Abstract
An increasing body of evidences from preclinical as well as epidemiological and clinical studies suggest a potential beneficial role of dietary intake of omega-3 fatty acids for cognitive functioning. In this narrative review, we will summarize and discuss recent findings from epidemiological, interventional and experimental studies linking dietary consumption of omega-3 fatty acids to cognitive function in healthy adults. Furthermore, affective disorders and schizophrenia (SZ) are characterized by cognitive dysfunction encompassing several domains. Cognitive dysfunction is closely related to impaired functioning and quality of life across these conditions. Therefore, the current review focues on the potential influence of omega-3 fatty acids on cognition in SZ and affective disorders. In sum, current data predominantly from mechanistic models and animal studies suggest that adjunctive omega-3 fatty acid supplementation could lead to improved cognitive functioning in SZ and affective disorders. However, besides its translational promise, evidence for clinical benefits in humans has been mixed. Notwithstanding evidences indicate that adjunctive omega-3 fatty acids may have benefit for affective symptoms in both unipolar and bipolar depression, to date no randomized controlled trial had evaluated omega-3 as cognitive enhancer for mood disorders, while a single published controlled trial suggested no therapeutic benefit for cognitive improvement in SZ. Considering the pleiotropic mechanisms of action of omega-3 fatty acids, the design of well-designed controlled trials of omega-3 supplementation as a novel, domain-specific, target for cognitive impairment in SZ and affective disorders is warranted.
Keywords: Bipolar disorder, cognition, depression, Omega-3 fatty acids, psychosis, schizophrenia.
INTRODUCTION
Overview
Cognitive functioning, including processes such as memory, language, attention, perception, problem solving and mental imagery is a core human ability which influence the behavior of all humans [1]. However, through accidents or age-related cognitive decline or dementia, certain cognitive functions are disturbed. Furthermore, several non-age-related psychiatric disorders, including mood disorders (i.e., major depressive disorder (MDD) and bipolar disorder (BD)) [2, 3] and schizophrenia (SZ) [4] are known to be associated with cognitive dysfunction encompassing several domains namely verbal memory, executive functions, attention and processing speed, with varying degrees of severity [5-7]. In mood disorders, evidences indicate that cognitive impairments persist in euthymic states [3, 5], while in SZ cognitive deficits are evident in first-episode, treatment-naïve patients [4]. Furthermore, cognitive dysfunction is closely related to impaired psychosocial functioning in MDD [6, 8], BD [9] and SZ [10].
Notwithstanding the exact mechanisms leading to cognitive dysfunction in SZ and mood disorders remain elusive, it has been postulated that neuroprogressive mechanisms related to immune-inflammatory aberrations, changes in tryptophan catabolite (TRYCAT) pathway, abnormalities in glutamatergic neurotransmission, impaired neurotrophic support, as well as oxidative and nitrosative stress (O&NS) might play a role [11-14]. Recently, novel compounds targeting these pathways have been tested and/or proposed as novel neuroprotector/procognitive agents for these disorders (reviewed in Refs. [15-17]).
While antipsychotic medications in SZ and antidepressant/mood stabilizer drugs in mood disorders target primarily psychotic and affective symptoms, the effects of anti-psychotics [18], antidepressants [6] and mood stabilizers [19] upon neurocognition are often suboptimal. Some first-line agents may even be detrimental in terms of cognitive function in mood disorders [19, 20] and SZ [21]. There are cognitive training programs specially developed targeting cognitive deficits mainly in psychosis (e.g., Cogpack® [22] or Cognitive Remediation [23, 24]). The review by Twamley and colleagues [25] showed that 14 out of 17 studies on the effects of cognitive training in SZ have small to medium effect sizes in improving cognitive functioning. Recent approaches also included physical training in traditional treatment concepts, with the hope that physical training may improve–beside psychopathological symptoms–cognitive functioning in psychiatric patient groups. Regarding the effects in SZ or affective disorders, current results are promising, but the overall number of controlled randomized trials that systematically examined the effects of physical exercise in SZ and MDD patients on cognitive symptoms is limited and effect sizes are largely unknown (see for review e.g., [26]). In sum, the treatment of cognitive dysfunction is a clear unmet need in SZ and mood disorders, and cognitive improvement is currently a treatment aim for these disorders [11-13, 27-30].
A promising approach to improve cognition focuses on the effects of nutrients and dietary supplementation. For instance, fatty acids – and predominantly n3 fatty acids - have been proposed as therapeutically valuable agents for widespread diseases as well as certain symptoms [31]. Due to their pleiotropic properties, omega-3 fatty acids, are currently under investigation as a treatment for arteriosclerosis, cancer, diabetes, hypertension, arthritis, dementia, psychiatric disorders and some autoimmune diseases [32-34]. However, the current review focuses on mental disorders which are accompanied by cognitive symptoms.
The overarching aims of this narrative review are two-fold. First, the major aim is to address the potential mechanisms related to the role of n3-PUFAs as potential cognitive enhancers. Second, to give we review preclinical and clinical evidences for cognitive effects – especially on memory – for n3-PUFAs for mood disorders and/or SZ. Finally, future research directions are discussed.
SEARCH STRATEGY
We searched a range of electronic databases, including PubMED, EMBASE and PsycINFO using the following search terms: ‘omega 3 fatty acids’, ‘cognition’, ‘memory’, ‘schizophrenia’, ‘bipolar disorder’, ‘depression’ and ‘mood disorders’. Studies were included if they contained original data on effects of n3-PUFAs in mood disorders and SZ and were published in English between 1990 and December, 2014. Both animal studies and clinical trials were considered for inclusion. The findings are classified with respect to the methods used in the specific studies.
POLYUNSATURATED FATTY ACIDS
Increasing evidence suggests that there are potential benefits of dietary supplementation to improve memory functioning [35, 36]. A special form of treatment that has attained attention in recent years is dietary supplementation with omega-3 fatty acids (n3-PUFAs, ω-3 fatty acids). n3-PUFAs have been proposed as an add-on therapy for several diseases with an inflammatory component [37]. Since the first studies related to the diet of Greenland Eskimos, omega-3 fatty acids have received increasing attention from researchers. Despite the high intake of total dietary fat by Greenland Eskimos, there was a remarkably low incidence of cardiovascular diseases; this surprising finding has been attributed to the anti-inflammatory and anti-atherogenic effects of n3-PUFAs [38].
Mammals are not able to synthesise all required fatty acids; they lack certain desaturase enzymes to produce longer unsaturated fatty acids. The only sources of these so-called essential fatty acids are dietary α-linolenic acid (ALA) and linoleic acid (LA). ALA is the precursor of n3-PUFAs, whereas LA is the precursor of the omega-6 (ω-6 or n-6) fatty acids [39]. Polyunsaturated fatty acids (PUFAs) are characterized by double bonds (-CH=CH-) in the carbon chain interrupted by a methylene group. Eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) are often referred to as very long-chain (n-3) PUFAs [40]. ALA is described with shorthand nomenclature as 18:3 (n-3), indicating that ALA consists of 18 carbon atoms, 3 double bonds and is considered an omega-3 fatty acid, because the first double bond is at the third position counting from the methyl-end [41] (see Fig. 1). Using shorthand nomenclature, DHA is labelled 22:6(n-3), EPA 20:5(n-3) and DPA 22:5(n-3) [41].
Fig. (1).
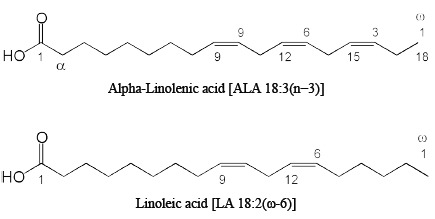
Molecular structures of the two precursors for omega-3 and omega-6 fatty acids: alpha-Linolenic acid (ALA) and Linolenic acid (LA).
METABOLISM OF FATTY ACIDS
In mammals, the only source for the precursors of the ω-3 and ω-6 fatty acid families is nutrition. In the liver, ALA and LA enter different metabolic pathways generating either n3-PUFAs (EPA, DPA, DHA) derived from the common precursor ALA, or arachidonic acid (ARA), which is derived from LA [42].
To a smaller amount, n3-PUFAs are metabolised in the cerebral microvasculature of the blood-brain-barrier, the astrocytes and the cerebral endothelium [43, 44].
In the human brain, DHA is the most abundant PUFA neuronal cell membrane and was guessed to account for 40% of the total brain membrane phospholipids fatty acid pool [45]. It is considered to be essential for efficient functioning of the mammalian central nervous system during perinatal development. Additionally, DHA affects brain-barrier function and plays a role in the regulation of neurotransmission systems like dopamine, serotonin, acetylcholine and norepinephrine, which in turn are affected in various mood and neuro-degenerative disorders [46]. The development of effective and innovative neuroimaging techniques has facilitated the in vivo investigation of the brain anatomy and functional circuitry and can be a useful tool to investigate the effectiveness of n3-PUFA supplementation on age-related memory problems and psychiatric disorders. Brain imaging methods usually include anatomical and functional techniques. The latter measures ongoing brain activity, e.g. through the use of brain mapping, event-related potentials (ERP) and functional magnetic resonance. The former on the other hand allows brain anatomical reconstruction through the use of magnetic resonance, computerized axial and positron emission tomography [47]. Diffusion tensor imaging (DTI) which is a MRI method sensitized to the motion of water molecules [48] which provides information on the microstructural integrity of white matter fiber tracts.
For the generation of long chain PUFAs, two different classes of enzymes are necessary: elongases, which elongate the carbon chain at the carboxyl-end, and desaturases which create carbon double bonds by removing two hydrogen atoms. Desaturases are classified with the delta symbol Δ and a number, indicating that a double bond is created at this position counting from the carboxyl-end. Animals, including humans, do not possess the Δ 15 desaturase enzyme, which is crucial for the synthesis of ALA [41]. Furthermore, humans cannot interconvert n6- and n3-PUFAs [45]. Consequently, the ratio of n6- to n3-PUFAs is not regulated by enzymatic activity but is the result of their proportions in the diet.
Since the same enzymes are involved in the generation of both, long chain n3-PUFAs, as well as long chain n6-PUFAs, ALA and LA and their respective metabolites compete for the same enzymatic machinery. In consequence, high levels of LA may inhibit the conversion of ALA to long chain n3-PUFAs and vice-versa.
Moreover, n3- and n6-PUFAs share the same enzymes responsible for converting fatty acids to lipid mediators, called eicosanoids: cyclooxygenases (COX) and lipoxygenases (LOX). ARA-derived eicosanoids generally are proinflammatory [49-51], whereas lipid mediators built up from EPA are thought to be less inflammatory or even anti-inflammatory [52]. Resolvins, docosatriens and neuroprotectins metabolised from DHA are also of anti-inflammatory action [53, 54], but may have additional neuroprotective mechanisms [55-57]. Fig. 2 shows the metabolic pathways for n-3 and n-6 fatty acids and their resulting eicosanoids with main biological/pathophysiological functions.
Fig. (2).
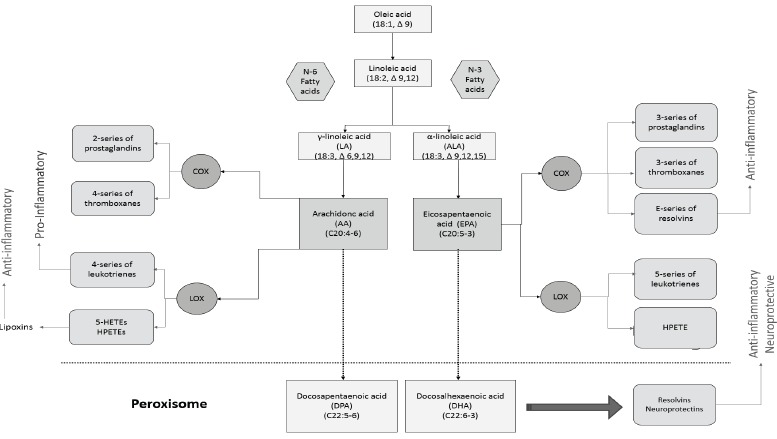
Metabolic pathways of n-3 and n-6 PUFAs and their resulting eicosanoids with main functions. Abbreviations: COX: cyclooxygenases, LOX: lipoxygenases, HETEs: hydroxyeicosatetraenoic acids, HPETE: hydroperoxyeicosatetraenoic acids, LTB4: leukotriene B4.
