Abstract
Background: Thromboangiitis obliterans (TAO), also called Buerger's disease, is a chronic peripheral vascular occlusive disease. It is an obliterative vasculitis characterized by arterial thrombosis and strongly associated with tobacco exposure. The pathogenesis and etiology of TAO are not well understood, but genetic factors may be important in its development. A case-control study was undertaken to identify genetic factors potentially involved in the pathogenesis of TAO in a Xinjiang Uyghur population of China, where TAO is common. Methods: We ascertained 177 TAO patients by clinical screening and 86 healthy individuals from the HAPMAP database. The genotypes of single-nucleotide polymorphisms (SNPs) of the participants were identified using the Affymetrix Genome-Wide Human SNP Array 6.0 to perform a genome wide association study (GWAS). The association between the SNPs and incidence of TAO was quantified using race stratification exposure. Results: Through a case-control GWAS study 26 SNPs were significantly associated with incidence of TAO following a Bonferroni correction. However, after genomic control correction for population stratification only three of these SNPS were highly significantly associated with TAO: rs376511 in IL17RC (OR = 24.4, 95% CI:8.68 − 68.62, p < 0.0001), rs7632505 in SEMA5B (OR = 29.47, 95% CI:7.16 − 121.3, p < 0.0001), and rs10178082 (OR = 18.09, 95% CI: 6.56 − 49.92, p < 0.0001) showed a significant risk of TAO in the Uyghur population. Conclusions: This study shows an association between these 3 SNPs and susceptibility to TAO in the Uyghur population, suggesting that polymorphisms in the IL-17RC and Sema 5B genes may pre-dispose individuals in this population to development of TAO. These findings require replication.
Introduction
Thromboangiitis obliterans (TAO or Buerger's disease) is a common chronic peripheral vascular occlusive disease in the Xinjiang region of Uyghur. It is an obliterative vasculitis characterized by arterial thrombosis and has been recognized to have a complex immune inflammatory basis. An association between TAO and tobacco exposure has been established, strong enough that it is a diagnostic criterion for the disease (Juergens et al., 1980; Matsushita et al., 1992).
Although TAO occurs throughout the world, it is quite uneven in its distribution. It is more prevalent in the Middle East and Far East than Western Europe and North America. Large numbers of patients were reported from the Mediterranean region (Mills and Porter, 1991; Olin, 2000), the Middle East (Papa and Adar, 1992), Eastern Europe (Nielubowicz et al., 1980), India (Kinare et al., 1976), and the Orient (McKusick and Harris, 1961; Shionoya, 1990). The incidence in North America is 8 to 12.6/100,000 population, which had declined, substantially in the last 25–30 years (Mills and Porter, 1991; Olin, 2000). Its incidence is highest among natives of India, Japan, Korea, Ceylon, and Ashkenazi Jews (Olin, 2000). Although it is very common, there are still no incidence data available in Xinjiang.
TAO often first appears in young people and is difficult to treat effectively. There is no cure, and patients often require limb amputation. Identifying the factors that lead to the development of TAO holds perhaps the greatest promise for prevention of the disease. Genetic factors are a likely candidate for one of the important contributors to the development of TAO in a segment of the population.
There are a few studies from the past that consider a possible correlation between susceptibility to TAO and genetic makeup. Recent reports also indicate that the genetic link is of current interest. For example, an increase in several human leukocyte antigen subtypes has been reported in patients with TAO by several investigators (subtypes A9, B5, A1, B8, and DR4) (McLoughlin et al., 1976; Smolen et al., 1978). Polymorphisms in genes for human leukocyte antigens and cluster of differentiation 14 (CD14) were associated with increased incidence of Buerger's disease in a Japanese population. CD14 is the main receptor for lipopolysaccharide from Gram-negative bacteria, and these workers suggested that genes involved in innate and adaptive immunity may, in part, determine susceptibility to Buerger's disease (Chen et al., 2007). Finally, the reference single-nucleotide polymorphism rs7744 located in the 3′-untranslated region of myeloid differentiation primary response protein 88 (MyD88) gene has been associated with the pathogenesis of TAO (Zhiyong Chen et al., 2011). Finally, guinea pig leukocyte antigen (GPla) 807 C/T polymorphism does not represent a risk factor for TAO disease itself but could be associated with premature onset of this disorder in predisposed individuals (Ostojic et al., 2007).
In this study, we have genotyped 26 single-nucleotide polymorphisms (SNPs) from a case–control study of TAO patients in China. The study included 177 patients with TAO and 86 control individuals. Genetic variants in case samples were genotyped using the Affymetrix Genome-Wide Human SNP Array 6.0. Statistical analysis of the association between SNPs and TAO was performed using the χ2 test and SNPStats software. Han Chinese in Beijing, China samples from HapMap Phase III were used as controls. Their genotype data in the PLINK format were downloaded from the HapMap file transfer protocol (FTP) site. After SNP analyzing and stratification by Uyghur race models, we found that rs376511 in interleukin 17 receptor C (IL17RC), rs7632505 in SEMA5B, and rs10178082 were associated with patients who had developed TAO. We consider that polymorphisms within IL17RC or semaphorin 5B (SEMA5B) may contribute to the occurrence or development of TAO in the Xinjiang Uyghur population. The functional significance of these polymorphisms will require further research.
