Abstract
The subjective experience of cognitive dysfunction (“fibrofog”) is common in fibromyalgia. This study investigated the relation between subjective appraisal of cognitive function, objective cognitive task performance, and brain activity during a cognitive task using functional magnetic resonance imaging (fMRI). Sixteen fibromyalgia patients and 13 healthy pain-free controls completed a battery of questionnaires, including the Multiple Ability Self-Report Questionnaire (MASQ), a measure of self-perceived cognitive difficulties. Participants were evaluated for working memory performance using a modified N-back working memory task while undergoing Blood Oxygen Level Dependent (BOLD) fMRI measurements. Fibromyalgia patients and controls did not differ in working memory performance. Subjective appraisal of cognitive function was associated with better performance (accuracy) on the working memory task in healthy controls but not in fibromyalgia patients. In fibromyalgia patients, increased perceived cognitive difficulty was positively correlated with the severity of their symptoms. BOLD response during the working memory task did not differ between the groups. BOLD response correlated with task accuracy in control subjects but not in fibromyalgia patients. Increased subjective cognitive impairment correlated with decreased BOLD response in both groups but in different anatomic regions. In conclusion, “fibrofog” appears to be better characterized by subjective rather than objective impairment. Neurologic correlates of this subjective experience of impairment might be separate from those involved in the performance of cognitive tasks.
Keywords: Fibromyalgia, Cognitive dysfunction, Neuroimaging, Cognitive testing
Highlights
-
•
Healthy and fibromyalgia volunteers performed the same on a working memory task.
-
•
BOLD brain measurements during the working task were also the same between groups.
-
•
Accuracy was related to cognitive appraisal in healthy volunteers but not in fibromyalgia.
-
•
Cognitive appraisal correlated with different patterns of BOLD activity in each group.
-
•
The feeling of cognitive impairment appears to be functionally separate from task performance.
1. Introduction
Perceived cognitive dysfunction (“fibrofog”) is an increasingly appreciated clinical complaint in fibromyalgia. Along with painful symptoms, over 50% of fibromyalgia patients experience distressing subjective cognitive impairment (Katz et al., 2004, Yunus et al., 1981). The most common complaints encompass the abilities to attend, concentrate, remember, use language, multi-task, and organize information. Dyscognition contributes significantly to both functional and work disability in fibromyalgia. Commensurate with its increasing recognition, its subjective evaluation has been incorporated into the 2010 preliminary American College of Rheumatology fibromyalgia diagnostic criteria (Wolfe et al., 2010).
Persons with fibromyalgia commonly perceive discordance between how they feel about their abilities and how they actually perform. In one seminal study, patients with rheumatoid arthritis, ankylosing spondylitis, and fibromyalgia were asked to rate their ability to perform several physical activities and were then videotaped actually performing these activities (Hidding et al., 1994). A striking discordance between self-reported ability and observed functional disability was observed in fibromyalgia that was not found in other patients with rheumatic disease. It is possible that the dyscognition of fibromyalgia may reflect a similar phenomenon, with discordance between the subjective experience of performing cognitive tasks and objective performance on those tasks.
To date, the entire body of science on dyscognition in fibromyalgia includes ~ 20 publications, mostly small population comparisons of fibromyalgia patients to controls performing cognitive tests (Ambrose et al., 2012). In summary, objective differences of small effect sizes have been demonstrated in performance on some neuropsychological tests of attention, executive function, and memory (Glass and Park, 2001). However, these findings often required experimental provocation (i.e. distraction, stress) to demonstrate effects (Dick et al., 2002, Glass, 2009, Glass et al., 2011, Leavitt and Katz, 2006, Leavitt and Katz, 2009, Reyes Del Paso et al., 2012, Seo et al., 2012) and effects are often not found with standard cognitive tests (Glass et al., 2011; Mohs et al.; Walitt et al., 2008).
Only a few studies have investigated the neurocognitive correlates of cognitive impairment in fibromyalgia. Using fMRI and a simple response inhibition task selected to ensure comparable performance (Go/No-Go task), Glass, et al. found that, compared to controls, fibromyalgia patients had less activation in several task-related brain areas and increased activation elsewhere in the brain (Glass et al., 2011). Using the 2back working memory task paradigm, Seo, et al. also found that fibromyalgia patients had less activation in brain areas related to working memory together with a small clinical difference in performance (88.26% vs. 95.56% accuracy) (Seo et al., 2012). More recently, Čeko, et al. reported finding no difference in performance or BOLD response between 28 fibromyalgia patients and 23 healthy controls on multiple levels of the N-back task (Čeko et al., 2015). All studies used methods that contrasted two different task difficulty levels (NoGo > Go; Nback > 0back). These papers present a variety of interpretations; 1) that decreases in task-related brain activity represent a deficit in task ability, perhaps due to overlapping networks leading to reduced resources to perform task) (Glass et al., 2011), 2) that impairment of the prefrontal cortex may lead to inappropriate organization of information (Seo et al., 2012), and 3) that there is no evidence of a measureable difference in Nback cognitive performance or compensatory neural recruitment in task-related brain networks (Čeko et al., 2015). All three studies adjusted for patient characteristics in their analyses but did not consider the discordance between the severity of subjective cognitive complaint and (objective) task performance.
Clinically, the objective consequences of dyscognition in fibromyalgia are not clear (Shmygalev et al., 2014). Cognitive deficits are not overtly obvious to medical observation, certainly far less than what is seen in pathologically-defined neuropsychiatric dementias such as Alzheimer's disease and vascular dementia. Objective differences noted on cognitive testing also correlate poorly with self-perception of cognitive deficits (Tesio et al., 2015). The dyscognition of fibromyalgia is perhaps better defined by the distressing intrusiveness of subjective cognitive symptoms rather than by a clinically-relevant cognitive deficit.
Here, we aimed to separate brain mechanisms related to subjective appraisal (subjective dyscognition) from those related to performance on a cognitive working memory task (objective dyscognition). We evaluated subjective cognitive function, as well as objective cognitive task performance, and brain activity during a cognitive task using BOLD functional magnetic resonance imaging (fMRI).
2. Materials and method
2.1. Subjects
Eighteen right-handed, female fibromyalgia patients and a group of 16 age-matched female right-handed healthy controls participated in this study. One fibromyalgia patient was excluded from the study because she was found to be pregnant during screening. One fibromyalgia patient and three healthy controls were unable to demonstrate understanding of the N-back task during the practice sessions and were excluded from analyses. All fibromyalgia patients (n = 16) met the 1990 American College of Rheumatology fibromyalgia criteria and did not have concomitant medical diagnoses. Healthy controls (n = 13) did not have any medical or psychiatric diagnoses, were not taking any medications, and did not meet fibromyalgia diagnostic criteria. Exclusion criteria for fMRI participation included left-handedness, pregnancy, cardiac pacemakers or other body metals, and claustrophobia. Behavioral data are missing on one patient for N-back task and one control for the N-back task and Multiple Ability Self-Report Questionnaire (MASQ).
