Abstract
AIM
To determine the parameters most informative in predicting the anatomical results of surgical treatment of idiopathic full-thickness macular hole (IMH).
METHODS
One hundred and sixty-two consecutive patients (170 eyes) after primary operation for IMH were enrolled. Outcomes were classified as anatomical success when both IMH closure and restoration of the outer retinal structure were achieved. “Prospective” group included 108 patients (115 eyes) followed with optical coherence tomography (OCT) and microperimetry for 1y after surgery. Potential prognostic criteria, except microperimetry data, were tested in “retrospective” group (54 patients, 55 eyes). Prognostic value of each parameter was determined using receiver operating characteristic (ROC) analysis. Combined predictive power of the best prognostic parameters was tested with the use of linear discriminant analysis.
RESULTS
IMH closure was achieved in 106 eyes (92%) in the prospective group and 49 eyes (89%) in the retrospective group. Despite anatomical closure, the outer retinal structure was not restored in two eyes in the first group and in one eye in the second group. Preoperative central subfield retinal thickness demonstrated the best discriminatory capability between eyes with anatomical success and failure: area under the ROC-curve (AUC) 0.938 (95% CI: 0.881-0.995), sensitivity 64% at fixed specificity 95% (cut-off value 300 µm) in the prospective group; sensitivity 57% and specificity 90% in the retrospective group. Other continuous parameters except tractional hole index (AUC: 0.796, 95% CI: 0.591-1.000) had significantly lower AUCs (P<0.05). The best combination of the parameters, established by discriminant analysis in the prospective group, could not confirm its predictive value in the retrospective group.
CONCLUSION
Preoperative central subfield retinal thickness is a strong and probably the best predictor of anatomical results of IMH surgical treatment.
Keywords: idiopathic full-thickness macular hole, optical coherence tomography, anatomical surgery outcome, prognosis, central subfield retinal thickness, receiver operating characteristic analysis
INTRODUCTION
Surgical treatment of idiopathic full-thickness macular hole (IMH) has demonstrated its safety and efficacy in a great number of studies. The modern vitrectomy technique with internal limiting membrane peeling provides anatomical closure, even of large IMH, in 90%-95%[1].
IMH size (minimum and/or base diameter), stage and duration of symptoms have long been established as the best predictors of anatomical closure of IMH[2]–[3]. Recent studies were mostly concerned with the prognosis of the functional effect of the operation using the results of the optical coherence tomography (OCT) separately or in combination with other data (duration of symptoms, preoperative visual acuity, age etc.)[4]–[11]. Few of these studies were also dealing with the prognosis of the anatomical closure of IMH and no new OCT predictors were found[4],[9].
OCT studies on retinal changes after IMH closure show gradual restoration of the outer retinal structure in most eyes[12]–[13]. However, in a small proportion of cases, despite IMH closure, no signs of structural or functional recovery could be found. Such eyes do not show the renewal of the inner segment ellipsoid and visual acuity does not improve in most cases or could even deteriorate. The loss of external limiting membrane integrity might be implicated in these cases[5],[7]–[8],[14]. Evidently, such cases represent a negative anatomical result of the surgery, but no attempts were made to predict them alongside with the absence of IMH closure.
The purpose of the present study was to determine the parameters most informative in predicting the anatomical results (including both IMH closure and restoration of the outer retinal structure) of IMH surgical treatment.
SUBJECTS AND METHODS
Subjects
One hundred and sixty-two patients with IMH (170 eyes) operated by two experienced surgeons-the authors of the study (Shkvorchenko DO and Sharafetdinov IK)-were enrolled. Exclusion criteria were: any concurrent visually significant ocular pathology, history of vitreous surgery, repeated operations for IMH. Patients with cataract, pseudophakia or high myopia were not excluded.
The first “prospective” group included 108 consecutive patients (115 eyes) operated from February 2010 to January 2013 who underwent comprehensive examinations for at least one year according to a planned schedule.
The second “retrospective” group included 54 consecutive patients (55 eyes) operated from February 2013 to August 2014. Selection of the second group of patients was carried out retrospectively on the basis of availability of both pre- and postoperative examinations, including high quality OCT evaluation. The absence of the restoration of the outer retinal structure should be confirmed by OCT performed at 6mo or later after surgery.
All patients underwent 3-port 25G vitrectomy with internal limiting membrane peeling, using combination of trypan blue and brilliant blue G, and air endotamponade. After surgery, patients were instructed to maintain a face-down position for 3-4d. Vitrectomy combined with phacoemulsification and intraocular lens (IOL) implantation was performed in 15 patients (15 eyes) of the prospective group and 20 patients (20 eyes) of the retrospective group. In 9 patients (9 eyes) of the first group and 3 patients (3 eyes) of the second group phacoemulsification with IOL implantation had been performed earlier.
