matrix metalloproteinase production by bone marrow mononuclear cells from normal individuals and patients with acute and chronic myeloid leukemia or myelodysplastic syndromes. Clin Cancer Res. 1999; 5:1115-1124. [PubMed] [Google Scholar]
- 59. Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. Journal of cellular physiology. 2007; 211:19-26. [DOI] [PubMed] [Google Scholar]
- 60. Alexander CM, Werb Z. Targeted disruption of the tissue inhibitor of metalloproteinases gene increases the invasive behavior of primitive mesenchymal cells derived from embryonic stem cells in vitro. J Cell Biol. 1992; 118:727-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chaudhary AK, Pandya S, Ghosh K, Nadkarni A. Matrix metalloproteinase and its drug targets therapy in solid and hematological malignancies: An overview. Mutat Res. 2013; 753:7-23. [DOI] [PubMed] [Google Scholar]
- 62. Reikvam H, Hatfield KJ, Oyan AM, Kalland KH, Kittang AO, Bruserud O. Primary human acute myelogenous leukemia cells release matrix metalloproteases and their inhibitors: Release profile and pharmacological modulation. European journal of haematology. 2010; 84:239-251. [DOI] [PubMed] [Google Scholar]
- 63. Patterson ML, Atkinson SJ, Knauper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001; 503:158-162. [DOI] [PubMed] [Google Scholar]
- 64. Jost M, Folgueras AR, Frerart F, Pendas AM, Blacher S, Houard X, Berndt S, Munaut C, Cataldo D, Alvarez J, Melen-Lamalle L, Foidart JM, Lopez-Otin C, Noel A. Earlier onset of tumoral angiogenesis in matrix metalloproteinase-19-deficient mice. Cancer Res. 2006; 66:5234-5241. [DOI] [PubMed] [Google Scholar]
- 65. Kudo Y, Iizuka S, Yoshida M, Tsunematsu T, Kondo T, Subarnbhesaj A, Deraz EM, Siriwardena SB, Tahara H, Ishimaru N, Ogawa I, Takata T. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J Biol Chem. 2012; 287:38716-38728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Friehs I, Margossian RE, Moran AM, Cao-Danh H, Moses MA, del Nido PJ. Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis. Basic research in cardiology. 2006; 101:204-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood). 2006; 231:20-27. [DOI] [PubMed] [Google Scholar]
- 68. Marchenko ND, Marchenko GN, Weinreb RN, Lindsey JD, Kyshtoobayeva A, Crawford HC, Strongin AY. Beta-catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. Int J Biochem Cell Biol. 2004; 36:942-956. [DOI] [PubMed] [Google Scholar]
- 69. Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer research. 2006; 26:3579-3583. [PubMed] [Google Scholar]
- 70. Buggins AG, Levi A, Gohil S, Fishlock K, Patten PE, Calle Y, Yallop D, Devereux S. Evidence for a macromolecular complex in poor prognosis CLL that contains CD38, CD49d, CD44 and MMP-9. Br J Haematol. 2011; 154:216-222. [DOI] [PubMed] [Google Scholar]
- 71. Hatfield KJ, Reikvam H, Bruserud O. The crosstalk between the matrix metalloprotease system and the chemokine network in acute myeloid leukemia. Current medicinal chemistry. 2010; 17:4448-4461. [DOI] [PubMed] [Google Scholar]
- 72. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000; 2:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liao D, Johnson RS. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007; 26:281-290. [DOI] [PubMed] [Google Scholar]
- 74. Yu XF, Han ZC. Matrix metalloproteinases in bone marrow: Roles of gelatinases in physiological hematopoiesis and hematopoietic malignancies. Histol Histopathol. 2006; 21:519-531. [DOI] [PubMed] [Google Scholar]
