Abstract
Although most mesotheliomas present with pleural effusions, it is controversial whether mesothelioma can be diagnosed with confidence in effusion cytology. Therefore, an ancillary marker of malignant mesothelial cells applicable in effusions would be clinically valuable. BRCA-1-associated protein (BAP1) is a tumor suppressor gene, which shows biallelic inactivation in approximately half of all mesotheliomas. We investigated whether loss of BAP1 expression by immunohistochemistry can be used to support a diagnosis of mesothelioma in effusion cytology. Immunohistochemistry for BAP1 was performed on cell blocks and interpreted blinded. 43 of 75 (57%) effusions associated with confirmed mesothelioma showed negative staining with positive internal controls. Of 57 effusions considered to have atypical mesothelial cells in the absence of a definitive diagnosis of mesothelioma, 8 cases demonstrated negative staining for BAP1. On follow-up six of these patients received a definitive diagnosis of mesothelioma in the subsequent 14 months (two were lost to follow-up immediately, and mesothelioma could not be excluded). Only 5 of 100 consecutive benign effusions were interpreted as BAP1 negative. One of these patients died soon after and mesothelioma could not be excluded. On unblinded review the four other patients with apparently negative BAP1 staining but no malignancy lacked convincing positive staining in non-neoplastic cells suggesting that BAP1 immunohistochemistry may have initially been misinterpreted. 47 effusions with adenocarcinoma were BAP1 positive. We conclude that loss of BAP1 expression, while not definitive, can be used to support the diagnosis of mesothelioma in effusion cytology. We caution that interpretation of BAP1 immunohistochemistry on cell block may be difficult and that convincing positive staining in non-neoplastic cells is required before atypical cells are considered negative. We also note that BAP1 loss is not a sensitive test as it occurs in only half of all mesotheliomas and cannot be used to exclude the diagnosis.
BRCA1-associated protein 1 (BAP1), encoded by the BAP1 gene at 3p21.1, is a nuclear ubiquitin hydrolase1, 2 involved in various cellular processes, including chromatin remodeling.1, 3 BAP1 behaves as a true tumor suppressor gene in accordance with the classic Knudson's two-hit model.1 The discovery of germline BAP1 mutations in families with high incidences of mesothelioma and other neoplasms has led to the recent recognition of the BAP1 tumor predisposition syndrome (OMIM #614327),4 which is inherited in an autosomal dominant manner and is characterised by uveal melanoma, mesothelioma, cutaneous melanocytic lesions, renal cell carcinoma, basal cell carcinoma, and possibly intrahepatic cholangiocarcinoma.1, 5, 6, 7, 8, 9, 10, 11 Sporadic BAP1 loss has also been reported in a range of tumors including uveal melanoma, mesothelioma, and cutaneous melanocytic neoplasms.12, 13, 14, 15
Pleural malignant mesothelioma is an uncommon neoplasm, which arises from the mesothelial lining of the pleura and is strongly associated with asbestos exposure. Patients usually present with non-specific symptoms such as dyspnea, chest wall pain, and pleural effusion, and are commonly diagnosed late in the disease process.16 Mesothelioma carries a poor prognosis, with 3- and 5-year survival rates well below 15%.17, 18 Although the association between mesothelioma and asbestos exposure is well established, only a minority of exposed individuals go on to develop mesothelioma,19 and mesothelioma has also been observed to occur in family clusters.20 These observations suggest a genetic predisposition to developing mesothelioma, and have led to the discovery of the association between germline BAP1 mutation and mesothelioma.4 Furthermore recent studies have identified an association between somatic BAP1 inactivation and mesothelioma, with double-hit inactivation of BAP1 reported in approximately half of all mesotheliomas.1, 13, 15, 21, 22, 23 Indeed, BAP1 appears to be the most commonly mutated gene in this neoplasm.13
Quite severe reactive atypia may occur in benign processes such as local infection, pneumothorax, collagen vascular disease, drug reactions, trauma, or inflammation, and may closely mimic mesothelioma cytologically.24 The definitive criterion for distinguishing malignant mesothelioma from benign processes remains the demonstration of an unequivocal invasive growth by atypical mesothelial cells—a feature that cannot be assessed in effusion cytology.24, 25, 26 Therefore, it is controversial whether cytological analysis of effusions can be used to make a diagnosis of mesothelioma even in the presence of extreme atypia.27
A large number of immunohistochemical markers performed on cell-block preparations from effusion cytology specimens have been proposed to support the diagnosis of mesothelioma. These ancillary markers include epithelial membrane antigen, p53, glucose transporter-1, and insulin-like growth factor-II mRNA-binding protein 3.28, 29, 30, 31, 32 Although these markers may be of assistance in borderline cases, to date they have not proven sufficiently sensitive or specific for widespread routine clinical use.29, 33 There is therefore an unmet clinical need for a highly specific marker of mesothelioma, which can be applied in cytology specimens.