Materials and Methods
Study participants
All patients with TAO who were admitted to the Department of Vascular Surgery in the People's Hospital of Xinjiang Uygur Autonomous Region, China, from October 2013 to January 2015 were included in the case–control study. The diagnosis of TAO was based on Shionoya's criteria (Shionoya, 1990). Arterial occlusive disease was diagnosed by means of arteriography or Doppler ultrasonography in all cases. Every case was diagnosed by the consensus of a team of four professors. Cases were excluded if the patient was younger than 15 years or older than 60 years, had received surgery with a joint endoscopic examination, did not have a clear diagnosis, was pregnant, or had one of the following diseases: chronic heart, lung, or brain disease, severe liver disease, diabetes, or heart failure. Han Chinese in Beijing, China (CHB) samples from HapMap Phase III were used as controls, and their genotype data in the PLINK format were downloaded from the HapMap FTP site. In addition, the controls were matched for age, gender, and educational level with cases.
We informed all participants about the experimental procedures and the purpose of the study. We obtained signed informed consent from each of them or from their guardians if the participant was younger than 18 years. The Human Research Committee of the People's Hospital of Xinjiang Uygur Autonomous Region for Approval of Research Involving Human Subjects approved the use of human tissue in this study.
Clinical and demographic data
A team including three doctors interviewed each patient to collect the necessary information, such as region, age, sex, tobacco use, alcohol use, ethnicity, education status, and family history of vascular disease. After the interview, we collected a 5-mL sample of venous blood from each participant.
Genotyping
DNA was isolated from blood using QIAamp® DNA Blood Mini Kit, DNA concentration above 50 ng/μL and processed on Affymetrix SNP 6.0 arrays, assessing genotype at 906,600 SNPs. The Birdseed v2 genotyping algorithm was used to make genotype calls. The initial genotype call rate among all individuals was 94.5% (range, 87.3–98.1%). SNPs were excluded from the analysis if they had no genotype for >5% of individuals, were not in Hardy–Weinberg equilibrium (HWE) among controls (using threshold p < 0.001), or had minor allele frequency (MAF) <5%. The minor allele was defined as the allele with lower frequency among the total sample of 177 individuals. The final data set contained 484,730 SNPs in 177 samples.
CHB samples from HapMap Phase III were used as controls, and their genotype data in the PLINK format were downloaded from the HapMap FTP site: ftp://ftp.ncbi.nlm.nih.gov/hapmap/phase_3/hapmap3_reformatted/.
Quality control
Every marker removed from a study is potentially an overlooked disease association, and thus, the impact of removing one marker is potentially greater than the removal of one individual. This led us to implement quality control on a per-individual basis before conducting quality control on a per-marker basis to maximize the number of markers remaining in the study. Details of the quality control are provided below.
Samples with any of the following were removed:
(1) Sex inconsistencies
We began by assessing genotype data from the X-chromosome to determine if it agreed with the reported sex. This should highlight plating errors. Unless the sample can be correctly identified using existing genotype data or unless it can be confirmed that sex was recorded incorrectly, individuals with discordant sex information were removed from further analysis.
(2) Elevated missing data rate
The genotype failure rate per individual is a measure of DNA sample quality. We excluded all individuals with a genotype failure rate >0.05.
(3) Outlying heterozygosity rate
The genotype heterozygosity rate per individual (excluding the sex chromosomes) is also a measure of DNA sample quality. An unusually high or unusually low proportion of heterozygous genotypes may be due to contamination or inbreeding, respectively. Thus, we excluded all samples with a heterozygosity rate >3 SD of the mean.
(4) Duplicated samples or DNA from related individuals
If either duplicate samples or samples from first- or second-degree relatives are present, a bias may be introduced in the study because the genotypes within families will be overrepresented. The sample would then no longer reflect the allele frequencies accurately.
The degree of recent shared ancestry for a pair of individuals (identity by descent, IBD) was estimated using a pruned set of SNPs set defined as having no SNP pair within a 50-kb window with an r2 > 0.2, excluding the sex chromosomes. The expectations are that the proportion of IBD of a pair will equal 1 for duplicate samples or monozygotic twins, 0.5 for first-degree relatives, 0.25 for second-degree relatives, and 0.125 for third-degree relatives. To ensure that no pair of individuals were duplicates or related, some individuals were excluded if the proportion of IBD for a pair was >0.1875.
(5) Divergent ancestry
In genetic studies, the parameter of interest is genotype distribution. It is possible that there will be an apparent association for an ancestrally important SNP because of allele frequency differences between the founder populations of the population under the study and control populations. In addition to carefully matching the study population with a control population with respect to population origin, potential stratification also must be examined and characterized during quality control. Efforts should then be made to remove or reduce the effect of population stratification through the removal of individuals of divergent ancestry.