- 75. Fingleton B. Matrix metalloproteinases as valid clinical targets. Curr Pharm Des. 2007; 13:333-346. [DOI] [PubMed] [Google Scholar]
- 76. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006; 70:1469-1480. [DOI] [PubMed] [Google Scholar]
- 77. Torii S, Goto Y, Ishizawa T, Hoshi H, Goryo K, Yasumoto K, Fukumura H, Sogawa K. Pro-apoptotic activity of inhibitory PAS domain protein (IPAS), a negative regulator of HIF-1, through binding to pro-survival Bcl-2 family proteins. Cell Death Differ. 2011; 18:1711-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001; 292:464-468. [DOI] [PubMed] [Google Scholar]
- 79. Willam C, Warnecke C, Schefold JC, Kugler J, Koehne P, Frei U, Wiesener M, Eckardt KU. Inconsistent effects of acidosis on HIF-alpha protein and its target genes. Pflugers Arch. 2006; 451:534-543. [DOI] [PubMed] [Google Scholar]
- 80. Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006; 59:15-26. [DOI] [PubMed] [Google Scholar]
- 81. Huang Y, Du KM, Xue ZH, Yan H, Li D, Liu W, Chen Z, Zhao Q, Tong JH, Zhu YS, Chen GQ. Cobalt chloride and low oxygen tension trigger differentiation of acute myeloid leukemic cells: Possible mediation of hypoxia-inducible factor-1alpha. Leukemia. 2003; 17:2065-2073. [DOI] [PubMed] [Google Scholar]
- 82. Chen GQ, Peng ZG, Liu W, Song LP, Jiang Y, Huang Y, Zhao Q. Hypoxia inducible factor-1alpha and leukemic cell differentiation. Sheng li xue bao : [Acta physiologica Sinica]. 2006; 58:5-13. [PubMed] [Google Scholar]
- 83. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011; 144:646-674. [DOI] [PubMed] [Google Scholar]
- 84. Hatfield KJ, Bedringsaas SL, Ryningen A, Gjertsen BT, Bruserud O. Hypoxia increases HIF-1alpha expression and constitutive cytokine release by primary human acute myeloid leukaemia cells. Eur Cytokine Netw. 2010; 21:154-164. [DOI] [PubMed] [Google Scholar]
- 85. Song LP, Zhang J, Wu SF, Huang Y, Zhao Q, Cao JP, Wu YL, Wang LS, Chen GQ. Hypoxia-inducible factor-1alpha-induced differentiation of myeloid leukemic cells is its transcriptional activity independent. Oncogene. 2008; 27:519-527. [DOI] [PubMed] [Google Scholar]
- 86. He M, Wang QY, Yin QQ, Tang J, Lu Y, Zhou CX, Duan CW, Hong DL, Tanaka T, Chen GQ, Zhao Q. HIF-1alpha downregulates miR-17/20a directly targeting p21 and STAT3: A role in myeloid leukemic cell differentiation. Cell Death Differ. 2013; 20:408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007; 12:853-859. [DOI] [PubMed] [Google Scholar]
- 88. Frolova O, Samudio I, Benito JM, et al. Regulation of HIF-1alpha signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther. 2012; 13:858-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Katoh Y, Katoh M. Comparative integromics on Angiopoietin family members. Int J Mol Med. 2006; 17:1145-1149. [PubMed] [Google Scholar]
- 90. Kawaguchi M, Sugaya M, Suga H, Miyagaki T, Ohmatsu H, Fujita H, Asano Y, Tada Y, Kadono T, Sato S. Serum levels of angiopoietin-2, but not angiopoietin-1, are elevated in patients with erythrodermic cutaneous T-cell lymphoma. Acta Derm Venereol. 2014; 94:9-13. [DOI] [PubMed] [Google Scholar]
- 91. Yu X, Seegar TC, Dalton AC, Tzvetkova-Robev D, Goldgur Y, Rajashankar KR, Nikolov DB, Barton WA. Structural basis for angiopoietin-1-mediated signaling initiation. Proc Natl Acad Sci U S A. 2013; 110:7205-7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Moss A. The angiopoietin:Tie 2 interaction: A potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013; 24:579-592. [DOI] [PubMed] [Google Scholar]