Given the high rate of BAP1 double-hit inactivation in mesothelioma and its correlation with loss of BAP1 expression as determined by immunohistochemistry in tissue specimens,21 we sought to investigate whether loss of expression of BAP1 as determined in cell-block preparations from pleural effusion specimens could be used to support a diagnosis of mesothelioma.
Materials and methods
The computerized database of the Department of Anatomical Pathology, Royal North Shore Hospital, Sydney, NSW, Australia, was searched for all cases of thoracic mesothelioma receiving a definitive histological tissue diagnosis between January 1991 and August 2014. The same database was searched to identify which of these patients also had effusion cytology specimens obtained at the time of, or before, primary tissue diagnosis. The results of immunohistochemical staining for BAP1 in these tissue biopsy samples has been previously reported.21 We also searched for all cases of thoracic mesothelioma receiving a definitive diagnosis on effusion cytology alone without confirmatory tissue biopsy diagnosis for the period June 1998 to August 2014. Although considered definitive cases of mesothelioma, because of the current controversy as to whether mesothelioma can be diagnosis by cytology alone, these cases were analyzed separately.
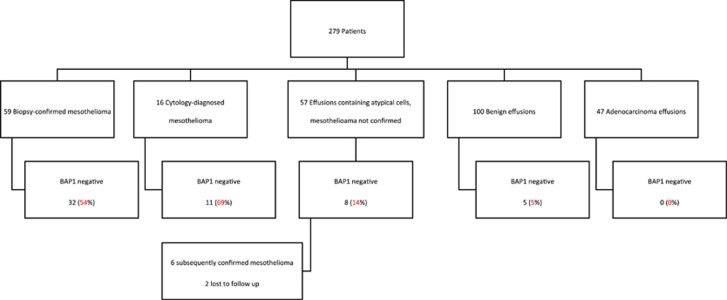
As control cohorts, we identified consecutive cases of benign effusions and effusions containing adenocarcinoma from the calendar year 2010. We then identified a cohort of cases containing atypical mesothelial cells from patients without a confirmed tissue diagnosis of mesothelioma by searching for all pleural effusions reported as containing atypical mesothelial cells from June 1998 to August 2014, including only patients who had never received a tissue diagnosis of mesothelioma in our department. These cases were screened by an experienced pathologist (AJG) and cytology scientist (AS) to both confirm the diagnosis and to check that sufficient material remained in the cell-block preparation to permit immunohistochemistry. The study cohorts are summarized in Figure 1.
Figure 1.
Flow chart summarizing the results of BAP1 immunohistochemistry in five different cohorts (n=279 patients). BAP1, BRCA1-associated protein 1.
Immunohistochemistry for BAP1 was performed on whole sections freshly cut from the cell-block preparations with a commercially available mouse monoclonal anti-BAP1 antibody (clone C-4, cat no sc-28383, Santa Cruz Biotechnology, USA) using previously described methods.21 Briefly, slides were sectioned at 4 μm onto positively charged slides (Super frost plus, Menzel-Glaser, Germany) and the slides were stained on an automated platform—the Leica Microsystems Bond III autostainer (Leica Microsystems, Mount Waverley, Victoria, Australia). Heat induced antigen retrieval was used for 30 min at 97 °C in the manufacturer's alkaline retrieval solution ER2 (VBS part no: AR9640) and the primary antibody was used at a dilution of 1 in 100. A biotin-free polymer-based detection system (Define, VBS part no: DS 9713) was employed.