(6) Population stratification
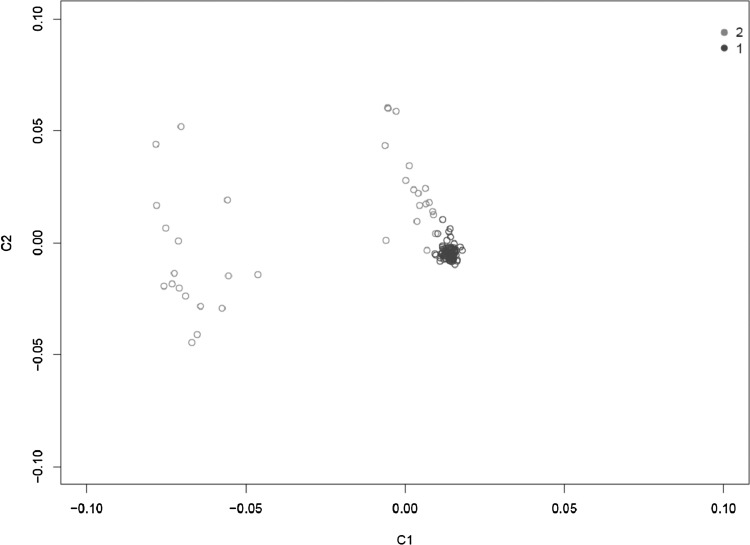
To evaluate differences in population race structure, a multidimensional scaling (MDS) method was performed using pruned SNPs to extract MDS dimensions and draw an MDS plot (seen in Fig. 4). Pairwise identity-by-state (IBS) distance was calculated, and IBS distance to each of the five nearest neighbors was transformed into a Z score. Individuals with a minimum Z score among the five nearest neighbors less than −4 were excluded from analysis as population outliers.
In the case of genetic studies, where the exposure of interest is genotype distribution, a signal of association will arise for an ancestrally informative SNP because of allele frequency differences between the founder populations that differentially comprise the cases and controls.
To assess population stratification, MDS analysis, based on pruned SNPs, was used to give a scatter plot in which each point is an individual and the two axes correspond to a reduced representation of the data in two dimensions C1 and C2.
A small number of individuals were excluded as outliers with a minimum Z score among the five nearest neighbors less than −4.
FIG. 4.
MDS plot.
SNPs were filtered if:
(1) There was an excessive missing genotype rate
The removal of low-quality markers is important to the success of a global-wide association (GWA) study because they can present as false positives and reduce the identification of true associations. Here, SNPs with missing genotype rate <0.05 were removed.
(2) There were different genotype-missing rates between the study and control populations.
Removing SNPs with substantial differences in missing genotype rate between the study and control populations is another means of reducing confounding and removing poorly genotyped SNPs. In studies where the study and control populations have been drawn from several different sources, it is wise to test for differences in call rate between these various groups before assuming that the combined population can be treated as a homogeneous group.
In this study, we excluded all SNPs with a significantly different (p < 0.00001) missing data rate between the study and control populations.
(3) There was a considerable deviation from HWE
Extensive deviation from HWE can indicate genotyping or genotype-calling error. However, it may also indicate selection, and if so, the study population may have deviations from HWE at markers associated with disease. Therefore, only control samples were used when we tested for deviations from HWE (Nagase et al., 2000).
In this study, SNPs with HWE p-values <0.001 were removed.
(4) Very low minor allele frequency
The small size of the heterozygote and rare homozygote clusters makes these variants difficult to call using current genotype-calling algorithms, and they frequently present as false positives in case–control association tests.
In this study, SNPs with MAF <0.05 in either population were removed.
Association analysis
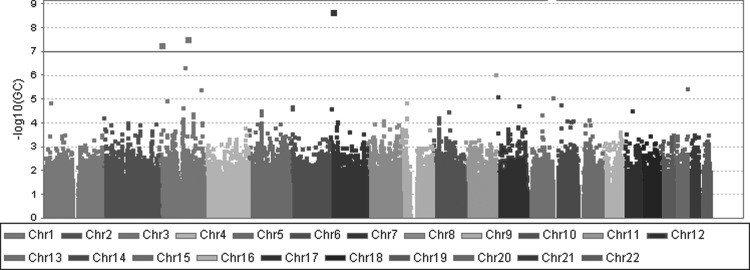
The association between each SNP and disease was estimated by the two-sided Cochran–Armitage trend test. Population stratification was investigated through the genomic control (GC) approach based on the median test statistic and quantile–quantile (Q–Q) plot analysis. Bonferroni correction was used to decrease the rate of the false positive due to multiple testing. Odds ratio (OR) and 95% confidence interval (CI) were calculated by multivariable unconditional logistic regression assuming a log-additive genetic model adjustment for age, gender, and educational level. In addition, the technique of GC was used to correct the result of the analysis using the trend test to control population stratification. The result of association analysis was shown in a Manhattan plot against the SNPs' chromosomal locations, as is shown in Figure 1.
FIG. 1.
Manhattan plot based on Cochran–Armitage trend tests with adjustment for GC. GC, genomic control.