In the presence of both fatty acid families in a ratio of 1-4:1 (n6:n3), Δ 5 and Δ 6 desaturases preferably generates n-3 rather than n-6 families [58]. Conversely, a western-type lifestyle may increase this ratio to 10:1 or even higher [59], and desaturases shifts towards producing more ARA and n6-PUFA metabolites [42], ultimately leading to a pro-inflammatory metabolic milieu.
In humans, the fraction of ALA converted to EPA is estimated to be about five percent; the amount of ALA converted to DHA is even less than 0.5 percent [60]. Interestingly, the conversion of ALA to longer-chain PUFAs seems to be higher in women than in men, providing a rationale for considering the influence of sex hormones, especially of oestrogen, on the metabolic pathways [61].
The ratio of n6/n3-PUFAs is hypothesized to be near 1:1 during human evolution [45]. The typical western-type diet has now reached a ratio of 10:1 to 20:1 (n6:n3 PUFAs) [59]. This ratio is further shifted towards n6-PUFAs with lower social status. Only few cultures with a low rate of industrialisation seem to have preserved a balanced n6/n3 ratio, most notably the Greenland Eskimos [62].
The rate of n6/n3-PUFAs in the diet as well as in neuronal of neuron cells plasma membranes may be estimated by the ratio in the membrane of thrombocytes and/or erythrocytes [63]. Aliments that are rich in ω-3 FAs are different kinds of seafood like trout, mackerel, salmon, herring, anchovy and sardine. Notable, herring and salmon contain higher amounts of the very long-chain PUFAs namely EPA and DHA [42]. Vegetarian sources of n3-PUFAs (mostly ALA) are wild plants, seeds and nuts. Particularly, rapeseed, sesame and walnuts are rich in n3-PUFAs. Linseed, safflower, sunflower and soy oil also contain high amounts of n6-PUFAs.
Taking into account that a re-balancing of n3/n6 requires dietary changes, the enrichment of natural foods (“functional foods”) has been proposed [64]. While n3-enriched functional foods may be a feasible means for reaching broad parts of the population, it brings with it some technical challenges, especially due to the fact that n3-PUFAs are highly sensitive to oxidation and may easily get rancid after short periods of time [64].
FATTY ACIDS AND BRAIN FUNCTIONING
Fatty acids are linked to various functional systems (e.g., immune system) and play a particularly important role for the maintenance of brain function and neural transmission [65]. N3-PUFAs are recognized as critical components of neurite membranes and are considered important for brain and retina development [66, 67]. A number of interventional studies have thus investigated the effects of dietary supplementation of n-3 PUFAs for the prevention of cognitive impairment and for the amelioration of cognitive performance across among healthy individuals [35, 68]. Further studies have shown an accumulation of n3-PUFAs in neural cells which may have beneficial effects on neuronal function, as well as anti-inflammatory and antioxidant activities [69, 70]. Furthermore, n3-PUFAs improves membrane fluidity [71], activates peroxisome-proliferator-activated receptors [72], and enhances neurotrophic support (e.g., increasingg BDNF signalling) [73]. These pleiotropic mechanisms of action provide support for possible neuroprotector and/or precognitive effects of n3-PUFAs for affective disorders and SZ (see Fig. 3).
Fig. (3).
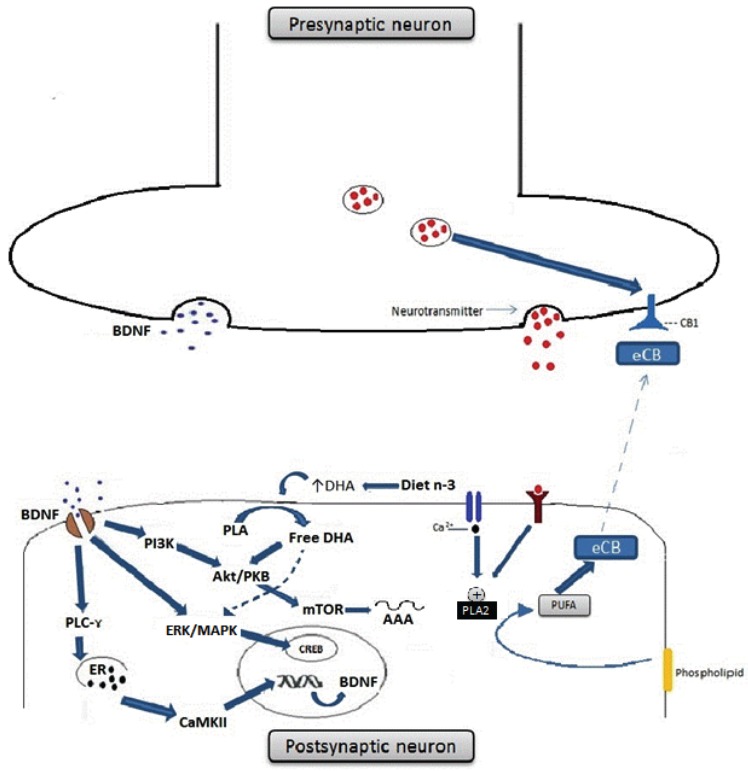
Omega-3 fatty acids enhance BDNF synthesis and signalling in neurons and also influence cannabinoid-mediated synaptic plasticity. (A) BDNF binds cognate TrKB receptors and activates PLC-γ, ERK/MAPK and Akt/PKB intracellular signalling pathways. Activation of PLC-γ leads to the release of calcium from the endoplasmatic reticulum and to the activation of CAMKII, which phosphorylates CREB and activates gene transcription. Activation of ERK/MAPK pathway also regulates transcription through CREB phosphorylation, whereas PI3K phosphorylates and activates Akt/PKB and mTOR, regulating translation. DHA enhances neurotrophic signalling by the PI3K/Akt BDNF signal transduction pathway. DHA also activates the MAPK pathway; activated MAPK then phosphorylates CREB which translocates into the nucleus and activates BDNF gene transcription. (B) Endocannabinoids (eCBs) are signalling lipids produced from PUFAs present in membrane phospholipids. PUFAs are released from the postsynaptic membrane by phospholipase A2 (PLA2) in response to neuronal activation. eCB released in the synaptic cleft can bind presynaptic cannabinoid receptor type 1 (CB1) on the presynaptic neuron. Activation of CB1 leads to inhibition of neurotransmitter release and synaptic activity. In rodents that are fed a n3-PUFA enriched diet, such eCB-mediated long-term depression (LTD) is inhibited at excitatory synapses, whereas rodents fed a diet chronically low in n-3 PUFAs has this form of eCB-mediated synaptic plasticity specifically interrupted. Abbreviations: BDNF- brain-derived neurotrophic factor; CAMKII- Calciumcalmodulin kinase II; CREB- cAMP-response element-binding protein; DHA-Docosahexaenoic acid; n-3-n-3 PUFAs; ERK- extracellularsignal- regulated kinases; MAPK- Mitogen-activated protein kinases; PI3K- Phosphoinositide 3-kinase; Akt/PKB- Protein kinase B; mTORMechanistic target of rapamycin.
A recent authoritative review by Bazinet & Laye highlighted that PUFAs and their mediators are responsible for certain processes within the central nervous system, namely: (i) the preservation of cell survival, structure and function of neurons, glial cells and endothelial cells; (2) the modulation of neuroinflammatory mechanisms; and (iii) the support and regulation of neurotransmission [31]. These mechanisms suggest a putative role for mood regulation and cognitive functioning, and, therefore, the PUFAs may be novel targets of interest for mood symptoms and cognitive deficits present in several psychiatric disorders, like affective disorders and schizophrenia. However, more research is needed to assess the brain uptake of fatty acids in normal and pathological conditions [31].
More recently, interactions between PUFAs and the endocannabinoid system have been reported (see Fig. 3) [74], which are of pathophysiological interest for inflammatory and neurological disorders. Furthermore, the function of PUFAs as carrier molecules for neurotransmitters may bring more possibilities for pharmacological applications [74]. The dietary supplementation of N-3 PUFAs stimulates the formation of specific n-3 PUFAs-derived conjugates like ethanolamine, dopamine, serotonin or other amines. Furthermore, it is involved in the synthesis of prototypic endocannabinoids like anandamide [71].
Docosahexaenoic acid (DHA) has potent anti-inflammatory properties. For example, the production of pro-inflammatory cytokines in the brain following systemic lipopolysaccharide (LPS) administration is attenuated in rodents with high levels of brain DHA [70, 75, 76]. The putative anti-inflammatory effects of DHA could result from a direct effect on invading macrophages or microglia. Indeed, in vitro and in vivo investigations have reported the DHA blocks microphage- and microglia-induced activation of NF-kappaB in the CNS of rodents with neuroinflammation [77, 78]. Furthermore, DHA may switch the microglia to a M2 anti-inflammatory phenotype [79]. Finally, in vivo, low-dietary consumption of n-3 PUFAs leads to microglia activation and to the production of pro-inflammatory cytokines in mice at weaning [80].
Compelling evidences also support omega-3 fatty acids’ antioxidant properties. For example, omega-3 fatty acids alone and in combination with lithium and aripiprazole reduced levels of superoxide dismutase (SOD), catalase (CAT), and lipid peroxidation products (thiobarbituric acid reactive substances) rodents submitted to the methylphenidate-induced mania model [81]. Furthermore, omega-3 fatty acids reversed the increased lipid peroxidation and protein carbonylation levels in rats submitted to the chronic mild stress model [82]. Omega-3 also prevented brain oxidative damage in the ketamine model of schizophrenia [83]. Taken together, these preclinical findings suggest that the antioxidant activity of omega-3 PUFAs may mitigate neuroprogression and cognitive dysfunction in mood and affective disorders.
PROCOGNITIVE EFFECTS OF OMEGA-3 FATTY ACIDS: PRECLINICAL EVIDENCES
Animal studies allow researchers to experimentally induce an isolated deficiency in nutrients and test for their effects on various physiological functions. An increasing body of experimental data seem to support a clear decline in memory functioning caused by a n3-PUFA restricted diet [84-88], which could be fully or partially restored by n3-PUFAs dietary supplementation. For example, Chung et al. [88] suggested that DHA plays a crucial role in the development and maintenance of proper spatial learning memory performance in rats. They showed that the recovery of brain DHA levels enhanced both spatial reference and working memory performance.
Accordingly, long-term nutrition with walnuts containing high amounts of ALA may strengthen memory function by enhancing the retention of learned tasks in rats [89]. Vinot and colleagues [90] investigated the impact of long-term, i.e. five months, dietary supplementation with tuna oil, which is rich in DHA, on behaviour, cognition and motor performance in adult non-human primates. Their results shown that n3-PUFAs supplementation lowered anxiety, spontaneous loco-motor activity and enhanced cognitive performance. However, it needs to be further investigated whether the reduction in anxiety is the reason for better cognitive performance or whether the intake of n3-PUFAs directly improved spatial reference memory in lemurs [90].
It has been hypothesized that EPA and DHA-rich cod liver oil may be helpful in preventing the deleterious effects of chronic restraint stress on recall and spatial memory [91]. Moreover, DHA supplementation has also been associated with improved working memory performance [92].
In animal research, experimentally induced memory impairments could be reversed by treatment with fish oil. It has been reported that a DHA-containing fish oil formulation was able to reverse ischemia-induced amnesia in rats and that this effect was maintained for up to two weeks after completion of treatment [93]. However, this study did not find effects on hippocampal damage caused by ischemia, which is inconsistent with other findings, e.g. [94] and needs to be further investigated.
