Abstract
AIM
To compare the effects of the surgical insult of cataract surgery on corneal inflammatory infiltration, neovascularization (NV) and lymphangiogenesis (LY) between the dry eye and non-dry eye in murine cataract surgery models.
METHODS
We established two groups of animals, one with normal eyes (non-dry eye) and the second with induced dry eyes. In both groups, we used surgical insults to mimic human cataract surgery, which consisted of lens extraction, corneal incision and suture. After harvesting of corneas on the 9th postoperative day and immunohistochemical staining, we compared NV, LY and CD11b+ cell infiltration in the corneas.
RESULTS
Dry eye group had significantly more inflammatory infiltration (21.75%±7.17% vs 3.65%±1.49%; P=0.049). The dry eye group showed significantly more NV (48.21%±4.02% vs 26.24%±6.01%; P=0.016) and greater levels of LY (9.27%±0.48% vs 4.84%±1.15%; P=0.007). In corneas on which no surgery was performed, there was no induction of NV in both the dry and non-dry group, but dry eye group demonstrated more CD11b+ cells infiltration than the non-dry eye group (0.360%±0.160% vs 0.023%±0.006%; P=0.068). Dry eye group showed more NV than non-dry eye group in both topical PBS application and subconjunctival PBS injection (P=0.020 and 0.000, respectively).
CONCLUSION
In a murine cataract surgery model, preexisting dry eye can induce more postoperative NV, LY, and inflammation in corneal tissue.
Keywords: angiogenesis, cataract surgery, dry eye disease, inflammation
INTRODUCTION
Cataract surgery can lead to dry eye even when dry eye symptoms and signs are absent or minimal preoperatively[1]–[6].The effects on cornea caused by cataract surgery which can lead to dry eye are greater in eyes that already have signs and symptoms of dry eye disease[3]–[4],[6]–[7].
In clinical practice, the majority of patients who have cataract surgery with intraocular lens (IOL) implantation are elderly. Furthermore, the incidence of dry eye in the elderly population is very high and one of the major risk factors for the development of dry eye has been identified as increased age[8]–[9]. Thus, we can assume that most of cataract surgeries are performed in patients with dry eye.
In our previous study, we reported that after ocular surface surgery the dry eye can be a pre-angiogenic milieu[10]. Here, we investigated the angiogenic and inflammatory effects of dry eye in a cataract surgery model. We hypothesized that the effects on cornea induced by cataract surgery would be amplified in the dry eye model compared with the non-dry eye model. In contrast to previous reports which focused on changes in the conjunctiva, we evaluate the cataract surgery-induced effects on corneal tissues.
SUBJECTS AND METHODS
Experimental Dry Eye Model
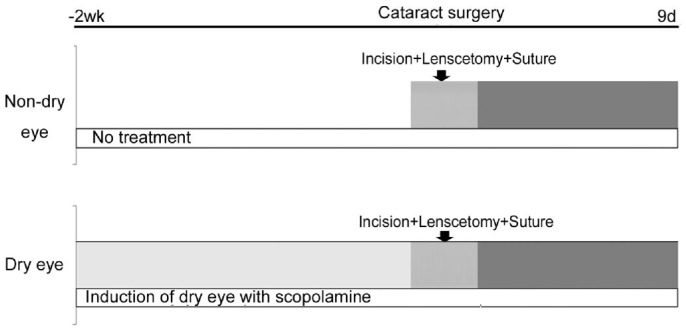
The experiments were performed in accordance with the regulations of ARVO (Association for Research in Vision and Ophthalmology) and were approved by the IACUC (Institutional Animal Care and Use Committee) of Catholic University of Korea, St. Vincent's Hospital. Eight- to ten-week-old female Balb/c mice (The Koatech Laboratory, Pyeongtak, Korea) were used. Mice were assigned to one of two groups, normal eye (non-dry eye,12 eyes) or anticholinergic-induced experimental dry eye model (12 eyes)[11] (Figure 1). In the dry eye group of mice, we started induction of dry eye 2wk before the cataract surgery. Dry eye was induced in the mice by pharmacologically applying a topical application of 1% atropine sulfate (Alcon Korea, Seoul, Korea) twice in the first 48h and subcutaneous injections of 0.2 mL of 10 mg/mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO, USA) three times a day, for the entire 23d duration of the experiment.