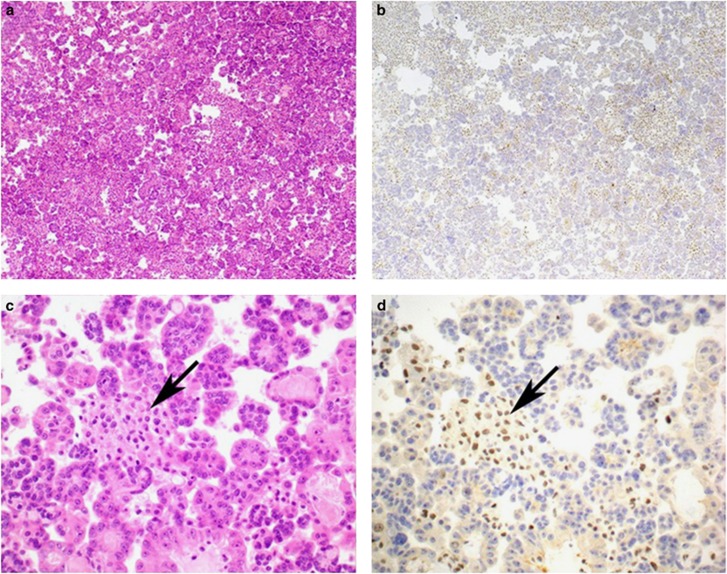
Immunohistochemistry was interpreted by a single pathologist (AJG) in conjunction with an H&E stained section cut in parallel with the immunohistochemistry section. At the time of interpreting the immunohistochemistry, the pathologist was blinded to the underlying diagnosis. The target cells for immunohistochemical assessment were the atypical cells in cases where they could be distinguished from background benign mesothelial cells and all mesothelial cells when this distinction could not be made. Negative staining was defined as completely absent nuclear staining in the target cells in the presence of a positive internal control, provided by non-neoplastic cells such as lymphocytes, stromal cells, or reactive mesothelial cells (illustrated in Figure 2). Positive staining was defined as positive nuclear staining in at least some target cells, (applying an arbitrary cutoff of at least 5% of the presumed target cells)—illustrated in Figures 3 and 4. If the target cells stained negatively but without a clear cut internal positive control, immunohistochemistry was considered non-contributory (that is, not negative, for the purpose of binary analysis).
Figure 2.
Serial H&E- (a and c) and BAP1- (b and d) stained sections from a pleural effusion associated with mesothelioma. All the neoplastic cells show completely negative staining for BAP1. Positive nuclear staining in non-neoplastic stromal and inflammatory cells (arrows) is noted and acts as an internal positive control. Original magnifications 100 × (a and b) and 400 × (c and d). BAP1, BRCA1-associated protein 1; H&E, hematoxylin and eosin.
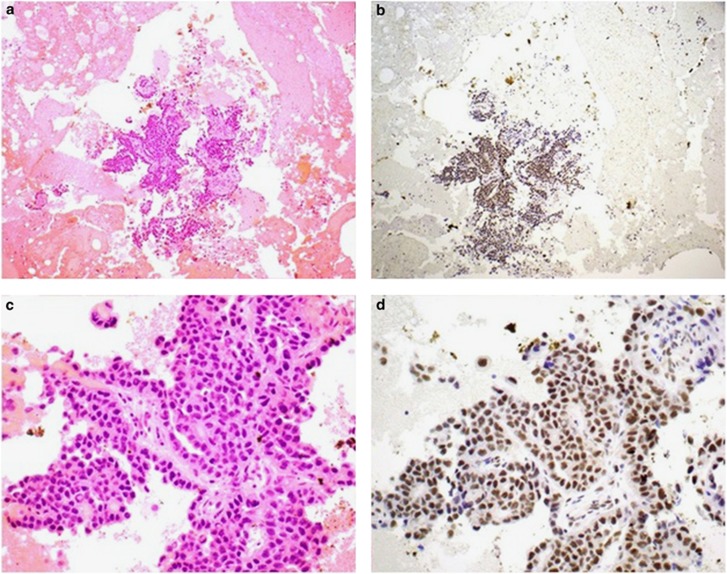
Figure 3.
