Abstract
AIM
To explore the expression of S100B in corneal epithelial cells under Aspergillus stimulation both in vivo and in vitro.
METHODS
Immortalized human corneal epithelial cells (HCECs) were exposed to inactive Aspergillus fumigatus (A. fumigatus) conidia at 0, 4, 8, 12, 16, and 24h respectively. The corneas of Wistar rats were exposed to active A. fumigatus at 0, 12, 24, 48h and the normal rat corneas were used for normal control. The mRNA level of S100B was evaluated by real time quantitative reverse transcription-polymerase chain reaction (qRT-PCR). S100B protein expression in cornea epithelium was detected by immunohistochemical/immunocytochemical staining (IHC/ICC).
RESULTS
Histopathology revealed a significant inflammatory cell infiltration in fungal keratitis human and rat cornea. Corneal epithelial cells didn't express or rarely express S100B at baseline. A. fumigatus significantly induced S100B mRNA expression in cultured corneal epithelial cells in a time depended manner in vitro, the mRNA began to rise significantly at 8h in vitro (P<0.05) and continue to rise as time prolonged (P<0.01). In vivo, S100B mRNA level was low in the normal corneas. However, it was increased in keratitis corneas from 12h after infection (P<0.05) and reached to a peak at 24h (P<0.001). Immunochemistry revealed an obvious staining in fungal keratitis corneas as well as immortalized HCECs compared to the normal ones respectively, indicating an increased expression of S100B protein.
CONCLUSION
S100B exists in corneal epithelial cells and is over-expressed under A. fumigatus stimulation. S100B may play an important role in the innate immune response of the corneal epithelium during A. fumigatus infection.
Keywords: S100B, cornea, Aspergillus fumigates, innate immunity
INTRODUCTION
Fungal keratitis is one of the most common factors which cause vision loss in the clinic work[1]. It usually causes cornea infiltration and ulceration, pain, photophobia and visual impairment that are difficult to treat. Polyenes, triazoles, and echinocandins are three main classes of antifungal agents with different limitations and side effects. Studies have been carried on for decades to explore the occurrence and development mechanism as well as effective agents of fungal keratitis, but it is still not fully understood. Aspergillus (A. flavus, A. fumigatus) and Fusarium (F. solani, F. oxysporum) species have been recently identified as the main etiologic agents of fungal keratitis[2]. Trauma in developing countries as well as contact lens wear in developed countries are known as the main reasons of human ocular fungi infection[3]–[4]. Recent findings have indicated that damage-associated molecular patterns (DAMPs) which are secreted from various cells participate in various human inflammation and immune responses[5]. As one member of DAMP, S100B belongs to the S100 family which consists of 24 members with calcium-binding capacity. Early basic research has already confirmed that high structural homology and function diversities exist between S100 members and some S100 proteins are involved in the anti-fungi and ocular immune process, even in the cornea[6]–[9]. S100B is also indicated to participate in the inflammation process through S100/RAGE axis in other tissues and S100B/RAGE axis has been proved to function together during aspergillosis[10]–[12]. However, studies between S100B and fungi in cornea were not found. Based on these results, we made a hypothesis that S100B participates in the cornea immune response during fungi invasion.
MATERIALS AND METHODS
Reagents
Sabouroud liquid culture was purchased from American Sigma Company; primers were from Invitrigen (Qingdao, Shandong Province, China); RNAiso Plus, reverse transcriptase polymerase chain reaction kits with gDNA Eraser (Perfect Real Time) and SYBR Premix Ex TaqTM (Tli RNaseH Plus) were purchased from TaKaRa (Dalian, Liaoning Province, China); Dulbecco's modified Eagle's medium (DMEM), Ham F-12, fetal bovine serum (FBS), insulin, human epidermal growth factor (EGF), penicillin and streptomycin were from American HyClone products; Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St Louis, Missouri, USA); 0.25% trypsin-EDTA solution was purchased from Solarbio (Beijing, China).
Fumigatus Conidia Antigens
Aspergillus fumigatus (A. fumigatus) strains (NO3.0772) was purchased from China General Microbiological Culture Collection Center. A. fumigatus was grown on Sabouraud glucose agar for 5-7d at 28°C. Fungal conidia was harvested by gently scraping the surface of the slants and then washed in sterile phosphate buffered saline (PBS). The conidia were harvested by centrifugation and inactivated by treatment with 70% ethanol for 12h. Inactive A. fumigatus was washed three times by PBS and was adjusted to 5×107 colony forming unit (CFU)/mL with DMEM.