Figure 1. Dry eye induction schedule.
Surgical Insults in the Dry Eye and Non-dry Eye Groups
In both the normal eye (non-dry eye) and the induced experimental dry eye model groups, we made the same surgical insults to mimic cataract surgery, including lens extraction, corneal incision and sutures (Figure 2).
Figure 2. Murine cataract surgery model.
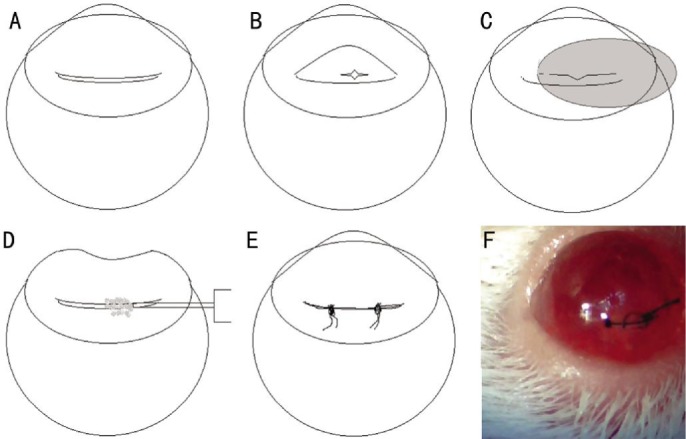
A: A curvilinear full thickness corneal incision; B: Puncture of the anterior capsule using a sharp needle; C: Removal of the lens material from the capsule; D: Injection of viscoelastics to reform the globe; E: Two corneal sutures of 10-0 nylon to close the wound; F: Representative picture.
In both groups, following pupil dilation, we made a trephine marking using a 2.0 mm corneal trephine from the limbal arcade and made a 1/3 circle curvilinear, full thickness corneal incision (Figure 2A). We then made a small puncture on the surface of the anterior lens capsule (Figure 2B) to remove the lens from the capsule using a 30-gauge needle (Figure 2C). After inserting a small amount of viscoelastics (sodium hyaluronate, 10 mg/mL) to prevent corneal shrinkage (Figures 2D and 2E), we reshaped the globe and applied two corneal sutures with 10-0 nylon to close the wound (Figure 2E). All sutures were removed 1wk post-surgery in both models.
Immunohistochemical Staining
After harvesting corneas on the 9th postoperative day, we performed immunohistochemical staining for neovascularization (NV), lymphangiogenesis (LY), and inflammatory infiltration.
The corneas were trimmed of any remaining limbus and iris. Immunohistochemical staining for vascular and lymphatic endothelial cells was performed on corneal flat mounts. Fresh corneas were dissected, rinsed in phosphate-buffered saline (PBS) for 30min and fixed in 100% acetone (Sigma) for 20min. After washing in PBST (0.1% Tween®20 in PBS), nonspecific binding was blocked with 3% bovine serum albumin (BSA) in PBS for 3 nights at 4°C. Incubation with 1:500 fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-mouse CD31 antibody (558738, BD Pharmingen) or 1:200 rabbit anti-LYVE-1 (ab14917, Abcam Inc., Cambridge, MA, USA) in 3% BSA in PBS at 4°C overnight was followed by 1:1000 goat anti-rabbit antibody-Alexa Fluor® 546 (A11071, Invitrogen Corporation, Carlsbad, CA, USA) for 1h with subsequent washes in PBST at room temperature. Corneas were mounted with the anti-fading agent Gelmount.
CD11b staining was done to compare inflammatory cell infiltration between the groups as follows. Harvested corneas were dissected, rinsed in PBS for 30min and fixed in 100% acetone for 20min. After washing in PBST, nonspecific binding was blocked with 3% BSA for 3 nights at 4°C. Overnight incubation with 1:100 Alexa Fluor® 647 rat anti-mouse CD11b antibody (557686, BD Pharmingen) in 3% BSA was done at 4°C. This was followed by washes in PBST at room temperature. Corneas were mounted with an anti-fading agent.