The result of association analysis was shown in Manhattan plot, drawn with Haploview 4.2 software. No marker was significant at the genome-wide level after Bonferroni correction (p adj. <0.05).The mean and median of trend χ2 values (mean = 0.959, median = 0.429) were very close to the expected values of 1.00 and 0.456, meaning no or no very strong stratification existed.
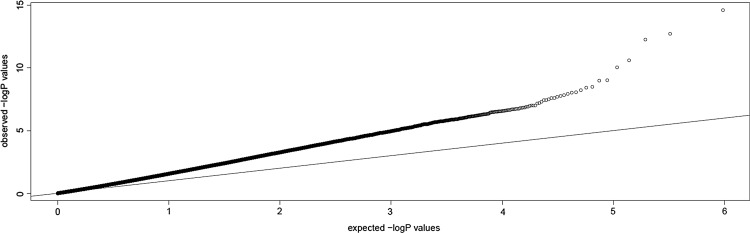
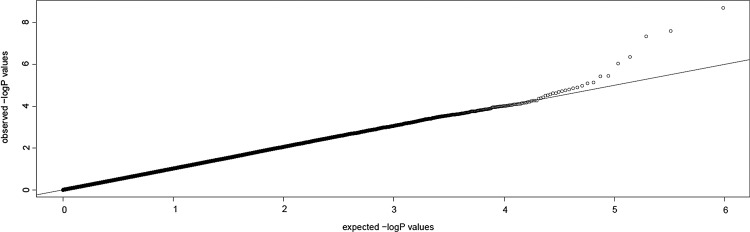
Many false positives of genetic correlation analysis were due to population stratification. So generally, we chose samples with the same genetic background. But actually, the control that has the normal large sample size cannot be found in Uyghur. So in this cohort, Han Chinese in Beijing, China samples from HapMap Phase III were used as controls. Considering the population stratification may exist, we use MDS to show the selected population genetic background. The distribution of both the case and control samples indicated that there may be a population stratification, and the deviation from the diagonal in the Q–Q plot before adjustment (as shown in Fig. 2) also suggests that there may be a population stratification, so we use GC to adjust the population stratification. After the adjustment, the Q–Q plot nearing the diagonal that shows the population stratification has been controlled (as shown in Fig. 3).
FIG. 2.
Q–Q plot based on Cochran–Armitage trend tests for GC. Q–Q, quantile–quantile.
FIG. 3.
Q–Q plot based on Cochran–Armitage trend tests with adjustment for GC.
Genomic inflation factors (λ) and Q–Q plots of the p-values from GWA analyses were used to assess population stratification. Bonferroni correction was used to decrease the rate of false-positive identification due to multiple testing.
Results
A total of 26 SNPs were closely related to TAO in this study. Chromosomal position, MAF, and HWE test results for all the SNPs are presented in Table 2. None of the SNPs displayed a significant deviation from HWE in the control population (p < 0.05), shown in Table 1.
Table 2.
Basic Information of Candidate SNPs
| Case | Control | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHR | SNP | A1 | A2 | A1A1 | A1A2 | A2A2 | A1A1 | A1A2 | A2A2 | χ2 | p | Position | Band | Gene(s) | Role |
| 1 | rs10430146 | G | A | 0 | 23 | 13 | 1 | 7 | 75 | 33.11 | 0.0001 | 27384315 | 1p36.11 | ||
| 2 | rs905965 | G | A | 9 | 19 | 8 | 1 | 27 | 56 | 28.35 | 0.0001 | 6911987 | 2p25.2 | ||
| 3 | rs376511 | A | G | 4 | 25 | 7 | 0 | 11 | 73 | 52.05 | 0.0001 | 9962816 | 3p25.3 | IL17RC | Intron |
| 3 | rs12629897 | T | C | 17 | 15 | 3 | 5 | 36 | 43 | 33.82 | 0.0001 | 38126640 | 3p22.2 | DLEC1 | Intron |
| 3 | rs13086279 | G | A | 7 | 18 | 11 | 2 | 12 | 70 | 31.52 | 0.0001 | 107640533 | 3q13.12 | LOC285205 | Intron |
| 3 | rs1846594 | G | A | 11 | 25 | 0 | 4 | 25 | 55 | 44.45 | 0.0001 | 112916203 | 3q13.2 | ||
| 3 | rs7632505 | G | A | 16 | 19 | 0 | 2 | 30 | 52 | 54.01 | 0.0001 | 122738307 | 3q21.1 | SEMA5B | Intron |
| 3 | rs891666 | A | T | 6 | 19 | 11 | 0 | 19 | 65 | 29.61 | 0.0001 | 150827190 | 3q25.1 | MED12L | Intron |
| 3 | rs2700474 | C | G | 5 | 20 | 10 | 0 | 20 | 63 | 28.42 | 0.0001 | 150885578 | 3q25.1 | MED12L | Intron |
| 3 | rs4266183 | G | A | 1 | 28 | 6 | 1 | 16 | 67 | 37.23 | 0.0001 | 186116859 | 3q27.3 | ||
| 5 | rs831781 | G | C | 14 | 17 | 4 | 4 | 34 | 46 | 30.56 | 0.0001 | 56347693 | 5q11.2 | ||
| 5 | rs331047 | G | A | 13 | 18 | 4 | 4 | 34 | 46 | 29.13 | 0.0001 | 56382328 | 5q11.2 | ||