Clinical Specimens
Corneas of fungal keratitis patients from department of ophthalmology were included to determine whether S100B participates in the inflammation process after fungi infection. Normal controls were the rest of normal peripheral corneal tissues after corneal transplantation. The study was conducted in agreement with the guidelines of the Declaration of Helsinki. Patients' and their families' consents were obtained before clinical materials were used for research purposes. Approval from the Hospital's Ethics Committee was also obtained before the beginning of research.
Immortalized Human Corneal Epithelial Cells Culture and Stimulation
Immortalized human corneal epithelial cells (HCECs) were obtained from Ocular Surface Laboratory of Zhongshan Ophthalmic Center as a gift and cultured in DMEM with 20% FBS, 100 U/mL penicillin G and 100 µg/mL streptomycin sulfate in a humidified atmosphere containing 5% CO2, 37°C. The medium was replaced every 2d before experiments start. HCEC suspensions of 1×105/mL were seeded into 12-well cell culture plates.
Immortalized HCECs were incubated in serum-free medium for 24h before inactive A.fumigatus stimulation. When cells grew to 80%-90% confluence, the experimental groups were added with inactive A.fumigatus, the normal control groups were added with serum-free medium. After 4, 8, 12, 16 and 24h incubation, cells were harvested for mRNA detection.
Animal Models
A total of 40 Wistar rats (both male and female) were purchased from Qingdao Institute of Drug Control (Qingdao, Shandong Province, China). Animals with cornea diseases were excluded. All the rats were randomly divided into control group and three A. fumigatus infection groups (12, 24, and 48h). The right eyes were chosen for experiments. The establishment and the evaluating criteria of animal model were in accordance with Zhao et al[1]. Rats were executed at 12, 24 and 48h respectively after the models' establishment. The cornea epithelium was scraped and preserved with RNAiso Plus in -80°C for mRNA detection. All studies were in compliance with the protocol approved by the animal care committee of the Affiliated Hospital of Qingdao University and were in compliance with institutional guidelines (Permit Number: 2012-0087).
Real Time Quantitative Reverse Transcription-polymerase Chain Reaction
According to the real time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) manufacturer's instructions, RNA was extracted from cultured immortalized HCECs and Wistar rats' corneal epitheliums by using RNAiso Plus reagent. The RNA quality and concentration were determined by spectrophotometer (Eppendorf, Hamburg, Germany). Complementary DNA was generated by reverse transcription of 2 µg total RNA. It was used for each 20 µL quantitative PCR reaction with SYBR Green using specific primers (Table 1). The thermocycler parameters were 95°C for 30s, followed by 40 cycles of 95°C for 5s, 60°C for 30s, and a final stage of 95°C for 15s, 60°C for 30s, 95°C for 15s. The S100B mRNA expression was quantified by RT-PCR. Housekeeping gene β-actin was used as an internal control. The ΔΔCT method was used for quantization of target gene products. Each experiment was repeated at least three times.
Table 1. Primers and production sizes.
| Gene | Primer sequence | Product size |
| Human β-actin | F: TGGCACCCAGCACAATGAA | 186 bp |
| R: TAAGTCATAGTCCGCCTAGAAGCA | ||
| Human S100B | F: ATGATGGAGACGGCGAATG | 200 bp |
| R: GCTACAACACGGCTGGAAAG | ||
| Rat β-actin | F: GGAGATTACTGCCCTGGCTCCTA | 150 bp |
| R: GACTCATCGTACTCCTGCTTGCTG | ||
| Rat S100B | F: GGGTGACAAGCACAAGCTGAA | 117 bp |
| R: AGCGTCTCCATCACTTTGTCCA |
Histopathology
Corneas from fungal keratitis patients and entire eyeballs from rat models were fixed by 4% paraformaldehyde for 24h. Following dehydrated in graded alcohol and cleared in xylene, samples were embedded in paraffin and cut into 3 µm transverse sections on slides, stored at room temperature. Slides were heated at 72°C for 10min on Leica HI1220 and then dewaxed in xylene, hydrated using graded ethanol, stained for histopathology by Haematoxylin and Eosin (H&E).
