Abstract
AIM
To evaluate if any association exists between central serous chorioretinopathy (CSCR) and the refractive status of the eye.
METHODS
This retrospective, institutional, case control study included 499 patients, wherein 262 patients diagnosed as acute CSCR, were compared with an age and gender matched control group of 237 patients. All patients were evaluated with a detailed systemic and ocular history, objective and subjective refractions for both eyes and complete ocular examination by a retina specialist, at all visits. Optical coherence tomography confirmed the diagnosis of CSCR.
RESULTS
The mean age was found to be 40±7y in the study group (Group 1) compared to 38±10y in the control group (Group 2). Most common refractive status in the study group, was emmetropia seen in 191 patients (72.9%), followed by hypermetropia seen in 47 patients (17.9%) and astigmatism seen in 21 patients (8.0%). Only 3 subjects (1.1%) had myopia, which was less than or equal to 1.0 D, compared to 70 subjects (29.5%) in the control group, suggesting a statistically significant lower incidence of CSCR among the myopic patients (P< 0.0001). With respect to the systemic factors, 26 (9.9%) patients were using systemic steroids in the study group (Group 1) compared to none in the control group (Group 2) suggesting a statistically significant association of CSCR with systemic steroid use (P<0.05). No other significant systemic risk factors were noted.
CONCLUSION
Though CSCR is a multifactorial disease, myopia serves as a protective factor for CSCR. Thus, myopic eyes are less likely to develop CSCR. Since both retinal pigment epithelium (RPE) and choriocapillaris are postulated in the pathogenesis of CSCR, chorio-retinal thinning and atrophy seen in myopic eyes are less likely to cause CSCR.
Keywords: central serous chorioretinopathy, refractive status, myopia
INTRODUCTION
Central serous chorioretinopathy (CSCR) is a disease in which a serous detachment of the neurosensory retina occurs over an area of leakage from the choriocapillaris through the retinal pigment epithelium (RPE)[1]. CSCR is the fourth most common retinopathy after age-related macular degeneration, diabetic retinopathy and retinal vein occlusions[1]. Acute CSCR includes cases with duration less than 3mo and chronic CSCR includes neurosensory detachments more than 3mo duration[1]. Bilateral involvement has been reported to occur in up to 40% of cases, although at time of diagnosis the rate is much lower at 4%[2]. Although it is commonly believed that CSCR is more prevalent in Asian compared with white populations, and least prevalent in black populations, the limited literature appears to suggest comparable rates across these ethnic groups[2].
Though various studies have evaluated systemic risk factors associated with CSCR, ocular factors associated with CSCR have not been studied[2]–[4]. Yannuzi et al[5] has described that most cases of CSCR have no refractive error or mild hypermetropia. However, no association between CSCR and emmetropia or myopia is known or proposed. This study was conceived on the observation of a large series of patients with CSCR who had no history of spectacle wear, who in other words were emmetropes, before the presenting episode. Also, it is a known fact that myopia has a protective effect on the onset and progression of other retinal diseases like diabetic retinopathy[6]–[7]. The aim of this study is to observe if any association exists between CSCR and the refractive status of the eye. Such an observation can throw further light on the pathogenesis of CSCR.
SUBJECTS AND METHODS
Subjects
This retrospective observational study conducted from January 2009 to January 2011 was approved by the Aravind Eye Hospital Institutional Review Board.
This study adhered to the tenets of the Declaration of Helsinki. All the patients who were diagnosed with acute CSCR in our tertiary care eye hospital, aged between 20 to 50y and who had refractive status available in their medical records pre- and post-CSCR, were included in this study. Patients with atypical CSCR, recurrent CSCR and chronic CSCR were excluded from the study. Patients who had any other co-existing retinal pathology, lenticular changes or cataract were also excluded from the study.