Fluorescent Microscopic Examination
After immunochemical staining for vascular endothelial cells and flat mounting of the corneas (9 eyes from each group), images of the corneal vasculature were captured using a camera attached to a fluorescent microscope (OLYMPUS BX51,Tokyo, Japan). NV and LY were quantified using Image J (National Institutes of Health) as described above. The total areas of NV and LY were calculated as follows: total NV (%)=(NV area/total cornea area)×100%; total LY (%)=(LY area/total cornea area)×100%. All images were analysed in blind method without knowledge of whether the eye was a dry-eye or non-dry eye.
Confocal Microscopic Examination
After harvesting corneas on the 9th postoperative day and immunohistochemical staining, we evaluated CD11b+ cell infiltration with a confocal microscope. To evaluate inflammatory infiltration with confocal microscope images, 3-4 sites from each cornea (3 eyes from each group) were chosen from both the dry eye and non-dry eye groups. A confocal microscope was used to quantify the area of inflammatory infiltration (LSM 510 META, Carl Zeiss, Germany). Horizontal sections (objective magnification: ×10) of 17-19 images were obtained from the top surface to the bottom of the cornea at 5 µm intervals and were stacked to create the final image stack. In each image stack, inflammatory infiltration was quantified by setting a threshold level of fluorescence above which cells were captured and processed using Image J (National Institutes of Health). The percentage area of CD11b+ cell infiltration was analyzed in each stack image using the pixel area. All images were analysed in blind method without knowledge of whether the eye was a dry eye or non-dry eye.
Comparison of Neovascularization and Inflammation Between Dry Eye Model and Non-dry Eye Model in Unoperated Eyes
We compared the NV and inflammation between dry eye and non-dry eye in unoperated eyes. In the dry eye group (8 eyes), after 2wk pharmacologic induction of dry eye, we harvested the corneas. In the non–dry eye group (8 eyes), no dry eye induction was performed in the duration of 2wk. After harvesting the corneas in both groups, the immunohistochemical double staining for CD11b and CD31 were performed. The fluorescent microscopic examination and the confocal microscopic examination were performed as described above.
Comparison of the Effect of Anti-inflammatory Drugs in Eyes with Cataract Surgery Between Dry Eye and Non-dry Eye Model
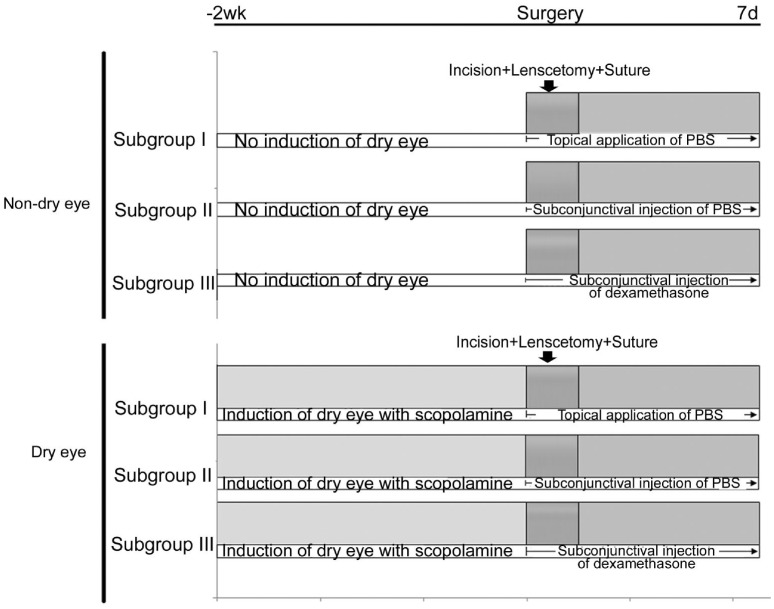
To simulate human cataract surgery, we added anti-inflammatory drugs (dexamethasone, 5 mg/mL) on eyes after cataract surgery in both dry eye and non-dry eye group. We made three subgroups (10 eyes in each subgroup) in both the dry eye and non-dry eye group, subgroup I: topical application of PBS; subgroup II: subconjunctival injection of PBS (10 µL); subgroup III: subconjunctival injection of dexamethasone (10 µL). In each subgroup, treatment was done daily until postoperative 7th day (Figure 3). After harvesting, the immunohistochemical double staining for CD11b and CD31 were performed. The fluorescent microscopic examination and the confocal microscopic examination were performed as described above.
Figure 3. Dry eye induction and treatment schedule in three subgroups.
