Abstract
AIM
To compare argon laser photocoagulation and intrastromal injection of voriconazole as adjunctive treatment modalities in cases of resistant mycotic corneal ulcers.
METHODS
Two groups each of them included 20 cases of resistant mycotic corneal ulcers. Both groups treated with local and systemic specific antimicrobial drugs guided with culture and sensitivity results. In one group argon laser photocoagulation was used as an adjunctive therapy to the specific antifungal drugs and in the other group, intrastromal injection of voriconazole was done besides the specific antifungal drugs. The 40 cases included in the study were proven according to culture and sensitivity to be 28 cases with pure fungal results and 12 cases with mixed (fungal and bacterial). In argon laser group, argon laser irradiation of the corneal ulcer was performed using argon laser 532 nm wavelength (Carl Zeiss LSL 532s AG; Meditec, Inc.) after fluorescein staining. In the other group, voriconazole solution (500 µg/mL) was prepared and injected in the corneal stroma. All cases were followed up for 3mo after healing was achieved.
RESULTS
Complete healing of the epithelial defect and resolution of stromal infiltration with no adverse effects were achieved in argon laser group in duration ranged from 2-4wk in 90% of cases. In voriconazole group 4 cases needed amniotic membrane graft due to thinning and 16 cases healed in duration ranged from 2-6wk (80% of cases).
CONCLUSION
Argon laser photocoagulation is superior to intrastromal voriconazole injection in treatment of resistant fungal corneal ulcers.
Keywords: mycotic keratitis, argon laser, voriconazole
INTRODUCTION
Fungal keratitis is a serious pathological corneal condition that commonly ends in great visual impairment. In most of the developing countries, fungal keratitis represents a major problem due to the poor socioeconomic status of many people, lack of proper medical care, unavailability of topical antifungal agents and difficulties in its clinical diagnosis, laboratory work[1]–[3].
Fungal corneal pathogens included four families: filamentous fungi, yeast, yeast like and dimorphic fungi. From the filamentous fungi, Aspergillus, fusarium and Cladosporium are the most common pathogens while Candida albicans is the most common member form of yeast family[4]–[5].
Mycotic keratitis occurs due to invasion by the pathogenic fungal strains and helped by poor host immunity and defense mechanisms due to local or systemic causes. Certain fungal strains have the ability to adhere to the cell wall and to produce proteolytic enzymes and toxins that destroy the host defense mechanisms[6]–[8].
Fungal corneal infections especially with filamentous agents are usually predisposed by trauma with vegetable or organic materials. On the other hand Candida albicans commonly attacks in the immune-compromised corneas[9]–[10].
Detection of pathogenic corneal fungi is based on culture and sensitivity tests using corneal swabs or biopsies. Other methods for fungus detection based on the types of enzymes produced by the invading fungus like immune-diffusion, electrophoresis and ELISA[11]–[12].
There are many antifungal agents used in different fungal infections, three major groups can be identified; polyenes: as amphotericin B and nystatin, azoles: as itraconazole and fuconazole and lastly pyrimidines: as flucytosine[13]–[14].
In the last few years argon laser photocoagulation was introduced as an adjunctive therapy in the treatment of resistant infected corneal ulcers including fungal cases with promising results[15].
Voriconazole is a recent triazole antifungal agent used orally and intravenously. Intrastromal voriconazole injection also is used nowadays to increase the effectiveness of antifungal therapy against mycotic pathogens that attack the cornea[16]–[17].
SUBJECTS AND METHODS
Patients included in this study attended the cornea unit in Department of Ophthalmology at Tanta University Hospital in Egypt for medical consultation in the period from August 2012 to August 2014.
Those patients were suffering from resistant mycotic corneal ulcers. They were divided into 2 groups each one included 20 cases of resistant fungal corneal ulcers with or without hypopyon level. Both groups were treated with local and systemic specific antifungal drugs guided with culture and sensitivity results. After one week of specific antifungal therapy with no obvious improvement, those patients were included in the study. The 40 cases of the study included 28 pure fungal ulcers and 12 mixed (fungal and bacterial) cases as proven with microbial culture and sensitivity results. Thirty-three cases of the included patients were associated with hypopyon. Informed consent was obtained from every participant in this study and ethical committee approval was obtained.