Methods
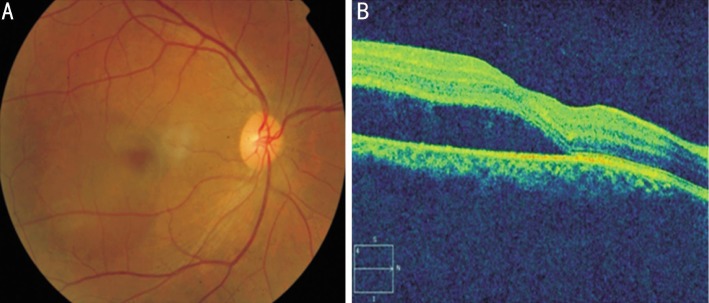
Of the 302 cases who presented with CSCR, 262 diseased eyes formed our study population. Only cases with adequate medical records to assess their refractive status before and after the resolution of CSCR were included in this study. Medical records of all 262 patients were examined. All patients underwent an extensive evaluation of their systemic and ocular history including present and past medications used and the spectacle history. Uncorrected visual acuity (UCVA), best corrected visual acuity (BCVA) and objective and subjective refractions were noted at baseline and periodically till the last follow-up visit. Each subject's refractive error was obtained with an auto refractor machine (Canon RK-5 Auto Ref-Keratometer; Canon, Inc., Ltd., Tokyo, Japan), after which subjective refraction by trained optometrists was performed to achieve BCVA. The final subjective refraction result was used in the analysis. Spherical equivalent (SE) was defined as sphere plus half negative cylinder. Refractive errors were defined as low myopia (SE between -0.5 D and -5 D), high myopia (SE >-5 D), and hyperopia (SE>1 D). The pre-CSCR refractive status was derived from the subjective and objective refractions done and the spectacle power prescribed as per the medical records available, in the visit prior to development of CSCR. The final refractive status was derived from the subjective and objective refractions done and the spectacle power prescribed as per the medical records available, in the visit after the resolution of CSCR. Slit-lamp examination of the anterior segment and a detailed fundus examination with slit-lamp biomicroscopy using 90 D lens and indirect ophthalmoscopy was performed by a retinal specialist. All the cases clinically diagnosed to have acute CSCR underwent optical coherence tomography (OCT) to confirm the neurosensory macular detachment, with no other macular pathology except pigment epithelial detachments (PED) (Figure 1). Fundus fluorescein angiography was performed only in patients who required intervention in the form of laser treatment or to rule out other retinal pathologies similar to CSCR.
Figure 1. General description.
A: Fundus photograph showing serous neurosensory macular elevation suggestive of CSCR; B: Optical coherence tomography showing neurosensory retinal detachment at the macula confirming the diagnosis.
A control group of 237 patients were randomly assembled from our tertiary care eye institute with ocular abnormalities other than CSCR, which was matched for age and gender. The control group was constructed so that the frequency of each diagnosis could not exceed 10% of the whole sample, although none of the diagnosis actually exceeded 9% of the total of the control group. These diagnoses included vitreous floaters, retinal vascular occlusive disease, ocular allergies, diabetic retinopathy, hypertensive retinopathy, anterior or posterior uveitis, retinal vasculitis, suspected glaucoma and other miscellaneous ocular disorders.
Statistical Analysis
The data obtained were analyzed with descriptive statistics. Chi-square analysis with continuity correction was used for categorical analysis. The Fisher exact test was used if the expected count in a cell was five or less. The independent-samples t-test was used to compare group means of continuous variables. Statistical analysis was performed with SPSS version 11.1 (SPSS, Chicago, Illinois, USA). For all tests, a P value of less than 0.05 was considered significant.
RESULTS
There were 262 patients in the study group (Group 1), analysed and compared with 237 patients in the control group (Group 2). The average age was found to be 40±7y in the study group as compared to 38±10y in the control group (Table 1). In the study group, 229 (87.4%) patients were males and 33 (12.6%) patients were females whereas in control group, 210 (88.6%) patients were males and 27 (11.4%) patients were females. There was no statistically significant difference with respect to age (P=0.16) and gender between the groups (P=0.67). In the study group, 252 (96.1%) patients had unilateral CSCR and 10 (3.8%) patients had bilateral CSCR.