Immunochemistry
Immunochemistry was performed for S100 protein detection with PV-9000 2-step plus®Poly-HRP antimouse/rabbit IgG detection system (ZSGB-BIO, Beijing, China). Corneal paraffin sections with thickness of 3 µm were dewaxed to water for immunology and histology chemistry. HCEC cells were cultured on slides in 6-well plate and exposed to A. fumigatus antigenic stimulation liquid at 0 and 24h after 90% confluence. After 4% paraformaldehyde for 15min, 1% Triton X-100 for 15min, cells were used for immunocytochemistry. After the heat induced epitope retrieval, sections were sequentially immersed in 3% hydrogen peroxide for 10min, S100B monoclonal antibody (1:100 dilution; Boster, Wuhan, Hubei Province, China ) at 37°C for 2h, polymer helper at 37°C for 20min, polyperoxidase-anti-mouse IgG at 37°C for 20min (PV-9000, ZSGB-BIO, Beijing, China). PBS buffer instead of S100B primary antibody was used as negative control. The reacting time was controlled under microscope. Brown particles were considered as positive criteria.
Statistical Analysis
Statistical analysis was performed by one-way analysis of variance, and further pairwise comparisons were made using SPSS 19.0 software. All of the results were presented as mean±SD. The level of statistical significance was set at P<0.05.
RESULTS
Significant Inflammatory Changes Occurred in Human and Rat Cornea after Aspergillus Fumigatus Infection
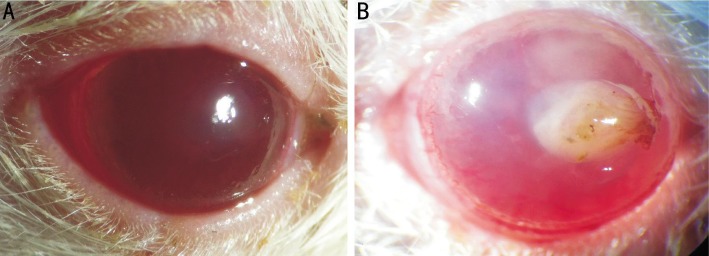
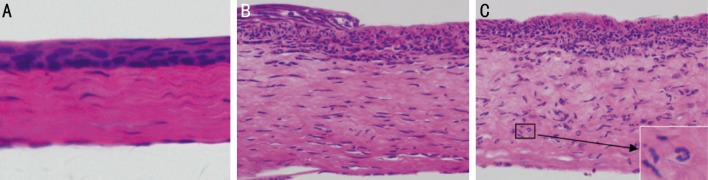
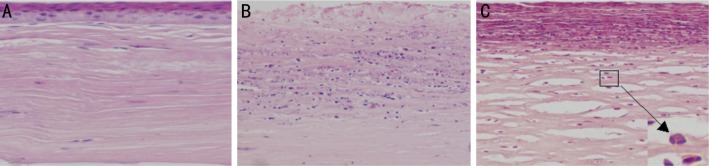
Fungal keratitis animal models were successfully established in Wistar rats with active A. fumigatus. Figure 1 shows the representative photomicrograph images of normal and infected rats' corneas. Corneal ulcer in rats appeared as early as 12h post infection. Limbal vascular congestion and corneal ulcer could be seen in keratitis corneas (Figure 1B). Figure 2 shows the corresponding H&E stained cornea sections. In histological sections, no obvious pathology change was observed in uninfected rats' corneas. The epithelial and stroma cells were arranged in neat rows, the transparent matrix was uniformly red dye with only few fibroblast nucleus (Figure 2A). On the contrary, a significant inflammatory cells infiltration in histopathology was observed in the infected rats' corneas compared to the normal ones. The infiltrating cells within the epithelium and stroma were mainly composed by neutrophils. In the central corneal ulcer, necrosis and ulcer formation occurred in the epithelium, collagen proliferation and derangement of fibroblasts occurred in the stomal layer with thickness increased (Figure 2C). In the peripheral corneal ulcer, the defect and residual epithelium area were visible at the same time. The infiltrating inflammatory cells were still clearly visible but less intensive compared to the central area (Figure 2B). Figure 3 shows the H&E stained cornea sections of normal and keratitis patients. The normal corneal tissue was regularly arranged and the epithelium was intact (Figure 3A). Similar to animal models, an obvious inflammatory change occurred in human corneal ulcer tissue. The epithelium in the ulcer area was loss while a significant increase of infiltrating inflammatory cells gathered both in the epithelial and stomal layer (Figure 3B). The main inflammatory cells were also neutrophils characterized by lobulated nucleus.