- 93. Maffei R, Martinelli S, Castelli I, Santachiara R, Zucchini P, Fontana M, Fiorcari S, Bonacorsi G, Ilariucci F, Torelli G, Marasca R. Increased angiogenesis induced by chronic lymphocytic leukemia B cells is mediated by leukemia-derived Ang2 and VEGF. Leukemia research. 2010; 34:312-321. [DOI] [PubMed] [Google Scholar]
- 94. Saito M, Watanabe J, Fujisawa T, Kamata Y, Nishimura Y, Arai T, Miyamoto T, Obokata A, Kuramoto H. Angiopoietin-1, 2 and Tie2 expressions in endometrial adenocarcinoma—the Ang2 dominant balance upregulates tumor angiogenesis in the presence of VEGF. Eur J Gynaecol Oncol. 2006; 27:129-134. [PubMed] [Google Scholar]
- 95. Thiyagarajan N, Acharya KR. Crystal structure of human angiogenin with an engineered loop exhibits conformational flexibility at the functional regions of the molecule. FEBS Open Bio. 2013; 3:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai). 2008;40:619-624. [DOI] [PubMed] [Google Scholar]
- 97. Martinelli S, Kanduri M, Maffei R, Fiorcari S, Bulgarelli J, Marasca R, Rosenquist R. ANGPT2 promoter methylation is strongly associated with gene expression and prognosis in chronic lymphocytic leukemia. Epigenetics. 2013; 8:720-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Maffei R, Martinelli S, Santachiara R, et al. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood. 2010; 116:584-592. [DOI] [PubMed] [Google Scholar]
- 99. Pavlov N, Badet J. (Angiogenin: Involvement in angiogenesis and tumour growth). Bull Cancer. 2001; 88:725-732. [PubMed] [Google Scholar]
- 100. Cheng CL, Hou HA, Jhuang JY, et al. High bone marrow angiopoietin-1 expression is an independent poor prognostic factor for survival in patients with myelodysplastic syndromes. Br J Cancer. 2011; 105:975-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hatfield KJ, Hovland R, Oyan AM, Kalland KH, Ryningen A, Gjertsen BT, Bruserud O. Release of angiopoietin-1 by primary human acute myelogenous leukemia cells is associated with mutations of nucleophosmin, increased by bone marrow stromal cells and possibly antagonized by high systemic angiopoietin-2 levels. Leukemia. 2008; 22:287-293. [DOI] [PubMed] [Google Scholar]
- 102. Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974; 249:123-127. [DOI] [PubMed] [Google Scholar]
- 103. Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D'Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Molecular biology of the cell. 2008; 19:994-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991; 252:1705-1708. [DOI] [PubMed] [Google Scholar]
- 105. Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991; 64:841-848. [DOI] [PubMed] [Google Scholar]
- 106. Nakashio A, Fujita N, Tsuruo T. Topotecan inhibits VEGF- and bFGF-induced vascular endothelial cell migration via downregulation of the PI3K-Akt signaling pathway. Int J Cancer. 2002; 98:36-41. [DOI] [PubMed] [Google Scholar]
- 107. Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine & growth factor reviews. 2005; 16:159-178. [DOI] [PubMed] [Google Scholar]
- 108. Smolej L, Andrys C, Maisnar V, Pour L, Maly J. Plasma concentrations of vascular endothelial growth factor and basic fibroblast growth factor in lymphoproliferative disorders. Acta Medica (Hradec Kralove). 2005; 48:57-58. [PubMed] [Google Scholar]
- 109. Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nature medicine. 2003; 9:604-613. [DOI] [PubMed] [Google Scholar]
- 110. Traer E, Javidi-Sharifi N, Agarwal A, Dunlap J, English I, Martinez J, Tyner JW, Wong M, Druker BJ. Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations. Blood. 2014; 123:1516-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]