This study was approved by the MedStar Health Institutional Review Board (protocol number 2010–050).
2.2. Measures of fibromyalgia impact and symptoms
Fibromyalgia Impact Questionnaire (FIQ) is a validated questionnaire designed to assess the spectrum of problems related to fibromyalgia and treatment responses (Burckhardt et al., 1991). It evaluates three domains: function, overall impact, and polysymptom burden related to pain, fatigue, sleep, and mood issues. The original FIQ is scored on a 0–100 scale with high scores indicating more severe symptoms. A 14% change in FIQ scores is considered the minimally clinical important difference (MCID) in fibromyalgia symptoms (Bennett, 2005). Patients enrolled in fibromyalgia clinical trials typically have FIQ scores of 50 or greater (Bennett, 2005, Hauser et al., 2013, Williams and Arnold, 2011). As FIQ scores represent the full spectrum of fibromyalgia complaints, FIQ score was used as a representative score of symptom burden in our imaging analyses.
Brief Pain Inventory (BPI) is a validated measurement of pain severity and pain interference (Tan et al., 2004). Both are scored on a 0–10 scales, with high scores indicating more severe pain. Patients enrolled in fibromyalgia clinical trials typically have BPI scores of about 6 (Hauser et al., 2013). A 2-3 point change is typically considered the MCID in pain (Keller et al., 2004, Williams and Arnold, 2011).
Multidimensional Fatigue Inventory (MFI) is a validated measure of fatigue severity that provides subscales of general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue (Smets et al., 1995). The MFI is scored on a 4–20 scale with high scores indicating more severe symptoms. There is no proposed MCID cut-off with the MFI. Patients enrolled in fibromyalgia clinical trials typically have MFI scores of approximately 14 (Hauser et al., 2013). In this study, scores are reported in terms of “general fatigue” and “mental fatigue” (Smets et al., 1995, Williams and Arnold, 2011). The MFI questions to determine mental fatigue are currently used in other tools to assess “fibrofog” (Boomershine, 2010).
2.3. Measures of subjective and objective dyscognition
Subjective dyscognition (subjective cognitive appraisal) was measured using the Multiple Ability Self-Report Questionnaire (MASQ), which assesses the subjective appraisal of cognitive difficulties in five cognitive domains: language, visual-perceptual ability, verbal memory, visual–spatial memory, and attention/concentration. The MASQ sub-scales are scored on an 8–40 scale with high scores indicating greater perceived difficulties. There is no proposed MCID cut-off with the MASQ. Patients enrolled in a fibromyalgia research clinic typically have MASQ subscales scores of approximately 20. In this study, subscale scores are reported in terms of “Verbal Memory”, “Language”, and “Attention/Concentration” (Seidenberg et al., 1994, Williams and Arnold, 2011) as these three domains are most highly related to the cognitive task during fMRI imaging (see below). The correlation coefficients for Verbal Memory, Language, and Attention/Concentration with 2back accuracy in healthy controls were − 0.68, − 0.59, and − 0.2, respectively. For this reason, Verbal Memory is used as a representative score of subjective cognitive appraisal in our imaging analyses.
2.4. Cognitive task during fMRI
Objective dyscognition was measured using a modified version of the N-back working memory task while undergoing fMRI. The modified N-back task used in this study has an increased overall cognitive demand compared to the classic N-back task. This task was employed previously by our group in other patient populations (Rayhan et al., 2013). Briefly, blocks of 9 pseudo-randomized uppercase letters (A, B, C, and D) were displayed for 1000 ms followed by 1500 ms of blank screen. Subjects responded using a four button box. In the 0back condition, subjects were asked to press the button that corresponds to the letter currently displayed on the screen. In the 2back condition, subjects were asked to press the button that corresponded to the letter that was presented two letters previously. This requires participants to simultaneously recall the letter shown two trials previously to the current displayed letter, push the button that corresponds to that previously shown letter, and keep track of the current sequence of presented letters to inform future answers. In the classic N-back task (Jaeggi et al., 2010) only a proportion of the displayed letters are “targets” (typically 30%); here subjects are required to continuously specify the current letter (0back) or the letter presented two previously (2back). Thus every letter is a “target” making this modified version of the N-back more attention-demanding than the classic N-back task.
Alternating blocks of 0back and 2back tasks were presented for 5 cycles. Immediately before each block, an instruction screen was presented to prepare the participant for each task. Between 0back and 2back blocks participants had a 8000 ms “rest” period in which a cross-hair was visually presented and there was no task. No feedback on performance was given. Stimuli were presented using the E-prime software (Psychology Software Tools, Pittsburgh, PA).
Accuracy was measured by subtracting the sum of misses and false positives from the number of condition items then dividing by the number of condition items. Reaction time was expressed in seconds.
Prior to entering the scanner, subjects were familiarized with the N-back paradigm through practice sessions on a standalone computer. Each participant was given 15 min to practice both tasks on a day prior to scanning and allowed to practice each task once on the day of scanning. This ensured that the subjects understood the directions and expectations during the sessions.
2.5. fMRI acquisition
All brain images were collected on a Siemens 3 T Tim Trio scanner equipped with a standard 12-channel head coil array. fMRI data were acquired using a T2⁎-weighted gradient-echo planar imaging (EPI) during the N-back task. The following acquisition parameters were used: repetition time (TR) 2500 ms, echo time (TE) 30 ms, 90 degrees flip angle, 205 ∗ x205-mm2 field of view (FOV) 64 × 64 matrix, and 47 slices (resolution 3.2 × 3.2 × 3.2 mm).
2.6. fMRI preprocessing
One patient did not complete the fMRI part of the study, and task performance data are missing for one healthy control. The imaging data were preprocessed and analyzed using SPM5 (Welcome Department of Imaging Neuroscience, London, UK; online at http:/www.fil.ion.ucl.ac.uk) in MATLAB (The Math Works Inc., Natick, MA). Preprocessing steps included spatial realignment to the first fMRI volume and six-parameter (three translations and three rotations) rigid-body correction for head motion, co-registration to the high-resolution (1.0 mm3) T1-weighted MPRAGE anatomical image, segmentation to identify voxels corresponding to gray matter, white matter, and cerebrospinal fluid, followed by warping to the corresponding tissue templates from SPM, spatial normalization into MNI space, and spatial smoothing using a Gaussian kernel of 8 mm. MPRAGE parameters were: TR/TE/TI = 1900/2.52/900 ms, flip angle = 90,176, 1.00 mm thick slices, FOV = 250 × 250 mm2; matrix = 246 × 256, and resolution = 1.02 × 0.98 × 1.00 mm3. To ensure that there were no significant group differences in head motion that might affect the fMRI analysis, mean total head motion (defined as the square root of the sum of squares of the six motion parameters) was compared between controls (0.08 ± 0.04) and patients (0.11 ± 0.07; p = 0.13) or between any of the individual six motion parameters (translations: x-direction p = 0.15, y-direction p = 0.81, z-direction p = 0.73; rotations: pitch p = 0.12, roll p = 0.81, yaw p = 0.24).
