Abstract
Aim: The purpose of this study was to evaluate the association of common polymorphisms in endothelial nitric oxide synthesis (eNOS; G894T) and renin–angiotensin–aldosterone system (angiotensin converting enzyme [ACE]—I/D, angiotensinogen—T704C, and angiotensin II receptor type 1—A1166C) as risk factors in the pathogenesis of coronary artery disease (CAD) in Bulgarian patients. Methods: This study included 171 patients with CAD and 123 control subjects. Polymerase chain reaction–restriction fragment length polymorphism was used for studying the single-nucleotide polymorphisms. Statistical analysis was performed using statistical software PASW for Windows. Results: A significantly higher percentage of the eNOS T894 allele was found in patients with acute coronary syndrome (ACS), compared to controls (p = 0.006) and patients with stable angina pectoris (SAP, p = 0.005). Results from a binary regression analysis suggested that eNOS T allele and ACE D allele carriers were more likely to develop ACS than controls (T allele odds ratio [OR] 2.585, p = 0.024; D allele OR 3.585, p = 0.046) and patients with SAP (T allele OR 2.955, p = 0.009; D allele OR 2.703, p = 0.05). Exploratory evaluation of gene–gene combinations showed a significant association between eNOS-G894T/ACE-I/D and ACS compared to controls (p = 0.022) and patients with SAP (p = 0.017). Conclusions: The eNOS G894T and ACE I/D polymorphisms are associated with an increased risk of developing ACS after adjusting for classical risk factors for atherosclerosis in the Bulgarian cohort.
Introduction
Coronary artery atherosclerosis (coronary artery disease [CAD]) is the leading cause of mortality and morbidity in both developed and developing countries. It is estimated that by 2020 CAD will be become a major cause of death worldwide (Tunstall-Pedoe et al., 2007). Atherosclerosis is a multifactorial disease that usually develops many years before any clinical symptoms are manifest. It is caused by a combination of several risk factors such as genetic predisposition, hyperlipoproteinemia, high cholesterol diet, alcohol intake, stress, smoking, and diabetes mellitus. (Black and Garbutt, 2002; Bonci et al., 2015; Peters and McEwen, 2015).
The renin–angiotensin–aldosterone system (RAAS) has been strongly implicated in the pathogenesis of cardiovascular disease and progressive renal disease. It is also a complex regulator of blood pressure, water hemostasis, and vascular tone (Sarkar et al., 2015). Angiotensin II (Ang II) is the effector substance of the RAAS and has a number of important effects, including vasoconstriction, renal proximal tubule sodium reabsorption, and aldosterone secretion. It also stimulates cellular proliferation, as well as matrix synthesis and accumulation (Ragia et al., 2010).
Angiotensinogen (AGT) is a key protein in the RAAS and its genetic variances have been linked with predisposition to essential hypertension (Jeunemaitre et al., 1992) and heart failure (Jiang et al., 2014). T704C (Met235Thr) is the most intensively studied polymorphism in this gene. The C704 allele has been associated with cardiovascular disease (Zakrewski-Jakubiak et al., 2008; Ragia et al., 2010) and in some studies (but not all) with increased blood levels of AGT as reviewed by Mondry et al. (2005).
Circulating AGT is primarily cleaved by the protease, renin, to yield a 10 amino acid peptide, angiotensin I, which in turn is processed by the angiotensin converting enzyme (ACE) to produce the active octapeptide Ang II (Firouzabadi et al., 2013). The ACE gene is highly polymorphic in the promoter and coding regions; ACE gene polymorphism based on the presence (insertion [I]) or absence (deletion [D]) within intron 16 of a 287-bp Alu repeat sequence is frequently studied, and results revealed that cases with DD genotype have a higher mortality of cardiovascular disease (Oro et al., 2007; Firouzabadi et al., 2013). ACE I/D polymorphism is associated with an increased level of circulating ACE. It is clear that higher angiotensin and ACE activity influence structure and function of arteries, as well as cardiac structures (Rahimi, 2012). The data on the studies of the I/D polymorphism showed an association with CAD risk in different populations (Kretowski et al., 2007; Zakrewski-Jakubiak et al., 2008; Miao and Gong, 2015).
The active molecule of RAAS–Ang II binds with high affinity to two distinct receptors, the type 1 and type 2 angiotensin receptors (AT1R and AT2R). The important cardiovascular actions of Ang II, including regulation of arterial blood pressure and water–salt balance, are mainly mediated by AT1R in target tissues such as the blood vessels, kidney, brain, and heart (Oro et al., 2007). Screen of the entire coding region and the 3′-untranslated region of the AT1R identified five frequent polymorphisms: +573, +1062, +1166, +1517, and +1878 (Duncan et al., 2001). The A to C substitution at position 1166 (A1166C) is one of the most often studied and has been associated with clinical events, such as myocardial infarction (MI) (Hirooka et al., 1992), hypertension (Bertrand, 2004), aortic stiffness (Creager and Roddy, 1994), and left ventricular mass changes (Di Mario et al., 2000).