In the first visit, careful ophthalmological examination using the slit lamp was done and detailed history was investigated to detect any predisposing factors that may be incriminated in such cases and so can be corrected if they are correctable. The previous medications taken by the patients were stopped for 1d and continued only the non-specific drugs like atropine sulphate and lubricants to give a chance for microbial culture and sensitivity to be done. Routine ocular ultrasonography was done for assessment of the posterior segment in order to detect its involvement that would change the approach of treatment.
The size of corneal ulcers as examined with the slit lamp ranged from 1.5-9.0 mm in both horizontal and vertical diameters.
The culture and sensitivity results showed multiple fungal isolates including aspergillus flavus, candida albicans, fusarium solani and others.
According to the culture and sensitivity results, the specific treatment was prescribed for each case. The topical antifungal drugs prescribed included voriconazole 1% (VFEND; Pfizer, Inc., New York, NY, USA) amphotericin B 0.15% (Fungizone; Bristol-Myers Squibb, New York, NY, USA) prepared as fortified drops, natamycin 5% (Natamet; Sun Pharmaceutical Industries Limited, Mumbai, India), fluconazole 0.2% (Diflucan; Pfizer, Inc., New York, NY, USA) taken directly from the vial, and itraconazole 1% (Itral; Jawa Pharmaceuticals, Lahore, Pakistan). The topical antibiotics prescribed according to culture and sensitivity results included gatifloxacin 0.5% (Zymer; Allergan, Inc., Irvine, CA, USA), ofloxacin 0.3% (Oflox; Allergan, Inc.), moxifloxacin 0.5% (Vigamox; Alcon Laboratories, Inc., Fort Worth, TX, USA), and tobramycin 0.3% (Tobrex; Alcon Laboratories, Inc.).
Every day follow up for one week was done in all cases of both groups. When improvement was achieved, we continued the medical treatment only and the case was excluded from the study.
After one week of specific therapy and no obvious improvement was noticed, the ulcer was considered resistant and included in the study. The 40 cases included in the study were divided into 2 groups; one group of 20 cases treated with intrastromal voriconazole and the other 20 cases included in the argon laser treatment group.
In the laser treatment group, a drop of benoxinate hydrochloride 0.4% (surface anesthesia) and a drop of fluorescein sodium 0.25% were instilled. Fluorescein sodium stained the epithelial defect of the treated corneas to allow the chance of argon laser beam energy to be absorbed by the corneal tissue otherwise the argon laser beam traverses the unstained cornea without absorption as in pan-retinal photocoagulation treatment for diabetic retinopathy. Argon laser therapy was done using argon green wavelength (Carl Zeiss LSL 532s AG; Meditec, Inc.). A spot size of 500 µm, pulse duration of 0.2s, and power of 900 mW were used. Number of shots varied from one case to another depending on the size of ulcer where we targeted the bed and edge of the ulcer during argon laser therapy with laser shots. Number of laser shots ranged from 18-163 shots as shown on the device counter including the missed shots due to unexpected eye movement in certain cases.
In the voriconazole treatment group, voriconazole powder was used to prepare a solution with concentration of 500 µg/mL. The prepared solution was loaded in a 1 mL tuberculin syringe with a 30 gauge needle. After topical anesthesia with benoxinate hydrochloride 0.4%, intrastromal injection was done under completely aseptic conditions in the operating room using the surgical microscope. The syringe needle was introduced obliquely to reach the mid stroma with bevel down. Then the drug was injected producing a wave of hydration in the area covered. Five divided injections were used to surround the corneal lesion. The total amount of the drug injected ranged from 0.05 mL to 0.1 mL. If no noticeable improvement was detected after 5d, the injection was repeated (3 times maximally).
Follow-up of each case was done daily till complete healing was achieved. In each case we assessed size of the epithelial defect, density and edge of infiltration, corneal edema, depth of the ulcer and hypopyon level. Then each case was followed up for 3mo to detect any relapse or recurrence.
Statistical Analysis
Statistical presentation and analysis of the present study was conducted, using the mean, standard deviation, Chi-square and t-test by SPSS V.20.