Statistical Analysis
Statistical analysis was performed using SPSS 11.5 (Chicago, IL, USA). NV and LY in each group were compared with the corresponding control group using an unpaired two-tailed t-test and a Mann-Whitney U test. P<0.05 was considered statistically significant.
RESULTS
Comparison of Postoperative Inflammatory Infiltration Between the Dry Eye and Non-dry Eye Groups
Comparison of the postoperative inflammatory infiltration between the dry eye (21.75%±7.17%) and non-dry eye groups (3.65%±1.49%) revealed that the dry eye group had significantly more inflammatory infiltration (P=0.049) (Figure 4).
Figure 4. Inflammatory infiltration after cataract surgery.
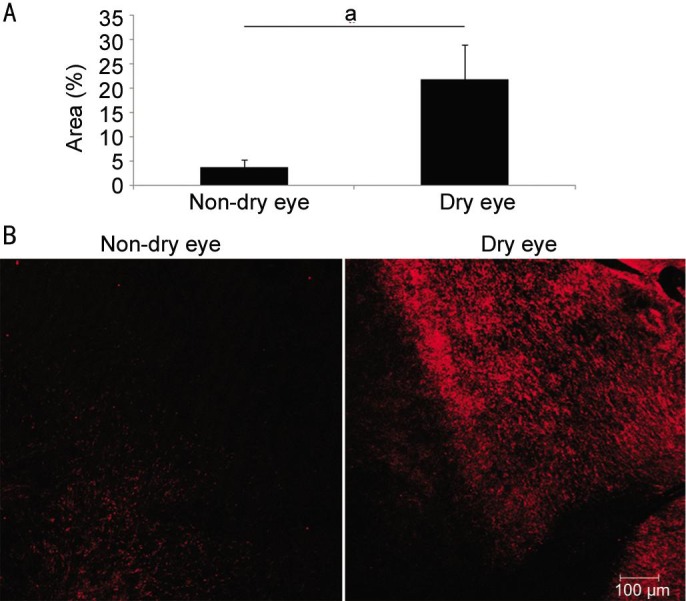
A: Comparison of CD11b+ inflammatory infiltration of cornea after cataract surgery between the dry eye and non-dry eye groups; B: Representative pictures. aP<0.05.
Comparison of Postoperative Neovascularization Between Groups
When postoperative NV was compared between dry eye (48.21%±4.02%) and non-dry eye groups (26.24%±6.01%), the dry eye group showed significantly more NV (P=0.016) (Figure 5).
Figure 5. Neovascularization after cataract surgery.
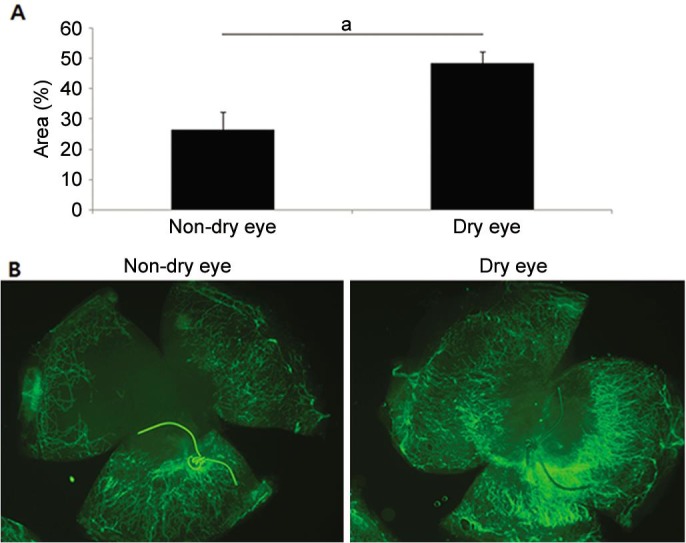
A: Comparison of neovascularization after cataract surgery between the dry eye and non-dry eye groups; B: Representative pictures. aP<0.05.
Comparison of Postoperative Lymphangiogenesis Between Groups
Comparison of postoperative LY between the dry eye (9.27%±0.48%) and non-dry eye groups (4.84%±1.15%) revealed that the dry eye group had significantly greater levels of LY (P=0.007) (Figure 6).
Figure 6. Lymphangiogenesis after cataract surgery.
