Abstract
Sweet syndrome (SS) is a rare inflammatory process presenting with painful erythematous skin eruptions, accompanied by fever and neutrophilia. It is associated with upper respiratory infection in fertile women (classic form), malignancy, infections, drugs and autoimmune diseases. Its pathogenesis remains to be determined. Nevertheless, cytokines may have a prominent role, due to a rapid response after corticosteroid administration. We describe a 32-year-old female with autoimmune hepatitis on azathioprine and prednisone, presenting with fever and inflammatory skin eruptions. Histologic examination of the skin lesions showed neutrophilic infiltrations of the dermis, confirming the diagnosis of SS. Concurrently, she tested borderline positive for recent CMV infection.
INTRODUCTION
Sweet syndrome (SS, or acute febrile neutrophilic dermatosis) is a ‘distinctive and fairly severe illness’ characterized by fever, peripheral neutrophilia and skin lesions [1]. Skin manifestations include non-pruritic, tender red papules or nodules that may evolve into plaques and resolve spontaneously or after treatment, without scarring. Most frequently, they are distributed asymmetrically in the face, neck and upper extremities [2]. The clinical entity ranges from the classic form, which presents in women between 30–50 years after upper respiratory infection to a more aggressive condition related to malignancy. Infection-related causes include bacterial (Staphylococcus species, Salmonella and Yersinia) and viral (HIV, CMV, HAV and HBV) infections. A drug-induced variant has also been described with azathioprine, furosemide, oral contraceptives, lithium and granulocyte colony stimulating factor being among the most well-established medications. Autoimmune diseases have also been associated with this.
The etiology of the syndrome is unknown. Altered immunologic response, septic process and hypersensitivity reaction are factors suggested to play a role [2]. Diagnosis is based on clinical and histopathologic criteria. Corticosteroids have been the standard of treatment. In the drug-induced form, there is a resolution of the lesions after drug withdrawal.
CASE REPORT
A 32-year-old Caucasian female presented in the emergency department of our institution complaining of a high fever accompanied by tender red maculopapular rash in her face, chest and her hands (Fig. 1), which started about 3 days ago. The patient had been diagnosed with autoimmune hepatitis a month previously and she was on taking a tapering dose of prednisone 20 mg/days and azathioprine 50 mg/days. Her previous medical history included type II diabetes mellitus well managed with metformin and sitagliptin.
Figure 1:
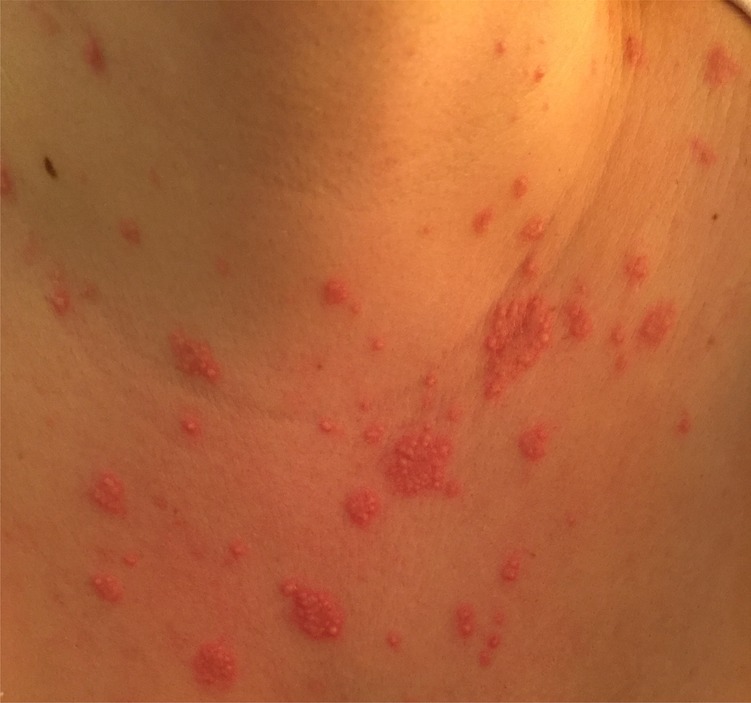
The tender red maculopapular rash in the patient's chest during her admission.