This study adhered to the tenets of the Declaration of Helsinki and had local ethics committee approval with informed consent obtained from all subjects.
Methods
Patients of the prospective group were examined before IMH surgery and at 1, 3, 6, and 12mo after the operation. Patients received complete ophthalmic examination, OCT and microperimetry. In the second group of patients the medical records were reviewed retrospectively with special attention to the data of the preoperative and the last postoperative visits that included OCT and complete ophthalmic examination.
OCT was performed in both groups of patients with the Cirrus HD-OCT 5000 (Carl Zeiss Meditec Inc., Dublin, CA, USA). The scan protocol was “Macular Cube 512×128” analyzed by “Macular Thickness Analysis”. Data acquisition and analysis software of Cirrus HD-OCT has been described in detail by many authors[7]–[8],[11]. The analysis protocol is fully automatic, and in most cases does not need manual correction. In a few eyes with relatively small stage 2-3 holes such correction was needed when an operculum prevented automatic measurement of the hole. In a prospective group of patients for better visualization we also used scan protocol “5 Line Raster” analyzed by “High Definition Images”.
The following preoperative OCT parameters were measured on a horizontal scan through the center of the hole: minimum diameter, base diameter, hole height (the distance from the pigment epithelium to the highest point of the hole margins), temporal and nasal arm length (the distance between the ends of the lines accepted as minimum and base diameters on either temporal or nasal side of the hole). Then four commonly used indices were calculated: the macular hole index (hole height/base diameter), the tractional hole index (hole height/minimum diameter), the hole form factor [(nasal+temporal arm length)/base diameter], the diameter hole index (minimum diameter/base diameter). For further analysis we also used the values provided by the “Macular Thickness Analysis” protocol-central subfield retinal thickness (1 mm in diameter), retinal thickness in the four inner subfields of the 6-mm OCT grid (Figures 1A, 2A) and cube volume.
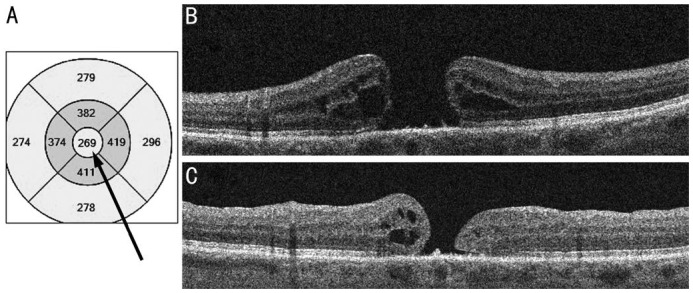
Figure 1. OCT in a case with poor prognosis based on central subfield retinal thickness below 300 µm (arrow).
A: OCT grid; B: Preoperative scan; C: Postoperative scan.
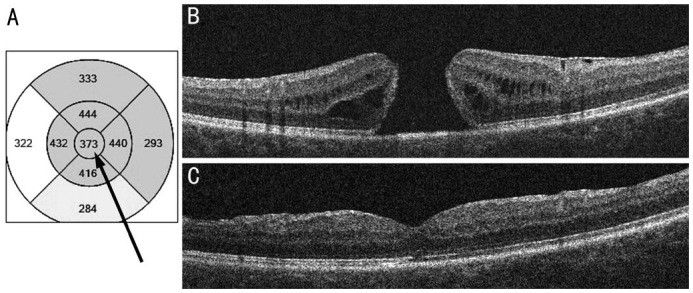
Figure 2. OCT in a case with good prognosis based on central subfield retinal thickness above 300 µm (arrow).
A: OCT grid; B: Preoperative scan; C: Postoperative scan taken 6mo after the operation.
Patients of the prospective group were examined with MP-1 microperimeter (Nidek Technologies, Vigonza, Italy) using the macula 8-degree program. Mean retinal sensitivities within the central 4 degrees and 8 degrees areas were determined.
Besides OCT and microperimetry data we studied prognostic significance of the age and sex of the patients, the stage of IMH according to Gass[15], best corrected visual acuity (BCVA) determined with both Snellen and ETDRS tables, refraction (sphere, cylinder), axial length. Duration of symptoms, as well as microperimetry data, was studied in the prospective group only.