Another study investigated the effects of central injection of interleukin (IL)-1ß on spatial memory in rats and the counteracting effects of EPA administration [95]. The intra-cerebroventricular injection of IL-1ß significantly impaired spatial working memory. In addition, rats were not able to retain information acquired only 50 minutes earlier. They also showed that IL-1ß only caused impairment in memory retrieval but encoding [95]. This deficit in memory seemed to be associated with an increase in corticosterone concentration. Moreover, the supplementation with ethyl-EPA attenuated the IL-1-β-induced memory impairment, which was attributed to reduction in corticosterone levels [95].
An important finding in the literature is that brain areas involved in neurogenesis, i.e. the hippocampus and olfactory bulbs, display “faster DHA recovery, a greater capability for DHA accumulation, and more resistance to DHA deprivation” [88] than other brain regions, such as the frontal cortex, visual cortex, and cerebellum. These findings are in line with findings from Tanabe and colleagues [85], who investigated the relationship between Fos expression in rat CA1 hippocampus triggered by DHA supplementation and improvement of spatial cognition. The Fos protein is a transcription factor and has been considered as a functional marker of neuronal activity [96]. Tanabe and colleagues [85] showed that the expression of Fos in the CA1 hippocampus increased when rats were supplemented with DHA and in addition Fos expression in this brain region correlated with spatial memory improvement.
n3-PUFA supplementation in rodents did not only facilitate the compensation of negative effects caused by a deficient diet but also showed promising results in normally fed animals. Gamosh et al. [97] showed that supraphysiological DHA supplementation (300mg/kg/day, over 10 weeks) increased DHA levels in the cerebral cortex and hippocampus of young, weaned rats.
Gama and collaborators [98] showed that omega-3 fatty acid implementation in a ketamine animal model of SZ prevented all, positive, negative and cognitive-like symptoms. The authors suggested that the results provides a rationale for the design of lage clinical trials to validate the use of n3-fatty acids to improve cognitive and psychopathological symptoms of the illness. Preclinical studies are summarized in Table 1.
Table 1.
Relevant studies concerning the effects of n3-PUFAs in animal research.
| Reference No. | Animal | Intervention | Experimental Behavioral Task | Duration | Results |
|---|---|---|---|---|---|
| [89] | Rat | Walnut | Memory function; by elevated plus maze (EPM) and radial arm maze (RAM) |
28 days | A significant improvement in learning and memory of walnut treated rats was observed |
| [157] | Rat | Fish oil | Spatial Learning; by water maze | 4 months | No effect on age-related deficits in memory |
| [91] | Male wistar rat | Cod liver oil | -Recall; passive avoidance situation -spatial reference and working memory; Barnes maze -locomotor activity, anxiety behavior; open field and elevated plus-maze |
21 days | Cod liver oil prevented the effects of chronic restraint stress on recall and the spatial memory |
| [158] | Male grey mouse lemur | Fish oil | -anxiety, reference spatial memory, locomotor activity monitoring, sensory-motor test; open field test | 5 months | ω3-supplemented animals exhibited lower anxiety level what was accompanied by better performances in a reference spatial memory task |
| [159] | BALBcByJ mouse | DHA, retinoid X receptor (RXR) agonist / antagonist | Promnemonic and antidespair activities; spontaneous alternation and forced swim test | DHA decreased despair behavior and improved working memory. The effects could be mimicked by RXR agonist, blocked by antagonist and inhibited by RXR knockout. | |
| [160] | Rat, infusion of Amyloid beta peptide (1-40) | EPA | cognition learning ability; eight-arm radial maze | 12 weeks | EPA significantly reduced the increase in the number of reference and working memory errors in the Abeta-infused rats |
| [161] |
Male Rat | Nonpurified or sunflower oil-based (ω-3) fatty acid-deficient diet alone or supplemented with (ω-3) fatty acids |
reference and working memory performance; Morris water maze | 102-130 days | Rats fed the fatty acid-deficient diet showed significantly poorer reference and working memory, and FO supplementation partially rescued both memory performances |
| [162] | Gerbil | Uridine, Choline, DHA | Working memory; Four-arm radial maze apparatus (radial maze, t-maze, y-maze, rotarod maze) |
4 weeks | DHA plus choline improved performance on the four-arm radial maze, T-maze, and Y-maze tests; coadministering UMP further enhanced these increases |
| [163] | Senescence-accelerated prone 8 (SAMP8) mouse | EPA, DHA | Learning, memory; T-maze training |
8 weeks | Dietary PUFA is associated with delay in cognitive decline |
| [164] |
Mouse | Fatty acid-deficient diet | spontaneous locomotor activity, anxiety related behavior; open field test, the elevated plus maze -locomotor activity, spatial task performance; Barnes circular maze |
7 weeks | The ω-3 fatty acid-deficient mice demonstrated impaired learning in the reference-memory version of the Barnes circular maze |
| [165] | Rat, infusion of Amyloid beta peptide (1-40) | DHA, Fatty acid-deficient diet | learning ability-related reference, working memory; 8-arm radial maze | 12 weeks | DHA significantly reduced the increase in the number of reference and working memory errors in the Abeta-infused rats |
| [166] | Rat | Fatty acid-deficient diet | -Motor activity; video image analyzer - anxiety-related behavior; elevated plus-maze -spatial task performance; Morris water maze | 9 weeks | Deficiency was associated with significantly reduced spatial learning |
| [167] |
Rat, pregnant, Testing of infants at PND77 | Diet of fish oil | Delayed spatial alternation; automated operant chambers | Delayed spatial alternation impairments in rats fed fish oil correlates with altered ω-6/ω-3 FA ratio | |
| [168] | Male wistar rat, administered IL-1beta |
EPA | Spatial memory; Morris water maze | 5 weeks | The effects of IL-1 were attenuated by the administration of E-EPA |
| [169] |
Rat | DHA | Spatial memory; eight-arm radial maze | 12 weeks | DHA administration significantly reduced the number of reference and working memory errors |
| [170] |
Spontaneously hypertensive rats (SHR), Wistar-Kyoto rats (WKY) |
DHA | spatial short-term memory; delayed-matching-to-place (DMP) version of the Morris water maze | 5 weeks | There was no effect of dietary supplementation on performance |
| [171] |
Male wistar rat | DHA | Spatial memory; eight-arm radial maze | 10 weeks | Administration of DHA significantly decreased the number of reference working memory error |
| [172] |
adult rat | Fatty acid-deficient diet | Learning; Morris water maze | The ω-3 fatty acid-deficient mice demonstrated impaired learning in the Morris water maze | |
| [137] |
Young (five week old) male rat | DHA | reference memory and working memory; partially (four of eight) baited eight-arm radial maze | 10 weeks | DHA administration significantly reduced the number of reference memory errors |
| [173] |
Male long-Evans rat | DHA, AA, LA, ALA, saturated fatty acids | Working memory; water maze | 3 - 6 weeks | The groups did not differ in the Morris water-maze, but on a test of working memory, the saturated fat group was impaired |
| [174] |
Senescence-accelerated prone 8 (SAMP8) mouse | LA, ALA | learning and memory; Sidman active avoidance task, light and dark discrimination learning test | 28 weeks | The group ALA showed greater improvement in learning in the Sidman active avoidance task than did the LA group. |
| [175] |
Rat | LA, ALA | Learning; water maze | Rats fed ALA-rich diet had a longer mean survival time and an increased learning ability in senescence | |
| [98] | Ketamine animal model of SZ; Wistar rats | Fatty acids | Locomotor activity, inhibitory avoidance, social interaction; open field task | 15 days | Prevented from positive, negative and cognitive symptoms |
CLINICAL STUDIES
Methodological Considerations
In the chapter below, we will focus on different issues pertaining to the design of clinical trials with n3-PUFAs in mental disorders.
Stratification Strategies
Before starting a clinical intervention trial, n3-PUFA intake (e.g. using food frequency questionnaires) and/or n3-PUFA tissue levels (e.g. in plasma or erythrocytes) should be assessed. Participants may then be stratified according to baseline n3-PUFA parameters. This approach would allow assessing the differential impact of n3-PUFA supplementation on psychopathological symptoms and cognitive functions in relation to n3 baseline status. It can be hypothesized that individuals with low n3-PUFA parameters at baseline may show the largest changes induced by in n3-PUFA supplementation. This stratification-based approach would also likely improve the statistical power and reduce the sample size necessary to achieve significant treatment effects due to the homogenization of participant groups.
Further baseline parameters potentially relevant to the intervention outcome may be the intake ratios of n3/n6 fatty acids or ratios of n3/total fat intake, as these parameters appear to affect lipid signalling in inflammatory systems [99].
Moreover, factors that may indirectly modulate downstream lipid processing, such as inflammation or atherosclerosis, should also be taken into account and controlled for. For instance, individuals with a favourable n3/n6 intake pattern, but with otherwise “pro-inflammatory” lifestyle factors, such as physical inactivity and cigarette smoking, may present a different response to n3-PUFA supplementation than those individuals without “pro-inflammatory” lifestyle factors. Depending on the respective hypothesis of intervention trials on omega-3 FA supplementation, complex scores comprising various lifestyle and metabolic factors, as well as genetic (e.g., APoE genotype) and inflammatory biomarkers (e.g. CRP, cytokines, etc.) could aid in the identification of participants who would be more likely to respond to n3-PUFA supplementation.
If the scientific goals of some trials focus on precise dosing of n3 supplements, markers of adherence to the study supplementation as well as markers of n3 uptake should prioritized (e.g. by measuring plasma or thrombocyte and erythrocyte membrane composition) [63].
Duration of Supplementation
Generally, it appears valid to assume that chronic diseases such as cardiovascular diseases, atherosclerosis, or psychiatric diseases, such as SZ or BD, may require long-term omega-3 supplementation. Epidemiological studies showing an association between chronic n3-PUFA intake and cardiovascular diseases or the incidence of MDD seem to support this notion [100].
Acute exacerbation of a depressive or psychotic episode, on the other hand, might require higher acute dosing of n3-PUFA supplementation to achieve therapeutic effects. However, considering that there is no empirical evidence to support this premise, clinical trials should to be designed to systematically assess possible relationships between “acuteness” of disease onset and differential effects of various omega-3 supplemental doses.
Different Forms of n3 Supplementation
It is still a matter of debate, whether natural sources of n3-PUFAs should be preferred over refined/distilled forms (e.g. fish oil capsules or EPA/DHA esters). Notwithstanding some may argue that natural foods account for the beneficial effects of n3-PUFA in large-scale epidemiological surveys, many natural n3-PUFA sources display potential hazards. For example, various forms of n3-PUFA-rich seafood and some fish have been found to be contaminated with relatively high levels of methyl-mercury and other pollutants (e.g., dioxine) that may counteract the health benefits.
Moreover, it cannot be ruled out that interventional effects based on natural foods (e.g. fish or n3-rich vegetables) may at least in part be explained by accompanying constituents such as antioxidants (e.g., such as in walnuts) or biologically active proteins/peptides (e.g., from fish). Finally, before designing an intervention trial, one needs to consider that different members of the n3-PUFA family (ALA vs. EPA vs. DHA) may have differential physiological and pharmacological effects [101]. Thus, the composition of the respective n3-PUFA supplement requires some attention.
Clinical Trials in Healthy Individuals
The effects of n3 fatty acid consumption on cognitive performance, including memory functioning, have been examined mostly in clinical trials focusing on the treatment of psychiatric and neurodegenerative diseases. Therefore, the available data on n3 fatty acids in healthy individuals is very limited. Up to date, six randomized, double-blind clinical trials on the use of n3 fatty acids in healthy adults have been published [35, 68, 102-106]. Longitudinal and cross-sectional studies hint at a beneficial effect of a lifelong high intake. A short additional supply of n3-PUFAs to a western-type diet in healthy adults may have small positive effects on the memory function, but results are equivocal. Epidemiologic studies suggest that a long-term, high intake of n3-PUFAs is associated with better memory function. However, these epidemiological surveys (mostly cross-sectional studies) do not allow the establishment of causal inferences.