On admission, she showed signs of systemic inflammatory response syndrome with hypotension, tachycardia and fever. The physical examination revealed no pathological signs, except from the rash and conjunctivitis in her right eye. Mild neutrophilia and increased inflammatory markers (Table 1) were found on initial laboratory evaluation. She was started on vancomycin and meropenem for possible infection and hydrocortisone.
Table 1:
Laboratory data on admission and Day 9
| Variable | Reference range | On admission | Day 9 |
|---|---|---|---|
| White blood count (109/l) | 4.37–9.68 | 10.38 | 6.60 |
| Neutrophils (%) | 42.5–73.2 | 93.3 | 83.3 |
| Lymphocytes (%) | 18.2–47.4 | 2.7 | 10.3 |
| Monocytes (%) | 4.3–11.0 | 3.1 | 6.4 |
| Eosinophils (%) | 0.0–3.0 | 0.8 | 0.0 |
| Basophils (%) | 0.0–0.7 | 0.1 | 0.0 |
| Haemoglobin (g/dl) | 10.6–13.5 | 13.1 | 11.5 |
| Haematocrit (%) | 37.0–47.0 | 38.8 | 38.6 |
| Mean cell volume (fl) | 77.0–93.0 | 77.9 | 88.1 |
| Platelets (109/l) | 150–450 | 519 | 472 |
| Urea (mg/dl) | 17–43 | 40 | 33 |
| Creatinine (mg/dl) | 0.51–0.95 | 1.06 | 0.61 |
| Albumin (g/dl) | 3.5–5.2 | 3.3 | 2.4 |
| Total bilirubin (mg/dl) | 0.3–1.2 | 0.56 | 0.43 |
| Alkaline phosphatase (U/l) | 30–120 | 88 | 124 |
| Gamma glutamyl transferase (U/l) | 9–38 | 61 | 122 |
| Aspartate aminotransferase (U/l) | 3–32 | 30 | 32 |
| Alanine aminotransferase (U/l) | 3–34 | 28 | 39 |
| C-reactive protein (mg/l) | 0.00–5.00 | 314.40 | 50.31 |
On Day 6, she showed no response to the treatment. Considering SS as a possible diagnosis, a punch skin biopsy from a chest lesion was scheduled and azathioprine was discontinued. The same day, CMV DNA tested low positive (150 copies/ml). Ganciclovir was initiated based on her immunocompromised state secondary to azathioprine. After 48 h, the skin rash improved significantly (Fig. 2) and inflammatory markers declined (Table 1).
Figure 2:
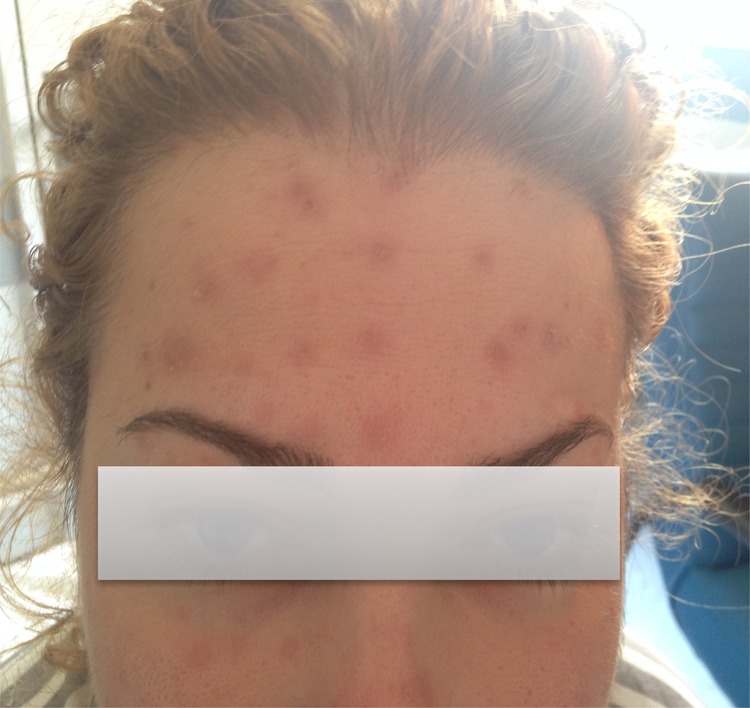
The patient's skin lesions subsided after cessation of azathioprine and administration of prednisone.