In addition to its function in the RAAS, ACE also plays a role in the reduction of bradykinin bioavailability. Bradykinin plays a crucial role in vascular health, through upregulation of endothelial nitric oxide synthesis (eNOS), which synthesizes the vasorelaxation factor NO (Verma, 2006). A number of polymorphisms were detected within the human eNOS gene, including variations in introns, in the 5′ flanking region, and in the coding sequence. One of these is the missense single-nucleotide change of G to T at position 894 (Glu298Asp) in exon 7 of the gene (Cherney et al., 2009). This polymorphism has been reported to be associated with reduced expression and activity of eNOS (Sawada et al., 2008) and is determined to be a risk factor for CAD (Abdel-Aziz and Mohamed, 2013).
In this study, we aimed to examine the relationship between the common polymorphisms in the eNOS gene (Glu298Asp) and the RAAS (ACE I/D, AT1R A1166C, AGT T704C) and their association in the development of cardiovascular diseases in Bulgarian patients.
Materials and Methods
Study population
The information and genetic samples provided by all individuals in this study were obtained with written informed consent with the approval of the Institutional Ethics Committees of the St. Anna Hospital (No. 54/28.02.2005).
In our study, 294 subjects (171 patients and 123 controls) were included. Patients were diagnosed with non-ST segment elevation acute coronary syndrome (unstable angina or non-ST elevation MI, n = 117) and stable angina pectoris (SAP, n = 54). Unstable angina patients had ischemic chest pain at rest within the preceding 48 h that had developed in the absence of an extracardiac precipitating cause with either ST segment depression of >0.1 mV or T-wave inversion in two or more contiguous leads on the present 12-lead ECG. Patients with non-ST elevation MI had similar diagnostic criteria with elevation of serum troponin T, without the evolution of pathological q-waves. The control group comprised 123 healthy volunteers in the same age of distribution, without angina symptoms and with normal physical examination and stress tests. Subjects with acute or chronic inflammatory diseases, malignancies, renal insufficiency, and severe liver disease, on immunosuppressive and antibiotic treatment, were excluded from the study. We also excluded patients with acute ST elevation MI, diabetes mellitus, and history of MI, surgical intervention, or major trauma within the preceding month. In patients with ACS, blood was collected after hospitalization to the intensive care unit at St. Anna Hospital before starting the anti-ischemic and anticoagulation therapy.
DNA preparation
Genomic DNA was extracted from peripheral blood samples, collected in ethylenediaminetetracetic acid anticoagulant tubes, by using sodium extraction protocol (Miller et al., 1998). The purity of the isolated DNA was confirmed with an A260/280 ratio of 1.80–2.00 for all samples tested, and the DNA quality was checked on agarose gel, followed by ethidium bromide staining.
Genotyping polymorphisms
To detect studied changes in the human's genome, we used polymerase chain reaction (PCR) and the restriction fragment length polymorphism (RFLP) assay. The detailed sequence information for single-nucleotide polymorphisms (SNPs) studied the following: eNOS Glu298Asp (rs1799983); ACE I/D (rs4646994); AGT T704C (rs699); and AT1R A1166C (rs5186) are available at http://ncbi.nlm.nih.gov/SNP/
The PCR was performed with specific oligonucleotide primers listed in Table 1. The table shows also the enzymes used for the RFLP analysis, annealing temperature and size of the products.
Table 1.
Sequences of the Polymerase Chain Reaction Primers and Enzymes Used for Restriction Fragment Length Polymorphism Analyses
| Polymorphisms | Primer sequence | Restriction enzyme/annealing temperature | Allele size (bp) |
|---|---|---|---|
| eNOS G894T | F:5′AAGGCAGGAGACAGTGGATGGA3′ | Mbo I/67°C | G = 248 |
| R:5′CCCAGTCAATCCCTTTGGTGCTCA3′ | T = 158; 90 | ||
| ACE I/D | F:5′CTGGAGACCACTCCCATCCTTTCT3′ | 65°C | I = 490 |
| R:5′GATGTGGCCATCACATTCGTCAGAT3′ | D = 190 | ||
| AGT T704C | F:5′CAGGGTGCTGTCCACACTGGACCCC3′ | Tth111I/67°C | T = 165 |
| R:5′CCGTTTGTGCAGGGCCTGGCTCTCT3′ | C = 141; 24 | ||
| AT1R A1166C | F:5′AGAAGCCTGCACCATGTTTTGAG3′ | Dde I/69°C | A = 410 |
| R:5′CCTGTTGCTCCTCTAACGATTTA3′ | C = 292; 118 |
eNOS, endothelial nitric oxide synthesis; ACE, angiotensin converting enzyme; AGT, angiotensinogen; AT1R, angiotensin II receptor type 1.