RESULTS
Slit lamp examination of patients of both groups revealed that the size of corneal ulcers ranged from 1.5-9.0 mm long in both vertical and horizontal meridians. The right eye was affected in 26 cases and the left eye in 14 cases. Age of the patients ranged from 24-63y in the argon laser group with a mean of 42±8.7y while it ranged from 27-59y in the voriconazole group with a mean of 40±6.4y. The argon laser group included 14 male and 6 female patients while the voriconazole group included 17 male and 3 female patients.
As regards duration of healing; in the argon laser group healing was achieved after about 2wk in 11 cases, 3wk in 2 cases and 4wk in 5 cases (Figures 1, 2). Two cases did not show improvement after argon laser therapy and amniotic membrane grafting (AMG) was done to help healing that was achieved after about 6wk from the first visit.
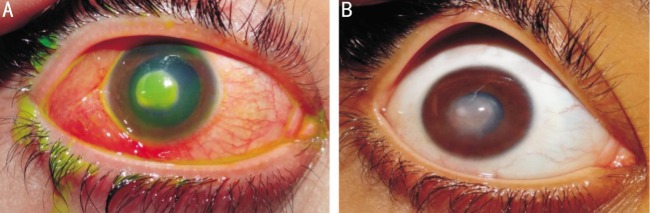
Figure 1. Resistant mycotic corneal ulcer associated with hypopyon before and after argon laser therapy.
Healing was achieved after 4wk [before (A) and after (B)].
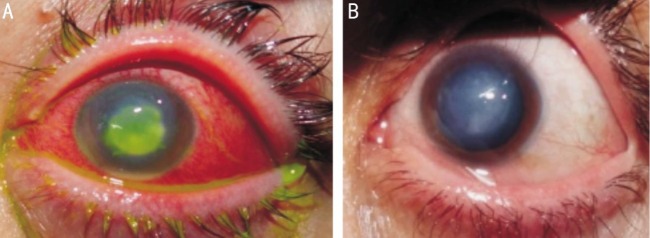
Figure 2. Resistant mycotic corneal ulcer associated with hypopyon related to contact lens wear before and after argon laser therapy.
Healing was achieved after 2wk [before (A) and after (B)].
In the voricanazole group, healing was achieved after about 2wk in 7 cases, 3wk in 5 cases, 4wk in 2 cases, 5wk in 1 case and 6wk in 1 case (Figures 3, 4). Four cases showed no improvement after repeated intrastromal voriconazole injections (maximally 3 times) and AMG was done to help healing.
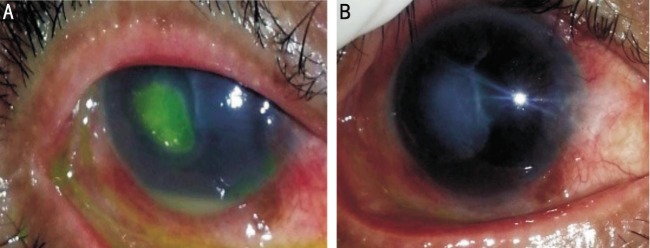
Figure 3. Resistant mycotic corneal ulcer associated with hypopyon before and after intrastromal voriconazole injection.
Healing was achieved after 2wk [before (A) and after (B)].
Figure 4. Resistant mycotic corneal ulcer associated with hypopyon before and after intrastromal voriconazole injection.
Healing was achieved after 4wk [before (A) and after (B)].
Statistically significant difference was obtained when comparing the healing results of both groups. Healing duration in laser group ranged from 2-4wk and in the voriconazole group ranged from 2-6wk (χ2=26.81, P=0.001). Two cases needed AMG in laser group while 4 cases needed AMG (χ2=3.922, P=0.047) (Tables 1, 2).