Statistical Analysis
Statistical analysis was performed using R software package version 3.1.2 (The R Foundation for Statistical Computing, http://www.r-project.org, accessed January, 30, 2015). Continuous variables were compared by Welch's t-test; categorical variables were compared by Chi-squared test with Yates' continuity correction and Fisher's exact test. Prognostic value of each parameter was determined using receiver operating characteristic (ROC) analysis. To test discriminatory capability between patients with anatomical success and failure, the areas under the receiver operating characteristic curves (AUCs) were calculated and compared. Additionally, sensitivities and corresponding cut-off values were extracted for each parameter at fixed specificity 95%. Combined predictive power of the best prognostic parameters was tested with the use of linear discriminant analysis. P<0.05 was considered as statistically significant.
RESULTS
Clinical and demographic characteristics of the prospective and retrospective groups of patients are presented in Table 1.
Table 1. Clinical and demographic characteristics of the prospective and retrospective groups of patients.
| Parameters | Prospective group (n=115) | Retrospective group (n=55) | P |
| Age (a) | 65.0±7.0 (47-77) | 67.3±7.2 (47-79) | <0.05 |
| Sex, M/F | 21/87 | 2/52 | <0.01 |
| Minimum diameter of the hole (µm) | 361.0±145.3 (58-800) | 414.0±156.0 (134-759) | <0.05 |
| Base diameter of the hole (µm) | 768.3±257.0 (130-1430) | 909.3±304.3 (260-1767) | <0.01 |
| BCVA before surgery (logMAR) | 0.76±0.33 (1.68-0.10) | 0.74±0.31 (1.68-0.22) | -a |
| Eyes with axial length≥26 mm | 6 | 2 | - |
| Duration of symptoms (mo) | 6.4±8.0 (1-72) | - | - |
| Stage by Gass, n (%) | Stage 2: 59 (51.3); Stage 3: 31 (27.0); Stage 4: 25 (21.7) | Stage 2: 27 (49.0); Stage 3: 14 (25.5); Stage 4: 14 (25.5) | - |
BCVA: Best corrected visual acuity. aThe absence of significant difference (P>0.05).
x±s
Minimum and base diameters of the hole, patient's age and proportion of women were higher in the retrospective group. These differences, especially the larger size of the holes posed an additional challenge to prognostic parameters established in the prospective group of patients.
Anatomical results of the surgery in both groups were similar, despite the large difference in the base diameter of the hole. IMH closure was achieved in 100 patients (106 eyes, 92%) in the prospective group and 48 patients (49 eyes, 89%) in the retrospective group. In these groups IMH was not closed in 9 and 6 eyes respectively. In the course of follow-up in two eyes in the first group and in one eye in the second group the outer retinal structure did not show any signs of restoration despite anatomical closure. These cases were also regarded as anatomical failure. So in total, 10 patients (11 eyes, 10%) in the prospective group and 7 patients (7 eyes, 13%) in the retrospective group were considered an anatomical failure.
In the prospective group seven variables were significantly different between patients with anatomical success and failure-central subfield retinal thickness and stage (P<0.000), duration of symptoms, minimum diameter, 8 degrees and 4 degrees retinal sensitivity (P<0.001), hole height (P<0.02). These and a number of other potential prognostic criteria were subjected to ROC-analysis (Table 2).
Table 2. AUC, sensitivity and cut-off value at fixed specificity 95% for the parameters potentially useful for the prediction of anatomical result of idiopathic macular hole surgery.
| Parameters | AUC (95% CI) | Sensitivity (%) at fixed specificity 95% | Cut-off valuea |
| Central subfield retinal thickness (µm) | 0.938 (0.881-0.995) | 64 | ≤300 |
| Stage by Gass | 0.820 (0.695-0.945) | 22 | -b |
| Tractional hole index | 0.796 (0.591-1.000) | 46 | ≤0.750 |
| Mean 4° retinal sensitivity (dB, n=107) | 0.785 (0.584-0.987) | 50 | ≤6.1 |
| Mean 8° retinal sensitivity (dB, n=107) | 0.777 (0.583-0.970) | 60 | ≤8.7 |
| Macular hole index | 0.749 (0.551-0.948) | 46 | ≤0.391 |
| Minimum diameter of the hole (µm) | 0.747 (0.532-0.963) | 46 | ≥555 |
| Height of the hole (µm) | 0.720 (0.572-0.869) | 29 | ≤347 |
| Duration of symptoms (mo) | 0.698 (0.524-0.872) | 20 | ≥13.0 |
| Base diameter of the hole (µm) | 0.665 (0.468-0.862) | 18 | ≥1132 |
| BCVA before surgery (LogMAR) | 0.618 (0.435-0.799) | 9 | ≥1.393c |
BCVA: Best corrected visual acuity. aCharacters ≤ or ≥ define a range of values predictive of the anatomical failure of the surgery; bCalculated fractional value is not applicable (only Stage 4 could be a cut-off value); cCorresponding decimal visual acuity: ≤0.04.