Serial H&E- (a and c) and BAP1- (b and d) stained sections from a pleural effusion associated with mesothelioma. In this case the atypical mesothelial cells show preserved positive nuclear staining for BAP1. Original magnifications 100 × (a and b) and 400 × (c and d). BAP1, BRCA1-associated protein 1; H&E, hematoxylin and eosin.
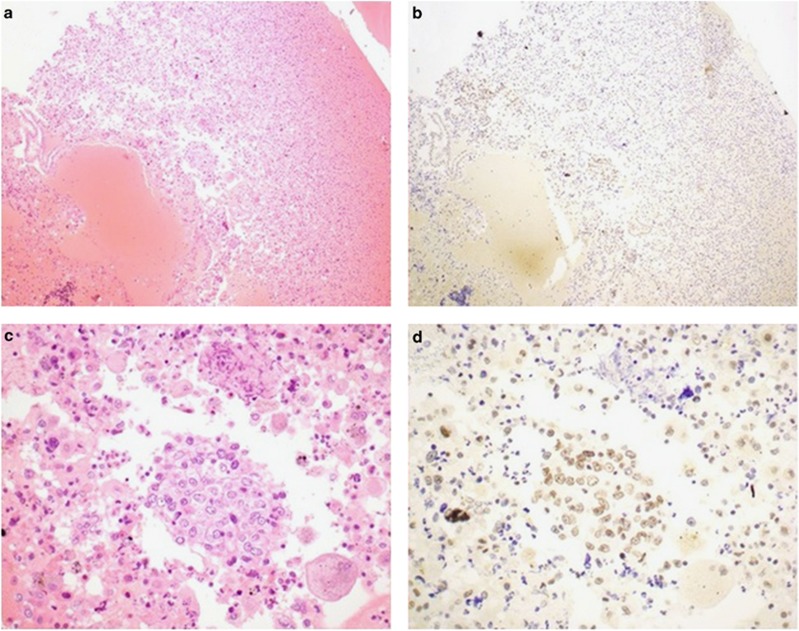
Figure 4.
Serial H&E- (a and c) and BAP1- (b and d) stained sections from a pleural effusion in which the mesothelial cells show considerable reactive atypia. In this case the atypical mesothelial cells show preserved positive nuclear staining for BAP1 and the patient was alive and disease free 2 years after presentation supporting a benign etiology. It is noted that both the atypical mesothelial cells and the non-neoplastic internal controls show less intense staining than other cases making interpretation difficult. Original magnifications 100 × (a and b) and 400 × (c and d). BAP1, BRCA1-associated protein 1; H&E, hematoxylin and eosin.
This study was approved by the Northern Sydney Local Health District medical ethics review board. Non-thoracic (that is, abdominal) mesotheliomas were excluded from this study.
Results
Immunohistochemistry for BAP1 was performed on effusion cell blocks from 279 patients, comprising 59 patients with mesothelioma confirmed on tissue biopsy, 16 cases in which the diagnosis of mesothelioma was made on cytology specimens only, 100 consecutive benign effusions, 47 consecutive effusions containing adenocarcinoma and 57 effusions containing atypical mesothelial cells only. The results of immunohistochemistry are summarized in Figure 1 and Tables 1 and 2. Briefly, in the cohort of 59 patients with biopsy confirmed mesothelioma, 32 (54%) showed negative staining for BAP1. The results of immunohistochemical staining in this cohort was concordant with the results of immunohistochemistry previously performed and reported21 on the accompanying tissue biopsies in all but two cases, both of which were interpreted as positive in the effusions but negative in all the neoplastic cells in the biopsies. On unblinded review of the slides this discrepancy was thought to be due to positive staining in reactive mesothelial cells in the effusion samples, while the neoplastic cells were either not identified or inconspicuous in the effusion samples and therefore negative staining was not appreciated. In the cohort of 16 patients in which the diagnosis of mesothelioma was made solely by cytology, 11 (69%) showed negative staining for BAP1. That is 43 of 75 (57%) of all patients with a definitive diagnosis of mesothelioma and material available in cell block demonstrated negative staining for BAP1 (Table 1).