Table 1. Subject demographics.
| Parameters | Group 1 (n=262) | Group 2 (n=237) | P |
| Age (a) | 40±7 | 38±10 | 0.16 |
| Gender | |||
| F | 33 (12.6%) | 27 (11.4%) | 0.67 |
| M | 229 (87.4%) | 210 (88.6%) |
The refractive status at presentation, based on the spectacle history and the spectacle power calculated prior to the onset of CSCR, of the 262 patients in the study group, 232 (88.5%) had emmetropia, 26 (9.9%) had hypermetropia, 1 (0.4%) had simple myopic astigmatism, and only 3 (1.1%) subjects had myopia. However, of the 237 patients in the control group, 123 (51.9%) had emmetropia, 37 (15.6%) had hypermetropia, 7 (3.0%) had simple myopic astigmatism, and 70 (29.5%) subjects had myopia. There was a statistically significant difference in number of subjects with myopia and emmetropia between the two groups (P<0.0001) suggesting inverse association of CSCR with myopia and direct association of CSCR with emmetropia (Table 2).
Table 2. Pre-CSCR refractive status (spectacle power).
| Refractive status (spectacle power) | Group 1 (n=262) | Group 2 (n=237) | P |
| Hypermetropia | 26 (9.9) | 37 (15.6) | |
| Myopia | 3 (1.1) | 70 (29.5) | <0.0001 |
| Simple myopic astigmatism | 1 (0.4) | 7 (3.0) | |
| Simple hypermetropic astigmatism | 0 (0.0) | 0 (0.0) | |
| Emmetropia | 232 (88.5) | 123 (51.9) | <0.0001 |
n (%)
The final refractive status, after refractive examination at last follow-up, of the 262 patients in study group, 191 (72.9%) had emmetropia, 47 (17.9%) had hypermetropia, 18 (6.9%) had simple myopic astigmatism, 3 (1.1%) had simple hypermetropic astigmatism, and only 3 (1.1%) subjects had myopia, which was less than or equal to 1.0 D. However, of the 237 patients in control group, 88 (37.1%) had emmetropia, 39 (16.5%) had hypermetropia, 29 (12.2%) had simple myopic astigmatism, 11 (4.6%) had simple hypermetropic astigmatism, and 70 (29.5%) subjects had myopia. There was a statistically significant difference in number of subjects with myopia and emmetropia between the two groups (P<0.0001), suggesting direct association of CSCR with Emmetropia and inverse association of CSCR with myopia (Table 3). The incidence of CSCR was more common among emmetropes and hypermetropes and rarely seen among myopes, only in low myopes of upto 1 D.
Table 3. Final (Post-CSCR) refractive status.
| Final refractive status | Group 1 (n=262) | Group 2 (n=237) | P |
| Hypermetropia | 47 (17.9) | 39 (16.5) | |
| Myopia | 3 (1.1) | 70 (29.5) | <0.0001 |
| Simple myopic astigmatism | 18 (6.9) | 29 (12.2) | |
| Simple hypermetropic astigmatism | 3 (1.1) | 11 (4.6) | |
| Emmetropia | 191 (72.9) | 88 (37.1) | <0.0001 |
n (%)
In relation to systemic history, of the 262 patients in study group, 26 (9.9%) patients were on systemic steroids, 21 (8.0%) patients had hypertension, 12 (4.6%) patients had diabetes mellitus and 203 (77.5%) patients had no significant systemic association. However of the 237 patients in control group, none of the patients were on systemic steroids, 29 (12.2%) patients had hypertension, 38 (16.0%) patients had diabetes mellitus and 170 (71.7%) patients had no significant systemic history. There was a statistically significant difference between the groups with respect to steroid use (P<0.0001), suggesting an association of CSCR with systemic steroid use (Table 4).