Histological examination (Fig. 3) confirmed the diagnosis of SS on Day 9. Pathological description reported diffuse polymorphonuclear infiltration of the reticular dermis with karyorrhexis accompanied by leukocytoklastic nuclear debris. Signs of vasculitis were absent. The patient was discharged after 11 days of hospitalization with prednisone and oral valganciclovir.
Figure 3:
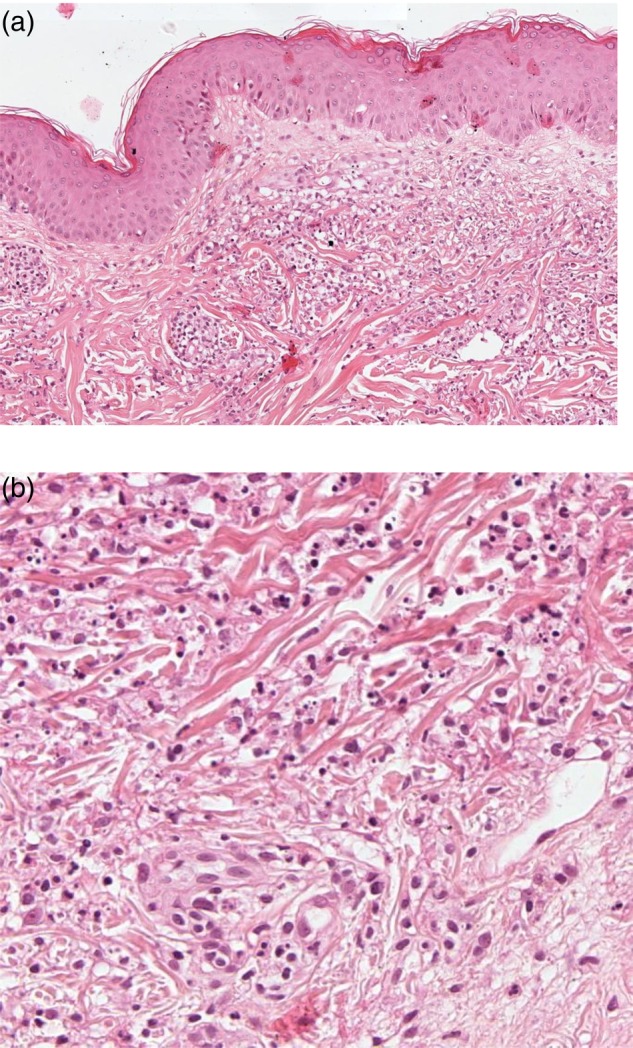
Histopathology of the patient's skin lesions, showing diffuse polymorphonuclear infiltration of the reticular dermis with karyorrhexis accompanied by leukocytoklastic nuclear debris and no signs of vasculitis [original magnification ×100 (a) and ×200 (b)].
DISCUSSION
Azathioprine has become the mainstay of treatment for various autoimmune diseases like autoimmune hepatitis, by inhibiting DNA and RNA synthesis. Azathioprine's adverse effects are classified into two categories: (i) the dose-independent, idiosyncratic early reactions (or allergic) and (ii) the dose-dependent, non-allergic [3].
A rare skin manifestation of the former, with an unknown pathogenesis, is Azathioprine-induced SS (AISS) [4]. It may be manifested within 4 weeks of its initiation to years after exposure. AISS is usually ignored, since it is indistinguishable from sepsis or an underlying disease's exacerbation. Azathioprine hypersensitivity syndrome (AHS) is another form of the allergic type reaction. The differentiation between AHS and AISS is very difficult, as both have very similar findings. It can be said that the hypotension is more evident in AHS; however, this is not conclusive.
Drug-induced SS (DISS) is usually indistinguishable from other types of SS, as it is characterized by clinical features typical of the classic form. Our patient fulfilled all the diagnostic criteria of DISS (Table 2), and denied any upper respiratory tract infection symptoms. Given the temporal association between azathioprine initiation and SS, we postulate that azathioprine was the culprit for this presentation. After reviewing the current biomedical literature, we identified 17 cases of AISS [5]. The majority was male (71%), ranging from 9 to 89 years (mean 47.2 years), with the rash onset after azathioprine initiation being between 5 and 28 days (mean 13.3 days) [6]. Approximately two-thirds (65%) of the cases were initially diagnosed as sepsis or exacerbation of an existing condition—most commonly inflammatory bowel disease [7]. First symptoms were fever and the typical SS rash (favoring face and trunk), with concomitant neutrophilia and elevated inflammatory markers. In all cases, the syndrome was related with the drug administration, while its cessation resulted in clinical improvement.