However, there are a few double-blind studies that investigated the effects of n3-PUFA supplementation on healthy individuals at different stages of life. Amongst young adults, findings are ambiguous, albeit studies reporting no cognitive benefits prevail. Fontani et al. [107], for example, reported an improvement in attention in a group of 33 individuals (mean 33 ± 7 years) following a 35-day long supplementation with n3-PUFAs. However, the sample showed significant improvements in only two of four attention tests which were employed in the study. In another study by Antypa and collaborators [108], a dosage of 2.3g n3-PUFA/d was administered for four weeks. Although small effects on reactivity and risky decision-making were observed, attention, memory, response inhibition and emotion recognition remained unaffected. Jackson et al. [109] reported a clinical trial of 159 individuals aged between 18-35 years who received supplementation with 1 or 2g DHA over a period of 12 weeks. Participants reported a reduction in mental fatigue at times of high cognitive demand, but testing showed no difference in cognition compared to placebo. A group of college students has been tested in a recent study by Karr and colleagues [110]; participants were treated with 480 mg DHA/720 mg EPA per day. No positive effects on verbal learning and memory, executive control and response inhibition were observed. The supplementation of n3 fatty acids during pregnancy, childhood and early adulthood may be safe, and positive effects on some cognitive domains were found occasionally [111, 112], but up to date, there is no consistent evidence for significant effects on memory and cognitive functioning.
Regardless of the increasing number of publications addressing the neuroprotective properties of n3-PUFAs [113, 114], there is still scarce data on the association between n3-PUFAs and memory functioning (using neuroimaging methods) in humans. Clinical and pathological studies have linked n3-PUFA deficiency to the neural disruption of memory functioning during brain development [115]. The longer the period between nutritional deficiency and the subsequent neurobehavioral assessment, the greater the risk for deleterious cognitive outcomes. Therefore, neuroimaging techniques should preferentially be employed in close temporal proximity to the time of nutritional insult [115]. Table 2 summarizes a handful of clinical trials of n3-PUFA supplementation in healthy human subjects.
Table 2.
Relevant studies concerning the effects of n3-PUFAs in healthy human adults. M = mean age.
| Reference No. | Design | Participants | Supplement | Duration | Results |
|---|---|---|---|---|---|
| [176] | RCT | 49 healthy (M=22-55 years) |
DHA (800mg) + EPA (1600mg) + other n-3-PUFAs (400mg) | 35 days | Improvement of attentional functions |
| [177] | RCT, double blind |
54 healthy (M=22 years) |
DHA (250mg) + EPA (1740), placebo | 4 weeks | Effect on reactivity and risky decision-making, but not on attention, memory, response inhibition and emotion recognition |
| [178] | RCT, double blind |
41 healthy (M=20 years) |
DHA (480mg) + EPA (720mg), placebo | 28 days | Not significant |
| [179] | RCT, double blind |
159 healthy (M=18-35 years |
DHA (1000mg or 2000 mg) placebo | 12 weeks | Not significant |
| [180] | RCT, double blind |
391 healthy (M= 65-90 years) |
DHA (1720 mg) + EPA (600mg), placebo | 18 months | Not ended |
| [68] | RCT, double blind |
867 healthy (M= 70-79 years) |
DHA (500 mg) + EPA (200mg), placebo |
24 months | Not significant |
| [181] | RCT, double blind |
485 healthy (M ≥ 55 years) |
DHA (900mg), placebo | 24 weeks | Improved learning and memory function |
| [182] | RCT | 50 healthy (M = 60.5 years) |
caloric restriction, relative increased intake of PUFA, control | 3 months | Not significant for PUFA |
| [183] | RCT, double blind |
49 healthy (M = 60-80 years) |
DHA (800 mg), lutein, combination of DHA and lutein | 4 months | Improvement of memory |
| [184] | Longitudinal study | 1449 healthy (M = 65-80 years) |
No supplement | 21 years | High intake of polyunsaturated fatty acids (PUFA) was associated with better semantic memory |
| [185] | RCT, double blind |
302 healthy (M =70 years) |
DHA/EPA (1800 or 400mg), placebo | 26 weeks | Not significant |
| [186] | Cross-sectional | 1613 healthy (M = 45-70 years) |
No supplement | Fatty fish and marine omega-3 PUFA consumption was associated with a reduced risk of impaired cognitive function | |
| [187] | Prospective | 5386 healthy (M > 55 years) |
No supplement | 2,1 years | Fish consumption was inversely related to incident dementia and in particular to Alzheimer's disease |
Omega-3 fatty acids supplementation: studies in psychiatric populations
Studies in Affective Disorders
Compelling evidences indicate that MDD patients present with cognitive impairments, including but not limited to memory deficits (vide Introduction). Frais and collaborators discussed the question of whether memory loss is a consequence of MDD or vice-versa [116]. It has been shown in animal and human trials that n3-PUFAs may improve memory performance. Patients with MDD present a lower intake of n3-PUFAs and a more limited metabolism than healthy controls [117].
Bipolar disorder is characterized by recurrent episodes of mania/hypomania and depression, and is linked with significant disability, morbidity and premature mortality [118]. The primary pharmacological intervention consists of mood stabilizers and antipsychotics. Findings suggest that n3-PUFAs may be crucially involved in the pathophysiology, treatment and prevention of BD [119]. Like MDD and SZ, individuals with BD present a disturbed lipid metabolism: the levels of DHA, ALA and EPA in erythrocytes of BD patients seem to be lower compared to healthy controls [120]. These findings, and the fact that first-degree relatives of patients showed a trend towards lower levels of n3-PUFAs, suggest that n3-PUFAs may play a role in the treatment of BD. In addition, an ecological study reported higher prevalence rates for bipolar spectrum disorders associated with lower per capita fish/seafood consumption [121]. Comparing BD versus SZ patients, patients with bipolar depression exhibited significantly lower levels of ALA and EPA compared to SZ patients [43]. Low levels of essential FAs seem to be a feature of many psychiatric disorders. Therefore it seems plausible that they may be involved in converging metabolic and signalling pathways that influence the pathophysiology of these diseases [43].
Earlier longitudinal studies with BD patients have investigated the neuroprotective properties of n3 PUFAs investigating T2 relaxation times [122] or looking at N-acetyl aspartate (NAA) [123], a marker of neuronal integrity that can be assessed by magnetic resonance spectroscopy. In the former study with BD female patients, the combination of DHA and EPA brought about greater membrane fluidity, as detected by reductions in T2 values, compared to controls in a 4-week study [122]. In the latter patients receiving EPA for 12 weeks showed increased NAA levels [123].
Additionally, research has shown disturbed fatty acid-related signal transduction as well [124]. N3-PUFAs have been suggested to enhance inhibitory signal transduction mechanisms comparable to mood stabilizers commonly used to treat BD. Moreover, there seems to be a role for BDNF levels, which are considered to be modulated by mood stabilizers as well as by n3-PUFAs.
Notwithstanding meta-analyses indicate that n3-PUFA supplementation may be effective for the treatment of affective symptoms in major depressive disorder [125] and bipolar depression [126], we found no published randomized controlled trial specifically addressing the effects of omega-3 in cognitive performance in either MDD or BD.
Studies in Schizophrenia Populations
Schizophrenia is a mental disorder characterised by positive symptoms like hallucinations and delusions, negative symptoms and cognitive dysfunction. A growing body of studies (e.g. [43]) suggest that abnormalities in phospholipid metabolism may be involved in the pathogenesis of SZ. Accordingly, there have been several investigations of the n3-PUFA content of membrane phospholipids in SZ. The results of these studies are partly inconsistent but generally points to a depletion of n6- and n3-PUFAs in erythrocytes and in neuronal cells of SZ patients. An associated biochemical abnormality in SZ is the elevated activity of phospholipase A2 (Phosphatidylcholin-2-acylhydrase) which releases fatty acids from membrane phospholipids.
Frais reported that levels of PUFAs and arachidonic acid correlated with the output of electrophysiological signals (N400) in unmedicated SZ patients, and that this association has been influenced by stimulus onset asynchrony of a semantic task [127]. The authors concluded that low fatty acids are associated with semantic memory and language dysfunctions in SZ; and this relationship might also influence N400 output.
N3-PUFAs are an essential component of cellular membranes and myelin sheaths around axon fibers, as described by neuropathological studies [128]. Peters and colleagues showed that n3-PUFA concentration in the brain of SZ patients were related to abnormal fractional anisotropy (FA) in Diffusion tensor imaging studies (DTI) across widespread areas of WM [128], including the corpus callosum, bilateral parietal, occipital, temporal and frontal lobes. Inverse correlations were found between n3-PUFA and radial diffusion in the WM tracts of the parietal, occipital, temporal frontal lobes. Increased radial diffusivity is supposed to reflect myelin breakdown [129, 130]. Hence, the results of the studies suggest that low n3-PUFA levels may be associated with myelin loss. To date, however, it is not yet fully understood how n3-PUFAs affect myelination processes. It has been proposed that the long-chain fatty acids may cross the membrane layer and thereby support myelin membrane stabilization [128, 131]. Alternatively, n3-PUFAs may influence myelination by stimulating the expression of myelin proteins [128, 132]. Importantly, disturbances in myelin metabolism has been a consistent finding in SZ [133].
A recent meta-analysis of randomized controlled trials of EPA supplementation in SZ failed to reveal therapeutic benefits compared to placebo for improvement in psychotic symptoms [134]. A single randomized-controlled trial investigated the effects of EPA on cognitive performance in SZ [135]. Fenton and coworkers studied group of N=87 patients meeting criteria for either schizophrenia or schizoaffective disorder with residual symptoms despite antipsychotic treatment in a 16-week randomized controlled trial. By the end of the trial participants randomized to ethyl EPA (3g/day) did not differ in ratings of residual symptoms or cognitive performance from patients randomized to placebo [135].
DISCUSSION
This narrative review addressed the potential beneficial effect of n3-PUFAs dietary supplementation on cognitive functioning in animal models and human adults, as well as potential reductions of cognitive dysfunction in affective disorders and SZ through N3-PUFA supplementation. MDD is often accompanied by the subjective feeling of reduced memory functioning. In moderate to severe forms of depression, this deterioration of cognition and concentration is apparent in neuropsychological testing [27]. The same phenomenon can be found in BD in an acute depressive or manic episode, but also as a cognitive decline between acute mood phases [27]. SZ is defined by positive symptoms, including acoustic hallucinations and delusions. The old label of “dementia praecox” is most appropriate to describe the negative symptoms, such as social retardation and loss of energy, that are accompanied by cognitive deficits [136]. In SZ and affective disorders, an associated impairment of the lipid metabolism was found; this raises the question that memory functions may be ameliorated by n3-PUFA supplementation in these disorders.
Evidence from animal research has demonstrated a favourable association between n3-PUFAs intake and memory function [85, 88, 89, 93, 137, 138]. However, these results failed to be unequivocally replicated in humans [69]. The current state of research indicates that an addition of n3-PUFA supplementation on top of a non-deficient diet most likely only has minimal enhancing effects on cognitive and memory functioning across the lifes pan [69]. However, this appraisal refers to the sum of studies that examined individuals who are considered to be healthy, i.e. have normal eating habits, a regular supply of n3-PUFAs and took additional n3-PUFA supplements for a limited time span only (usually weeks to months). On the other hand, a few longitudinal and cross-sectional population-based studies indicate that a n3-fatty acid rich diet may be associated with better memory performance [36, 139]. As these studies were non-interventional, the nature of the relationship remains unclear. People eating more fish could generally follow a healthier life style and demonstrated a better general health status [140, 141]. They might care more about their nutrition, be more physically and cognitively active, and have higher education levels; these confounding factor were not controlled for in all studies. The life style of Inuits (Greenland Eskimos) seems to differ from a predominantly sedentary lifestyle of average members of western societies, in more aspects than just differences in n3-PUFA intake [45].