2.7. Statistical analysis of behavioral data
Data are presented as means ± SD. Statistical analysis of subjective data (pain, FIQ, BPI, MASQ, fatigue) was performed using independent sample t-tests in SPSS version 21. Pearson correlation analyses were used to determine the association between N-back accuracy on the 2back task and subjective scores. Correlational analyses were not possible with 0back accuracy scores, since participants in both groups had near perfect performance at this N-back level.
2.8. Statistical analysis of fMRI data
The preprocessed fMRI data were entered into a first–level individual analysis that compared the BOLD response during the 2back task with the 0back task (2back > 0back contrast). Additionally, contrasts for both conditions compared to the implicit baseline were computed. For these analyses, the time courses of the six motion parameters were included as regressors of no interest.
The contrast images were then entered into a second-level analysis to evaluate group differences in BOLD response during task performance (2back > 0back) across the whole brain. Group differences were also examined in 0back > baseline and 2back > baseline to account for possible influence of different baseline fMRI levels to task-related fMRI activity. Finally, we investigated the relationship between fMRI activity during task performance on the 2back task and task accuracy, and between fMRI activity during task performance on the 2back task and MASQ, per group, as well as group differences. Results were considered significant at a voxel-wise threshold of p < 0.01 and cluster-corrected for multiple comparisons (p < 0.05) using random-field theory (RFT).
3. Results
3.1. Demographics and clinical data
The characteristics of the fibromyalgia and healthy control groups are shown in Table 1. There were no significant differences in age between the groups (p = 0.87). The BPI, total FIQ, and fatigue scores (general, total) for fibromyalgia subjects were clinically and statistically different from controls (p < 0.001). All of the above measures were significantly positively correlated in patients (p < 0.03). To avoid issues of multiple correlations, total FIQ score was used as the compound measure of fibromyalgia impact and symptoms in further analyses as it is a comprehensive and accurate measure of fibromyalgia patients' overall condition. The MASQ scores revealed lower ability in fibromyalgia patients compared to controls (difference of 8.6 points for verbal memory and attention/concentration; 6.5 points for language, p < 0.001). The three subscales of MASQ were highly positively correlated (p < 0.001). The MASQ Verbal Memory score, which most closely reflects the processes involved in the fMRI task, was used as the measure of subjective cognitive appraisal for further analyses. Clinically, these data characterize a fibromyalgia group with substantial pain, functional disability, and subjective cognitive complaints compared to controls.
Table 1.
Sample characteristics.
| Characteristics | Patients (n = 16) mean (SD) | Controls (n = 13) mean (SD) | p-value |
|---|---|---|---|
| Age | 44.9 (± 10.2) | 44.2 (± 11.2) | 0.87 |
| Pain: | |||
| BPI severity (scale range: 0–10) | 4.59 (± 2.26) | 0.71 (± 1.10) | < 0.001 |
| BPI interference (0–10) | 4.42 (± 2.84) | 0.26 (± 0.61) | < 0.001 |
| FIQ impact (0–100) | 53.5 (± 12.8) | 18.8 (± 9.65) | < 0.001 |
| Fatigue: | |||
| MFI general fatigue (4–20) | 17.3 (± 2.53) | 8.38 (± 3.20) | < 0.001 |
| MFI mental fatigue (4–20) | 14.3 (± 4.16) | 6.19 (± 2.36) | < 0.001 |
| Subjective cognitive appraisal: | |||
| MASQ verbal memory (8–40) | 21.7 (± 7.55) | 13.1 (± 3.12) | < 0.001 |
| MASQ attention/concentration (8–40) | 21.8 (± 5.70) | 13.1 (± 4.26) | < 0.001 |
| MASQ language (8–30) | 18.6 (± 5.72) | 12.1 (± 3.08) | < 0.001 |
| N-back accuracy (%) | (n = 15) | (n = 12) | |
| 0-back | 94.8 (± 7.12) | 96.5 (± 4.07) | 0.65 |
| 2-back | 51.8 (± 23.6) | 64.7 (± 26.2) | 0.19 |
| N-back reaction time (ms) | (n = 15) | (n = 12) | |
| 0-back | 719 (± 169) | 670 (± 129) | 0.39 |
| 2-back | 690 (± 310) | 576 (± 252) | 0.31 |
BPI, Brief Pain Inventory; FIQ, Fibromyalgia Impact Score; MFI, Multidimensional Fatigue Inventory; MASQ, Multiple Ability Self-Report Questionnaire.
There was no significant difference between groups on either 0back or 2back task accuracy (0back p = 0.65, 2back p = 0.19) or reaction time (0back p = 0.39, 2back p = 0.31).