(n=115)
According to Table 2, central subfield retinal thickness was the most promising prognostic factor showing highest values both of AUC and sensitivity at fixed specificity 95%. Its AUC was significantly (P<0.05) larger as compared to AUCs of other parameters, with the exception of stage and tractional hole index. But the stage of the hole, though having the second highest value of AUC, had a very low sensitivity at fixed specificity 95%, which hindered its use as a prognostic criterion.
Using the calculated cut-off values for fixed specificity 95% we tested the prognostic capabilities of the parameters with the higher AUCs in retrospective group of patients. Once again, the central subfield retinal thickness showed the best predictive power: sensitivity 57% (4 out of 7 eyes) and specificity 90% (43 out of 48 eyes). No other parameter demonstrated better results though the differences were not statistically significant.
Figures 1 and 2 show illustrative examples of prognosis based on central subfield retinal thickness below and above the critical value of 300 µm. The patients in the figures are quite similar. They are 67 and 61-year-old women with 12 and 14mo duration of symptoms, minimum diameter of the hole 643 and 604 µm, base diameter of the hole 896 and 1254 µm respectively. But an operation was successful only in the second case with central subfield retinal thickness 373 µm.
With the use of linear discriminant analysis the best combination of the potential prognostic parameters was established in the prospective group of patients. Besides central subfield retinal thickness, final set of the parameters included stage (by Gass[15]), minimum and base diameters of the hole, its height and BCVA (logMAR). Compared to central subfield retinal thickness this combination of the parameters improved results in the prospective group, but showed low sensitivity in the retrospective group of patients revealing only 2 out of 7 eyes with anatomical failure (the differences were not significant).
DISCUSSION
OCT parameters and indices are considered the best predictors of anatomical and functional results of IMH surgery[4]–[11],[13],[16]–[17]. The prognosis of IMH closure most often was based on minimum diameter or/and base diameter of the hole[4],[9],[16]–[17], and such indices as the hole form factor[13],[16] and the macular hole index[9]. Central subfield retinal thickness was measured only after surgery to find its correlation with the functional result[18].
The results of the present study suggest that preoperative central subfield retinal thickness is a strong and possibly the best predictor of anatomical results of IMH surgery. It could predict both the anatomical closure of the hole and the restoration of the outer retinal structure. Given that the central subfield area is a constant, the mean retinal thickness in this area (central subfield retinal thickness) directly reflects the volume of tissues available for restoration of the fovea after the surgery. It seems logical that the reduction of this “reserve” volume below the critical value is a significant unfavorable prognostic sign.
The central subfield retinal thickness is the single prognostic parameter obtained directly from the OCT analysis results. In case of its low value the review of OCT printout would require immediate attention of the surgeon. For other prognostic OCT parameters additional measurements and, in case of indices, some simple calculations should be performed. The measurements do not take much time, but are subjective and could sometimes bring inconsistent results due to wrong scan selection or measurement errors.
As it was mentioned in the methods section, we performed manual correction of OCT results in cases with the operculum preventing automatic measurement of the hole. But according to our data this manual correction did not influence the prognosis in the few cases where it was performed, and thus was not necessary.
Central subfield retinal thickness demonstrated slightly lower predictive power in patients of the retrospective group operated later in time. It could be explained by the differences of the groups as shown in Table 1, but also by the increasing experience of the surgeons and improving of the surgical techniques that provided for the better results in more difficult cases.
This study has several limitations. The minimum diameter of the hole did not exceed 800 µm, so the larger holes need further study. Additionally we could not double-check the predictive power of the retinal sensitivity in the retrospective group because microperimetry was not performed in this group of patients. However, according to the results of ROC analysis, the retinal sensitivity should be regarded as a very promising prognostic factor that deserves further study. All the measurements were performed on the Cirrus HD-OCT device. We did not compare its measurements with OCT devices by other manufacturers. Still, most modern spectral-domain OCTs are able to provide the information on the central subfield retinal thickness though the measurements algorithms could vary. As a result the cut-off values might slightly differ with other devices, but, presumably, it should not influence on the prognostic value of the measurements.