Notwithstanding these methodical issues, the significant associations between cognitive health and n3-PUFA intake in numerous epidemiological studies may be explained by long-lasting dietary habits reflecting lifelong higher intake of n3-PUFAs. Considering the potential roles of chronic PUFA intake for brain development and brain ageing over several decades, these beneficial-effects may only partially be replicated in clinical intervention trials that usually last no more than several weeks up to months. Considering n3-PUFA’s known beneficial effects on neuronal viability and function by mediation of inflammation, oxidation, vascular function, brain phosphatides levels and regulation of apoptosis [69], it is safe to assume that a favourable lifelong balance of dietary n6/n3-PUFAs may considerably contribute to decreasing cumulative organ damage. Regarding memory function it could thus prevent cumulative organ damage in the brain. This could explain the significantly lower risk of cognitive and memory impairment in people with a high dietary fish intake.
Affective disorders are often accompanied by memory impairments. As shown in SZ, patients suffering from affective disorders show a disturbed lipid metabolism [43]. More recently, early-stage bipolar disorder individuals were found to have lowered phospholipases A2 activities compared to healthy controls [142] Hence, increased n3-PUFAs may represent a feasible preventive measure [43]. Randomized clinical trials as well as meta-analyses suggest that n3-PUFA supplementation may be effective in treating symptoms of patients suffering from a clinically manifest depression (e.g. [141, 143]. However, to date, no randomized controlled trial had addressed the putative effects of n3-PUFAs as cognitive enhancers for affective disorders. Clearly, the findings of this review indicate that this could be an effective therapeutic strategy which should be tested future trials.
Schizophrenia is a disabling chronic disease that brings with it a reduced cognitive function as a prominent characteristic. Many studies showed lower levels of n3-PUFAs in schizophrenic patients in comparison with healthy controls. This could be due to unhealthy dietary habits as severely affected schizophrenic patients tend to have an unhealthy lifestyle. With regard to the neuroprotective properties of n3-PUFAs, this deficiency could also be a vulnerability factor [43]. This theory is underpinned by the finding of a disturbed lipid metabolism and of the fact that the strength of positive symptoms is negatively correlated to n3-PUFA intake [43]. However, a balanced diet of n6/n3-PUFAs may be beneficial only at an early stage of the disease as shown by [144]. N3-PUFA supply as a prevention method may decrease the vulnerability in a high risk cohort. The published results of EPA supply in an established SZ showed little benefit [134]. A single randomized controlled trial failed to demonstrate cognitive benefits associated with ethyl EPA supplementation [135]. However, this trial included participants with chronic schizophrenia and lasted 16-weeks. Thus, the effects of omega-3 supplementation for cognitive dysfunction in SZ need to be better scrutinized in future studies (e.g., in early-stage SZ).
Limitations and Safety Concerns
In general, the risk of n3-PUFA fatty acid supplementation is considered to be very low as intervention trials reported no serious adverse reactions at the applied n3-PUFA doses [145]. Nonetheless, some potential risks related to n3-PUFA intake need to be considered. In addition to algae and nuts, one important natural n3-PUFA source is fish, which might be contaminated with toxins because of the pollution of the oceans. There is also the risk of accumulating methyl-mercury, dioxins and polychlorinated biphenyls. These toxins may have the potential to increase the risk for cancer or harm an unborn child when fish intake by the mother is increased in order to prevent postpartum depression. However, a review indicates that eating fish once or twice a week is not worrisome [146]. A more intensified fish diet should focus on fish species with lower propensity for toxin accumulation.
The problem of pollution does also apply to n3-PUFA supplements, such as fish oil capsules. Some, but not all of commercially available fish-oils are tested for contaminations. Some products claim to be virtually pollutant-free due to specific distillation processes. Supplements for intervention trials need to display very high levels of purity and should be certified by health agencies (for example, the US Food and Drug Administration).
High doses of s n3-PUFAs may prolong bleeding time [147, 148]. There have been no reports of serious adverse effects like spontaneous bleeding, but the reduced aggregation of thrombocytes should be kept in mind, especially in individuals prone to falls (e.g. frail elderly individuals) or with regard to planned surgery or in subjects with coagulation dustrubances. As a precautionary instrument, in vivo bleeding time should be assessed at baseline and during n3-PUFA supplementation.
In general, studies suggest potential weight reductions during n3-PUFA supplementation which might be explained by metabolic effects related to n3-PUFA induced PPAR-Gamma modulation. Further studies are needed to investigate n3-PUFA’s effects on the uptake, plasma binding and metabolism of psychiatric medications [149].
A potential problem with n3-PUFA supplementation is posed by the question whether supra-physiological doses may adversely affect physiological systems following long-term supplementation. Supplementation of seemingly natural and healthy nutrients has a history of serious negative effects. For example, the SELECT study (Selenium and Vitamin E Cancer Prevention Trial) by the Southwest Oncology Group was aimed to show a prevention of cancer by the intake of vitamin E and selenium as an addition to the ATBC (Alpha-Tocopherol, Beta-Carotene Cancer Prevention) trial. Contrary to expectations, the intake of vitamin E showed a trend towards an elevated risk of prostate carcinoma [150], while dietary supplementation with selenium increased the risk for diabetes [151]. As a consequence of these findings, the study had to be discontinued. Based on these experiences, it may be recommended that clinical trials on n3-PUFA should utilize large supra-physiological doses over longer periods of time in combination with shifting n3/n6 or n3/total fat ratios towards ratios that are regarded as safe and beneficial.
However, an important question pertains to the dosing of n3-PUFAs in intervention trials. The dose necessary to elicit therapeutic benefits may depend on baseline tissue levels of n3-PUFAs: low tissue levels of DHA, the endogenous generation of which is strongly limited from its n3 precursors, may require higher initial supplemental doses of DHA and EPA supplementation, compared to conditions with normal n3-PUFA tissue levels at baseline. A relative reduction of n3-PUFAs may also be postulated in medical conditions with very high inflammatory activity, such as rheumatoid arthritis (RA). Indeed, trials with n3-PUFA doses of up to 7g/d (on average 3.5g/d) showed reduced inflammation markers and improved clinical symptoms in RA [152].
Nevertheless, human trials in healthy individuals suggest that moderate supplemental doses of n3-PUFAs are already more than sufficient to elicit a significant anti-inflammatory effect and that higher supplementation doses do not reveal any further anti-inflammatory benefits in healthy adults [153]. This finding is of particular interest as it may hint towards a possible explanation why interventional studies with higher n3-PUFA doses appeared to be of no better or even worse effect in improving psychiatric or cognitive symptoms than similar but lower-dosed trials: In fact, it could be shown that while two low-dose trials (600 mg – 2000 mg/d of DHA and EPA combined) [154] revealed significant beneficial effects on depression scores, a high-dosed trial (6g/d) [155] on the other hand lead to no such benefits. Thus, these findings call for caution regarding the use of high doses of supplemental n3-PUFA in interventional trials for psychiatric diseases. Since a substantially lower dose may be required for this group of patients than for patients with highly active inflammatory and autoimmune disorders.
CONCLUSION
In conclusion, the potential beneficial effect of n3-PUFAs on memory function is still a matter of debate. We can conclude that there is some very promising but still inconclusive evidence for a relevant clinical benefit from n3-PUFA supplementation for cognitive enhancement in affective disorders and SZ. Based on results of existing clinical trials in healthy volunteers as well as animal research, however, there appears to be a need for larger, controlled clinical trials with n3-PUFAs aiming at cognitive improvement in mood and psychotic disorders. Ideally, these studies would need to be performed in patients and individuals stratified for various genetic risk factors, inflammatory parameters and baseline n3-PUFA levels, as a means to avoid critical confounding factors.
The conventional psychiatric diagnostic categories have been recently challenged by the US National Institute of Health Research Domain Criteria (RDoC) [156]. In this diagnostic framework, emphasis is placed on domain-specific, neuroscientifically-informed elements which spare convential classification boundaries. This review indicates that supplementation with n3-PUFAs may prove novel, domain-specific, agents for memory improvement.
ACKNOWLEDGEMENTS
GSA is supported by a postdoctoral fellowship by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brazil). AFC is supported by a research fellowship award from CNPq.
LIST OF ABBREVIATIONS
- AD
= Alzheimer’s disease
- ALA
= alpha linolenic acid
- ARA
= arachidonic acid
- Aβ
= Amyloid-beta
- BD
= bipolar disorder
- BDNF
= Brain-derived neurotrophic factor
- COX
= cyclooxygenases
- CT
= clinical trial
- DHA
= docosahexaenoic acid
- EPA
= eicosapentaenoic acid
- ERP
= event-related potentials
- FA
= fatty acid
- FO
= fish oil
- GLA
= gamma linolenic acid
- HETEs
= hydroxyeicosatetraenoic acids
- HPETE
= hydroperoxyeicosatetraenoic acids
- LA
= linoleic acid
- LOX
= lipoxygenases
- LTB4
= leukotriene B4
- LTM
= long term memory
- MCI
= mild cognitive impairment
- MDD
= depressive disorder
- n3 / n6
= omega-3, omega-6
- NAA
= N-acetyl aspartate
- PUFA
= polyunsaturated fatty acid
- RCT
= randomised clinical trial
- SFA
= saturated fatty acid
- STM
= short term memory
- SZ
= schizophrenia
- WM
= working memory
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Fareed M., Afzal M. Estimating the inbreeding depression on cognitive behavior: a population based study of child cohort. 2014. [DOI] [PMC free article] [PubMed]
- 2.Rock P.L., Roiser J.P., Riedel W.J. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 2013:1–12. doi: 10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- 3.Bourne C., Aydemir Ö., Balanzá-Martínez V., Bora E., Brissos S., Cavanagh J.T., Clark L., Cubukcuoglu Z., Dias V.V., Dittmann S., Ferrier I.N., Fleck D.E., Frangou S., Gallagher P., Jones L., Kieseppä T., Martínez-Aran A., Melle I., Moore P.B., Mur M., Pfennig A., Raust A., Senturk V., Simonsen C., Smith D.J., Bio D.S., Soeiro-de-Souza M.G., Stoddart S.D., Sundet K., Szöke A., Thompson J.M., Torrent C., Zalla T., Craddock N., Andreassen O.A., Leboyer M., Vieta E., Bauer M., Worhunsky P.D., Tzagarakis C., Rogers R.D., Geddes J.R., Goodwin G.M. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr. Scand. 2013;128(3):149–162. doi: 10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- 4.Fatouros-Bergman H., Cervenka S., Flyckt L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. 2014. [DOI] [PubMed]
- 5.Bora E., Harrison B.J., Yücel M., Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol. Med. 2013;43(10):2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre R.S., Cha D.S., Soczynska J.K., Woldeyohannes H.O., Gallaugher L.A., Kudlow P., Alsuwaidan M., Baskaran A. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress. Anxiety. 2013;30(6):515–527. doi: 10.1002/da.22063. [DOI] [PubMed] [Google Scholar]