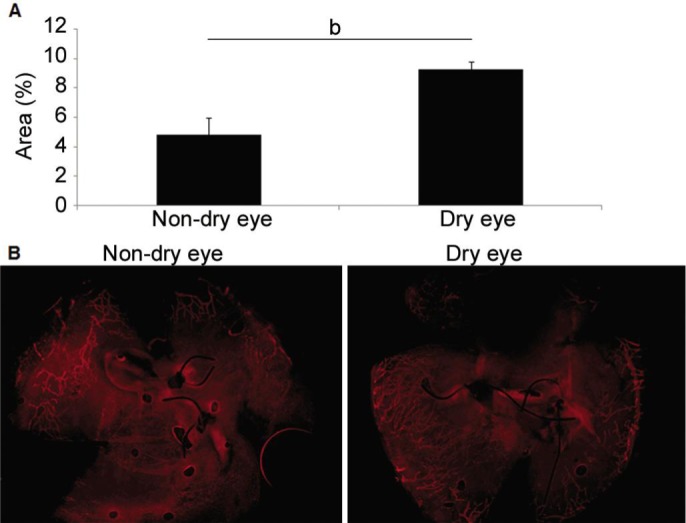
A: Comparison of lymphangiogenesis after cataract surgery between the dry eye and non-dry eye groups; B: Representative pictures. bP<0.01.
Comparison of Neovascularization and Inflammation Between Dry Eye and Non-dry Eye Model in Unoperated Eyes
In corneas on which no surgery was performed, there was no induction of NV in both the dry and non-dry group (Figure 7A), but dry eye group demonstrated more CD11b+ cells infiltration than the non–dry eye group (P=0.068) (Figure 7B, 7C). The percentage area of CD11b+ infiltration was 0.023%±0.006% in the non–dry eye group and 0.360%±0.160% in the dry eye group.
Figure 7. Neovascularization in unoperated eyes.
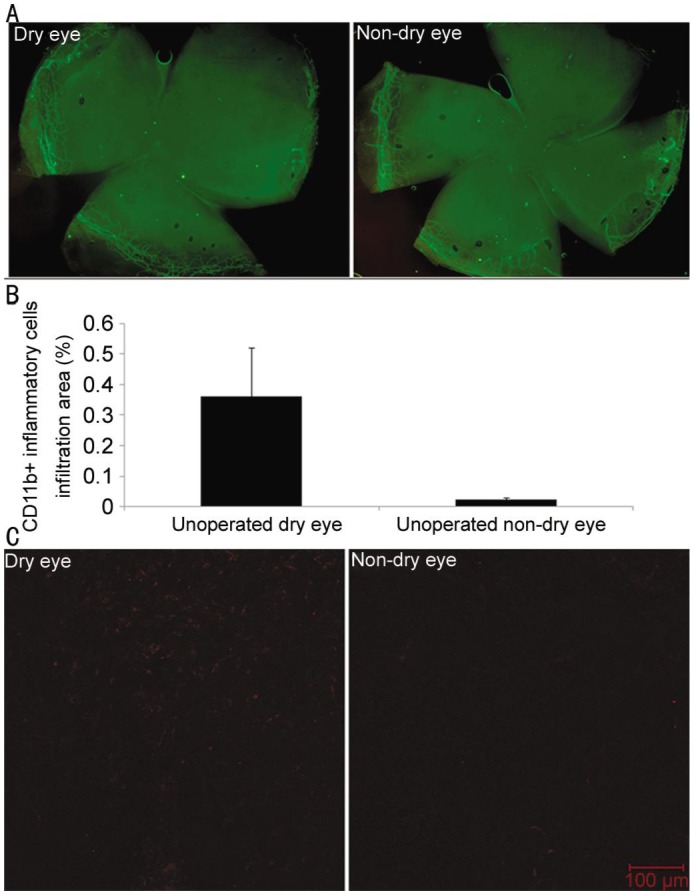
A: Comparison of neovascularization between dry eye and non-dry eye model in unoperated eyes (representative pictures); B: Comparison of inflammation between dry eye and non-dry eye model in unoperated eyes (CD11b+ cells infiltration); C: Comparison of inflammation between dry eye and non-dry eye model in unoperated eyes (representative pictures).