Figure 1. Photomicrographs of normal and fungal keratitis rat models.
A: Normal rat cornea was transparent; B: Representative cornea ulcer is seen in fungal infection rat cornea.
Figure 2. Histological sections of normal and fungal keratitis corneas in rats.
A: Regular tissue structure is seen in normal corneal tissue; B: A mass of inflammatory cells infiltration is seen in keratitis corneal tissue (×200 magnification); C: The characteristic inflammatory cells were neutrophils (×400 maginfication).
Figure 3. Histological sections of human normal and fungal keratitis corneas.
A: Regular tissue structure is seen in normal corneal tissue; B: Characteristic inflammatory cells infiltration is seen in keratitis corneal tissue (×200 magnification); C: Cells infiltration were mainly composed by neutrophils (×400 maginfication).
Up-regulated Gene Expression of S100B in Corneal Epithelial Cells under Aspergillus Fumigatus Stimulation both in Vitro and in Vivo
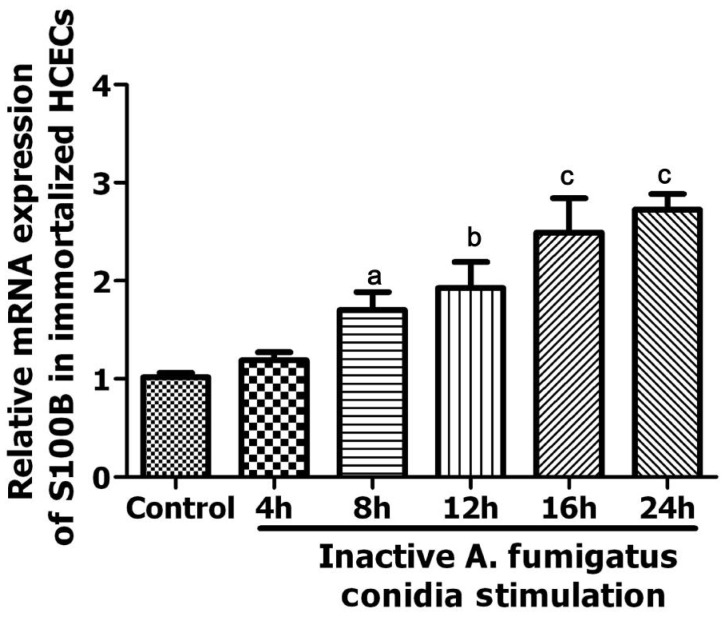
With immortalized HCECs in vitro, qRT-PCR analysis revealed that A. fumigatus conidia induced the expression of S100B in a time-depended manner. As it is shown in Figure 4, A. fumigatus conidia significantly up-regulated S100B mRNA level at 8h (P<0.05) and then kept on rising gradually as the stimulation continued (12h: P<0.01; 16h: P=0.001; 24h: P<0.001). There was no significant statistical differences in untreated HCECs between the corresponding time points (P>0.05) (data were not shown).
Figure 4. Expression of S100B mRNA in untreated and A. fumigatus stimulated immortalized HCECs groups.
aP<0.05 vs control group; bP<0.01 vs control group; cP<0.001 vs control group.
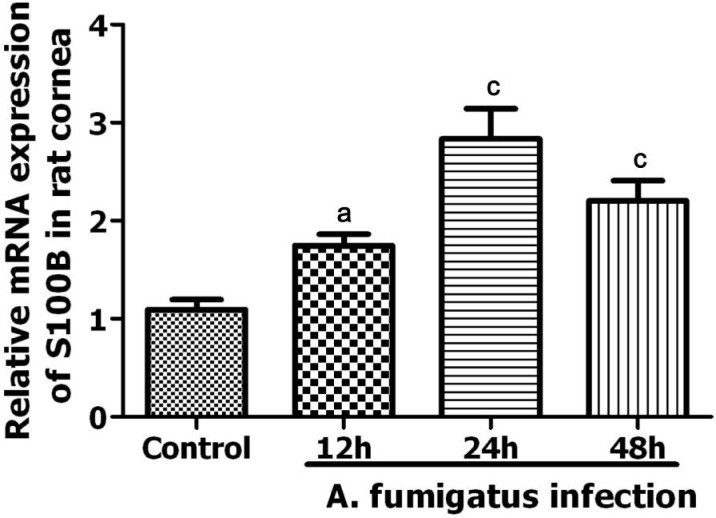
In fungal keratitis animal models, RT-PCR also detected a significant increase of S100 mRNA in infected corneal epithelia (Figure 5). The level of S100 mRNA increased at 12h after fungi invasion (P<0.05) and reached to maximum level at 24h (P<0.001). There was still significant difference at 48h after A. fumigatus infection though S100B mRNA level declined (P=0.001).