- 7.Bora E. Developmental trajectory of cognitive impairment in bipolar disorder: Comparison with schizophrenia. Eur. Neuropsychopharmacol. 2014 doi: 10.1016/j.euroneuro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre R.S., Soczynska J.Z., Woldeyohannes H.O., Alsuwaidan M.T., Cha D.S., Carvalho A.F., Jerrell J.M., Dale R.M., Gallaugher L.A., Muzina D.J., Kennedy S.H. The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr. Psychiatry. 2015;56:279–282. doi: 10.1016/j.comppsych.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 9.Depp C.A., Mausbach B.T., Harmell A.L., Savla G.N., Bowie C.R., Harvey P.D., Patterson T.L. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14(3):217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fett A.K., Viechtbauer W., Dominguez M.D. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. 2011. [DOI] [PubMed]
- 11.Davis J., Moylan S., Harvey B.H., Maes M., Berk M. Neuroprogression in schizophrenia: Pathways underpinning clinical staging and therapeutic corollaries. Aust. N. Z. J. Psychiatry. 2014;48(6):512–529. doi: 10.1177/0004867414533012. [DOI] [PubMed] [Google Scholar]
- 12.Moylan S., Maes M., Wray N.R., Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol. Psychiatry. 2013;18(5):595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 13.Berk M., Kapczinski F., Andreazza A.C., Dean O.M., Giorlando F., Maes M., Yücel M., Gama C.S., Dodd S., Dean B., Magalhães P.V., Amminger P., McGorry P., Malhi G.S. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes B.S., Steiner J., Berk M., Molendijk M.L., Gonzalez-Pinto A., Turck C.W., Nardin P., Gonçalves C.A. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol. Psychiatry. 2015;20(9):1108–1119. doi: 10.1038/mp.2014.117. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K., Malchow B., Falkai P., Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(5):367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 16.Dodd S., Maes M., Anderson G. Putative neuroprotective agents in neuropsychiatric disorders. 2013. [DOI] [PubMed]
- 17.Carvalho A.F., Miskowiak K.K., Hyphantis T.N., Kohler C.A., Alves G.S., Bortolato B., G Sales P.M., Machado-Vieira R., Berk M., McIntyre R.S. Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol. Disord. Drug Targets. 2014;13(10):1819–1835. doi: 10.2174/1871527313666141130203627. [DOI] [PubMed] [Google Scholar]
- 18.Citrome L. Unmet needs in the treatment of schizophrenia: new targets to help different symptom domains. J. Clin. Psychiatry. 2014;75(Suppl. 1):21–26. doi: 10.4088/JCP.13049su1c.04. [DOI] [PubMed] [Google Scholar]
- 19.Dias V.V., Balanzá-Martinez V., Soeiro-de-Souza M.G., Moreno R.A., Figueira M.L., Machado-Vieira R., Vieta E. Pharmacological approaches in bipolar disorders and the impact on cognition: a critical overview. Acta Psychiatr. Scand. 2012;126(5):315–331. doi: 10.1111/j.1600-0447.2012.01910.x. [DOI] [PubMed] [Google Scholar]
- 20.Popovic D., Vieta E., Fornaro M., Perugi G. Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with SSRi antidepressants. J. Affect. Disord. 2015;173:211–215. doi: 10.1016/j.jad.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Kasper S., Resinger E. Cognitive effects and antipsychotic treatment. Psychoneuroendocrinology. 2003;28(Suppl. 1):27–38. doi: 10.1016/S0306-4530(02)00115-4. [DOI] [PubMed] [Google Scholar]
- 22.Marker K.R. COGPACK. The Cognitive Training Package Manual. software m, editor. Heidelberg & Ladenburg1987-2012. [Google Scholar]
- 23.Mogami T., Ikezawa S., Kaneko K., Pu S., Nakagome K. [Outcome studies of cognitive remediation for schizophrenia]. Nihon Shinkei Seishin Yakurigaku Zasshi. 2011;31(5-6):245–249. [Outcome studies of cognitive remediation for schizophrenia]. [PubMed] [Google Scholar]
- 24.Wykes T., Reeder C., Landau S., Everitt B., Knapp M., Patel A., Romeo R. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br. J. Psychiatry. 2007;190:421–427. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]
- 25.Twamley E.W., Jeste D.V., Bellack A.S. A review of cognitive training in schizophrenia. Schizophr. Bull. 2003;29(2):359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- 26.Malchow B., Reich-Erkelenz D., Oertel-Knöchel V., Keller K., Hasan A., Schmitt A., Scheewe T.W., Cahn W., Kahn R.S., Falkai P. The effects of physical exercise in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(6):451–467. doi: 10.1007/s00406-013-0423-2. [DOI] [PubMed] [Google Scholar]
- 27.Torres I.J., Boudreau V.G., Yatham L.N. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr. Scand. Suppl. 2007;(434):17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 28.Heinrichs R.W., Zakzanis K.K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037/0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 29.Millan M.J., Agid Y., Brüne M., Bullmore E.T., Carter C.S., Clayton N.S., Connor R., Davis S., Deakin B., DeRubeis R.J., Dubois B., Geyer M.A., Goodwin G.M., Gorwood P., Jay T.M., Joëls M., Mansuy I.M., Meyer-Lindenberg A., Murphy D., Rolls E., Saletu B., Spedding M., Sweeney J., Whittington M., Young L.J. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 30.Markowitsch H.J. Gedächtnisstörungen. Stuttgart: Kohlhammer; 1999. [Google Scholar]
- 31.Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15(12):771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 32.Cabré E., Mañosa M., Gassull M.A. Omega-3 fatty acids and inflammatory bowel diseases - a systematic review. Br. J. Nutr. 2012;107(Suppl. 2):S240–S252. doi: 10.1017/S0007114512001626. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y.H., Bae S.C., Song G.G. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. 2012. [DOI] [PubMed]
- 34.Baxheinrich A., Stratmann B., Lee-Barkey Y.H., Tschoepe D., Wahrburg U. Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of α-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br. J. Nutr. 2012;108(4):682–691. doi: 10.1017/S0007114512002875. [DOI] [PubMed] [Google Scholar]
- 35.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A., Salem N., Jr, Stedman M., MIDAS Investigators Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6(6):456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Eskelinen M.H., Ngandu T., Helkala E.L., Tuomilehto J., Nissinen A., Soininen H., Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int. J. Geriatr. Psychiatry. 2008;23(7):741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y.H., Bae S.C., Song G.G. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch. Med. Res. 2012;43(5):356–362. doi: 10.1016/j.arcmed.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Bang H.O., Dyerberg J., Sinclair H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980;33(12):2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 39.Patterson E., Wall R., Fitzgerald G.F. Health implications of high dietary omega-6 polyunsaturated Fatty acids. 2012. [DOI] [PMC free article] [PubMed]
- 40.Calder P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2012;668(Supplement 1 ):S50–S58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 41.Calder P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012;142(3):592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 42.Patterson E. Health implications of high dietary omega-6 polyunsaturated Fatty acids. 2012. [DOI] [PMC free article] [PubMed]
- 43.Perica M.M., Delas I. Essential fatty acids and psychiatric disorders. Nutr. Clin. Pract. 2011;26(4):409–425. doi: 10.1177/0884533611411306. [DOI] [PubMed] [Google Scholar]
- 44.Moore S.A., Yoder E., Spector A.A. Role of the blood-brain barrier in the formation of long-chain omega-3 and omega-6 fatty acids from essential fatty acid precursors. J. Neurochem. 1990;55(2):391–402. doi: 10.1111/j.1471-4159.1990.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 45.Simopoulos A.P. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011;44(2):203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 46.Karr J.E., Alexander J.E., Winningham R.G. Omega-3 polyunsaturated fatty acids and cognition throughout the lifespan: a review. 2011. [DOI] [PubMed]
- 47.Hüsing B., Jäncke L., Tag B. Impact Assessment of Neuroimaging: Final Report, vdf Hochschulverlag AG, 2006. 2006.
- 48.Pierpaoli C., Basser P.J. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 49.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calder P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012;142(3):592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 51.Kidd P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007;12(3):207–227. [PubMed] [Google Scholar]
- 52.Simopoulos A.P. Health effects of omega-3 polyunsaturated fatty acids. 2001.
- 53.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong S., Gronert K., Devchand P.R., Moussignac R.L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003;278(17):14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 55.Marcheselli V.L., Hong S., Lukiw W.J., Tian X.H., Gronert K., Musto A., Hardy M., Gimenez J.M., Chiang N., Serhan C.N., Bazan N.G. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278(44):43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee P.K., Marcheselli V.L., Serhan C.N. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. 2004. [DOI] [PMC free article] [PubMed]
- 57.Serhan C.N. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8(2):115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Un D. Biological Significance of Essential Fatty Acids. Japi; 2006. p. 54. [PubMed] [Google Scholar]
- 59.Simopoulos A.P. Omega-3 fatty acids and athletics. Curr. Sports Med. Rep. 2007;6(4):230–236. [PubMed] [Google Scholar]
- 60.Plourde M., Cunnane S.C. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007;32(4):619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 61.Burdge G.C., Calder P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005;45(5):581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 62.Bang H.O., Dyerberg J., Hjøorne N. The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 1976;200(1-2):69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 63.Fekete K., Marosvölgyi T., Jakobik V., Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89(6):2070S–2084S. doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- 64.Taneja A., Singh H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu. Rev. Food Sci. Technol. 2012;3:105–123. doi: 10.1146/annurev-food-022811-101130. [DOI] [PubMed] [Google Scholar]
- 65.Balanzá-Martínez V., Fries G.R., Colpo G.D., Silveira P.P., Portella A.K., Tabarés-Seisdedos R., Kapczinski F. Therapeutic use of omega-3 fatty acids in bipolar disorder. Expert Rev. Neurother. 2011;11(7):1029–1047. doi: 10.1586/ern.11.42. [DOI] [PubMed] [Google Scholar]
- 66.Zicker S.C., Jewell D.E., Yamka R.M., Milgram N.W. Evaluation of cognitive learning, memory, psychomotor, immunologic, and retinal functions in healthy puppies fed foods fortified with docosahexaenoic acid-rich fish oil from 8 to 52 weeks of age. J. Am. Vet. Med. Assoc. 2012;241(5):583–594. doi: 10.2460/javma.241.5.583. [DOI] [PubMed] [Google Scholar]
- 67.Yavin E., Himovichi E., Eilam R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162(4):1011–1022. doi: 10.1016/j.neuroscience.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Dangour A.D., Allen E., Elbourne D., Fasey N., Fletcher A.E., Hardy P., Holder G.E., Knight R., Letley L., Richards M., Uauy R. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2010;91(6):1725–1732. doi: 10.3945/ajcn.2009.29121. [DOI] [PubMed] [Google Scholar]
- 69.Itua I., Naderali E.K. Review: omega-3 and memory function: to eat or not to eat. Am. J. Alzheimers Dis. Other Demen. 2010;25(6):479–482. doi: 10.1177/1533317510376943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orr S.K., Trépanier M.O., Bazinet R.P. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88(1):97–103. doi: 10.1016/j.plefa.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 71.Meijerink J., Balvers M., Witkamp R. N-Acyl amines of docosahexaenoic acid and other n-3 polyunsatured fatty acids - from fishy endocannabinoids to potential leads. Br. J. Pharmacol. 2013;169(4):772–783. doi: 10.1111/bph.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajjar T., Meng G.Y., Rajion M.A., Vidyadaran S., Othman F., Farjam A.S., Li T.A., Ebrahimi M. Omega 3 polyunsaturated fatty acid improves spatial learning and hippocampal peroxisome proliferator activated receptors (PPARα and PPARγ) gene expression in rats. BMC Neurosci. 2012;13:109. doi: 10.1186/1471-2202-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kou W., Luchtman D., Song C. Eicosapentaenoic acid (EPA) increases cell viability and expression of neurotrophin receptors in retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cells. Eur. J. Nutr. 2008;47(2):104–113. doi: 10.1007/s00394-008-0703-1. [DOI] [PubMed] [Google Scholar]