| 6 | rs6929018 | T | C | 7 | 24 | 4 | 3 | 24 | 57 | 31.76 | 0.0001 | 6873698 | 6p25.1 | ||
| 6 | rs494238 | G | A | 10 | 17 | 9 | 1 | 23 | 60 | 31.02 | 0.0001 | 7023654 | 6p25.1 | ||
| 7 | rs1859604 | T | G | 5 | 16 | 14 | 0 | 11 | 73 | 31.01 | 0.0001 | 7687826 | 7p21.3 | RPA3 | Intron |
| 7 | rs10178082 | T | A | 22 | 11 | 2 | 2 | 22 | 59 | 62.65 | 0.0001 | 10706912 | 7p21.3 | ||
| 9 | rs7863803 | A | G | 1 | 22 | 12 | 0 | 11 | 71 | 32.91 | 0.0001 | 27924555 | 9p21.2 | ||
| 10 | rs2456672 | C | T | 9 | 26 | 0 | 5 | 32 | 43 | 30.21 | 0.0001 | 68243362 | 10q21.3 | CTNNA3 | Intron |
| 11 | rs2851117 | T | C | 0 | 8 | 27 | 35 | 36 | 13 | 41.97 | 0.0001 | 133705905 | 11q25 | SPATA19 | Downstream |
| 12 | rs7248 | A | C | 6 | 15 | 14 | 0 | 9 | 75 | 34.96 | 0.0001 | 10375302 | 12p13.2 | GABARAPL1 | 3′ UTR |
| 12 | rs11110833 | G | A | 6 | 28 | 2 | 4 | 25 | 55 | 32.11 | 0.0001 | 101930143 | 12q23.2 | ||
| 13 | rs9528951 | C | T | 8 | 18 | 10 | 0 | 22 | 59 | 29.21 | 0.0001 | 66203063 | 13q21.32 | ||
| 13 | rs9550256 | A | T | 16 | 15 | 5 | 4 | 29 | 51 | 34.71 | 0.0001 | 114494675 | 13q34 | FAM70B | Intron |
| 14 | rs8017647 | T | C | 6 | 17 | 12 | 1 | 11 | 72 | 32.42 | 0.0001 | 32456358 | 14q12 | ||
| 17 | rs8072266 | C | T | 1 | 24 | 11 | 0 | 15 | 68 | 30.34 | 0.0001 | 42639727 | 17q21.31 | FZD2 | Downstream |
| 20 | rs1006644 | A | G | 17 | 11 | 7 | 3 | 24 | 57 | 37.43 | 0.0001 | 60230289 | 20q13.33 | CDH4 | Intron |
A1, minor allele code based on whole samples; A2, major allele code based on whole samples; p, asymptotic p-value.
Table 1.
Basic Information of Candidate SNPs
| MAF | HWE | 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | A1/A 2 | CHR | Case | Control | p | OR | Lower | Upper | p | |
| 1 | rs10430146 | G/A | 1 | 0.32 | 0.05 | 0.20 | 12.50 | 4.79 | 32.61 | 0.0001 |
| 2 | rs905965 | G/A | 2 | 0.51 | 0.17 | 0.45 | 6.17 | 2.87 | 13.28 | 0.0001 |
| 3 | rs376511 | A/G | 3 | 0.46 | 0.07 | 1.00 | 24.40 | 8.68 | 68.62 | 0.0001 |
| 4 | rs12629897 | T/C | 3 | 0.70 | 0.27 | 0.59 | 7.12 | 3.29 | 15.41 | 0.0001 |
| 5 | rs13086279 | G/A | 3 | 0.44 | 0.10 | 0.15 | 6.66 | 3.08 | 14.38 | 0.0001 |
| 6 | rs1846594 | G/A | 3 | 0.65 | 0.20 | 0.51 | 13.80 | 4.96 | 38.42 | 0.0001 |
| 7 | rs7632505 | G/A | 3 | 0.73 | 0.20 | 0.50 | 29.47 | 7.16 | 121.30 | 0.0001 |
| 8 | rs891666 | A/T | 3 | 0.43 | 0.11 | 0.59 | 7.26 | 3.22 | 16.35 | 0.0001 |
| 9 | rs2700474 | C/G | 3 | 0.43 | 0.12 | 0.60 | 7.48 | 3.24 | 17.25 | 0.0001 |
| 10 | rs4266183 | G/A | 3 | 0.43 | 0.11 | 1.00 | 14.06 | 5.34 | 37.03 | 0.0001 |
| 11 | rs831781 | G/C | 5 | 0.64 | 0.25 | 0.57 | 6.32 | 2.97 | 13.46 | 0.0001 |
| 12 | rs331047 | G/A | 5 | 0.63 | 0.25 | 0.57 | 6.11 | 2.87 | 13.02 | 0.0001 |
| 13 | rs6929018 | T/C | 6 | 0.54 | 0.18 | 0.72 | 7.69 | 3.33 | 17.76 | 0.0001 |
| 14 | rs494238 | G/A | 6 | 0.51 | 0.15 | 0.68 | 6.28 | 2.98 | 13.23 | 0.0001 |
| 15 | rs1859604 | T/G | 7 | 0.37 | 0.07 | 1.00 | 8.66 | 3.58 | 20.92 | 0.0001 |
| 16 | rs10178082 | T/A | 7 | 0.79 | 0.16 | 1.00 | 18.09 | 6.56 | 49.92 | 0.0001 |
| 17 | rs7863803 | A/G | 9 | 0.34 | 0.07 | 1.00 | 12.01 | 4.70 | 30.70 | 0.0001 |
| 18 | rs2456672 | C/T | 10 | 0.63 | 0.26 | 1.00 | 8.67 | 3.41 | 22.02 | 0.0001 |
| 19 | rs2851117 | T/T | 11 | 0.11 | 0.63 | 0.48 | 0.08 | 0.03 | 0.21 | 0.0001 |
| 20 | rs7248 | A/C | 12 | 0.39 | 0.05 | 1.00 | 10.21 | 4.05 | 25.71 | 0.0001 |
| 21 | rs11110833 | G/A | 12 | 0.56 | 0.20 | 0.51 | 8.47 | 3.50 | 20.49 | 0.0001 |
| 22 | rs9528951 | C/T | 13 | 0.47 | 0.14 | 0.34 | 6.73 | 3.05 | 14.84 | 0.0001 |