Table 1. BAP1 expression in patients with confirmed mesothelioma.
| Method of diagnosis, n | BAP1 negative, n (%) | BAP1 normal expression, n (%) |
|---|---|---|
| Tissue biopsy confirmation, 59 | 32 (54) | 27 (46) |
| Cytology diagnosis only, 16 | 11 (69) | 5 (31) |
Table 2. Patient characteristics of non-mesothelioma patients.
| Diagnosis and BAP1 status | n (%) | Age at diagnosis, median (range) | Male, n (%) |
|---|---|---|---|
| Cellular atypia in the absence of definitive diagnosis | 57 (100) | 81 (24–95) | 29 (51) |
| BAP1 loss on Cytology | 8 (14) | 82 (68–90) | 5 (9) |
| BAP1 normal expression on Cytology | 49 (86) | 81 (24–95) | 24 (42) |
| Benign effusions | 100 (100) | 76 (25–96) | — |
| BAP1 loss on Cytology | 5 (5) | 77 (25–83) | — |
| BAP1 normal expression on Cytology | 95 (95) | 76 (36–96) | — |
| Adenocarcinoma effusions | 47 (100) | 69 (35–94) | 16 (34) |
| BAP1 loss on Cytology | 0 | — | — |
| BAP1 normal expression on Cytology | 47 (100) | 69 (35–94) | 16 (34) |
The results of BAP1 immunohistochemistry in patients with benign, atypical, or adenocarcinoma-related pleural effusions are presented in Table 2. All 47 patients with adenocarcinoma in pleural effusions demonstrated positive staining for BAP1 in malignant cells. Of 100 consecutive patients with benign pleural effusions, 5 (5%) were interpreted as showing negative staining for BAP1 when scored blinded. The immunohistochemically stained slides on these patients then underwent unblinded review and further clinical information was sought, which is presented in Table 3. Briefly, it was thought that one of the patients was likely to have had unbiopsied mesothelioma at the time of diagnosis (asbestos exposure, suggestive imaging, and died 12 months later of unknown cause) and this patient was thought to show true negative staining on review. There was no known clinical evidence of mesothelioma at 4-year follow-up for any of the remaining four patients (three of whom had other epithelial neoplasms all of which showed positive BAP1 staining on tissue biopsy). When the immunohistochemistry from these cases underwent unblinded review it was appreciated that in areas where the neoplastic cells were interpreted as negative, there was quite weak or absent staining in the internal positive controls in the non-neoplastic cells. That is, on unblinded review, it was thought that the BAP1 staining may have initially been misinterpreted and perhaps should have been classified as non-contributory rather than negative.
Table 3. Details of five patients with benign effusions and negative BAP1 expression.