3.2. Relationship between cognitive task performance and subjective cognitive appraisal
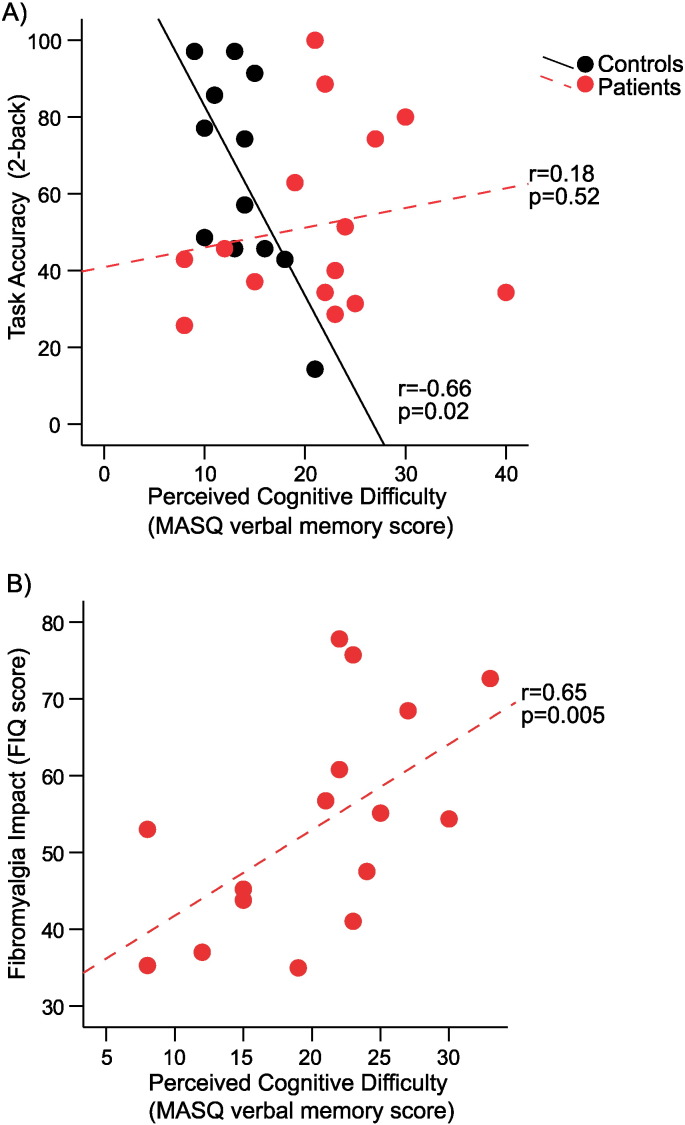
In controls, the appraisal of verbal memory faculty as measured by the MASQ verbal memory score was negatively associated with 2back accuracy (r = − 0.66, p = 0.02; Fig. 1A), with subjects who reported less cognitive difficulty demonstrating better task performance. However, no such association was found for fibromyalgia patients (r = 0.18, p = 0.52; Fig. 1A). Thus, while controls demonstrated the expected correlation between subjective cognitive appraisal and task performance, in fibromyalgia patients, subjective cognitive appraisal was not related to cognitive performance.
Fig. 1.
Relationship between perceived cognitive difficulty and task performance and fibromyalgia symptoms.
A) Perceived cognitive difficulty (MASQ verbal memory score) was negatively associated with Nback task performance (2back accuracy) in controls (n = 12, r = − 0.66, p = 0.02), but not in fibromyalgia patients (n = 15, r = 0.18, p = 0.52); controls, black; patients, red. B) In patients, perceived cognitive difficulty (MASQ verbal memory score) was positively associated with the severity of fibromyalgia (FIQ score), n = 16, r = 0.65, p = 0.005. (For interpretation of the references to color in this figure, the reader is referred to the online version of this chapter.)
3.3. Relationship of task performance and subjective cognitive appraisal with fibromyalgia impact
In patients, no significant association was found between cognitive performance (2back accuracy) on the N-back task and fibromyalgia impact (FIQ r = 0.19, p = 0.55). However, the FIQ score in patients was significantly correlated with subjective cognitive appraisal (MASQ Verbal Memory r = 0.65, p = 0.005, Fig. 1B; in controls this correlation was not significant, r = 0.07, p = 0.82). Thus, patients with greater symptoms severity reported greater perceived cognitive difficulties.
3.4. N-back task-related BOLD response
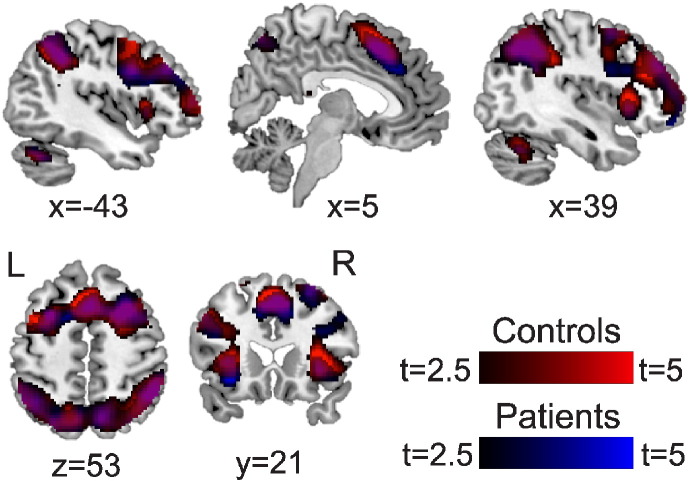
N-back task-related activation (2back > 0back contrast) was observed in fibromyalgia patients and healthy controls in fronto-parietal brain areas typically associated with working memory (dorsolateral and ventrolateral prefrontal cortices (DLPFC, VLPFC), superior and inferior parietal lobules (SPL, IPL), supplemental motor area (Bernstein et al., 2003), anterior cingulate cortex (ACC), insula, precuneus, cerebellum) (p < 0.05 cluster-corrected; Fig. 2, Table 2). There were no significant differences between groups in the 2back > 0back contrast.
Fig. 2.
BOLD response for the 2back > 0back contrast in healthy controls and fibromyalgia patients.
Both groups demonstrated increased fMRI activations in 2back > 0back in a widespread fronto-parietal network of brain regions typically activated in working memory tasks, including bilateral DLPFC, VLPFC, parietal cortices, precuneus, SMA, ACC, and insula (p < 0.05 cluster-corrected). Controls n = 13, red; patients n = 15, blue. Colorbars are t-values from 2.5 to 5. (For interpretation of the references to color in this figure, the reader is referred to the online version of this chapter.)
Table 2.
BOLD response during the N-back task (2 > 0back contrast).
| Region | Peak t-value | Peak MNI coordinate | Cluster volume (voxels) | Cluster p-value |
|---|---|---|---|---|
| Controls (n = 13): | ||||
| SMA (mid), PMC (L), DLPFC (R) | 8.4 | 0, 16, 54 | 5660 | < 0.001 |
| IPL (L), IPL (R) SMG (R) | 8.4 | − 36, − 56, 40 | 7316 | < 0.001 |
| DLPFC (R), ACC (mid) | 7.6 | 44, 36, 32 | 2459 | < 0.001 |
| VLPFC/FrOP (L), DLPFC (L) | 7.1 | − 42, 54, 8 | 697 | < 0.022 |
| Insula (L) | 6.4 | − 30, 22, 4 | 622 | < 0.029 |
| Patients (n = 15): | ||||
| IPL (L), IPL (R) | 7.4 | 44, − 46, 40 | 7196 | < 0.001 |
| DLPFC (R), SMA (mid), ACC (R) | 7.2 | 28, 14, 54 | 8354 | < 0.001 |
| Insula (L), VLPFC (L), DLPFC (L) | 4.9 | − 30, 22, − 8 | 565 | 0.036 |
| Cerebellum | 4.9 | − 36, − 54, − 34 | 650 | 0.026 |
| Controls > patients: | ||||
| No significant clusters | ||||
| Patients > controls: | ||||
| No significant clusters | ||||
The threshold for these analyses was set at a voxel-wise p < 0.01. Significant clusters corrected for multiple comparisons at p < 0.05 using random field theory (RFT) are reported. Included in these analyses were n = 13 controls and n = 15 patients.