In conclusion, the present study showed that preoperative central subfield retinal thickness is a strong and probably the best and the most practical predictor of anatomical results (including both IMH closure and restoration of the outer retinal structure) of IMH surgical treatment.
Acknowledgments
Conflicts of Interest: Shpak AA, None; Shkvorchenko DO, None; Sharafetdinov IK, None; Yukhanova OA, None.
REFERENCES
- 1.Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 2.Kang HK, Chang AA, Beaumont PE. The macular hole: report of an Australian surgical series and meta-analysis of the literature. Clin Experiment Ophthalmol. 2000;28(4):298–308. doi: 10.1046/j.1442-9071.2000.00329.x. [DOI] [PubMed] [Google Scholar]
- 3.Ip MS, Baker BJ, Duker JS, Reichel E, Baumal CR, Gangnon R, Puliafito CA. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120(1):29–35. doi: 10.1001/archopht.120.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Gupta B, Laidlaw DA, Williamson TH, Shah SP, Wong R, Wren S. Predicting visual success in macular hole surgery. Br J Ophthalmol. 2009;93(11):1488–1491. doi: 10.1136/bjo.2008.153189. [DOI] [PubMed] [Google Scholar]
- 5.Ooka E, Mitamura Y, Baba T, Kitahashi M, Oshitari T, Yamamoto S. Foveal microstructure on spectral-domain optical coherence tomographic images and visual function after macular hole surgery. Am J Ophthalmol. 2011;152(2):283–290.e1. doi: 10.1016/j.ajo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Shimozono M, Oishi A, Hata M, Kurimoto Y. Restoration of the photoreceptor outer segment and visual outcomes after macular hole closure: spectral-domain optical coherence tomography analysis. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1469–1476. doi: 10.1007/s00417-011-1681-1. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117(9):1815–1824. doi: 10.1016/j.ophtha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology. 2012;119(7):1438–1446. doi: 10.1016/j.ophtha.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Wakely L, Rahman R, Stephenson J. A comparison of several methods of macular hole measurement using optical coherence tomography, and their value in predicting anatomical and visual outcomes. Br J Ophthalmol. 2012;96(7):1003–1007. doi: 10.1136/bjophthalmol-2011-301287. [DOI] [PubMed] [Google Scholar]
- 10.Alkabes M, Padilla L, Salinas C, Nucci P, Vitale L, Pichi F, Burès-Jelstrup A, Mateo C. Assessment of OCT measurements as prognostic factors in myopic macular hole surgery without foveoschisis. Graefes Arch Clin Exp Ophthalmol. 2013;251(11):2521–2527. doi: 10.1007/s00417-013-2347-y. [DOI] [PubMed] [Google Scholar]
- 11.Matsumiya W, Kusuhara S, Shimoyama T, Honda S, Tsukahara Y, Negi A. Predictive value of preoperative optical coherence tomography for visual outcome following macular hole surgery: effects of imaging alignment. Jpn J Ophthalmol. 2013;57(3):308–315. doi: 10.1007/s10384-013-0232-1. [DOI] [PubMed] [Google Scholar]
- 12.Christensen UC, Krøyer K, Sander B, Larsen M, la Cour M. Prognostic significance of delayed structural recovery after macular hole surgery. Ophthalmology. 2009;116(12):2430–2436. doi: 10.1016/j.ophtha.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Michalewska Z, Michalewski J, Nawrocki J. Continuous changes in macular morphology after macular hole closure visualized with spectral optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1249–1255. doi: 10.1007/s00417-010-1370-5. [DOI] [PubMed] [Google Scholar]
- 14.Landa G, Gentile RC, Garcia PM, Muldoon TO, Rosen RB. External limiting membrane and visual outcome in macular hole repair: spectral domain OCT analysis. Eye (Lond) 2012;26(1):61–69. doi: 10.1038/eye.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119(6):752–759. doi: 10.1016/s0002-9394(14)72781-3. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86(4):390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter AB, Folgar FA, Weissbrot J, Wald KJ. Macular hole surgery prognostic success rates based on macular hole size. Ophthalmic Surg Lasers Imaging. 2012;43(3):184–189. doi: 10.3928/15428877-20120102-05. [DOI] [PubMed] [Google Scholar]
- 18.Chalam KV, Murthy RK, Gupta SK, Brar VS, Grover S. Foveal structure defined by spectral domain optical coherence tomography correlates with visual function after macular hole surgery. Eur J Ophthalmol. 2010;20(3):572–577. doi: 10.1177/112067211002000306. [DOI] [PubMed] [Google Scholar]