Statistical analyses
The Student's t test was used to compare the quantitative parameters in the groups with a normal distribution such as age, cholesterol, and triglyceride levels. The comparison of the categorical variables was performed by the Pearson's χ2 test and Fisher's test. The probability values of p < 0.05 were considered statistically significant. To test for the Hardy–Weinberg equilibrium, the expected genotype frequencies were calculated from the allele frequencies. Results are presented as mean ± standard deviation or percentage.
Binary logistic regression analysis was performed considering the diagnosis as the dependent variable and different risk factors as covariates in the model. With the backward stepwise model, we assess the effect of different factors, such as obesity, sex, hypertension, smoking status, family history, age as a continuous variable, and the studied polymorphisms in eNOS, ACE, AGT, and AT1R genes, using the dominant model, assessing the effect of carrying the polymorphisms (as hetero- or homozygotes). Binary logistic regression was also applied to evaluate whether the studied polymorphisms are related to the risk of developing SAP and ACS. The diagnosis was used as a dependent variable and of the dominant model of the polymorphism, and other risk factors, such as obesity and current smoking status, were used as covariates. Odds ratio (OR) and the confidence interval (95% CI) were also calculated. For all analyses, a probability value under 5% (two-tailed) was considered to be significant. PASW for Windows (SPSS, Inc.) was used to perform all statistical analyses.
Results
The baseline clinical characteristics of patients with CAD (SAP and ACS) and control subjects included in the analysis are presented in Table 2. The prevalence of classical atherogenic risk factors, such as hypertension, family history, obesity, and smoking status, was significantly higher among the patients (ACS and SAP), and there was no significant difference between the two patient groups (Table 2).
Table 2.
Baseline Characteristics of the Studied Subjects
| Variable | Controls (n = 123) | Patients with SAP (n = 54) | P1 | Patients with ACS (n = 117) | P2 | P3 |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 55.7 ± 13.7 | 60 ± 9.8 | 0.219 | 60.3 ± 9.3 | 0.169 | 0.818 |
| Sex, male (%) | 57 (46.3%) | 40 (74.1%) | 0.001 | 69 (59%) | 0.050 | 0.056 |
| Smoking status (%) | 57 (46.3%) | 36 (66.7%) | 0.013 | 72 (61.5%) | 0.018 | 0.518 |
| Cholesterol (mean ± SD) | 5.29 ± 1.3 | 5.32 ± 1.1 | 0.895 | 6.04 ± 1.6 | <0.001 | 0.002 |
| Triglycerides (%) | 1.98 ± 1.36 | 1.89 ± 1.07 | 0.660 | 1.9 ± 1.05 | 0.657 | 0.898 |
| Hypertension (%) | 66 (53.7%) | 44 (81.5%) | <0.001 | 92 (78.6%) | <0.001 | 0.668 |
| Obesity (%) | 17 (13.8%) | 31 (57.4%) | <0.001 | 64 (54.7%) | <0.001 | 0.741 |
| Family predisposition (%) | 9 (7.3%) | 14 (25.9%) | 0.001 | 44 (37.6%) | <0.001 | 0.134 |
ACS, acute coronary syndrome; SAP, stable angina pectoris; P1, comparing between controls and patients with SAP; P2, comparing between controls and patients with ACS; P3, comparing between patients with ACS and SAP; SD, standard deviation.
Table 3 shows the genotype and allelic distribution of the studied polymorphisms in control groups and patient groups. Genotype frequencies were in agreement with those predicted from the Hardy–Weinberg equilibrium in control subjects and patients within both groups: SAP and ACS, as well as the overall study group. The genotype and allelic frequencies for the control group do no differ from those observed in the European ethnic group (http://ncbi.nlm.nih.gov/SNP/) and, thus, provide us with the ability to use them for accurate comparison.
Table 3.