Table 1. Percentage and duration of healing of mycotic corneal ulcers in both argon laser and voriconazole groups.
| Duration | Laser | Voriconazole | χ2 | P |
| 2wk | 11 (55) | 7(35) | 26.810 | 0.001a |
| 3wk | 2 (10) | 5 (25) | ||
| 4wk | 5 (25) | 2 (10) | ||
| 5wk | 0 (0) | 1 (5) | ||
| 6wk | 0 (0) | 1 (5) |
aStatistical significance.
n (%)
Table 2. Percentage of healing of mycotic corneal ulcers in both argon laser and voriconazole groups with AMG.
| Amniotic membrane grafting | Laser | Voriconazole | χ2 | P |
| Healing without AMG | 18 (90) | 16 (80) | 3.922 | 0.047a |
| Healing with AMG | 2 (10) | 4 (20) |
aStatistical significance.
n (%)
As regards the visual acuity results, we found that at time of first presentation, all cases of both groups ranged from hand motion to 0.2 (decimal system). No visual acuity improvement was achieved after healing in 9 cases (45%) in laser group while 7 cases (35%) in the voriconazole group did not show visual acuity improvement. One line gain was achieved in 7 cases (35%) and 11 cases (55%) and two or more line gain was achieved in 4 cases (20%) and 2 cases (10%) in laser group and voriconazole group respectively with statistically significant difference (χ2=9.031, P=0.011) (Table 3).
Table 3. Visual acuity results after healing of mycotic corneal ulcers in both argon laser and voriconazole groups.
| Visual acuity improvement | Laser | Voriconazole | χ2 | P |
| No improvement | 9 (45) | 7 (35) | 9.031 | 0.011a |
| One line gain | 7 (35) | 11(55) | ||
| Two or more line gain | 4 (20) | 2 (10) |
aStatistical significance.
n (%)
DISCUSSION
Resistant mycotic corneal ulcers represent a great challenge to ophthalmologists all over the world. Surgical interference was the way to solve this problem in many instances. In the United States, penetrating keratoplasty was done in 34% of cases according to certain reports and in 47% of the cases in other reports[18]–[19].
Many antifungal drugs were used for the treatment of resistant mycotic corneal ulcers according to culture and sensitivity results. In many instances, topical use of those drugs was not sufficient alone to treat those cases and so adjunctive treatments were described. From these adjunctive strategies: subconjunctival injections of antifungal agents as fluconazole and amphotrericin B, intrastromal injection of voriconazole, cross linking, AMG and conjunctival flaps[20]–[25].
Different laser types are currently used in many ophthalmic procedures like argon, YAG, excimer, and femtosecond lasers. Argon laser absorption by the ocular tissue targets is maximally achieved by melanin and hemoglobin present in the retina not in the cornea. To be absorbed by the cornea, it needs an exogenous dye that can absorb the energy in the argon laser beam. Emission and excitation of fluorescein dye in a diluted alkaline solution can be best achieved by wavelength around 500 nm. Argon laser used in our study is produced by the machine (Carl Zeiss LSL 532s AG; Meditec, Inc.), so this argon laser with 532 nm can be absorbed by fluorescein dye when staining the corneal epithelial defect producing its thermal damaging effect. Over-heating of corneal tissues causes suppression of cellular enzymes (40°C -45°C), damage of the cellular proteins (above 60°C) and damage of DNA (above 70°C). Over-heating damage affects both the host tissue and the organism itself[15],[26].
The ulcer bed debridement is frequently done in cases of fungal keratitis to enhance penetration of antifungal drugs. Argon laser photocoagulation may produce similar effect to debridement where it produces over-heating of the ulcer bed and so shrinkage of the thick area of keratitis. In addition, argon laser also produces a fungicidal effect due to its thermal damage of the infected tissue. It has been reported that the temperature in corneal tissue rises over 90 degrees due to argon-tissue interaction[15],[27].
To the best of our knowledge, there are no reports that had investigated the use of argon laser photocoagulation in the treatment of resistant fungal corneal ulcers.
We (the same authors) published a report in 2014 that evaluated the argon laser photocoagulation as adjunctive therapy for resistant infected corneal ulcers and we found similar results to what we found in the present study as regards healing and visual acuity[28].
Voriconazole is a recent triazole antifungal agent. It has the broadest spectrum coverage of antifungal drugs and good intraocular penetration. It is commercially available as oral and intravenous preparations[29].
There are many studies that evaluated the use of voriconazole in treatment of fungal keratitis as topical drops and intrastromal injections. Prakash et al [17] reported successful use of intrastromal voriconazole injection in three patients of fungal keratitis.