SMA, supplemental motor area; PMC, premotor cortex; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; SMG, supramarginal gyrus; VLPFC, ventrolateral prefrontal cortex; FrOP, frontal operculum; ACC, anterior cingulate cortex; mid, midline; L, left hemisphere; R, right hemisphere.
To assess the contribution of baseline activation, which might be altered in the patient population, group differences were also evaluated between fibromyalgia patients and controls for each condition separately relative to the implicit baseline (2back > baseline, 0back > baseline). No significant group differences were found.
3.5. Nback-related BOLD response and objective task performance
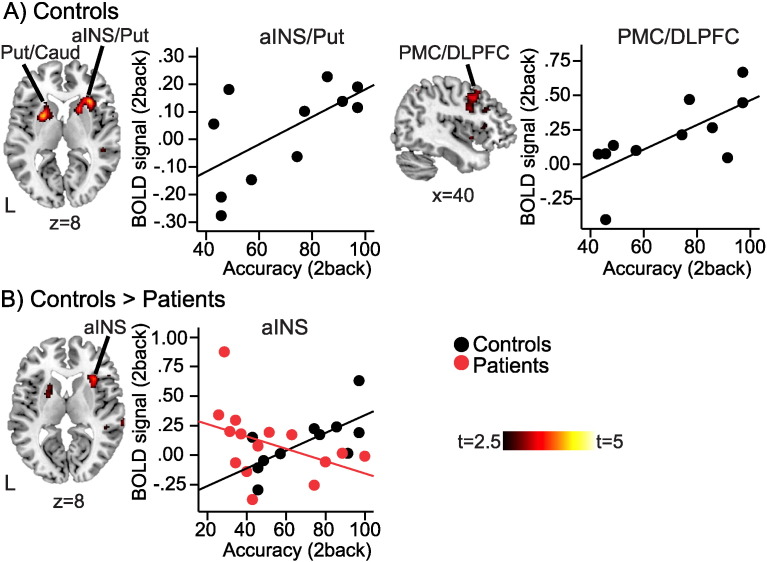
Controls showed a positive association between BOLD response and task accuracy during the 2back in the right anterior insula/putamen, right frontal operculum (FrOP), left putamen, left caudate, right premotor cortex (PMC) and right DLPFC (p < 0.05 cluster-corrected, Fig. 3A, Table 3). Fibromyalgia patients showed no such association in any region. Between-group comparison showed significantly stronger association between 2back accuracy and BOLD response in controls compared to patients in the right anterior insula (Fig. 3B, Table 3).
Fig. 3.
Relationship between BOLD response and task performance.
A) In controls, BOLD response during 2back was significantly positively correlated with 2back task accuracy in right anterior insula (Freeman et al., 2011), bilateral putamen (Put), left caudate (Caud), and right premotor cortex/dorsolateral prefrontal cortex (PMC/DLPFC); p < 0.05 cluster-corrected. There were no significant clusters in fibromyalgia patients. B) The positive correlation between BOLD response and task performance was significantly stronger in controls vs. patients in the right anterior insula (Freeman et al., 2011); p < 0.05 cluster-corrected. Displayed on MNI standard brain. Scatter plots are showing mean BOLD signal (a.u.) in respective cluster plotted against task accuracy (%). Controls n = 12, black circles; patients n = 15, red circles; L, left hemisphere. Colorbars are t-values from 2.5 to 5. (For interpretation of the references to color in this figure, the reader is referred to the online version of this chapter.)
Table 3.
Positive association between NBack-related BOLD response and task accuracy during the 2back task.
| Region | Peak t-value | Peak MNI coordinate | Cluster volume (voxels) | Cluster p-value |
|---|---|---|---|---|
| Controls (n = 12): | ||||
| aINS (R), FrOP (R), Putamen (R) | 4.4 | 32, 22, 8 | 1447 | 0.004 |
| Putamen (L), Caudate (L) | 4.2 | − 24, 6, 6 | 584 | 0.047 |
| PMC (R), DLPFC (R) | 3.5 | 32, − 2, 32 | 629 | 0.040 |
| Patients (n = 15): | ||||
| No significant clusters | ||||
| Controls > patients: | ||||
| aINS (R) | 3.7 | 32, 22, 8 | 648 | 0.038 |
| Patients > controls: | ||||
| No significant clusters | ||||
The threshold for these analyses was set at a voxel-wise p < 0.01. Significant clusters corrected for multiple comparisons at p < 0.05 using random field theory (RFT) are reported. Included in these analyses were n = 12 controls (performance data missing for one subject) and n = 15 patients.
aINS, anterior insula; FrOP, frontal operculum; PMC, premotor cortex; DLPFC, dorsolateral prefrontal cortex; L, left hemisphere; R, right hemisphere.
3.6. Nback-related BOLD response and subjective cognitive appraisal
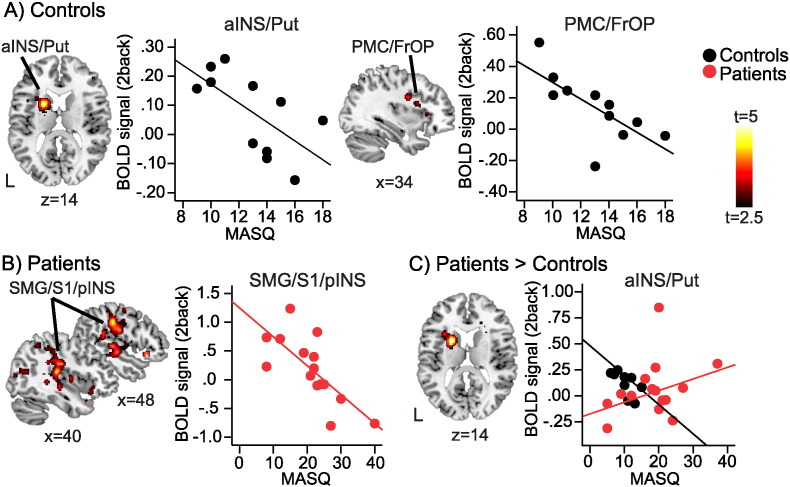
Controls showed a negative association between BOLD response during the 2back task and subjective cognitive appraisal (as measured by the verbal memory score on the MASQ) in the left anterior insula/putamen, and right PMC/FrOP (p < 0.05 cluster-corrected; Fig. 4A, Table 4); more cognitive complaint was associated with lower task-related activations in these regions.
Fig. 4.
Relationship between BOLD response and perceived cognitive difficulty.