| Age | Gender | Clinical features | Conclusion after unblinded review |
|---|---|---|---|
| 82 | M | Known asbestos exposure. Progressive pleural thickening on imaging. Died of unknown cause 12 months after effusion. | Like mesothelioma (true negative BAP1 immunohistochemistry). |
| 80 | F | Known asbestos exposure with pleural plaques. Alive with no evidence of mesothelioma 4 years after biopsy. | False-negative BAP1 immunohistochemistry. |
| 67 | M | Diagnosed with adenocarcinoma of unknown primary site (BAP1 immunohistochemically positive) 1 month later. | False-negative BAP1 immunohistochemistry. |
| 83 | M | Recent Whipples resection for BAP1 immunohistochemically positive pancreatic adenocarcinoma. | False-negative BAP1 immunohistochemistry. |
| 25 | F | Recently underwent oophorectomy for BAP1-positive borderline mucinous ovarian tumor. | False-negative BAP1 immunohistochemistry. |
Of the 57 pleural effusions that contained atypical mesothelial cells, 8 (14%) cases showed BAP1 loss. Further follow-up was sought on these patients and is presented in Table 4. Briefly, six patients were confirmed to have mesothelioma on tissue biopsies or autopsies performed at other institutions over the subsequent 2–14 months. Tissue from these biopsies was not available for review to confirm the BAP1 status on the mesotheliomas. The remaining two patients with atypical mesothelial cells in pleural effusion and negative staining for BAP1 died of unknown cause shortly after the effusions were sampled (2 and 9 months later). Therefore, mesothelioma could be neither definitively excluded nor confirmed as the cause of their pleural effusions.
Table 4. Follow-up details of BAP1-negative patients with atypical mesothelial cells on initial investigation.
| Age | Gender | Subsequent diagnosis of mesotheioma | Time from surgery to final mesothelioma diagnosis and/or death |
|---|---|---|---|
| 74 | M | No | Immediately lost to follow-up. Died of unknown cause 9 months later. |
| 90 | F | No | Immediately lost to follow-up. Died of unknown cause 2 months later. |
| 86 | F | Yes | Died of mesothelioma 14 months later. |
| 68 | F | Yes | Biopsy confirmed mesothelioma 7 months later. |
| 80 | M | Yes | Biopsy confirmed mesothelioma 2 months later. Died of mesothelioma 8 months later. |
| 84 | M | Yes | Biopsy confirmed mesothelioma 2 months later. Died of mesothelioma 4 months later. |
| 77 | M | Yes | Biopsy confirmed mesothelioma 7 months later. |
| 84 | M | Yes | Biopsy confirmed mesothelioma 7 months later. |
Discussion
Loss of immunohistochemical staining for BAP1 in the presence of an internal positive control in non-neoplastic cells has been proven to be a reliable marker of BAP1 double-hit inactivation in tissue samples from mesothelioma and uveal melanoma.1, 13, 22 Given that double-hit inactivation of BAP1 has been reported as a key driver event in approximately half of all mesotheliomas,1, 13, 15, 22, 23 loss of immunohistochemical staining for BAP1 is an attractive ancillary marker for mesothelioma with the potential to be highly specific, albeit not particularly sensitive. For example, recently Sheffield et al29 found loss of BAP1 immunohistochemical staining in 7 of 26 (27%) tissue biopsy samples from mesothelioma, but in none of 49 benign mesothelial proliferations. Similarly, we recently reported the loss of BAP1 immunohistochemical staining in 106 of 229 (46%) tissue biopsy samples of mesothelioma, but did not investigate BAP1 staining in benign pleural disease.21 However, to date BAP1 expression has not been studied in cytology samples in pleural effusion specimens, which is particularly important given both the propensity for mesothelioma to present with recurrent effusions and also the potential difficulty of interpreting BAP1 immunohistochemistry in cell-block preparations particularly given that the significance is placed on negative staining, which may occur artefactually.
In this study, when assessing BAP1 immunohistochemistry blinded as to the underlying diagnosis, we found negative staining in the presence of an internal positive control in 43 of 75 (57%) mesotheliomas comprising 32 of 59 (54%) cases with tissue biopsy confirmation and 11 of 16 (69%) cases where the diagnosis was made based solely on cytology. Given that we propose BAP1 immunohistochemistry as an ancillary marker in atypical cases, our group of 57 cases containing atypical mesothelial cells on which, even after review, we were unable to make a definitive diagnosis of mesothelioma, is particularly important. In this cohort, eight cases (14%) demonstrated negative staining, and six of these patients went on to have a confirmed diagnosis of mesothelioma while two of the cases (both of which had suggestive imaging) died shortly after meaning that mesothelioma could not be excluded. That is, our findings clearly indicate that negative staining for BAP1 in effusion cytology supports a diagnosis of mesothelioma.