Sharma et al [21] reported successful treatment of 10 cases of deep resistant fungal keratitis out of 12 cases after intrastromal voriconazole injection as adjunctive therapy to topical voriconazole 1% eye drops with success rate of 83.33%[21].
Recently, more than one study reported that fungal corneal ulcers caused by Fusarium are resistant to intrastromal voriconazole injections even if repeated and we found similar results to those reports where 2 of the 4 resistant cases that needed AMG in our study were caused by Fusarium[30]–[31].
In our study, the parameters of argon laser used was adjusted to a power of 900 mW, spot size of 500 µm and a pulse duration of 0.2s. During the procedure we noticed blanching of the corneal stroma and small bubble cavitations in the stroma.
In our study, complete healing was achieved in 90% of cases in argon laser group (18 cases) and duration of healing ranged from about 2 to 4wk. While in the voriconazole group, healing was achieved only in 16 cases (80%) in a longer duration ranged from 2-6wk with statistically significant difference (χ2=26.81, P=0.001) as shown in Table 1. Two case of argon laser group (10%) needed AMG to achieve complete healing in comparison to 4 cases (20%) of voriconazole group with statistically significant difference (χ2=3.922, P=0.047) as shown in Table 2.
As regards visual acuity results, improvement with two or more line gain (decimal system) was achieved in 4 cases (25%) and 9 cases (45%) did not show any improvement in argon laser group while only 2 cases of voriconazole group showed similar improvement and 7 cases (35%) did not improve at all. Statistical analysis of visual acuity results showed significant results (χ2=9.031, P=0.011) as shown in Table 3.
Acknowledgments
Conflicts of Interest: Khater MM, None; El-Shorbagy MS, None; Selima AA, None.
REFERENCES
- 1.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PA. Fungal infections of the cornea. Eye (Lond) 2003;17(8):852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 3.Ou JI, Acharya NR. Epidemiology and treatment of fungal corneal ulcers. Int Ophthalmol Clin. 2007;47(3):7–16. doi: 10.1097/IIO.0b013e318074e727. [DOI] [PubMed] [Google Scholar]
- 4.Tuli SS. Fungal keratitis. Clin Ophthalmol. 2011;5:275–279. doi: 10.2147/OPTH.S10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas P. Tropical ophthalmomycoses. In: Seal D, Pleyer U, editors. Ocular infection. Investigation and treatment in practice. 2nd ed. New York: Informa Healthcare; 2007. pp. 271–305. [Google Scholar]
- 6.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Nath R, Baruah S, Saikia L, Devi B, Borthakur AK, Mahanta J. Mycotic corneal ulcers in upper Assam. Indian J Ophthalmol. 2011;59(5):367–371. doi: 10.4103/0301-4738.83613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57(4):273–279. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Thomas PA, Leck AK, Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br J Ophthalmol. 2005;89(12):1554–1558. doi: 10.1136/bjo.2005.076315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitani A, Shiraishi A, Uno T, Miyamoto H, Hara Y, Yamaguchi M, Ohashi Y. In vivo and in vitro investigations of fungal keratitis caused by Colletotrichum gloeosporioides. J Ocul Pharmacol Ther. 2009;25(6):563–565. doi: 10.1089/jop.2009.0069. [DOI] [PubMed] [Google Scholar]
- 12.Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16(4):730–797. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rautaraya B, Sharma S, Kar S, Das S, Sahu SK. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol. 2011;11:39. doi: 10.1186/1471-2415-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FlorCruz NV, Peczon IV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2012;2:CD004241. doi: 10.1002/14651858.CD004241.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrino F, Carrasco MA. Argon laser phototherapy in the treatment of refractory fungal keratitis. Cornea. 2013;32(1):95–97. doi: 10.1097/ICO.0b013e318256140e. [DOI] [PubMed] [Google Scholar]