A) In controls, BOLD response during 2back was significantly negatively correlated with the MASQ verbal score in the left anterior insula /putamen (aINS/Put), and right premotor cortex/frontal operculum (PMC/FrOP); p < 0.05 cluster-corrected. B) In patients, BOLD response during 2back was significantly negatively correlated with the MASQ verbal score in the right supramarginal gyrus/primary somatosensory cortex/posterior insula (SMG/S1/pINS0; p < 0.05 cluster-corrected. C) The negative correlation between BOLD response and MASQ verbal score was significantly stronger in patients vs. controls in the left anterior insula/putamen (aINS/Put); p < 0.05 cluster-corrected. Displayed on MNI standard brain. Scatter plots are showing mean BOLD signal (a.u.) in respective cluster plotted against MASQ verbal memory score. Controls n = 12, black circles; patients n = 15, red circles; L, left hemisphere. Colorbars are t-values from 2.5 to 5. (For interpretation of the references to color in this figure, the reader is referred to the online version of this chapter.)
Table 4.
Negative association between NBack-related BOLD response during the 2back task and perceived cognitive difficulty (MASQ verbal memory score).
| Region | Peak t-value | Peak MNI coordinate | Cluster volume (voxels) | Cluster p-value |
|---|---|---|---|---|
| Controls (n = 12): | ||||
| Putamen (L), aINS (L) | 4.8 | − 22, 8, 14 | 579 | 0.044 |
| PMC (R), FrOP (R) | 3.8 | 34, − 2, 30 | 709 | 0.028 |
| Patients (n = 15): | ||||
| SMG/S1/pINS (R) | 5.1 | 66, − 30, 30 | 7928 | < 0.001 |
| Occipital/IPL (R), | 5.2 | 48, − 80, 8 | 1014 | 0.011 |
| Inferior temporal (L), Fusiform (L) | 4.5 | − 42, − 40, − 6 | 1788 | 0.001 |
| Fusiform (R) | 4.4 | 30, − 34, − 16 | 1193 | 0.006 |
| Occipital (L) | 4.3 | − 36, − 74, 16 | 1299 | 0.005 |
| Controls > patients: | ||||
| No significant clusters | ||||
| Patients > controls: | ||||
| Putamen (L), aINS (L) | − 22, 6, 14 | 611 | 0.040 | |
The threshold for these analyses was set at a voxel-wise p < 0.01. Significant clusters corrected for multiple comparisons at p < 0.05 using random field theory (RFT) are reported.
aINS, anterior insula; PMC, premotor cortex; FrOP, frontal operculum; SMG, supramarginal gyrus; S1, primary somatosensory cortex; pINS, posterior insula; IPL, inferior parietal lobule; L, left hemisphere; R, right hemisphere.
Fibromyalgia patients also demonstrated a negative association between subjective cognitive appraisal and BOLD response, but in a separate set of brain regions, including the right supramarginal gyrus/primary somatosensory cortex (SMG/S1) and posterior insula, right IPL, left inferior temporal cortex/fusiform gyrus, and bilateral occipital cortex (p < 0.05 cluster-corrected; Fig. 4B, Table 4). Patients displayed significantly less negative correlation between subjective cognitive appraisal and fMRI activity during working memory task compared to healthy controls in the left putamen/anterior insula (Fig. 4C, Table 4).
4. Discussion
The experience of cognitive dysfunction contributes prominently to the symptoms that comprise the current concept of fibromyalgia. This study evaluated subjective cognitive appraisal, objective task performance, and brain activity during task performance. These measurements describe a clinical picture of “fibrofog” in which subjective impairment is clinically considerable, yet distinguishable from a difficult working memory task performance in which objective impairment was minimal and no differences in BOLD activity were observed between groups during task performance. However, a dissociation between task accuracy and task-related BOLD response was observed in fibromyalgia patients but not in controls. Further, increased subjective cognitive appraisal was accompanied by a unique pattern of brain activation during task performance. Simply put, fibromyalgia patients appear to have a “disconnect” between the subjective experience of cognitive problems and the objective reality of cognitive performance. The amount of disconnect also appears to be related to the severity of clinical symptoms.
These results are consistent with Čeko et al. (Čeko et al., 2015), in that fibromyalgia patients performed similar to controls on the 2back task. Our version of 2back appears to be more difficult than that used by Seo et al., leading to much lower scores in both groups. We observed a 15% difference in performance that did not reach significance. Expected brain activations associated with the 2back were equivalent between the groups after subtracting the easier condition (0back). Therefore, “fibrofog” does not appear to be an inability to properly activate task-related brain networks.
While group differences in the objective 2back task performance and related BOLD response were not demonstrated, the groups differed dramatically in the perception of their cognitive function (cognitive appraisal). MASQ scores demonstrate that fibromyalgia patients have a ~ 8.7 point (27.2%) increase in perceived dyscognition in each cognitive domain tested. These differences appear to be clinically meaningful (Williams and Arnold, 2011). This suggests that there is an important discordance between subjective dyscognition and objective cognitive performance. This discordance is all the more striking when subjective cognitive appraisal is correlated with objective performance. Subjective cognitive appraisal is predictive of 2back task accuracy in healthy volunteers (Pearson correlation r = − 0.66, p = 0.02) but not predictive in fibromyalgia patients (Pearson correlation r = 0.35, p = 0.27). This discordance also appears to have a neurobiological correlate. Activity in the right PMC/DLPFC, right anterior insula, and bilateral putamen correlated with task accuracy in healthy volunteers; task accuracy could not be attributed to any discrete brain activation in fibromyalgia patients.
The lack of relationship between subjective experience and objective performance is a key observation for understanding “fibrofog”. Similar discordances are observed in many other symptomatic aspects of fibromyalgia in which perceived deficits are reported to be much greater than those found on functional testing (Hidding et al., 1994). In this way, the dyscognition of fibromyalgia appears similar to the “brain fog” described in Gulf War Illness and other war-related illnesses, (Binder et al., 1999, Hyams et al., 1996, Wallin et al., 2009) chronic fatigue syndrome, (Capuron et al., 2006, Ocon, 2013) post-Lyme disease syndrome, (Hassett et al., 2009) postural orthostatic tachycardia syndrome, (Freeman et al., 2011) the “chemo brain” in cancer survivors, (Tannock et al., 2004, Wang et al., 2015) the “brain fag” historically described in neurasthenia, (Ross, 2004) and (perhaps) the ethnographic illness “brain fag” documented in male African teenagers (Ola et al., 2009). These disorders are characterized by distressing subjective cognitive impairment without clinically obvious cognitive deficits.