| 23 | rs9550256 | A/T | 13 | 0.65 | 0.22 | 1.00 | 6.29 | 3.09 | 12.80 | 0.0001 |
| 24 | rs8017647 | T/C | 14 | 0.41 | 0.08 | 0.39 | 7.90 | 3.43 | 18.22 | 0.0001 |
| 25 | rs8072266 | C/T | 17 | 0.36 | 0.09 | 1.00 | 10.08 | 4.11 | 24.70 | 0.0001 |
| 26 | rs1006644 | A/G | 20 | 0.64 | 0.18 | 0.72 | 6.15 | 3.12 | 12.12 | 0.0001 |
SNP, single-nucleotide polymorphism; A1/A2, tested allele (minor allele by default); CHR, chromosome; OR, odds ratio; 95% CI lower, lower bound of 95% confidence interval for odds ratio; 95% CI upper, upper bound of 95% confidence interval for odds ratio; STAT, coefficient Wald statistic; HWE, Hardy–Weinberg equilibrium; HWEp, p-value for H–W; p, asymptotic p-value for Wald statistic.
When we conducted quality control, one sample had a genotype failure rate >0.05. No sample had an outlying heterozygosity rate, and no pair of individuals were duplicated or related, according to the criteria established. Individuals were split into two subgroups, as can be seen from the MDS plot and shown in Figure 4. Thus, population stratification was further assessed and corrected during association analysis. After quality control, 484,730 autosomal SNPs in 177 individuals with TAO and 86 healthy individuals were included in the association analysis.
After race stratification and Bonferroni correction, 26 markers were significant at the genome-wide level (p adj. <0.05). However, both the genomic inflation factor (λ) and the analysis indicated a strong population stratification effect on the association result. The genomic inflation was 1.74197, and the Q–Q plot deviated from the expected line from the beginning.
After GC correction for population stratification, only three markers, specifically, rs10178082, rs7632505, and rs376511, from the original 26 were still significant at the genome-wide level after Bonferroni correction (p adj. <0.05). Furthermore, the Q–Q plot did not indicate systematic increase in false positives due to population stratification. The observed distribution did not deviate from expected, except in its extreme right tail.
Discussion
TAO (OMIM: 211480) is a complex inflammatory disease that primarily affects small blood vessels, causing acute cellular thrombosis. Its diagnosis and therapy are a puzzle for medical staff and patients. Tobacco use or exposure is central in the initiation and progression of the disease, and a genetic predisposition for TAO has been suggested. Although numerous other risk factors are implicated in TAO, the relationship between smoking and TAO is strong. We suggest that understanding the pathogenesis of TAO requires investigating the interaction between the genetic factors and tobacco exposure. Previous studies showed that the prevalence is much higher in comparison to that in Han Chinese in the south of Xinjiang. The statistic data from the Department of Vascular Surgery in the People's Hospital of Xinjiang Uygur Autonomous Region (which is one of the biggest hospitals in this region) show that Uyghur patients accounted for 54.3% in all TAO patients from 2005 to 2011. Nearly 98.75% were males; the Uygur-to-Han Chinese ratio was 4.34:1. But the ethnic differences in the onset of risk factors have not yet been systematic, multifaceted, multiangle research. This may help us discover the occurrence and development of the molecular mechanism and signal transduction pathways for further understanding. This was the goal of this study.