- 16.Jurkunas UV, Langston DP, Colby K. Use of voriconazole in the treatment of fungal keratitis. Int Ophthalmol Clin. 2007;47(2):47–59. doi: 10.1097/IIO.0b013e318036bd47. [DOI] [PubMed] [Google Scholar]
- 17.Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146(1):56–59. doi: 10.1016/j.ajo.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Patel A, Hammersmith K. Contact lens-related microbial keratitis: recent outbreaks. Curr Opin Ophthalmol. 2008;19(4):302–306. doi: 10.1097/ICU.0b013e3283045e74. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan L, Sharma S, Nalamada S, Sahu SK, Das S, Garg P. Natamycin in the treatment of keratomycosis: correlation of treatment outcome and in vitro susceptibility of fungal isolates. Indian J Ophthalmol. 2011;59(6):512–514. doi: 10.4103/0301-4738.86328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dev S, Rajaraman R, Raghavan A. Severe fungal keratitis treated with subconjunctival fluconazole. Am J Ophthalmol. 2006;141(4):783; author reply 783–784. doi: 10.1016/j.ajo.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Sharma N, Agarwal P, Sinha R, Titiyal JS, Velpandian T, Vajpayee RB. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: case series. Br J Ophthalmol. 2011;95(12):1735–1737. doi: 10.1136/bjo.2010.192815. [DOI] [PubMed] [Google Scholar]
- 22.Carrasco MA, Genesoni G. Treatment of severe fungal keratitis with subconjunctival amphotericin B. Cornea. 2011;30(5):608–611. doi: 10.1097/ICO.0b013e3181fb826d. [DOI] [PubMed] [Google Scholar]
- 23.Galperin G, Berra M, Tau J, Boscaro G, Zarate J, Berra A. Treatment of fungal keratitis from Fusarium infection by corneal cross-linking. Cornea. 2012;31(2):176–180. doi: 10.1097/ICO.0b013e318221cec7. [DOI] [PubMed] [Google Scholar]
- 24.Yildiz EH, Nurozler AB, Ozkan Aksoy N, Altiparmak UE, Onat M, Karaguzel H. Amniotic membrane transplantation: indications and results. Eur J Ophthalmol. 2008;18(5):685–690. doi: 10.1177/112067210801800504. [DOI] [PubMed] [Google Scholar]
- 25.Sandinha T, Zaher SS, Roberts F, Devlin HC, Dhillon B, Ramesh K. Superior fornicial conjunctival advancement pedicles (SFCAP) in the management of acute and impending corneal perforations. Eye (Lond) 2006;20(1):84–89. doi: 10.1038/sj.eye.6701814. [DOI] [PubMed] [Google Scholar]
- 26.Krauss JM, Puliafito CA, Steinert RF. Laser interactions with the cornea. Surv Ophthalmol. 1986;31(1):37–53. doi: 10.1016/0039-6257(86)90050-0. [DOI] [PubMed] [Google Scholar]
- 27.Loh AR, Hong K, Lee S, Mannis M, Acharya NR. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28(8):856–859. doi: 10.1097/ICO.0b013e318199fa77. [DOI] [PubMed] [Google Scholar]
- 28.Khater MM, Selima AA, El-Shorbagy MS. Role of argon laser as an adjunctive therapy for treatment of resistant infected corneal ulcers. Clin Ophthalmol. 2014;8:1025–1030. doi: 10.2147/OPTH.S59928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siatiri H, Daneshgar F, Siatiri N, Khodabande A. The effects of intrastromal voriconazole injection and topical voriconazole in the treatment of recalcitrant Fusarium keratitis. Cornea. 2011;30(8):872–875. doi: 10.1097/ICO.0b013e3182100993. [DOI] [PubMed] [Google Scholar]
- 30.Kalaiselvi G, Narayana S, Krishnan T, Sengupta S. Intrastromal voriconazole for deep recalcitrant fungal keratitis: a case series. Br J Ophthalmol. 2015;99(2):195–198. doi: 10.1136/bjophthalmol-2014-305412. [DOI] [PubMed] [Google Scholar]
- 31.Niki M, Eguchi H, Hayashi Y, Miyamoto T, Hotta F, Mitamura Y. Ineffectiveness of intrastromal voriconazole for filamentous fungal keratitis. Clin Ophthalmol. 2014;8:1075–1079. doi: 10.2147/OPTH.S63516. [DOI] [PMC free article] [PubMed] [Google Scholar]