Genotypic and Allelic Distributions of the Studied Polymorphisms: Endothelial Nitric Oxide Synthesis G894T, Angiotensin Converting Enzyme I/D, Angiotensinogen T704C, and Angiotensin II Receptor Type 1 A1166C in Controls and Patients
| eNOSa,b—n (%) | ACE—n (%) | AGT—n (%) | AT1R—n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | GG | GT | TT | II | ID | DD | TT | TC | CC | AA | AC | CC |
| Controls (n = 123) | 74 | 37 | 12 | 26 | 59 | 38 | 56 | 53 | 14 | 67 | 52 | 4 |
| (60.1) | (30.1) | (9.8) | (21.1) | (48) | (30.9) | (45.5) | (43.0) | (11.6) | (54.4) | (42.3) | (3.3) | |
| G—185 (75.2%) | I—111 (45.1%) | T—165 (67.1%) | A—186 (75.6%) | |||||||||
| T—61 (24.8%) | D—135 (54.9%) | C—81 (32.9%) | C—60 (24.4%) | |||||||||
| SAP (n = 54) | 33 | 19 | 2 | 15 | 18 | 21 | 25 | 24 | 5 | 29 | 24 | 1 |
| (61.1) | (35.2) | (3.7) | (27.8) | (33.3) | (38.9) | (46.3) | (44.4) | (9.3) | (53.7) | (44.4) | (1.9) | |
| G—85 (78.7%) | I—48 (44.4%) | T—74 (68.5%) | A—82 (75.9%) | |||||||||
| T—23 (21.3%) | D—60 (55.6%) | C—34 (31.5%) | C—26 (24.1%) | |||||||||
| ACS (n = 117) | 43 | 63 | 11 | 16 | 56 | 45 | 44 | 59 | 14 | 69 | 40 | 8 |
| (36.8) | (53.7) | (9.4) | (13.7) | (47.9) | (38.4) | (37.6) | (50.4) | (12.0) | (59.0) | (34.2) | (6.8) | |
| G—149 (63.7%) | I—88 (37.6%) | T—147 (62.8%) | A—178 (76.0%) | |||||||||
| T—85 (36.3%) | D—146 (62.4%) | C—87 (37.2%) | C—56 (24.0%) | |||||||||
Genotype distribution χ2 = 18.386, p = 0.001.
Allele distribution χ2 = 11.362, p = 0.003.
When the ACS, SAP, and control groups were compared with respect to eNOS G894T polymorphism in exon 7, the GG genotype was found to be the most prevalent in the control group (60.1%) and in the group of patients with SAP (61.1%) compared to the patients with ACS (36.8%), whereas the heterozygous genotypes were found more frequently (53.7%) (χ2 = 18.386, p = 0.001). Comparing the same group with respect to the allele distribution, we noted the increase of the T allele in the group of patients with ACS (36.3%) compared with the same allele in the other two groups—control subjects (24.8%) and group of patients with SAP (21.3%) (χ2 = 11.362, p = 0.003). We did not observe significant differences in the distribution of the genotypes and alleles of the other studied polymorphisms.
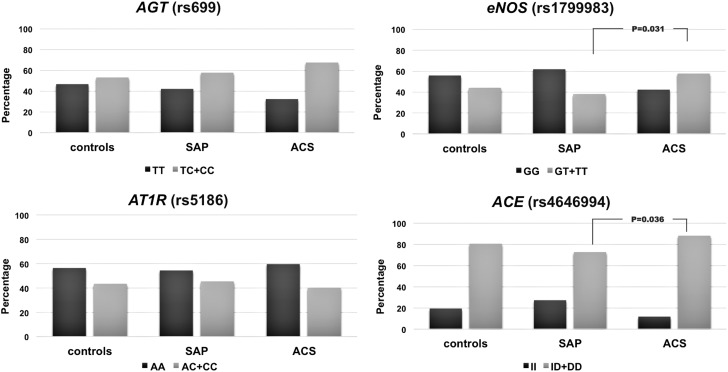
Using the dominant genetic model (wild-type homozygous genotype vs. heterozygous + polymorphic homozygous genotypes), we analyzed the distribution of the studied polymorphisms in dependence of the baseline clinical characteristics of controls and patients—SAP and ACS groups. In the ACS group of patients with obesity, we noted an increase in the percentage of subjects carrying at least one eNOS T894 allele (64.6%, p = 0.001) compared with controls (26.7%) and patients with SAP (26.7%). In the smokers group, again, the eNOS polymorphisms showed a higher percentage of people who smoked and suffered from ACS (41.7%, p = 0.022) compared with controls and SAP patients (respectively 35.5% and 36.5%). Figure 1 shows the distribution of the studied polymorphisms under the dominant genetic model in the group of subjects with hypertension. Compared with the patients who develop SAP, the ones with ACS had a significantly higher percentage of eNOS—GT + TT (p = 0.031) and ACE—ID + DD genotypes (p = 0.036).
FIG. 1.
Distribution of the studied polymorphisms in the group of subjects with hypertension.
Thereafter, using the binary logistic regression, we assessed the association of the polymorphic alleles in all subjects, with susceptibility to SAP and ACS (Table 4). Unadjusted odds ratio for carrying T894 allele of the eNOS and D allele of ACE gene and the occurrence of ACS compared with SAP was 2.724 and 2.567, respectively (Table 4). After adjustment for age, sex, smoking status, hypertension, obesity, family predisposition, cholesterol and triglyceride levels the analyses were performed comparing health subjects (Table 5A) and patients with SAP (Table 5B) with the group of subjects with ACS. The results showed an increase in the risk of developing ACS when carrying T894 and D allele of eNOS and ACE genes in combination with other risk factors. We did not find a significant contribution of any of the studied polymorphisms when comparing controls and patients with SAP.