This discordance is in contradistinction to neuropsychiatric dementias with demonstrable pathology such as Alzheimer's disease, Parkinson's disease, Wernicke's encephalopathy, Korsakoff Syndrome, and vascular dementia, where the cognitive impairment is clinically obvious. In these disorders, cognitive impairment is measured easily using objective tests and ratings from relatives (McGlone et al., 1990). In fact, a common clinical scenario in neuropsychiatric dementia is the reverse of what is seen in fibromyalgia: clearly discernible cognitive deficits accompanied by minimization, confabulation, or outright denial of these cognitive deficits by the afflicted (Weinstein et al., 1994). Unlike fibromyalgia, these progressive dementias are associated also with obvious physical disability and early mortality (Wolfe et al., 2013). The dyscognition of fibromyalgia appears to be more a problem of subjective cognitive appraisal – the privileged homeostatic sensation that accompanies cognitive tasks – than of actual cognitive performance.
Differences in cognitive appraisal, however, do appear to be associated with BOLD response during a cognitive task. Lower subjective dyscognition in controls was associated with decreased activation of the left putamen, left anterior insula, and right PMC/FrOP. A more widespread pattern was observed in fibromyalgia, with patients with the highest levels of dyscognition demonstrating decreased activity in the right SMG/S1 and posterior insula, right fusiform gyrus, right IPL, left inferior temporal cortex, and bilateral fusiform gyrus and occipital cortex. Negative correlations between cognitive appraisal and activation were found in the left putamen/anterior insula of controls but not in fibromyalgia patients. It is possible that these differences in task-related activations may contribute to the experience of “fibrofog”.
Neurologic activity measured during cognitive tasks likely represents the entire experience of the task, action and subjective experience, rather than being only a surrogate of ability. Fibromyalgia patients demonstrate equivalent performance and BOLD response in the task-related network when compared to matched controls, but also demonstrate differing patterns of activity outside of the task network that correlate with cognitive appraisal. These patterns may be interpreted in a variety of ways. Task performance may lead to secondary, reflexive activations or deactivations that provide a sense of cognitive appraisal. Alternatively, these differences may not be related to task performance but rather reflect differences in perception. It may be that patients with increased cognitive complaints were more sensitive to the scanning environment or more concerned about their ability to adequately perform, each with their own neurobiological correlates. Our data cannot differentiate between these possibilities, but do suggest that approaches that focus solely on task performance and its associated activation patterns may not provide sufficient answers to the nature of dyscognition in fibromyalgia. Future approaches will need to compare the similarities and differences of subjective and objective performance and their neurobiologic correlates in tasks with a range of difficulties.
4.1. Limitations
This study has several limitations. The findings are from a comparative cohort study with a relatively small sample size. A true but small difference in performance might be demonstrated in a larger sample. Overall subjective dyscognition was measured but not task-related subjective dyscognition. Performance accuracy was measured only in the scanning environment.
It is possible that the lack of difference in objective cognitive function reported here is due to specifics in the study design, such as the use of the N-back task. It is possible that other task designs may have found objective differences in performance. However, N-back testing represents a difficult cognitive task that requires attention, recall, quick reactions, and persistence; all aspects of tasks described as problematic by persons with fibromyalgia. N-back testing is sensitive to cognitive impairment, as demonstrated in both Parkinson's disease (Miller et al., 2009) and schizophrenia (Perlstein et al., 2001). We assert that if “fibrofog” is associated with a gross deficit in working memory, the N-back paradigm used in this study would have captured it. While a single negative finding cannot conclusively demonstrate that there is no objective cognitive deficit in fibromyalgia, these results suggest that any such deficit would be clinically insignificant.
It is also possible that the poor association between subjective symptom reporting and objective cognitive performance is related to the influence of psychiatric variables, such as anxiety and depression. However, psychological issues are such an essential part of the clinical phenomenon of fibromyalgia that they cannot be meaningfully separated. In the National Health Interview Survey, 62.7% of persons meeting fibromyalgia criteria self-report a diagnosis of depression and 64.8% report being “often anxious” (Walitt et al., 2015). In recognition that fibromyalgia cannot be readily disassociated from psychiatric comorbidity, feeling depressed was added to the ACR diagnostic definition in 2011 (Wolfe et al., 2011).
5. Conclusion
In summary, these results suggest that “fibrofog” is better characterized by subjective rather than objective cognitive impairment. This difference in cognitive appraisal appears to have neurologic correlates. We opine that these differences reflect both the sensory and behavioral aspects of task performance rather than a singular deficit in cognitive ability in fibromyalgia patients.
Acknowledgments
The authors have no conflicts of interest to report in relation to this manuscript. This manuscript was funded by grants from the American College of Rheumatology Research and Education Foundation and the MedStar Health Research Institute. Infrastructural support was provided by Georgetown Howard Universities Center for Clinical and Translational Science (GHUCCTS). This research was supported (in part) by the Intramural Research program of the NIH, National Center for Complementary and Integrative Health (NCCIH).
References
- Ambrose K.R., Gracely R.H., Glass J.M. Fibromyalgia dyscognition: concepts and issues. Reumatismo. 2012;64(4):206–215. doi: 10.4081/reumatismo.2012.206. [DOI] [PubMed] [Google Scholar]
- Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 2005;23(5 Suppl. 39):S154–S162. [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Binder L.M., Storzbach D., Anger W.K. Subjective cognitive complaints, affective distress, and objective cognitive performance in Persian Gulf War veterans. Arch. Clin. Neuropsychol. 1999;14(6):531–536. [PubMed] [Google Scholar]
- Boomershine C.S. The FIBRO system: a rapid strategy for assessment and management of fibromyalgia syndrome. Ther. Adv. Musculoskelet Dis. 2010;2(4):187–200. doi: 10.1177/1759720X10374437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt C.S., Clark S.R., Bennett R.M. The Fibromyalgia Impact Questionnaire: development and validation. J. Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- Capuron L., Welberg L., Heim C. Cognitive dysfunction relates to subjective report of mental fatigue in patients with chronic fatigue syndrome. Neuropsychopharmacology. 2006;31(8):1777–1784. doi: 10.1038/sj.npp.1301005. [DOI] [PubMed] [Google Scholar]