Table 4. Systemic history.
| Systemic association | Group 1 (n=262) | Group 2 (n=237) | P |
| Steroid | 26 (9.9) | 0 (0.0) | <0.0001 |
| Hypertension | 21 (8.0) | 29 (12.2) | |
| Diabetes mellitus | 12 (4.6) | 38 (16.0) | |
| None | 203 (77.5) | 170 (71.7) |
n (%)
DISCUSSION
The term “CSCR” was coined by Duggan who opined that permanent pigmentary changes are characteristic of choroidal and not retinal lesions and the transudate is subretinal originating in the choriocapillaries[2]. CSCR occurs preferentially in healthy men between 25-55 years of age.
Type A personality, cushing's syndrome, systemic hypertension, systemic steroid use and obstructive sleep apnea may be associated with CSCR[2]. The pathogenesis here is thought to be elevated circulating cortisol and epinephrine, which affect the autoregulation of the choroidal circulation. Furthermore, Tewari et al[8] demonstrated that patients with CSCR showed impaired autonomic response with significantly decreased parasympathetic activity and significantly increased sympathetic activity. Corticosteroids have a direct influence on the expression of adrenergic receptor genes and thus contribute to the overall effect of catecholamines on the pathogenesis of CSCR. Consequently, multiple studies have conclusively implicated the effect of corticosteroids in the development of CSCR[2]–[4].
The pathophysiology of CSCR remains poorly understood despite advances in imaging techniques and numerous studies of the disease. The choroid is thought to be hyperpermeable in CSCR, possibly as a result of stasis, ischemia, or inflammation[9]. The staining of the inner choroid seen on mid-phase indocyanine green angiography (ICGA) is the primary evidence of choroidal hyperpermeability[10]. The primary role of the choroid is further supported by the enhanced depth imaging (EDI) OCT finding of the thickened choroid in both eyes of patients with CSCR[11]. Hyperpermeable choroidal vessels are thought to produce increased tissue hydrostatic pressure, which promotes the formation of retinal PEDs, overwhelms the barrier function of the RPE, and leads to areas of fluid accumulation between the retina and the RPE[12]. RPE dysfunction plays a significant role in the pathogenesis of CSCR. This is perhaps most easily appreciated in cases of diffuse retinal pigment epithelium (DRPE), in which widespread loss of RPE cells is apparent on clinical exam and fundus autofluorescence (FAF), and where the loss of RPE barrier and pumping functions in the setting of an engorged choroid results in chronic subretinal fluid[13]. The focal areas of leakage through the RPE are characteristic of classic CSCR. These pinpoint leaks were seen as focal defects in the RPE that were thought to be primarily responsible for the accumulation of subretinal fluid. Spectral domain OCT studies of the RPE in CSCR have shown RPE defects in the location of a fluorescein leakage in some patients[14]. Another OCT-based study of the RPE showed that RPE abnormalities are present in nearly all-asymptomatic, fluid-free contralateral eyes of CSCR patients[15].
The strong association between CSCR and gluco-corticosteroid use suggests a role in pathogenesis. Both serum glucocorticoid[16] and serum catecholamine levels[17] are elevated in active CSCR. Glucocorticoids could potentially impact the course of the disease by affecting the choroid, Bruch's membrane, or the RPE[18].
Carvalho-Recchia et al[19] showed in a series that 52% of patients with CSCR had used exogenous steroids within 1mo of presentation as compared with 18% of control subjects. However, in our series 26 patients (9.9%) in study group were on systemic steroids compared to none in our control group, which was statistically significant (P<0.0001). Most of them were using steroids for allergic conditions. Haimovici et al[4] evaluated systemic risk factors for CSCR in 312 patients and 312 control subjects. Systemic steroid use [odds ratio (OR), 37.1] and pregnancy (OR, 7.1) were most strongly associated with CSCR. The other risk factors included antibiotic use, alcohol use, untreated hypertension, and allergic respiratory disorders. In our study, none of these other systemic risk factors were noted to be statistically significant. However, 21 (8%) patients had hypertension, 12 (4.5%) patients had diabetes mellitus and 203 (77.4%) patients had no systemic disease.