Figure 5. Expression of S100B mRNA in normal and A. fumigatus infected rat models.
aP<0.05 vs control group; cP<0.001 vs control group.
S100B Protein Participates in Corneal Epithelial Immune Response During Aspergillus Fumigatus Infection both in Vitro and in Vivo
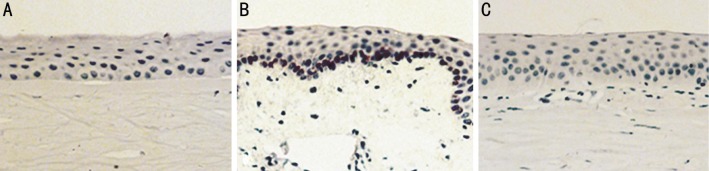
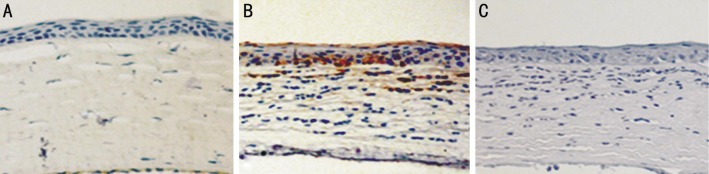
Brown staining indicates the expression of S100B protein. There was no obvious staining in normal cornea epithelium as well as immortalized HCECs. In vivo, an obvious positive staining was demonstrated in corneal epithelium which mainly focused on the basal layer epithelial cells in fungal keratitis corneal samples. However, S100B protein could hardly be detected in normal corneal epithelium both in human and animal models (Figures 6, 7).
Figure 6. S100B expression in human corneal epithelia evaluated by immunohistochemistry.
Brown staining indicates the expression of S100B protein. A: There was no obvious staining in normal cornea epithelium; B: Brown staining was only obvious in the basal layer epithelial cells of fungal keratitis cornea; C: Isotype showed negative staining (×200 maginfication).
Figure 7. S100B expression in Rat model cornea evaluated by immunohistochemistry.
Brown staining indicates the expression of S100B protein. A: There was no obvious staining in normal cornea epithelium; B: Brown staining was also obvious in fungal keratitis cornea and mostly located at the basal layer; C: Isotype showed negative staining (×200 maginfication).
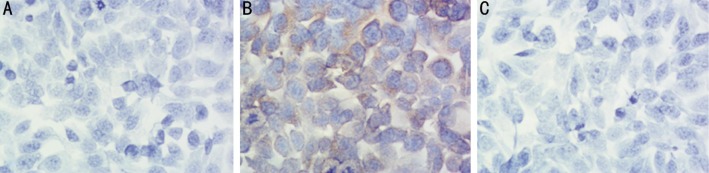
In vitro, immortalized HCECs were cultured on slides. Strong staining of S100B protein was observed in inactive A. fumigatus stimulated cells. The positive S100B expression located in the cytoplasm and mainly around the nucleus (Figure 8). As indicated in normal corneal epithelium, no obvious staining was found in the normal control cells without stimulation.
Figure 8. S100B expression in immortalized HCECs stained by immunocytochemistry.
Brown staining indicates the expression of S100B protein. A: There was no obvious staining in immortalized HCECs; B: Positive protein expression of S100B was observed in inactive A. fumigatus stimulated cells, brown particles located in the cytoplasm and mainly around the nucleus; C: Isotype showed negative staining (×400 maginfication).