We successfully identified and genotyped 26 SNPs in 263 participants, including 177 individuals with TAO and 86 unafflicted individuals from a Xinjiang Uyghur population. We identified three SNPs that were associated with increased risk for TAO and suggest that polymorphism of the genes where these SNPs are located may play an important role in the risk of TAO in the Xinjiang Uyghur population. Two of these genes are IL17RC and SEMA5B. Theoretically, our results are the first to identify an association between these genes and TAO in this population.
IL-17 is an inflammatory cytokine produced primarily by a unique lineage of CD4 T cells that are critical in the pathogenesis of many autoimmune diseases (Harrington et al., 2005). IL-17 enhances cytokine and growth factors' release from resident cells; these factors include IL-6, IL-8, IL-11, granulocyte macrophage colony-stimulating factor, and vascular endothelial growth factor (Chang et al., 2006, 2011; de Beaucoudrey et al., 2008; Claudio et al., 2009). The biological activity of IL-17 is dependent on IL-17R, a receptor complex composed of IL-17 receptor A (IL17RA) and IL17RC. This situation, perhaps, represents a new paradigm for the interactions between the family of IL-17 ligands and their receptors (Aggarwal et al., 2003).
IL17RC (cytogenetic locations: 3p25.3; OMIM: 610925), an rIL-17R family member sharing 23% amino acid sequence identity with IL-17RA, is an essential component of IL-17R (Park et al., 2005). The human IL17RC gene, located on chromosome 3, contains 19 exons and spans 16,550 bp within the chromosomal region 3p25.3–3p24.1. The gene encodes a single-pass type I transmembrane protein (IL17RC), which was characterized as an integral part of the IL-17R complex (Lett et al., 2009). The IL-17R complex mediates IL-17 effector function through the IL-17 cytokine–IL-17R complex signaling axis (Kolls and Linden, 2004). Both the dysfunctions in IL17RC and the IL-17 cytokine–IL-17R signaling axis are implicated in a number of human diseases. As expected, given its central role in regulating inflammation, dysfunctions in the IL-17 cytokine–IL-17R signaling axis can promote the production of several proinflammatory cytokines, such as tumor necrosis factor-α, IL-1β, and IL-6. These could play important roles in TAO as IL-17 and IL17RC may contribute to endothelial dysfunction, which is implicated in TAO. The rs376511 polymorphism may impair the function of the IL17RC gene and accelerate endothelial dysfunction, leading to the occurrence and progression of TAO.
SEM5B (OMIM: 609298) inhibits the proliferation, migration, and tube formation of endothelial cells. It was first cloned by Adams et al. (1996) and is expressed in human embryonic and stem cells (Langrish et al., 2005). It also acts in the nervous system to inhibit axon development (Allen and Gaffen, 2010), where it is proteolytically processed from its transmembrane form (Browne et al., 2012). The semaphorin family is still being identified but is characterized by the sema domain, consisting of ∼500 amino acids, which is essential for protein activity and receptor-binding specificity (Feiner et al., 1997). Several semaphorin family members exert antiangiogenic functions (Kessler et al., 2004; Basile et al., 2006; Guttmann-Raviv et al., 2007) and contain domains with specific peptide sequences that are able to bind the endothelial cell surface receptor CD36 (Deborah et al., 2000). This confers the ability to inhibit angiogenesis in vitro and in vivo (Chen et al., 2000). The proposal that SEM5B affects the vascular endothelial cell is a new idea, in need of further exploration.
The results may be influenced by some limitations. First, large samples of replication are required. Generally, the GWAS report needs repeated verification, including a first step of chip screening and a second step of replication to the selected sites in large sample. But due to the undeveloped economic and medical care system, combined with the limited knowledge of TAO, most patients were misdiagnosis or missed diagnosis, and also, it covers a vast geography in the Xinjiang region, and it is sometimes far more difficult to find such large samples for replication. Second, TAO as an autoimmune inflammatory disease results in complex factors. We could not assess the risk according to stratification of behaviors (e.g., smoking, type of cigarette, the amount and frequency of smoking, cotinine level), environmental factors (weather, cold exposure, etc.), and other risk factors (age, sex, etc.) of TAO. In addition, this still needs further large-scale researches with more detailed individual data, with a different behavior- and environment-combined background warranted to further validate gene–gene, gene–behavior, and gene–environment interactions on gene and TAO risk. In addition, we still have a long way to go.
Conclusions
In conclusion, TAO is the most common peripheral vascular disease in Xinjiang. This research mainly focuses on the preliminary screening of the TAO susceptibility genes and sets a theoretical basis for validating biological function of these SNPs for the next step. Our data identified for the first time the relationship between TAO and three SNPs in Xinjiang Uyghur. They may serve as risk factors of this disease in the Xinjiang Uyghur population. Further studies may also reveal the role of these polymorphisms in TAO.