In our study, 191 patients (72.9%) had emmetropia, 47 patients (17.9%) had hypermetropia, 21 patients (7.9%) had astigmatism. Importantly, only 3 of the 262 patients (1.1%) with CSCR had myopia, that too only -1.0 D. Studies so far, evaluating the risk factors for CSCR, have not looked into the ocular factors, especially the refractive status of the patients.
Jain et al[6] showed the beneficial effect of myopia on diabetic retinopathy. The incidence of diabetic retinopathy in myopia was significantly lower as compared with emmetropes and hypermetropes. The authors also found that the degree of myopic refractive error in cases with retinopathy was always less than 5 D, while no cases had retinopathy who had myopia of 5 or more dioptres. No such relationship was noted in cases of hypermetropes. Recently, Lim et al[7] has proved in a large population based study that myopic refraction and longer axial length are associated with a lower risk of diabetic retinopathy. Although the mechanisms underlying the protective effect of myopia on diabetic retinopathy currently are unclear, most theories have centered on pathologic changes associated with axial globe elongation.
Yzer et al[20] reported 6 cases of CSCR in myopic patients whose choroidal thickness was high compared to the expected choroidal thickness for their myopic axial lengths. However, the underlying systemic risk factors were not looked into in these patients.
Basic pathophysiology in CSCR involves choriocapillary hyperpermiability that leads to leakage and secondary dysfunction of the overlying RPE. Myopic eyes frequently show anatomical changes of choroidal and RPE thinning and atrophy. Scleral elongation and deformation of the posterior pole may underlie the decreased ocular pulse and retinal blood flow frequently seen with increasing myopia[7], in contrast to the increased chorio-capillary perfusion (hyperpermeability) seen in CSCR on ICGA. Chorio-retinal thinning in high myopia, also may be protective both by reducing the metabolic demands of the retina and by facilitating diffusion of oxygen through the retina[21].
Axial myopia is characterized by retinal arterioles that are longer than those in “normal” or emmetropic eyes. On retinal photographs these myopic vessels have been shown to narrow by 1.3% per diopter of increasing myopia[22]. This is equivalent to a 3.9% narrowing per millimeter increase in axial length, in which a 1-mm axial length increase equates to 3 D of myopia[23]. Hence, myopia results in blood flowing through a longer arteriolar tree in the retina and choroid on its course to the capillary bed. Each millimeter increase in axial myopia over emmetropia (23 mm) would result in a 4.2% increase in vessel length. The normal pressure attenuation for blood flowing from the optic nerve to vessels measuring 30-40 microns in diameter has been calculated to be 15 mm Hg. Hence, for every millimeter increase in axial length, there would be an additional 0.6 mm Hg of blood pressure attenuation from the optic nerve to this retino-choroidal arteriolar capillary level. Just as low blood pressure protects the retina in subjects with diabetes[24], so would the presumed lowering of the hydrostatic pressure in the chorio-capillary bed caused by the elongation of the retino-choroidal arteriolar tree in myopic individuals, protects against CSCR similar to the protection seen in diabetic retinopathy. From this perspective, future therapies may be imagined.
Our study has shown that myopia, especially in higher degrees, is a protective factor in CSCR. However, since the disease has multifactorial etio-pathogenesis, myopic patients can still develop CSCR depending on the systemic factors, but to a lesser extent compared to other refractive status of the eye.
In conclusion, this study provides evidence to support the clinical impression that myopic eyes are less likely to have CSCR. Since both choriocapillaries and RPE are postulated in pathogenesis of CSCR, chorio-retinal thinning and atrophy seen in myopic eyes are less likely to cause CSCR. These findings contribute to further insights into the pathogenic pathways for CSCR. With the emergence of newer techniques of choroidal imaging and various techniques for choroidal thickness assessment like enhanced depth OCT, this hypothesis that myopia in higher degrees protects against CSCR, could be further proven in larger prospective studies.