However, benign pleural effusions greatly outnumber effusions due to mesothelioma, and, therefore, for an ancillary marker of malignant mesothelioma to have real clinical utility, it must be highly specific. It is concerning that 5 of 100 (5%) benign effusions were thought to show negative staining for BAP1 when interpreted blinded to the underlying diagnosis. Although one of these patients, who died 12 months later, may have had mesothelioma, the remaining four patients had no evidence of mesothelioma at up to 4 years follow-up. That is these four patients would have been given a false-positive diagnosis of mesothelioma if the diagnosis was based solely on negative staining for BAP1. On unblinded review, it was thought that the negative staining may have been artefactual because staining in the internal positive controls was relatively weak or absent close to the atypical mesothelial cells, which were interpreted as negative. However, the fact remains that these cases were interpreted as negative even when the presence of an internal positive control was required when they were initially examined blinded as to the outcome. Therefore, because BAP1 immunohistochemistry may be difficult to interpret with certainty in pleural effusion specimens, loss of BAP1 expression even when interpreted by experienced observers, can only be used to support a diagnosis of mesothelioma in the appropriate clinical and morphological context and is not completely definitive when interpreted in isolation. From a practical point of view, we would, therefore, recommend BAP1 immunohistochemistry only be undertaken on cases that contain atypical mesothelial cells in which the diagnosis of mesothelioma is being strongly considered morphologically and in these cases loss of BAP1 expression can be interpreted as supporting the diagnosis of mesothelioma.
We note that none of 47 pleural effusions containing confirmed adenocarcinoma demonstrated loss of staining for BAP1, whereas BAP1 loss was found in 43 of 75 (57%) mesotheliomas. Therefore, in addition to supporting a diagnosis of mesothelioma over reactive mesothelial change in the appropriate context, loss of expression of BAP1 can also be used to support a malignancy as being mesothelial rather than epithelial. However, although BAP1 loss is rare in most epithelial malignancies, it has been reported in some cholangiocarcinomas and renal carcinomas and is common in some non-epithelial malignancies such as uveal melanomas.1, 5, 6, 7, 8, 9, 10, 11 Therefore, this potential role for BAP1 immunohistochemistry should be considered in the appropriate clinical context based on which other neoplasms are in the differential diagnosis.
In conclusion, loss of BAP1 expression in effusion cytology is strongly supportive of a diagnosis of mesothelioma. Although it is not definitive, it can be used to support the diagnosis of malignancy in atypical mesothelial proliferations and at the very least negative staining for BAP1 in mesothelial cells in effusion cytology specimens should result in a high index of suspicion for the diagnosis of mesothelioma. We caution that BAP1 immunohistochemistry may be difficult to interpret in effusion specimens and that the presence of an internal positive control in non-neoplastic cells is an absolute pre-requisite before significance is placed on negative staining. Furthermore, we note that only approximately half of mesotheliomas will show the loss of staining for BAP1 and, therefore, positive staining for BAP1 cannot be used to exclude a diagnosis of mesothelioma.
Acknowledgments
This project was supported by the Sydney Vital, Translational Cancer Research, through a Cancer Institute NSW competitive grant.
The authors declare no conflict of interest.
References
- 1Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology 2013;45:116–126. [DOI] [PubMed] [Google Scholar]
- 2Battaglia A. The importance of multidisciplinary approach in early detection of BAP1 tumor predisposition syndrome: clinical management and risk assessment. Clin Med Insights Oncol 2014;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Jiao Y, Pawlik TM, Anders RA et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4OMIM: Online Mendelian Inheritance in Man [homepage on the Internet] Baltimore: Johns Hopkins University School of Medicine; 2015 [updated 11 July 2013; cited 28 May 2015]. Available from http://omim.org/entry/614327.