- 74.Meijerink J., Balvers M., Witkamp R. N-Acyl amines of docosahexaenoic acid and other n-3 polyunsatured fatty acids - from fishy endocannabinoids to potential leads. Br. J. Pharmacol. 2013;169(4):772–783. doi: 10.1111/bph.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mingam R., Moranis A., Bluthé R.M., De Smedt-Peyrusse V., Kelley K.W., Guesnet P., Lavialle M., Dantzer R., Layé S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur. J. Neurosci. 2008;28(9):1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delpech J.C., Madore C., Joffre C., Aubert A., Kang J.X., Nadjar A., Layé S. Transgenic increase in n-3/n-6 fatty acid ratio protects against cognitive deficits induced by an immune challenge through decrease of neuroinflammation. Neuropsychopharmacology. 2015;40(3):525–536. doi: 10.1038/npp.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Smedt-Peyrusse V., Sargueil F., Moranis A., Harizi H., Mongrand S., Layé S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008;105(2):296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 78.Figueroa J.D., Cordero K., Baldeosingh K., Torrado A.I., Walker R.L., Miranda J.D., Leon M.D. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J. Neurotrauma. 2012;29(3):551–566. doi: 10.1089/neu.2011.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hjorth E., Zhu M., Toro V.C., Vedin I., Palmblad J., Cederholm T., Freund-Levi Y., Faxen-Irving G., Wahlund L.O., Basun H., Eriksdotter M., Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 2013;35(4):697–713. doi: 10.3233/JAD-130131. [DOI] [PubMed] [Google Scholar]
- 80.Madore C., Nadjar A., Delpech J.C. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. 2014. [DOI] [PubMed]
- 81.Arunagiri P., Rajeshwaran K., Shanthakumar J., Tamilselvan T., Balamurugan E. Combination of omega-3 Fatty acids, lithium, and aripiprazole reduces oxidative stress in brain of mice with mania. Biol. Trace Elem. Res. 2014;160(3):409–417. doi: 10.1007/s12011-014-0067-8. [DOI] [PubMed] [Google Scholar]
- 82.de Mello A.H., Gassenferth A., Schraiber Rde.B., Souza Lda.R., Florentino D., Danielski L.G., Cittadin-Soares Eda.C., Fortunato J.J., Petronilho F., Quevedo J., Rezin G.T. Effects of omega-3 on behavioral and biochemical parameters in rats submitted to chronic mild stress. Metab. Brain Dis. 2014;29(3):691–699. doi: 10.1007/s11011-014-9577-5. [DOI] [PubMed] [Google Scholar]
- 83.Zugno A.I., Chipindo H.L., Volpato A.M., Budni J., Steckert A.V., de Oliveira M.B., Heylmann A.S., da Rosa Silveira F., Mastella G.A., Maravai S.G., Wessler P.G., Binatti A.R., Panizzutti B., Schuck P.F., Quevedo J., Gama C.S. Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience. 2014;259:223–231. doi: 10.1016/j.neuroscience.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 84.Moriguchi T., Greiner R.S., Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75(6):2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 85.Tanabe Y., Hashimoto M., Sugioka K., Maruyama M., Fujii Y., Hagiwara R., Hara T., Hossain S.M., Shido O. Improvement of spatial cognition with dietary docosahexaenoic acid is associated with an increase in Fos expression in rat CA1 hippocampus. Clin. Exp. Pharmacol. Physiol. 2004;31(10):700–703. doi: 10.1111/j.1440-1681.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- 86.Lim S.Y., Hoshiba J., Moriguchi T., Salem N., Jr N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr. Res. 2005;58(4):741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- 87.Fedorova I., Hussein N., Di Martino C., Moriguchi T., Hoshiba J., Majchrzak S., Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77(5-6):269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung W.L., Chen J.J., Su H.M. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 2008;138(6):1165–1171. doi: 10.1093/jn/138.6.1165. [DOI] [PubMed] [Google Scholar]
- 89.Haider S., Batool Z., Tabassum S., Perveen T., Saleem S., Naqvi F., Javed H., Haleem D.J. Effects of walnuts (Juglans regia) on learning and memory functions. Plant Foods Hum. Nutr. 2011;66(4):335–340. doi: 10.1007/s11130-011-0260-2. [DOI] [PubMed] [Google Scholar]
- 90.Vinot N., Jouin M., Lhomme-Duchadeuil A., Guesnet P., Alessandri J.M., Aujard F., Pifferi F. Omega-3 fatty acids from fish oil lower anxiety, improve cognitive functions and reduce spontaneous locomotor activity in a non-human primate. PLoS One. 2011;6(6):e20491. doi: 10.1371/journal.pone.0020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trofimiuk E., Braszko J.J. Long-term administration of cod liver oil ameliorates cognitive impairment induced by chronic stress in rats. Lipids. 2011;46(5):417–423. doi: 10.1007/s11745-011-3551-3. [DOI] [PubMed] [Google Scholar]
- 92.Wietrzych-Schindler M., Szyszka-Niagolov M., Ohta K. Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. 2011. [DOI] [PubMed]
- 93.Fernandes J.S., Mori M.A., Ekuni R., Oliveira R.M., Milani H. Long-term treatment with fish oil prevents memory impairments but not hippocampal damage in rats subjected to transient, global cerebral ischemia. Nutr. Res. 2008;28(11):798–808. doi: 10.1016/j.nutres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Bas O., Songur A., Sahin O. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. 2007. [DOI] [PubMed]
- 95.Song C., Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J. Lipid Res. 2004;45(6):1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Kovács K.J. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 1998;33(4):287–297. doi: 10.1016/S0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 97.Gamoh S., Hashimoto M., Hossain S., Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin. Exp. Pharmacol. Physiol. 2001;28(4):266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 98.Gama C.S., Canever L., Panizzutti B., Gubert C., Stertz L., Massuda R., Pedrini M., de Lucena D.F., Luca R.D., Fraga D.B., Heylmann A.S., Deroza P.F., Zugno A.I. Effects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophrenia. Schizophr. Res. 2012;141(2-3):162–167. doi: 10.1016/j.schres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Simopoulos A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002;21(6):495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 100.Sanhueza C., Ryan L., Foxcroft D.R. Diet and the risk of unipolar depression in adults: systematic review of cohort studies. J. Hum. Nutr. Diet. 2013;26(1):56–70. doi: 10.1111/j.1365-277X.2012.01283.x. [DOI] [PubMed] [Google Scholar]
- 101.Peet M., Brind J., Ramchand C.N., Shah S., Vankar G.K. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr. Res. 2001;49(3):243–251. doi: 10.1016/S0920-9964(00)00083-9. [DOI] [PubMed] [Google Scholar]
- 102.Swainson R., Hodges J.R., Galton C.J., Semple J., Michael A., Dunn B.D., Iddon J.L., Robbins T.W., Sahakian B.J. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement. Geriatr. Cogn. Disord. 2001;12(4):265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- 103.van de Rest O., Geleijnse J.M., Kok F.J., van Staveren W.A., Dullemeijer C., Olderikkert M.G., Beekman A.T., de Groot C.P. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 104.Johnson E.J., McDonald K., Caldarella S.M., Chung H.Y., Troen A.M., Snodderly D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008;11(2):75–83. doi: 10.1179/147683008X301450. [DOI] [PubMed] [Google Scholar]
- 105.Delis D.C., Kramer J.H., Kaplan E. The California Verbal Learning Test. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 106.Danthiir V., Burns N.R., Nettelbeck T., Wilson C., Wittert G. The older people, omega-3, and cognitive health (EPOCH) trial design and methodology: a randomised, double-blind, controlled trial investigating the effect of long-chain omega-3 fatty acids on cognitive ageing and wellbeing in cognitively healthy older adults. Nutr. J. 2011;10:117. doi: 10.1186/1475-2891-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fontani G., Corradeschi F., Felici A., Alfatti F., Migliorini S., Lodi L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Invest. 2005;35(11):691–699. doi: 10.1111/j.1365-2362.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 108.Antypa N., Van der Does A.J., Smelt A.H., Rogers R.D. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J. Psychopharmacol. (Oxford) 2009;23(7):831–840. doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]
- 109.Jackson P.A., Deary M.E., Reay J.L., Scholey A.B., Kennedy D.O. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18-35 years. Br. J. Nutr. 2012;107(8):1232–1243. doi: 10.1017/S000711451100403X. [DOI] [PubMed] [Google Scholar]
- 110.Karr J.E., Alexander J.E., Winningham R.G. Omega-3 polyunsaturated fatty acids and cognition throughout the lifespan: a review. Nutr. Neurosci. 2011;14(5):216–225. doi: 10.1179/1476830511Y.0000000012. [DOI] [PubMed] [Google Scholar]
- 111.Dunstan J.A., Simmer K., Dixon G. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch. Dis. Child Fetal Neonatal. 2008;93:F45–50. doi: 10.1136/adc.2006.099085. [DOI] [PubMed] [Google Scholar]
- 112.Judge M.P., Harel O., Lammi-Keefe C.J. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am. J. Clin. Nutr. 2007;85(6):1572–1577. doi: 10.1093/ajcn/85.6.1572. [DOI] [PubMed] [Google Scholar]
- 113.Cao D.H., Xu J.F., Xue R.H., Zheng W.F., Liu Z.L. Protective effect of chronic ethyl docosahexaenoate administration on brain injury in ischemic gerbils. Pharmacol. Biochem. Behav. 2004;79(4):651–659. doi: 10.1016/j.pbb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 114.Cao D., Li M., Xue R., Zheng W., Liu Z., Wang X. Chronic administration of ethyl docosahexaenoate decreases mortality and cerebral edema in ischemic gerbils. Life Sci. 2005;78(1):74–81. doi: 10.1016/j.lfs.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 115.Georgieff M.K. Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 2007;85(2):614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 116.Frais A.T. Depression and the causal role of specific memory system degenerations: link may be supported by reported therapeutic benefits of Omega 3 fatty acids. Med. Hypotheses. 2007;69(1):67–69. doi: 10.1016/j.mehy.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 117.Park Y., Kim M., Baek D., Kim S.H. Erythrocyte n-3 polyunsaturated fatty acid and seafood intake decrease the risk of depression: case-control study in Korea. Ann. Nutr. Metab. 2012;61(1):25–31. doi: 10.1159/000339264. [DOI] [PubMed] [Google Scholar]
- 118.Ketter T.A. Diagnostic features, prevalence, and impact of bipolar disorder. 2010. [DOI] [PubMed]
- 119.Sublette M.E., Ellis S.P., Geant A.L., Mann J.J. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry. 2011;72(12):1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balanzá-Martínez V., Fries G.R., Colpo G.D., Silveira P.P., Portella A.K., Tabarés-Seisdedos R., Kapczinski F. Therapeutic use of omega-3 fatty acids in bipolar disorder. Expert Rev. Neurother. 2011;11(7):1029–1047. doi: 10.1586/ern.11.42. [DOI] [PubMed] [Google Scholar]
- 121.Noaghiul S., Hibbeln J.R. Cross-national comparisons of sea food consumption and rates of bipolar disorders. . Am. J. Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 122.Hirashima F., Parow A.M., Stoll A.L. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am. J. Psychiatry. 2004;161:1922–1924. doi: 10.1176/appi.ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 123.Frangou S., Lewis M., Wollard J., Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J. Psychopharmacol. (Oxford) 2007;21(4):435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 124.Horrobin D.F., Bennett C.N. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot. Essent. Fatty Acids. 1999;60(4):217–234. doi: 10.1054/plef.1999.0037. [DOI] [PubMed] [Google Scholar]
- 125.Grosso G., Pajak A., Marventano S., Castellano S., Galvano F., Bucolo C., Drago F., Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9(5):e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarris J., Mischoulon D., Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. 2012. [DOI] [PubMed]
- 127.Condray R., Yao J.K., Steinhauer S.R. Semantic memory in schizophrenia: association with cell membrane essential fatty acids. 2008. [DOI] [PMC free article] [PubMed]
- 128.Peters B.D., Szeszko P.R., Radua J., Ikuta T., Gruner P., DeRosse P., Zhang J.P., Giorgio A., Qiu D., Tapert S.F., Brauer J., Asato M.R., Khong P.L., James A.C., Gallego J.A., Malhotra A.K. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr. Bull. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song S.K., Sun S.W., Ramsbottom M.J. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 130.Sun S.W., Liang H.F., Le T.Q., Armstrong R.C., Cross A.H., Song S.K. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32(3):1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- 131.Sastry P.S. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res. 1985;24(2):69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 132.Salvati S., Natali F., Attorri L., Di Benedetto R., Leonardi F., Di Biase A., Ferri F., Fortuna S., Lorenzini P., Sanchez M., Ricceri L., Vitelli L. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J. Neurosci. Res. 2008;86(4):776–784. doi: 10.1002/jnr.21537. [DOI] [PubMed] [Google Scholar]