DISCUSSION
Rats and mice are commonly used as keratitis animal models[1],[13]–[14]. In this study, we successfully established a keratitis animal model of rats under A. fumigatus invasion. Cornea infection in patients and animals with active A. fumigatus were characterized by significant inflammatory cells infiltration, tissue destruction and ulcer formation. In our study, we demonstrated that the inflammatory cells in cornea were mainly composed by neutrophils during infection. This change was consistent with the pathologic findings of Leal and Pearlman[15] that neutrophils contribute to 95% of the cells in the early stage and 70% in the later stage of infection. By comparing the central and peripheral regions of the ulcer, we also found that the more severe the tissue damage was, the more inflammatory cells infiltrated. It may be explained by an immune response that inflammatory cells were chemotactic to eliminate the pathogens and amplify the inflammatory response. Meanwhile, enzymes and toxins from inflammatory cells aggravated the tissue damage, resulting in a much more severe structural damage where more inflammatory cells aggregated.
S100B and some other S100 proteins can be secreted or released by cytokines (e.g. interferon-γ) and toll-like receptor ligands, to regulate cell functions, mostly in immune cells (such as monocytes/macrophages/microglia, neutrophils, lymphocytes, mast cells) and other cell types (like epithelial and endothelial cells, neurons, astrocytes, Schwann cells, articular chondrocytes)[16], participating in the innate and adaptive immune responses, cell migration and chemotaxis, tissue development and repair, and leukocyte and tumor cell invasion.
Recent findings proved that S100 alarmins kept a close relationship with anti-fungi immunity[7],[17]. However, as a major member of S100 family, there's no relevant research in human corneas between fungi and S100B. To investigate whether S100B participates in the cornea anti-fungi innate immune response, we stimulated immortalized HCECs cells with inactive A. fumigatus conidia and rat models with active A.fumigatus colonies.
In our study, we not only proved the existence of S100B in human corneal epithelial cells but also confirmed a close correlation between S100B and cornea fungi immunity for the first time. The existence of S100B in human corneal epithelia is line with a relevant cornea study in rabbit[18]. S100B expression was rare in normal corneal tissues both in gene as well as protein level, indicating that it was not constitutively expressed under normal circumstances. However, after fungi invasion, corneal epithelial cells up-regulated S100B expression. To explore the protein expression of S100B, immunochemistry method was adopted. We found an obvious staining in fungal keratitis corneal sections compared to normal control ones. The epithelial cells with S100B positive staining mainly focus at the base of corneal epithelium tissues and S100B protein was mainly located in the cytoplasm of corneal cells. This result is in keeping with a previous study of S100B functions[19]. We also explored the mRNA changes of S100B under different conditions both in vitro and in vivo. In our in vitro study, immortalized HCECs were included. We found a meaningful increase of S100B mRNA expression at 8h after inactive A. fumigatus stimulation and continued to increase as time prolonged. There was no difference between different normal control groups at corresponding time points. In fungal keratitis animal models, we found a similar result of S100B gene expression. S100B mRNA began to increase at 12h after exposure to A. fumigatus and reached to a peak point at 24h. There was a decline at 48h after A. fumigatus invasion. Our protein and mRNA results are in accordance with Guglielmo Sorci et al's[20] reseach in which S100B showed a similar dynamic change in mice lung tissue and was believed to integrate pathogen- and danger-sensing pathways together. It is known that the early innate immune response occurs within the 96h after pathogen infection, and all of our results conform to this time point[21]. So we believe that S100B participate the cornea innate immune response to fungi infection. In conclusion, our research found that S100B could also be expressed by human corneal epithelial cells and its expression could be significantly induced by A. fumigatus infection. This strongly supports our hypothesis that S100B may serve as an important mediator in the early innate immune response of corneal resistance to fungi infection. Relevant research found that inhibition of RAGE or S100B decreases the infiltration of immune/inflammatory cells, indicating an important role of S100/RAGE axis in the immune response[22]. Team of Donato et al[23] found that various signal pathways are involved in S100B's biological effect to mediate the production of inflammatory cytokines and chemotactic factors in other cell types[24]. Our preliminary results have made an understanding of S100B in corneal anti-fungi innate immunity, further studies are needed to explore whether S100B functions in the same pathway during cornea fungi infection.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81170825, No.81470609); Specialized Research Fund for the Doctoral Program of Higher Education (No.20123706110003); The Youth Natural Science Foundation of Shandong Province (No.ZR2013HQ007); The Key Project of Natural Science Foundation of Shandong Province (No.ZR2012HZ001).