Comparison of Postoperative Neovacularization and Inflammation in Subgroups Between Dry Eye and Non-dry Eye Model
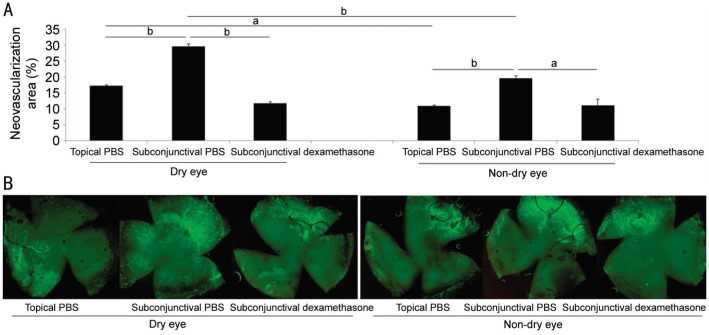
Figure 8A and 8B show the comparison of NV. In both dry eye and non-dry eye group, subconjunctival injection of PBS (subgroup II) showed more NV than topical application of PBS (subgroup I)(P=0.000 and 0.001, respectively). Dry eye group showed more NV than non-dry eye group in both topical PBS application (subgroup I) and subconjunctival PBS injection (subgroup II) (P=0.020 and 0.000, respectively).
Figure 8. Neovascularization after cataract surgery in three treatment subgroups.
A: Comparison of neovacularization after cataract surgery in three subgroups between dry eye and non-dry eye model; B: Representative pictures. aP<0.05, bP<0.01.
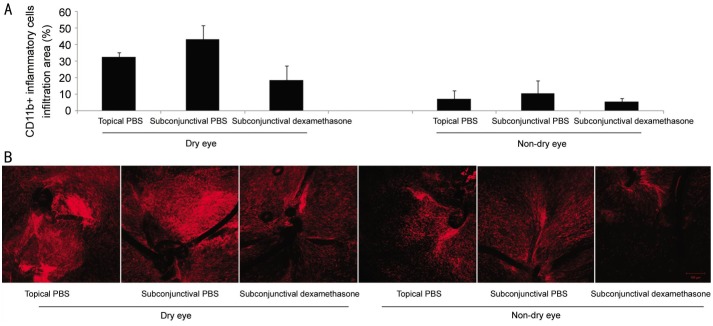
Figure 9A and 9B show the comparison of inflammatory infiltration. In dry eye and non-dry eye group, subconjunctival injection of PBS showed a tendency of more inflammation than topical application of PBS. Dry eye group showed a tendency of more inflammatory infiltration than non-dry eye group in both topical PBS application (subgroup I) and subconjunctival PBS injection (subgroup II).
Figure 9. Inflammatory infiltration after cataract surgery in three treatment subgroups.
A: Comparison of inflammation after cataract surgery in three subgroups between dry eye and non-dry eye model; B: Representative pictures.
DISCUSSION
Cataract surgery can lead to dry eye[1]–[5]. The mechanisms resulting in exacerbation of surface disease after cataract surgery likely include several factors: an increase in inflammatory mediators due to postoperative inflammation, exposure to microscopic light, toxicity from the use of benzalkonium chloride containing eye drops and damage to the corneal nerves from limbal incisions[2]–[4],[12].The effects of cataract surgery that can lead to dry eye are greater in eyes with pre-existing dry eye disease[3]–[4],[7],[12]. Patients with pre-existing severe ocular surface disease are at significantly greater risk of complication after cataract surgery[2],[7],[13].
In this study, we investigated whether there were differences in post-cataract surgery inflammation and LY in corneal tissue between dry eyes and non-dry eyes. In the development of a cataract surgery model, we attempted to mimic cataract surgery procedures performed in humans, including corneal incision, suture and lens extraction with an intact posterior lens capsule. However, there were still several differences in our model system compared with human cataract surgery. We did not implant an IOL and thoroughly clean up the retained cortex and viscoelastics. Consequently, the lensectomy which was performed in this study would induce more postoperative intracameral inflammation than would do in human cataract surgery.
Clinically, in cases of human cataract surgery, we typically do not evaluate inflammation of the cornea, but instead evaluate inflammation of the anterior chamber[14]–[15]. However, in contrast to non-dry eye surgeries, cases of dry eye postoperative corneal inflammation and intracameral inflammation induced by the cataract surgery can be problematic and require intensive treatment. Thus, in dry eye cases, postoperative anti-inflammatory treatment are important in decreasing both intracorneal and intracameral inflammation.