Acknowledgments
Conflicts of Interest: Manayath GJ, None; Arora S, None; Parikh H, None; Shah PK, None; Tiwari S, None; Narendran V, None.
REFERENCES
- 1.Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 2.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Experiment Ophthalmol. 2013;41(2):201–214. doi: 10.1111/j.1442-9071.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 3.Gemenetzi M, Salvo GD, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24(12):1743–1756. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 4.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S, Central Serous Chorioretinopathy Case- Control Study Group Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–249. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzi LA, Gitter KA, Schatz H. Central serous chorioretinopathy. In: Yannuzi LA, Gitter KA, Schatz H, editors. The macula: a comprehensive text and atlas. Baltimore MD: Williams and Wilkins; 1979. pp. 145–165. [Google Scholar]
- 6.Jain IS, Luthra CL, Das T. Beneficial effect of myopia on diabetic retinopathy. Indian J Ophthalmol. 1965;13(3):88–94. [PubMed] [Google Scholar]
- 7.Lim LS, Lamoureux E, Saw SM, Tay WT, Mitchell P, Wong TY. Are myopic eyes less likely to have diabetic retinopathy? Ophthalmology. 2010;117(3):524–530. doi: 10.1016/j.ophtha.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 8.Tewari HK, Gadia R, Kumar D, Venkatesh P, Garg SP. Sympathetic-parasympathetic activity and reactivity in central serous chorioretinopathy: a case-control study. Invest Ophthalmol Vis Sci. 2006;47(8):3474–3478. doi: 10.1167/iovs.05-1246. [DOI] [PubMed] [Google Scholar]
- 9.Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149(3):361–363. doi: 10.1016/j.ajo.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, Guyer DR, Slakter JS, Sorenson JA, Orlock DA. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203–213. doi: 10.1097/00006982-199616030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 12.Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa DL, Huang SJ, Klancnik JM, Jr, Aizman A. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. 2003. Retina. 2012;32(Suppl. 1):288–298. doi: 10.1097/iae.0b013e31823f99a9. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson B, Noble J, Forooghian F, Meyerle C. Central Serous Chorioretinopathy: Update on Pathophysiology and Treatment. Surv Ophthalmol. 2013;58(2):103–126. doi: 10.1016/j.survophthal.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto H, Gomi F, Wakabayashi T, Sawa M, Tsujikawa M, Tano Y. Morphologic changes in acute central serous chorioretinopathy evaluated by Fourier-domain optical coherence tomography. Ophthalmology. 2008;115(9):1494–1500. doi: 10.1016/j.ophtha.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Gupta P, Gupta V, Dogra MR, Singh R, Gupta A. Morphological changes in the retinal pigment epithelium on spectral-domain OCT in the unaffected eyes with idiopathic central serous chorioretinopathy. Int Ophthalmol. 2010;30(2):175–181. doi: 10.1007/s10792-009-9302-2. [DOI] [PubMed] [Google Scholar]
- 16.Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81(11):962–964. doi: 10.1136/bjo.81.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110(4):698–703. doi: 10.1016/S0161-6420(02)01975-9. [DOI] [PubMed] [Google Scholar]
- 18.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47(5):431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho-Recchia CA, Yannuzzi LA, Negrão S, Spaide RF, Freund KB, Rodriguez-Coleman H, Lenharo M, Iida T. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–1837. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 20.Yzer S, Fung AT, Barbazetto I, Yannuzzi LA, Freund KB. Central serous chorioretinopathy in myopic patients. Arch Ophthalmol. 2012;130(10):1339–1340. doi: 10.1001/archophthalmol.2012.850. [DOI] [PubMed] [Google Scholar]
- 21.Ursekar TN. Classification, etiology and pathology of myopia. Indian J Ophthalmol. 1983;31(6):709–1711. [PubMed] [Google Scholar]
- 22.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: the Reykjavik Eye Study. Acta Ophthalmol Scand. 2007;85(4):361–366. doi: 10.1111/j.1600-0420.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 24.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]