- 133.Mighdoll M.I., Tao R., Kleinman J.E., Hyde T.M. Myelin, myelin-related disorders, and psychosis. Schizophr. Res. 2015;161(1):85–93. doi: 10.1016/j.schres.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 134.Fusar-Poli P., Berger G. Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J. Clin. Psychopharmacol. 2012;32(2):179–185. doi: 10.1097/JCP.0b013e318248b7bb. [DOI] [PubMed] [Google Scholar]
- 135.Fenton W.S., Dickerson F., Boronow J., Hibbeln J.R., Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am. J. Psychiatry. 2001;158(12):2071–2074. doi: 10.1176/appi.ajp.158.12.2071. [DOI] [PubMed] [Google Scholar]
- 136.Hahlweg K. Schizophrenie. in Störungen im Erwachsenenalter - Spezielle Indikationen - Glossar (Lehrbuch der Verhaltenstherapie Bd 2, 3 Aufl, S 407-434). Edited by Schneider JMS. Berlin: Springer; 2009. [Google Scholar]
- 137.Gamoh S., Hashimoto M., Sugioka K., Shahdat Hossain M., Hata N., Misawa Y., Masumura S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93(1):237–241. doi: 10.1016/S0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 138.Hashimoto M., Tanabe Y., Fujii Y., Kikuta T., Shibata H., Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr. 2005;135(3):549–555. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- 139.Kalmijn S., Launer L.J., Ott A., Witteman J.C., Hofman A., Breteler M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997;42(5):776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 140.Logan A.C. Omega-3 fatty acids and major depression: a primer for the mental health professional. Lipids Health Dis. 2004;3:25–32. doi: 10.1186/1476-511X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Timonen M., Horrobin D., Jokelainen J. Fish consumption and depression: the Northern Finland 1966 birth cohort study. 2004. [DOI] [PubMed]
- 142.Ikenaga E.H., Talib L.L., Ferreira A.S., Machado-Vieira R., Forlenza O.V., Gattaz W.F. Reduced activities of phospholipases A2 in platelets of drug-naïve bipolar disorder patients. Bipolar Disord. 2015;17(1):97–101. doi: 10.1111/bdi.12229. [DOI] [PubMed] [Google Scholar]
- 143.Edwards R., Peet M., Shay J. Omega-3 polyunsaturated fatty acid levels in the diet and in the red blood cell membranes of depressed patients. 1998. [DOI] [PubMed]
- 144.Amminger G.P., Schäfer M.R., Papageorgiou K. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. 2010. [DOI] [PubMed]
- 145.Sydenham E., Dangour A.D., Lim W.S. Omega 3 fatty acid for the prevention of cognitive decline and dementia. 2012. [DOI] [PubMed]
- 146.Mozaffarian D., Rimm E.B. Fish intake, contaminants, and human health: evaluating the risks and the benefits. 2006. [DOI] [PubMed]
- 147.Emsley R., Myburgh C., Oosthuizen P., van Rensburg S.J. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am. J. Psychiatry. 2002;159(9):1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- 148.Emsley R., Niehaus D.J., Koen L., Oosthuizen P.P., Turner H.J., Carey P., van Rensburg S.J., Maritz J.S., Murck H. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr. Res. 2006;84(1):112–120. doi: 10.1016/j.schres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 149.Brandt H.M., Popish S.J., Lott R.S. Effect of omega-3 fatty acids on valproate plasma protein binding. Ann. Clin. Psychiatry. 2010;22(4):280–282. [PubMed] [Google Scholar]
- 150.Klein E.A., Thompson I.M., Jr, Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., Minasian L.M., Ford L.G., Parnes H.L., Gaziano J.M., Karp D.D., Lieber M.M., Walther P.J., Klotz L., Parsons J.K., Chin J.L., Darke A.K., Lippman S.M., Goodman G.E., Meyskens F.L., Jr, Baker L.H. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dunn B.K., Richmond E.S., Minasian L.M., Ryan A.M., Ford L.G. A nutrient approach to prostate cancer prevention: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutr. Cancer. 2010;62(7):896–918. doi: 10.1080/01635581.2010.509833. [DOI] [PubMed] [Google Scholar]
- 152.Calder P.C. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘Nutrition and autoimmune disease’ PUFA, inflammatory processes and rheumatoid arthritis. Proc. Nutr. Soc. 2008;67(4):409–418. doi: 10.1017/S0029665108008690. [DOI] [PubMed] [Google Scholar]
- 153.Kiecolt-Glaser J.K., Belury M.A., Andridge R., Malarkey W.B., Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav. Immun. 2011;25(8):1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nemets H., Nemets B., Apter A. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. 2006. [DOI] [PubMed]
- 155.Keck P.E.J., Mintz J., McElroy S.L. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. 2006. [DOI] [PubMed]
- 156.Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sergeant S., McQuail J.A., Riddle D.R., Chilton F.H., Ortmeier S.B., Jessup J.A., Groban L., Nicolle M.M. Dietary fish oil modestly attenuates the effect of age on diastolic function but has no effect on memory or brain inflammation in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66(5):521–533. doi: 10.1093/gerona/glr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vinot N., Jouin M., Lhomme-Duchadeuil A. Omega-3 fatty acids from fish oil lower anxiety, improve cognitive functions and reduce spontaneous locomotor activity in a non-human primate. 2011. [DOI] [PMC free article] [PubMed]
- 159.Wietrzych-Schindler M., Szyszka-Niagolov M., Ohta K. Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. 2011. [DOI] [PubMed]
- 160.Hashimoto M., Hossain S., Tanabe Y., Kawashima A., Harada T., Yano T., Mizuguchi K., Shido O. The protective effect of dietary eicosapentaenoic acid against impairment of spatial cognition learning ability in rats infused with amyloid beta(1-40). J. Nutr. Biochem. 2009;20(12):965–973. doi: 10.1016/j.jnutbio.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 161.Chung W.L., Chen J.J., Su H.M. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 2008;138(6):1165–1171. doi: 10.1093/jn/138.6.1165. [DOI] [PubMed] [Google Scholar]
- 162.Holguin S., Huang Y., Liu J., Wurtman R. Chronic administration of DHA and UMP improves the impaired memory of environmentally impoverished rats. Behav. Brain Res. 2008;191(1):11–16. doi: 10.1016/j.bbr.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Petursdottir A.L., Farr S.A., Morley J.E., Banks W.A., Skuladottir G.V. Effect of dietary n-3 polyunsaturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63(11):1153–1160. doi: 10.1093/gerona/63.11.1153. [DOI] [PubMed] [Google Scholar]
- 164.Fedorova I., Hussein N., Di Martino C., Moriguchi T., Hoshiba J., Majchrzak S., Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77(5-6):269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hashimoto M., Hossain S., Agdul H. Docosahexaenoic acid-induced amelioration on impairment of memory learning in amyloid beta-infused rats relates to the decreases of amyloid beta and cholesterol levels in detergent-insoluble membrane fractions. 2005. [DOI] [PubMed]
- 166.Lim S.Y., Hoshiba J., Moriguchi T., Salem N., Jr N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr. Res. 2005;58(4):741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- 167.Roegge C.S., Widholm J.J., Engeseth N.J., Wang X., Brosch K.O., Seegal R.F., Schantz S.L. Delayed spatial alternation impairments in adult rats following dietary n-6 deficiency during development. Neurotoxicol. Teratol. 2005;27(3):485–495. doi: 10.1016/j.ntt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 168.Song C., Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J. Lipid Res. 2004;45(6):1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- 169.Tanabe Y., Hashimoto M., Sugioka K., Maruyama M., Fujii Y., Hagiwara R., Hara T., Hossain S.M., Shido O. Improvement of spatial cognition with dietary docosahexaenoic acid is associated with an increase in Fos expression in rat CA1 hippocampus. Clin. Exp. Pharmacol. Physiol. 2004;31(10):700–703. doi: 10.1111/j.1440-1681.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- 170.Clements K.M., Girard T.A., Xing H.C., Wainwright P.E. Spontaneously hypertensive and Wistar Kyoto rats differ in delayed matching-to-place performance and response to dietary long-chain polyunsaturated fatty acids. Dev. Psychobiol. 2003;43(1):57–69. doi: 10.1002/dev.10121. [DOI] [PubMed] [Google Scholar]
- 171.Gamoh S., Hashimoto M., Hossain S., Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin. Exp. Pharmacol. Physiol. 2001;28(4):266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 172.Moriguchi T., Greiner R.S., Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75(6):2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 173.Wainwright P.E., Xing H.C., Ward G.R., Huang Y.S., Bobik E., Auestad N., Montalto M. Water maze performance is unaffected in artificially reared rats fed diets supplemented with arachidonic acid and docosahexaenoic acid. J. Nutr. 1999;129(5):1079–1089. doi: 10.1093/jn/129.5.1079. [DOI] [PubMed] [Google Scholar]
- 174.Umezawa M., Ohta A., Tojo H., Yagi H., Hosokawa M., Takeda T. Dietary alpha-linolenate/linoleate balance influences learning and memory in the senescence-accelerated mouse (SAM). Brain Res. 1995;669(2):225–233. doi: 10.1016/0006-8993(94)01250-L. [DOI] [PubMed] [Google Scholar]
- 175.Yamamoto N., Okaniwa Y., Mori S., Nomura M., Okuyama H. Effects of a high-linoleate and a high-alpha-linolenate diet on the learning ability of aged rats. Evidence against an autoxidation-related lipid peroxide theory of aging. J. Gerontol. 1991;46(1):B17–B22. doi: 10.1093/geronj/46.1.B17. [DOI] [PubMed] [Google Scholar]
- 176.Fontani G., Corradeschi F., Felici A., Alfatti F., Migliorini S., Lodi L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Invest. 2005;35(11):691–699. doi: 10.1111/j.1365-2362.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 177.Antypa N., Van der Does A.J., Smelt A.H., Rogers R.D. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J. Psychopharmacol. (Oxford) 2009;23(7):831–840. doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]
- 178.Karr J.E., Grindstaff T.R., Alexander J.E. Omega-3 polyunsaturated fatty acids and cognition in a college-aged population. Exp. Clin. Psychopharmacol. 2012;20(3):236–242. doi: 10.1037/a0026945. [DOI] [PubMed] [Google Scholar]
- 179.Jackson P.A., Deary M.E., Reay J.L. No effect of 12 weeks' supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18-35 years. Br. J. Nutr. 2012;107:1232–1243. doi: 10.1017/S000711451100403X. [DOI] [PubMed] [Google Scholar]
- 180.Danthiir V., Burns N.R., Nettelbeck T., Wilson C., Wittert G. The older people, omega-3, and cognitive health (EPOCH) trial design and methodology: a randomised, double-blind, controlled trial investigating the effect of long-chain omega-3 fatty acids on cognitive ageing and wellbeing in cognitively healthy older adults. Nutr. J. 2011;10:117. doi: 10.1186/1475-2891-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A., Salem N., Jr, Stedman M., MIDAS Investigators Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6(6):456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 182.Witte A.V., Fobker M., Gellner R., Knecht S., Flöel A. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA. 2009;106(4):1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Johnson E.J., McDonald K., Caldarella S.M., Chung H.Y., Troen A.M., Snodderly D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008;11(2):75–83. doi: 10.1179/147683008X301450. [DOI] [PubMed] [Google Scholar]
- 184.Eskelinen M.H., Ngandu T., Helkala E.L., Tuomilehto J., Nissinen A., Soininen H., Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int. J. Geriatr. Psychiatry. 2008;23(7):741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 185.van de Rest O., Geleijnse J.M., Kok F.J., van Staveren W.A., Hoefnagels W.H., Beekman A.T., de Groot L.C. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2008;88(3):706–713. doi: 10.1093/ajcn/88.3.706. [DOI] [PubMed] [Google Scholar]
- 186.Kalmijn S., van Boxtel M.P., Ocke M. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. 2004. [DOI] [PubMed]
- 187.Kalmijn S., Feskens E.J., Launer L.J., Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997;145(1):33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]