There have been several reports regarding changes in the objective signs and histopathologic findings associated with dry eye after cataract surgery[1]–[2],[16]. These previous reports of histopatholic changes usually describe findings in the conjunctiva, such as impression cytology of the conjunctiva, rather than findings in the cornea. Clinically, one of the noteworthy complications in cornea after cataract surgery in patients with severe dry eye is sterile corneal melt[7],[13],[17]–[18]. In our cataract surgery model, we evaluated inflammation and lymphangiogenesis in corneal tissue according to the level of dry eye. Our evaluation of the effects of cataract surgery on cornea tissue rather than on the conjunctiva in eyes with dryness is novel and importance, in that the cornea is the main organ tissue directly related to visual acuity after cataract surgery[6],[19]–[20].
We determined that the level of corneal inflammation after cataract surgery correlated with the dryness of the eye, and was therefore an additional important condition that surgeons should pay attention to, in addition to postoperative intracameral inflammation. In our results, in unoperated eyes, corneas in dry eye group demonstrated more inflammatory infiltration than non-dry eye group (Figure 7). The amplified corneal inflammation and NV after cataract surgery in dry eye group might be caused by the surgical insults added on the dry eye-insults (Figures 8, 9). LY due to the local inflammatory reaction around the suture material following human cataract surgery was not a common condition[21]–[22]. However, as there were emerging data relating dry eye and LY, we should consider the postoperative lymphangiogenic response in cornea after cataract surgery in dry eye patients[23]–[25]. Given our results, we suggest that dry eye patient corneas need more postoperative anti-inflammatory, and possibly anti-lymphangiogenic treatment than non-dry eye corneas after cataract surgery. With postoperative subconjunctival injection of dexamethasone in our cataract surgery model, there was not a significant difference of NV and inflammation between dry eye and non-dry eye. However, we anticipate the difference in the effect of anti-inflammatory drugs between dry eye and non-dry eye model with longer duration of dry eye induction as in the chronic dry eye disease of human.
Currently, several preoperative and postoperative treatments were utilized in the clinic to decrease eye dryness after cataract surgery, such as artificial tears, secretagogues and anti-inflammatory agents[6],[8],[12],[26]–[27]. To prevent postoperative dry eye after cataract surgery, especially in preexisting dry eye cases, we suggest an emphasis on the importance of applying the postoperative anti-inflammatory treatment in order to decrease corneal and intracameral inflammation.
Acknowledgments
Cho YK designed the study, performed the animal work, wrote and revised the manuscript. Kwon JW and Chung YW assisted the animal work and revised the manuscript. Kwon JW and Chung YW contributed equally to the work and therefore should be considered equivalent. La TY, Choi JA and Jee DH assisted revision of the manuscript.
Foundation: Supported by the St.Vincent's Hospital, Research Institute of Medical Science Foundation (No. SVHR-2015-13).
Conflicts of Interest: Kwon JW, None; Chung YW, None; Choi JA, None; La TY, None; Jee DH, None; Cho YK, None.