Acknowledgments
This work was supported by the Xinjiang Nature Science Foundation-funded project no. 2012211A089. We thank all the participants for their cooperation in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams RH, Betz H, Puschel AW. (1996) A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev 57:33–45 [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie MH, et al. (2003) Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278:1910–1914 [DOI] [PubMed] [Google Scholar]
- Allen WH, Gaffen SL. (2010) IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol 32:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JR, Castilho RM, Williams VP, Gutkind JS. (2006) Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A 103:9017–9022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne K, Wang W, Liu RQ, et al. (2012) Transmembrane semaphorin5B is proteolytically processed into a repulsive neural guidance cue. J Neurochem 123:135–146 [DOI] [PubMed] [Google Scholar]
- Chang SH, Park H, Dong C. (2006) Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem 281:35603–35607 [DOI] [PubMed] [Google Scholar]
- Chang SH, Reynolds JM, Pappu BP, et al. (2011) Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity 35:611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Herndon ME, Lawler J. (2000) The cell biology of thrombospondin-1. Matrix Biol 19:597–614 [DOI] [PubMed] [Google Scholar]
- Chen Z, Nakajima T, Inoue Y, et al. (2011) A single nucleotide polymorphism in the 3′-untranslated region of MyD88 gene is associated with Buerger disease but not with Takayasu arteritis in Japanese. J Hum Genet 56:545–547 [DOI] [PubMed] [Google Scholar]
- Chen Z, Takahashi M, Naruse T, et al. (2007) Synergistic contribution of CD14 and HLA loci in the susceptibility to Buerger disease. Hum Genet 122:367–372 [DOI] [PubMed] [Google Scholar]
- Claudio E, Sønder SU, Saret S, et al. (2009) The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol 182:1617–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaucoudrey L, Puel A, Filipe-Santos O, et al. (2008) Mutations in STAT3 and IL12RB1 impair the development of humanIL-17-producing T cells. J Exp Med 205:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborah AS, Donny LFC, Wilson SC. (2000) Interleukin-1β and tumor necrosis factor-α decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res 86:1259–1265 [DOI] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. (1997) Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron 19:539–545 [DOI] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, et al. (2007) Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem 282:26294–26305 [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, et al. (2005) Interleukin 17-producing CD4_ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132 [DOI] [PubMed] [Google Scholar]
- Juergens JL, Spittell JA, Jr, Fairbairn JF II, et al. (1980) Thromboangiitis obliterans (Buerger's disease TAO). In: Peripheral Vascular Diseases; WB Saunders Co, Philadelphia, PA, pp. 467–491 [Google Scholar]
- Kessler O, Shraga-Heled N, Lange T, et al. (2004) Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res 64:1008–1015 [DOI] [PubMed] [Google Scholar]
- Kinare SG, Kher YR, Rao G, et al. (1976) Pattern of occlusive peripheral cardiovascular disease in India. Angiology 27:165–180 [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. (2004) Interleukin-17 family members and inflammation (review). Immunity 21:467–476 [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, et al. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett RL, Wang W, O'Connor TP. (2009) Semaphorin 5B is a novel inhibitory cue for corticofugal axons. Cereb Cortex 19:1408–1421 [DOI] [PubMed] [Google Scholar]
- Matsushita M, Shionoya S, Matsumoto T. (1992) Urinary cotinine measurement in patients with Buerger's disease: effects of active and passive smoking on the disease process. J Vasc Surg 14:53–58 [PubMed] [Google Scholar]
- McKusick VA, Harris WS. (1961) Buerger's syndrome in the Orient. Bull Johns Hopkins Hosp 109:241–291 [Google Scholar]
- McLoughlin GA, Helsby CR, Evans CC. (1976) Association of HLA-A9 and HLA-B5 with Buerger's disease. Br Med J 2:1165–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JL, Porter JM. (1991) Buerger's disease (thromboangiitis obliterans). Ann Vasc Surg 5:570–572 [DOI] [PubMed] [Google Scholar]
- Nagase T, Kikuno R, Ishikawa K, et al. (2000) Prediction of the coding sequences of unidentified human genes. XVII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 7:143–150 [DOI] [PubMed] [Google Scholar]
- Nielubowicz J, Rosnowski A, Pruszynski B, et al. (1980) Natural history of Buerger's disease. J Cardiovasc Surg 21:529–540 [PubMed] [Google Scholar]
- Olin JW. (2000) Thromboangiitis obliterans (Buerger's disease). N Engl J Med 343:864–869 [DOI] [PubMed] [Google Scholar]
- Ostojic L, Zelenika D, Zotz RB, et al. (2007) Platelet receptor HPA-1 polymorphism of αIIbbβ3 and 807 C/T polymorphism of α2β1 and Buerger's disease. Angiology 58:169–174 [DOI] [PubMed] [Google Scholar]
- Papa MZ, Adar T. (1992) A critical look at thromboangiitis obliterans (Buerger's disease). Perspect Vasc Surg 5:1–21 [Google Scholar]
- Park H, Li ZX, Yang XO, et al. (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya S. (1990) Etiology, pathology, pathophysiology, clinical manifestation, diagnosis, Buerger's disease. In: Pathology, Diagnosis and Treatment.University of Nagoya Press, Nagoya (Japan), pp. 38–198 [Google Scholar]
- Smolen JS, Youngchaiyud U, Weidinger P. (1978) Autoimmunological aspects of thromboangiitis obliterans (Buerger's disease). Clin Immunol Immunopathol 11:168–177 [DOI] [PubMed] [Google Scholar]