- 5Pilarski R, Cebulla CM, Massengill JB et al. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer 2014;53:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 2012;44:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Abdel-Rahman MH, Pilarski R, Cebulla CM et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet 2011;48:856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8De La Fouchardiere A, Cabaret O, Savin L et al. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet e-pub ahead of print 31 July 2014; doi:doi: 10.1111/cge.12472. [DOI] [PubMed]
- 9Testa JR, Cheung M, Pei J et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Wadt KA, Aoude LG, Johansson P et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet e-pub ahead of print 15 September 2014; doi:10.1111/cge.12501. [DOI] [PubMed]
- 11Wiesner T, Obenauf AC, Murali R et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011;43:1018–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Wiesner T, Murali R, Fried I et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol 2012;36:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Nasu M, Emi M, Pastorino S et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Koopmans AE, Verdijk RM, Brouwer RWW et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol 2014;27:1321–1330. [DOI] [PubMed] [Google Scholar]
- 15Bott M, Brevet M, Taylor BS et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21. 1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Van der Bij S, Koffijberg H, Burgers J et al. Prognosis and prognostic factors of patients with mesothelioma: a population-based study. Br J Cancer 2012;107:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Baud M, Strano S, Dechartres A et al. Outcome and prognostic factors of pleural mesothelioma after surgical diagnosis and/or pleurodesis. J Thorac Cardiovasc Surg 2013;145:1305–1311. [DOI] [PubMed] [Google Scholar]
- 19Carbone M, Ly BH, Dodson RF et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol 2012;227:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Carbone M, Emri S, Dogan AU et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer 2007;7:147–154. [DOI] [PubMed] [Google Scholar]
- 21Farzin M, Toon CW, Clarkson A et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology 2015;47:302–307. [DOI] [PubMed] [Google Scholar]
- 22Betti M, Casalone E, Ferrante D et al. Inference on germline BAP1 mutations and asbestos exposure from the analysis of familial and sporadic mesothelioma in a high-risk area. Genes Chromosomes Cancer 2015;54:51–62. [DOI] [PubMed] [Google Scholar]
- 23Yoshikawa Y, Sato A, Tsujimura T et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103:868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Henderson DW, Shilkin KB, Whitaker D. Reactive mesothelial hyperplasia vs mesothelioma, including mesothelioma in situ: a brief review. Am J Clin Pathol 1998;110:397–404. [DOI] [PubMed] [Google Scholar]
- 25Husain AN, Colby T, Ordonez N et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647–667. [DOI] [PubMed] [Google Scholar]
- 26Husain AN, Colby TV, Ordóñez NG et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2009;133:1317–1331. [DOI] [PubMed] [Google Scholar]
- 27Henderson DW, Reid G, Kao SC et al. Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J Clin Pathol 2013;66:847–853. [DOI] [PubMed] [Google Scholar]
- 28Shen J, Pinkus GS, Deshpande V et al. Usefulness of EMA, GLUT-1, and XIAP for the cytologic diagnosis of malignant mesothelioma in body cavity fluids. Am J Clin Pathol 2009;131:516–523. [DOI] [PubMed] [Google Scholar]
- 29Sheffield BS, Hwang HC, Lee AF et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977–982. [DOI] [PubMed] [Google Scholar]
- 30Monaco SE, Shuai Y, Bansal M et al. The diagnostic utility of p16 FISH and GLUT-1 immunohistochemical analysis in mesothelial proliferations. Am J Clin Pathol 2011;135:619–627. [DOI] [PubMed] [Google Scholar]
- 31Kato Y, Tsuta K, Seki K et al. Immunohistochemical detection of GLUT-1 can discriminate between reactive mesothelium and malignant mesothelioma. Mod Pathol 2007;20:215–220. [DOI] [PubMed] [Google Scholar]
- 32Shi M, Fraire AE, Chu P et al. Oncofetal protein IMP3, a new diagnostic biomarker to distinguish malignant mesothelioma from reactive mesothelial proliferation. Am J Surg Pathol 2011;35:878–882. [DOI] [PubMed] [Google Scholar]
- 33Lee AF, Gown AM, Churg A. IMP3 and GLUT-1 immunohistochemistry for distinguishing benign from malignant mesothelial proliferations. Am J Surg Pathol 2013;37:421–426. [DOI] [PubMed] [Google Scholar]