Table 4.
Nonadjusted Odds Ratio for Contribution of the Studied Polymorphisms to Development of the Disease
| Controls vs. SAP | Controls vs. ACS | SAP vs. ACS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | OR | CI 95% | P | OR | CI 95% | p | OR | CI 95% | p |
| eNOS G894T (GT + TT)a | 0.783 | 0.406–1.513 | 0.467 | 2.133 | 1.195–3.807 | 0.010 | 2.724 | 1.342–5.529 | 0.006 |
| ACE I/D (ID + DD)b | 0.658 | 0.318–1.363 | 0.260 | 1.688 | 0.797–3.578 | 0.172 | 2.567 | 1.108–5.947 | 0.028 |
| AGT (TC + CC)c | 0.994 | 0.528–1.870 | 0.984 | 1.389 | 0.789–2.433 | 0.251 | 1.398 | 0.711–2.750 | 0.332 |
| AT1R (AC + CC)d | 1.024 | 0.542–1.935 | 0.941 | 0.834 | 0.478–1.457 | 0.524 | 0.841 | 0.414–1.612 | 0.552 |
As reference is used GG genotype.
As reference is used II genotype.
As reference is used MM genotype.
As reference is used AA genotype.
All significant differences are shown in bold.
OR, odds ratio; 95% CI, 95% confidential interval.
Table 5A.
Results of Stepwise Backward Computed Binary Logistic Regression Model—Adjusted Odds Ratio as a Dependent Variable Compared Groups with Acute Coronary Syndrome Versus Controls (as a Reference)
| Controls vs. ACS | |||
|---|---|---|---|
| Factor | OR | CI 95% | p |
| eNOS G894T (GT + TT)a | 2.585 | 1.133–5.897 | 0.024 |
| ACE I/D (ID + DD)b | 3.585 | 1.018–9.159 | 0.046 |
| Obesity | 9.450 | 3.741–23.873 | <0.001 |
| Age | 1.077 | 1.035–1.120 | <0.001 |
| Sex (male) | 4.120 | 1.676–10.125 | 0.002 |
| Smoking status | 2.470 | 1.053–5.790 | 0.038 |
| Family predisposition | 6.020 | 1.947–18.612 | 0.002 |
| Cholesterol levels | 1.560 | 1.063–2.289 | 0.023 |
| Triglyceride levels | 0.719 | 0.513–1.007 | 0.055 |
As reference is used GG genotype.
As reference is used II genotype.
Covariates included in the analysis at first step were as follows: studied polymorphisms as recessive genetic model; obesity; age; sex; family predisposition; hypertension; diabetes status; smoking status; and cholesterol and triglycerides levels.
Table 5B.
Results of Stepwise Backward Computed Binary Logistic Regression Model—Adjusted Odds Ratio as a Dependent Variable Compared Groups of Acute Coronary Syndrome Versus Stable Angina Pectoris (as a Reference)
| SAP vs. ACS | |||
|---|---|---|---|
| Factor | OR | CI 95% | P |
| eNOS G894T (GT + TT)a | 2.955 | 1.307–6.683 | 0.009 |
| ACE I/D (ID + DD)b | 2.703 | 0.982–7.438 | 0.054 |
| Hypertension | 0.274 | 0.72–1.043 | 0.058 |
| Cholesterol levels | 1.395 | 0.967–2.013 | 0.075 |
As reference is used GG genotype.
As reference is used II genotype l.
Finally, we performed an exploratory study using dominant genetic models comparing the combinations of the studied polymorphisms and their distribution in healthy controls and in patient groups. Table 6 shows the frequency of the patients having T894 allele of eNOS gene in combination with D allele—ACE in the ACS group—54.9% compared with controls (p = 0.003, OR 2.401, 95% CI 1.343–4.293) and the SAP group (p = 0.001, OR 3.475, 95% CI 1.645–7.342). There was also a significantly increased frequency of patients having a combination of eNOS T894 and AGT C704 allele in the ACS group compared with controls (p = 0.007, OR 2.330, 95% CI 1.245–4.360) and patients with SAP (p = 0.040, OR 2.240, 95% CI 1.027–4.887).
Table 6.