- Čeko M., Gracely J.L., MA Fitzcharles, Seminowicz D.A., Schweinhardt P., Bushnell M.C. Is a responsive default mode network required for successful working memory task performance? J. Neurosci. 2015;35 doi: 10.1523/JNEUROSCI.0264-15.2015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick B., Eccleston C., Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47(6):639–644. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F.B. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011;161(1–2):46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Glass J.M. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum. Dis. Clin. N. Am. 2009;35(2):299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Glass J.M., Park D.C. Cognitive dysfunction in fibromyalgia. Curr. Rheumatol. Rep. 2001;3(2):123–127. doi: 10.1007/s11926-001-0007-4. [DOI] [PubMed] [Google Scholar]
- Glass J.M., Williams D.A., Fernandez-Sanchez M.L. Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J. Pain. 2011;12(12):1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett A.L., Radvanski D.C., Buyske S. Psychiatric comorbidity and other psychological factors in patients with “chronic Lyme disease”. Am. J. Med. 2009;122(9):843–850. doi: 10.1016/j.amjmed.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W., Urrutia G., Tort S. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst. Rev. 2013;1 doi: 10.1002/14651858.CD010292. [DOI] [PubMed] [Google Scholar]
- Hidding A., van Santen M., De Klerk E. Comparison between self-report measures and clinical observations of functional disability in ankylosing spondylitis, rheumatoid arthritis and fibromyalgia. J. Rheumatol. 1994;21(5):818–823. [PubMed] [Google Scholar]
- Hyams K.C., Wignall F.S., Roswell R. War syndromes and their evaluation: from the U.S. Civil War to the Persian Gulf War. Ann. Intern. Med. 1996;125(5):398–405. doi: 10.7326/0003-4819-125-5-199609010-00007. [DOI] [PubMed] [Google Scholar]
- Jaeggi S.M., Buschkuehl M., Perrig W.J. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18(4):394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Katz R.S., Heard A.R., Mills M. The prevalence and clinical impact of reported cognitive difficulties (fibrofog) in patients with rheumatic disease with and without fibromyalgia. J. Clin. Rheumatol. 2004;10(2):53–58. doi: 10.1097/01.rhu.0000120895.20623.9f. [DOI] [PubMed] [Google Scholar]
- Keller S., Bann C.M., Dodd S.L. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain. 2004;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Leavitt F., Katz R.S. Distraction as a key determinant of impaired memory in patients with fibromyalgia. J. Rheumatol. 2006;33(1):127–132. [PubMed] [Google Scholar]
- Leavitt F., Katz R.S. Normalizing memory recall in fibromyalgia with rehearsal: a distraction-counteracting effect. Arthritis Rheum. 2009;61(6):740–744. doi: 10.1002/art.24559. [DOI] [PubMed] [Google Scholar]
- McGlone J., Gupta S., Humphrey D. Screening for early dementia using memory complaints from patients and relatives. Arch. Neurol. 1990;47(11):1189–1193. doi: 10.1001/archneur.1990.00530110043015. [DOI] [PubMed] [Google Scholar]
- Miller K.M., Price C.C., Okun M.S. Is the N-back task a valid neuropsychological measure for assessing working memory? Arch. Clin. Neuropsychol. 2009;24(7):711–717. doi: 10.1093/arclin/acp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs R., Mease P., Arnold L.M. The effect of duloxetine treatment on cognition in patients with fibromyalgia. Psychosom. Med. 2012;74(6):628–634. doi: 10.1097/PSY.0b013e31825b9855. [DOI] [PubMed] [Google Scholar]
- Ocon A.J. Caught in the thickness of brain fog: exploring the cognitive symptoms of Chronic Fatigue Syndrome. Front. Physiol. 2013;4:63. doi: 10.3389/fphys.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola B.A., Morakinyo O., Adewuya A.O. Brain Fag Syndrome — a myth or a reality. Afr. J. Psychiatry. 2009;12(2):135–143. doi: 10.4314/ajpsy.v12i2.43731. [DOI] [PubMed] [Google Scholar]
- Perlstein W.M., Carter C.S., Noll D.C. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am. J. Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Rayhan R.U., Raksit M.P., Timbol C.R. Prefrontal lactate predicts exercise-induced cognitive dysfunction in Gulf War Illness. Am. J. Transl. Res. 2013;5(2):212–223. [PMC free article] [PubMed] [Google Scholar]
- Reyes Del Paso G.A., Pulgar A., Duschek S. Cognitive impairment in fibromyalgia syndrome: the impact of cardiovascular regulation, pain, emotional disorders and medication. Eur. J. Pain. 2012;16(3):421–429. doi: 10.1002/j.1532-2149.2011.00032.x. [DOI] [PubMed] [Google Scholar]
- Ross Z. 2004. Women on the Verge: The Culture of Neurasthenia in Nineteenth-Century America. (University BoTotLSJ, editor) [Google Scholar]
- Seidenberg M., Haltiner A., Taylor M.A. Development and validation of a Multiple Ability Self-Report Questionnaire. J. Clin. Exp. Neuropsychol. 1994;16(1):93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- Seo J., Kim S.H., Kim Y.T. Working memory impairment in fibromyalgia patients associated with altered frontoparietal memory network. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0037808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmygalev S., Dagtekin O., HJ Gerbershagen. Assessing cognitive and psychomotor performance in patients with fibromyalgia syndrome. Pain Ther. 2014 doi: 10.1007/s40122-014-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets E.M., Garssen B., Bonke B. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Tan G., Jensen M.P., Thornby J.I. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J. Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tannock I.F., Ahles T.A., Ganz P.A. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J. Clin. Oncol. 2004;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Tesio V., Torta D.M., Colonna F. Are fibromyalgia patients cognitively impaired? objective and subjective neuropsychological evidence. Arthritis Care Res. 2015;67(1):143–150. doi: 10.1002/acr.22403. [DOI] [PubMed] [Google Scholar]
- Walitt B., Roebuck-Spencer T., Bleiberg J. Automated neuropsychiatric measurements of information processing in fibromyalgia. Rheumatol. Int. 2008;28(6):561–566. doi: 10.1007/s00296-007-0487-2. [DOI] [PubMed] [Google Scholar]
- Walitt B., Nahin R.L., Katz R.S. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin M.T., Wilken J., Alfaro M.H. Neuropsychologic assessment of a population-based sample of Gulf War veterans. Cogn. Behav. Neurol. 2009;22(3):155–166. doi: 10.1097/WNN.0b013e3181b278e8. [DOI] [PubMed] [Google Scholar]
- Wang X.M., Walitt B., Saligan L. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine. 2015;72(1):86–96. doi: 10.1016/j.cyto.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Edwin A., Friedland Robert P., Wagner Elizabeth E. Denial/unawareness of impairment and symbolic behavior in Alzheimer's disease. Neuropsychiatry Neuropsychol. Behav. Neurol. 1994;7:176–184. [Google Scholar]
- Williams D.A., Arnold L.M. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ) Arthritis Care Res. 2011;63(Suppl. 11):S86–S97. doi: 10.1002/acr.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F., Clauw D.J., Fitzcharles M.A. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Clauw D.J., Fitzcharles M.A. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Hassett A.L., Walitt B. Mortality in fibromyalgia: a study of 8,186 patients over thirty-five years. Arthritis Care Res. 2013;63(1):94–101. doi: 10.1002/acr.20301. [DOI] [PubMed] [Google Scholar]
- Yunus M., Masi A.T., Calabro J.J. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin. Arthritis Rheum. 1981;11(1):151–171. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]