REFERENCES
- 1.Oh T, Jung Y, Chang D, Kim J, Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012;56(2):113–118. doi: 10.1007/s10384-012-0117-8. [DOI] [PubMed] [Google Scholar]
- 2.Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(9 Suppl 1):S16–S20. doi: 10.1097/ICO.0b013e31812f67ca. [DOI] [PubMed] [Google Scholar]
- 3.Ram J, Gupta A, Brar G, Kaushik S, Gupta A. Outcomes of phacoemulsification in patients with dry eye. J Cataract Refract Surg. 2002;28(8):1386–1389. doi: 10.1016/s0886-3350(02)01387-1. [DOI] [PubMed] [Google Scholar]
- 4.Ram J, Sharma A, Pandav SS, Gupta A, Bambery P. Cataract surgery in patients with dry eyes. J Cataract Refract Surg. 1998;24(8):1119–1124. doi: 10.1016/s0886-3350(98)80107-7. [DOI] [PubMed] [Google Scholar]
- 5.Han KE, Yoon SC, Ahn JM, Nam SM, Stulting RD, Kim EK, Seo KY. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am J Ophthalmol. 2014;157(6):1144–1150. doi: 10.1016/j.ajo.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Movahedan A, Djalilian AR. Cataract surgery in the face of ocular surface disease. Curr Opin Ophthalmol. 2012;23(1):68–72. doi: 10.1097/ICU.0b013e32834d90b7. [DOI] [PubMed] [Google Scholar]
- 7.Jones RR, Maguire LJ. Corneal complications after catarct surgery in patients with rheumatoid arthritis. Cornea. 1992;11(2):148–150. doi: 10.1097/00003226-199203000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Miyake G, Ota I, Miyake K, Zako M, Iwaki M. Effects of topical diquafosol pretreatment on intraoperative corneal wetting. J Cataract Refract Surg. 2014;40(10):1682–1688. doi: 10.1016/j.jcrs.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Versura P, Giannaccare G, Campos EC. Sex-Steroid Imbalance in Females and dry eye. Curr Eye Res. 2015;40(2):162–175. doi: 10.3109/02713683.2014.966847. [DOI] [PubMed] [Google Scholar]
- 10.Cho YK, Archer B, Amabti BK. Dry eye predisposes to corneal neovascularization and lymphangiogenesis after corneal injury in a murine model. Cornea. 2014;33(6):621–627. doi: 10.1097/ICO.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, Saban DR, Kodati S, Stern ME, Dana R. Effect of dessiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunipathogenesis. Invest Ophthalmol Vis Sci. 2013;54(4):2457–2464. doi: 10.1167/iovs.12-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afsharkhamseh N, Movahedan A, Motahari H, Djalilian AR. Cataract surgery in patients with ocular surface disease: An update in clinical diagnosis and treatment. Saudi J Ophthalmol. 2014;28(3):164–167. doi: 10.1016/j.sjopt.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen KL. Sterile corneal perforation after cataract surgery in Sjögren's syndrome. Br J Ophthalmol. 1982;66(3):179–182. doi: 10.1136/bjo.66.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Hazazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12(1):4–8. doi: 10.1097/00055735-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chee SP, Ti SE, Sivakumar M, Tan DT. Postoperative inflammation: extracapsular cataract extraction versus phacoemulsification. J Cataract Refract Surg. 1999;25(9):1280–1285. doi: 10.1016/s0886-3350(99)00161-3. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez MA, Arriola-Vilalobos P, Torralbo-Jimenez P, Giron N, de la Heras B, Herrero Vanrell R, Alvarez-Barrientos A, Benitez-del-Castillo JM. The effect of preservative-free HP-Guar on dry eye after phacoemulsification: a flow cytometry study. Eye (Lond) 2010;24(8):1331–1337. doi: 10.1038/eye.2010.24. [DOI] [PubMed] [Google Scholar]
- 17.Insler MS, Boutros G, Boulware DW. Corneal ulceration following cataract surgery in patients with rheumatoid arthritis. J Am Intraocul Implant Soc. 1985;11(6):594–597. doi: 10.1016/s0146-2776(85)80145-2. [DOI] [PubMed] [Google Scholar]
- 18.Radtke N, Meyers S, Kaufman HE. Sterile corneal ulcers after cataract surgery in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96(1):51–52. doi: 10.1001/archopht.1978.03910050015003. [DOI] [PubMed] [Google Scholar]
- 19.Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133(2):181–186. doi: 10.1016/s0002-9394(01)01365-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchia T, Negishi K, Tsubota K. Functional visual acuity measurement in cataract and intraocular lens implantation. Curr Opin Ophthalmol. 2011;22(1):31–36. doi: 10.1097/ICU.0b013e3283414f36. [DOI] [PubMed] [Google Scholar]
- 21.Bar-Sela SM, Spierer O, Spierer A. Suture-related complications after congenital cataract surgery: Vicryl versus Mersilene sutures. J Cataract Refract Surg. 2007;33(2):301–304. doi: 10.1016/j.jcrs.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Bar-Sela SM, Spierer O, Spierer A. The removal of 10/0 polyester (Mersilene) sutures after small incision congenital cataract surgery. Eur J Ophthalmol. 2008;18(1):82–86. doi: 10.1177/112067210801800114. [DOI] [PubMed] [Google Scholar]
- 23.Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128(7):819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. 2012;130(1):84–89. doi: 10.1001/archophthalmol.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004;2(2):124–130. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]