Distribution of Paired Combinations of the Studied Polymorphisms in Dominant Genetic Model
| Combinations of the alleles | Controls (%) | Patients with SAP (%) | P1 | Patients with ACS (%) | P2 | P3 |
|---|---|---|---|---|---|---|
| eNOS T allele | 33.6 | 27.8 | 0.406 | 54.9 | 0.006 | 0.027 |
| ACE D allele | ||||||
| eNOS T allele | 43.3 | 39.3 | 0.697 | 56.3 | 0.109 | 0.233 |
| AGT C allele | ||||||
| eNOS T allele | 35.0 | 39.3 | 0.124 | 36.8 | 0.090 | 0.429 |
| AT1R C allele | ||||||
| ACE D allele | 43.3 | 39.3 | 0.697 | 56.3 | 0.109 | 0.233 |
| AGT C allele | ||||||
| ACE D allele | 35.0 | 39.3 | 0.124 | 36.8 | 0.090 | 0.429 |
| AT1R C allele | ||||||
| AGT C allele | 23.3 | 28.6 | 0.581 | 23 | 0.303 | 0.701 |
| AT1R C allele | ||||||
P1, comparison between controls and patients with SAP; P2, comparison between patients with ACS and SAP; P3, comparison between controls and patients with ACS.
All significant differences are shown in bold.
Discussion
Using association studies, assessing the link between the genetic polymorphisms and complex diseases remains controversial. However, to understand the genetic etiology of complex human traits, estimation of allele or genotype distribution of polymorphisms in candidate genes is an efficient method to evaluate their associations with the diseases (Morton and Collins, 1998). In the present study, we have analyzed polymorphisms in four genes, in which proteins are involved in blood pressure homeostasis, for their possible association with CAD in Bulgarian patients.
To the best of our knowledge, this is the first study to explore the connection between polymorphisms in eNOS and RAAS genes in the pathogenesis of CAD in the Bulgarian population. Investigations of the relationship between eNOS G894T (Glu298Asp) gene polymorphism and CAD, with regard to the relevance of its genetic background, have given various results. Several studies have shown an association of T allele with an increased risk of developing MI (Rudnicki and Mayer, 2009) and ischaemic heart disease (IHD) (Casas et al., 2004). In their meta-analysis study, Luo et al. (2014) summarized the results from 34 studies involving 8229 cases and 12,839 controls and showed that eNOS G894T polymorphism is associated with an increased risk of MI in the Asian population and no association was found in non-Asians. In contrast, the meta-analysis of Li et al. (2010) found that T894 allele is associated with CAD in Europeans. This result is consistent with the findings from the present study, where we found a significant prevalence of the T allele in patients with ACS compared to SAP and controls (36.3%, p = 0.003). This suggests the possible link between the polymorphism and the disease.
Since Rigat et al. (1990) first described it, the ACE I/D polymorphism has been intensively studied for its contribution in the pathogenesis of essential hypertension, ischemic and idiopathic dilated cardiomyopathy, CAD, autosomal dominant polycystic kidney disease, congenital anomalies of the kidney and urinary tract in children, and so on (Schiavello et al., 2001; Rudnicki and Mayer, 2009; Firouzabadi et al. 2013; Kostadinova et al., 2014). Using the dominant genetic model, we observed the prevalence of the ACE ID + DD genotypes among patients with ACS, compared with SAP or controls. According to the literature, the presence of D allele is associated with increase plasma concentration and activity of the ACE enzyme, and also with increased sensitivity to Ang II, which is believed to be the basis for MI, ventricular hypertrophy, hypertension, and so on (Hara et al., 2014).
We also studied polymorphisms in AT1R and AGT, which had been previously investigated for their connection with hypertension and cardiovascular and renal diseases. The results for association of the A1166C polymorphism in the study of Tiret et al. (1998) demonstrated the relationship of the polymorphism with hypertension in women, whereas Schmidt et al. (1997) were not able to find any connection between the C1166 allele and hypertension in the German population. This shows that the AT1R gene polymorphism is likely to be more risk prevalent among high-risk groups. In the present study, the results showed no association of the polymorphisms with ACS and SAP. Barbalic et al. (2006) also failed to find an association between polymorphisms in AGT and AT1R genes; they explain this result with the small sample size that lacks the statistical power to detect differences, which could be true about the current study as well.
Using binary logistic regression analysis, we examined the involvement of the polymorphisms, included in the present study, in developing the disease. When the analysis was performed, only considering the polymorphisms, the data indicated a significantly higher risk of developing ACS in subjects who are carriers of T894 (eNOS) and D allele (ACE). However, developing of ischemic heart disease is a multifactor process and should be considered not only for the relationship with individual polymorphism, which is one of the major limitations of some of the previous studies, but also in association with different risk factors, including family predisposition and environmental factors. We, therefore, perform the binary regression analysis again with adjustment for other risk factors, such as age, sex, obesity, hypertension, family predisposition, and current smoking status. The results showed that the polymorphisms in eNOS and ACE in combination with other risk factors have an even higher risk of developing ACS. This suggests that the presence of T894 and D alleles could be used as predictive factors for predisposition to the disease.