Conflicts of Interest: Zhang J, None; Zhao GQ, None; Qu J, None; Che CY, None; Lin J, None; Jiang N, None; Zhao H, None; Wang XJ, None.
REFERENCES
- 1.Zhao GQ, Wang X, Hu LT, Lin J, Che CY, Jiang N. Expression of substance P in experimental fungal keratitis. Zhonghua Yan Ke Za Zhi. 2011;47(5):443–450. [PubMed] [Google Scholar]
- 2.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB, Colby K, Tuli SS, Patel SR, Lee SM, Irvine J, Stulting RD, Mauger TF, Schein OD. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117(12):2263–2267. doi: 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Pouwels SD, Heijink IH, ten Hacken NH, Vandenabeele P, Krysko DV, Nawijn MC, van Oosterhout AJ. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7(2):215–226. doi: 10.1038/mi.2013.77. [DOI] [PubMed] [Google Scholar]
- 6.Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 7.Yano J, Noverr MC, Fidel PL., Jr Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine. 2012;58(1):118–128. doi: 10.1016/j.cyto.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong L, Lan W, Lim RR, Chaurasia SS. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul Surf. 2014;12(1):23–31. doi: 10.1016/j.jtos.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Deng Q, Sun M, Yang K, Zhu M, Chen K, Yuan J, Wu M, Huang X. MRP8/14 enhances corneal susceptibility to Pseudomonas aeruginosa Infection by amplifying inflammatory responses. Invest Ophthalmol Vis Sci. 2013;54(2):1227–1234. doi: 10.1167/iovs.12-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heizmann CW, Ackermann GE, Galichet A. Pathologies involving the S100 proteins and RAGE. Subcell Biochem. 2007;45:93–138. doi: 10.1007/978-1-4020-6191-2_5. [DOI] [PubMed] [Google Scholar]
- 11.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha C, Giovannini G, Pierini A, Bell AS, Sorci G, Riuzzi F, Donato R, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. Genetically-determined hyperfunction of the S100B/RAGE axis is a risk factor for aspergillosis in stem cell transplant recipients. PLoS One. 2011;6(11):e27962. doi: 10.1371/journal.pone.0027962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou Shousha M, Santos AR, Oechsler RA, Iovieno A, Maestre-Mesa J, Ruggeri M, Echegaray JJ, Dubovy SR, Perez VL, Miller D, Alfonso EC, Bajenaru ML. A novel rat contact lens model for Fusarium keratitis. Mol Vis. 2013;19:2596–2605. [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Hu Y, Chen S, Dong C, Zhang J, Li Y, Yang J, Han X, Zhu X, Xu G. Role of activated macrophages in experimental Fusarium solani keratitis. Exp Eye Res. 2014;129:57–65. doi: 10.1016/j.exer.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Leal SM, Jr, Pearlman E. The role of cytokines and pathogen recognition molecules in fungal keratitis - Insights from human disease and animal models. Cytokine. 2012;58(1):107–111. doi: 10.1016/j.cyto.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57. [PMC free article] [PubMed] [Google Scholar]
- 17.Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PL., Jr The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS One. 2012;7(9):e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yew DT, Lam TK, Tsang D, Au YK, Li WW, Tso MO. Changes of cytochemical markers in the conjunctival and corneal epithelium after corneal debridement. Cell Mol Neurobiol. 2000;20(4):465–482. doi: 10.1023/A:1007071014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60(6):540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 20.Sorci G, Giovannini G, Riuzzi F, Bonifazi P, Zelante T, Zagarella S, Bistoni F, Donato R, Romani L. The danger signal S100B integrates pathogen- and danger-sensing pathways to restrain inflammation. PLoS Pathog. 2011;7(3):e1001315. doi: 10.1371/journal.ppat.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Che CY, Li XJ, Jia WY, Li N, Xu Q, Lin J, Wang Q, Jiang N, Hu LT, Zhao GQ. Early expression of surfactant proteins D in Fusarium solani infected rat cornea. Int J Ophthalmol. 2012;5(3):297–300. doi: 10.3980/j.issn.2222-3959.2012.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 23.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B's double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793(6):1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi R, Kastrisianaki E, Giambanco I, Donato R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem. 2011;286(9):7214–7226. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]