Table 2:
Diagnostic criteria for DISS (all five criteria are required)
| 1. | Abrupt onset of painful erythematous plaques or nodules |
| 2. | Histopathologic evidence of a dense neutrophilic infiltrate without evidence of vasculitis |
| 3. | Temperature >38°C |
| 4. | Temporal relation of drug ingestion and onset of symptoms or recurrence of symptoms with drug rechallenge |
| 5. | Resolution temporally related to drug withdrawal or after treatment with corticosteroids |
The relationship between SS and autoimmune hepatitis has been described only once in the English literature [8]. In that case, a female patient of 18 years old without an apparent medical history presented with fever, sore throat, arthralgias and a diffuse painful erythematous rash mainly on the trunk, extremities and face. On clinical examination, she had hepatosplenomegaly and mild icterus. Liver function tests and cholestatic enzymes were mildly increased. Furthermore, antinuclear (ANA) and anti-smooth muscle (anti-Sm) antibodies were positive. Percutaneous liver biopsy showed evidence of autoimmune hepatitis and the skin punch biopsy revealed findings consistent with SS. She was started on azathioprine and prednisone. On the 15th month after the initiation of the treatment she had an SS recurrence and was treated again with azathioprine and a tapering dose of prednisone. She did not have a recurrence at 4.5 years of follow-up.
In our case, the patient was first diagnosed with autoimmune hepatitis based on liver biopsy findings, while ANA and anti-Sm were negative. A month after the diagnosis of autoimmune hepatitis and while she was on tapering dose of prednisone and azathioprine, she was diagnosed with SS based on the punch biopsy of the lesions. After 6 months of the SS diagnosis, she has not have any recurrence of SS.
This is a case report of drug-induced SS due to azathioprine in an immunocompromised patient with autoimmune hepatitis and a recent CMV infection. The question that arises is whether the recent CMV infection had triggered a further immune response enhancing the ability of azathioprine to induce SS. Due to the lack of knowledge between SS–CMV interplay, we are unable to come to a safe conclusion. A second question is if there is a link between SS and autoimmune hepatits. Further studies are, however, needed to address this adequately.
SS triad is the set of fever, neutrophilia and dermatological findings in association with infection, malignancy, drugs and autoimmune disease. We report a case of a fertile female with autoimmune hepatitis that presented in our hospital with the typical manifestations of the syndrome. She was taking azathioprine and corticosteroids and had a borderline recent CMV infection. While this is a typical case of DISS, a further investigation on the association of autoimmune hepatitis, CMV infection and SS is warranted in the future.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No funding to report.
ETHICAL APPROVAL
This case report was approved by the ethical committee of the Nicosia General Hospital under the supervision of the Cyprus Ministry of Health.
CONSENT
A consent form was signed by the patient.
GUARANTOR
E.X. is a guarantor of this study.
ACKNOWLEDGEMENTS
We thank Dr G. Georgiou, histopathologist at the General Hospital of Nicosia, for kindly give us permission to use the histopathology figures of the patient.
REFERENCES
- 1.Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol 1964;76:349–56. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PR. Sweet's syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis SM et al. . Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 2004; 14:181–7. [DOI] [PubMed] [Google Scholar]
- 4.Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol 2006;55:369–89. [DOI] [PubMed] [Google Scholar]
- 5.Choonhakarn C, Chaowattanapanit S. Azathioprine-induced Sweet's syndrome and published work review. J Dermatol 2013;40:267–71. [DOI] [PubMed] [Google Scholar]
- 6.Bidinger JJ, Sky K, Battafarano DF, Henning JS. The cutaneous and systemic manifestations of azathioprine hypersensitivity syndrome. J Am Acad Dermatol 2011;65:184–91. [DOI] [PubMed] [Google Scholar]
- 7.Paoluzi OA, Crispino P, Amantea A, Pica R, Iacopini F, Consolazio A et al. . Diffuse febrile dermatosis in a patient with active ulcerative colitis under treatment with steroids and azathioprine: a case of Sweet's syndrome: case report and review of literature. Dig Liver Dis 2004;36:361–6. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Helwig K, Komar MJ. Sweet's syndrome in association with probable autoimmune hepatitis. J Clin Gastroenterol 1999;2:349–50. [DOI] [PubMed] [Google Scholar]