To investigate if there is a combined effect of the polymorphisms included in this study, we evaluate the distribution of the biallelic pairs in the dominant genetic model. The combination of eNOS T894 and ACE D allele has a significantly higher percentage in ACS than in controls and in patients with SAP, suggesting that this combination might have a role in the pathogenesis of CAD. The ECTIM study (Poirier et al., 1998) observed an increased percentage of AT1R variances in ACE DD patients with MI, but in the study of Plat et al. (2009), the investigators failed to confirm an association between the two and MI. In the current study, we did not detect significant differences in the distribution of this and the other allelic combination variances between the studied groups. We did not investigate the role of other risk factors as stress or alcohol intake, and therefore, their influence on the development of the disease and the association with the studied polymorphisms could not be excluded. A further study on a bigger set of patients from the Bulgarian population is needed to confirm the polymorphisms as a risk factor for CAD.
In conclusion, we examined the distribution of polymorphisms in eNOS gene—Glu298Asp, ACE ID, AGT T704C, and A1166C in AT1R gene in Bulgarians with IHD (SAP and ACS). Using the binary logistic regression model, we demonstrated the association between eNOS Glu298Asp and ACE I/D polymorphisms, independently and in a combination with other risk factors, with increased risk of developing the disease. Our observations could be useful for identifying the patients more susceptible to the disease and to ACS in particular and may help in the treatment and management.
Acknowledgment
This work was supported by the National Science Fund of Bulgaria (Grant No. G2/2004).
Author Disclosure Statement
No competing financial interests exist.
References
- Abdel-Aziz TA, Mohamed RH. (2013) Association of endothelial nitric oxide synthase gene polymorphisms with classical risk factors in development of premature coronary artery disease. Mol Biol Rep 40:3065–3071 [DOI] [PubMed] [Google Scholar]
- Barbalic M, Skaric-Juric T, Cambien F, et al. (2006) Gene Polymorphisms of the renin-angiotensin system and early development of hypertension. Am J Hypertens 19:837–842 [DOI] [PubMed] [Google Scholar]
- Bertrand ME. (2004) Provision of cardiovascular protection by ACE inhibitors: a review of recent trials. Curr Med Res Opin 20:1559–1569 [DOI] [PubMed] [Google Scholar]
- Black P, Garbutt L. (2002) Stress, inflammation and cardiovascular disease. J Psychosom Res 52:1–23 [DOI] [PubMed] [Google Scholar]
- Bonci E, Chiesa C, Versacci P, et al. (2015) Association of non-alcoholic fatty liver disease with subclinical cardiovascular changes: a systematic review and meta-analysis. Biomed Res Int.2015:213737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas JP, Bautista LE, Humphries SE, et al. (2004) Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation 109:1359–1365 [DOI] [PubMed] [Google Scholar]
- Cherney DZ, Scholey JW, Zhou J, et al. (2009) Endothelial nitric oxide synthase gene polymorphisms and the renal hemodynamic response to L-arginine. Kidney Int 75:327–332 [DOI] [PubMed] [Google Scholar]
- Creager MA, Roddy MA. (1994) Effects of captopril and enalapril on endothelial function in hypertensive patients. Hypertension 24:499–505 [DOI] [PubMed] [Google Scholar]
- Di Mario C, Strikwerda S, Gil R, et al. (2000) Long-term changes in the response of conductance and resistance coronary vessels to endothelium-dependent and independent vasodilators. A double-blind placebo-controlled study of the effect of a 6-month treatment with cilazapril. Ital Heart J 1:674–683 [PubMed] [Google Scholar]
- Duncan J, Scholey J, Miller J. (2001) Angiotensin II type 1 receptor gene polymorphisms in humans: physiology and pathophysiology of the genotypes. Curr Opin Nephrol Hypertens 10:111–116 [DOI] [PubMed] [Google Scholar]
- Firouzabadi N, Tajik N, Bahramali E, et al. (2013) Gender specificity of a genetic variant of angiotensin-converting enzyme and risk of coronary artery disease. Mol Biol Rep 40:4959–4965 [DOI] [PubMed] [Google Scholar]
- Hara M, Sakata Y, Nakatani D, et al. (2014) Renin–angiotensin–aldosterone system polymorphisms and 5-year mortality in survivors of acute myocardial infarction. Int Heart J 55:190–196 [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Imaizumi T, Masaki H, et al. (1992) Captopril impaired endothelium-dependent vadodilation in hypertensive patients. Hypertension 20:175–180 [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. (1992) Molecular basis of human hypertension: role of angiotensinogen. Cell 71:169–180 [DOI] [PubMed] [Google Scholar]
- Jiang W, He H, Yang Z. (2014) The angiotensinogen gene polymorphism is associated with heart failure among Asians. Sci Rep 4:4207–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretowski A, McFann K, Hokanson JE, et al. (2007) Polymorphisms of the renin-angiotensin system genes predict progression of subclinical coronary atherosclerosis. Diabetes 56:863–871 [DOI] [PubMed] [Google Scholar]
- Kostadinova E, Miteva L, Stanilova S. (2014) Genetic polymorphism in angiotensin-converting enzyme gene and congenital anomalies of the kidney and urinary tract. Trakia J Sci 12:191–196 [Google Scholar]
- Li J, Wu X, Li X, et al. (2010) The endothelial nitric oxide synthase gene is associated with coronary artery disease: a meta-analysis. Cardiology 116:271–278 [DOI] [PubMed] [Google Scholar]
- Luo J, Wen J, Zhou H, et al. (2014) Endothelial nitric oxide synthase gene g894t polymorphism and myocardial infarction: a meta-analysis of 34 studies involving 21068 subjects. Plos One 9:e87196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Gong H. (2015) Correlation of ACE gene deletion/insertion polymorphism and risk of pregnancy-induced hypertension: a meta-analysis based on 10,236 subjects. J Renin Angiotensin Aldosterone Syst DOI: 10.1177/1470320315588872 [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. (1998) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondry A, Loh M, Liu P, et al. (2005) Polymorphisms of the insertion/deletion ACE and M235T AGT genes and hypertension: surprising new findings and meta-analysis of data. BMC Nephrol 6:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NE, Collins A. (1998) Tests and estimates of allelic association in complex inheritance. Proc Natl Acad Sci U S A 95:11389–11393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro C, Hongwei Q, Walter GT. (2007) Type 1 angiotensin receptor pharmacology: signaling beyond G proteins. Pharmacol Ther 113:210–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, McEwen B. (2015) Stress habituation, body shape and cardiovascular mortality. Neurosci Biobehav Rev 56:139–150 [DOI] [PubMed] [Google Scholar]
- Plat AW, Stoffers HE, Klungel OH, et al. (2009) The contribution of six polymorphisms to cardiovascular risk in a Dutch high-risk primary care population: the HIPPOCRATES project. J Hum Hypertens 23:659–667 [DOI] [PubMed] [Google Scholar]
- Poirier O, Georges JL, Ricard S, et al. (1998) New polymorphisms of the angiotensin II type 1 receptor gene and their associations with myocardial infarction and blood pressure: the ECTIM study. J Hypertens 16:1443–1447 [DOI] [PubMed] [Google Scholar]
- Ragia G, Nikolaidis E, Tavridou A, et al. (2010) Renin-angiotensin-aldosteron system gene polymorphyisms in coronary artery bypass graft surgery patients. J Renin Angiotensin Aldosterone Syst 11:136–145 [DOI] [PubMed] [Google Scholar]
- Rahimi Z. (2012) ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathol 1:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, et al. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86:1343–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M, Mayer G. (2009) Significance of genetic polymorphisms of the rennin-angiotensin-aldosterone system in cardiovascular and renal disease. Pharmacogenomics 10:463–476 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Gupta V, Kumar A, et al. (2015) M235T polymorphism in the AGT gene and A/G substitution in the REN gene correlate with end-stage renal disease. Nephron 129:104–108 [DOI] [PubMed] [Google Scholar]
- Sawada T, Kishimoto T, Osaki Y, et al. (2008) Relation of the Glu298Asp polymorphism of the nitric oxide synthase gene to hypertension and serum cholesterol in Japanese workers. Prev Med 47:167–171 [DOI] [PubMed] [Google Scholar]
- Schiavello T, Burke V, Bogdanova N, et al. (2001) Angiotensin-converting enzyme activity and the ACE Alu polymorphism in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 16:2323–2327 [DOI] [PubMed] [Google Scholar]
- Schmidt S, Beige J, Walla-Friedel M, et al. (1997) A polymorphism in the gene for the angiotensin II type 1 receptor is not associated with hypertension. J Hypertens 15:1385–1388 [DOI] [PubMed] [Google Scholar]
- Tiret L, Blanc H, Ruidavets JB, et al. (1998) Gene polymorphisms of the rennin-angiotensin system in relation to hypertension and parental history of myocardial. J Hypertens 16:37–44 [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H, Vanuzzo D, Hobbs , et al. (2007) Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet 355:688–700 [DOI] [PubMed] [Google Scholar]
- Verma S. (2006) Restoration of endothelial function with ACE inhibitors. Medicographia 28:326–332 [Google Scholar]
- Zakrewski-Jakubiak M, de Denus S, Dube M, et al. (2008) Ten renin-angiotensin system-ralted gene polymorphisms in maximally treated Canadian Caucasian patients with heart failure. Br J Clin Phamacol 65:742–751 [DOI] [PMC free article] [PubMed] [Google Scholar]