Abstract
Full-length (42 kDa) CCAAT/enhancer binding protein alpha (C/EBP alpha) (p42) has been implicated in the transcriptional activation of adipocyte genes including the 422(aP2) and C/EBP alpha genes during differentiation of 3T3-L1 preadipocytes. We have identified a 30-kDa isoform (p30) of C/EBP alpha that is expressed by 3T3-L1 adipocytes, mouse adipose tissue, and rat liver. In vitro translation of wild-type C/EBP alpha mRNA or transient transfection with a wild-type C/EBP alpha vector gave rise to similar levels of p42 and p30. Mutational analysis revealed that p30 is an alternative translation product initiated at the third in-frame methionine codon of the C/EBP alpha message. p30C/EBP alpha binds to the C/EBP sites within and activates reporter gene expression driven by the 422(aP2) and C/EBP alpha gene promoters. Although transfection of 3T3-L1 preadipocytes with a strong p30C/EBP alpha expression vector is insufficient to induce differentiation, this vector advances the differentiation program. Unlike p42C/EBP alpha, which inhibits cell proliferation, p30C/EBP alpha is not antimitotic. Thus, the N-terminal 12-kDa segment of full-length C/EBP alpha contains an amino acid sequence necessary for antimitotic activity. During differentiation of 3T3-L1 preadipocytes and during hepatocyte development, the cellular p42C/EBP alpha/p30C/EBP alpha ratio changes, raising the possibility of a regulatory role.
Full text
PDF
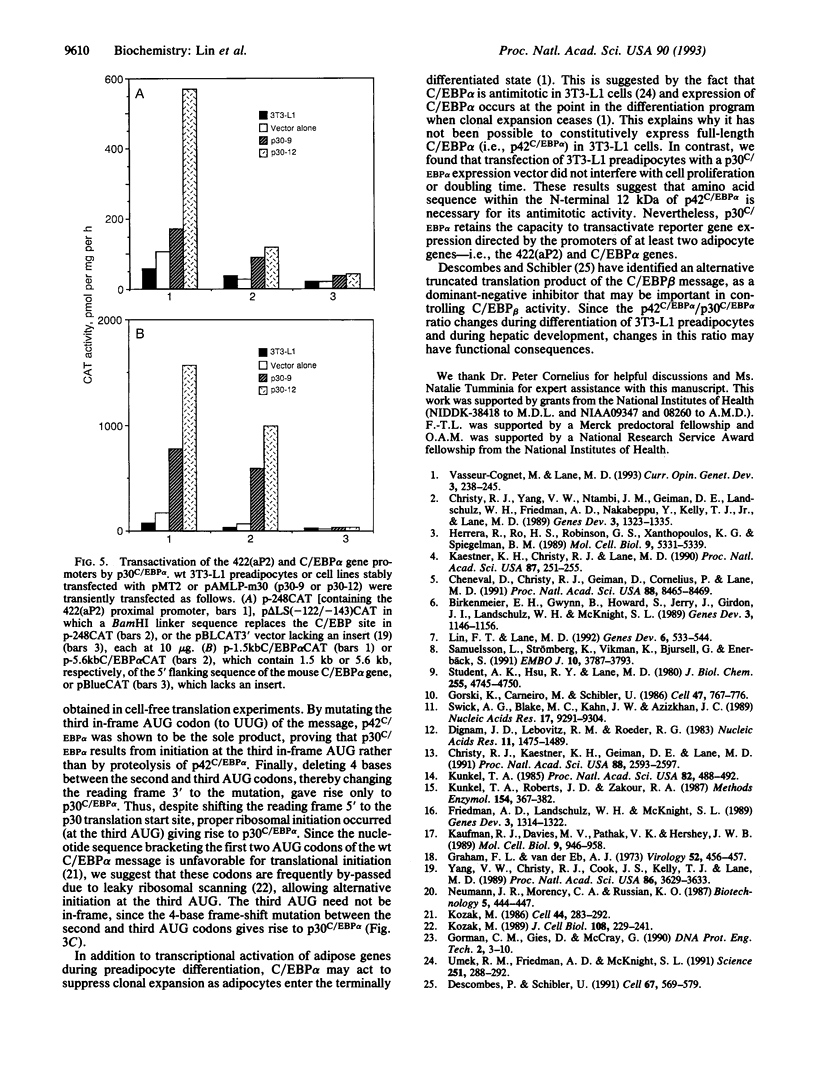
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Cheneval D., Christy R. J., Geiman D., Cornelius P., Lane M. D. Cell-free transcription directed by the 422 adipose P2 gene promoter: activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8465–8469. doi: 10.1073/pnas.88.19.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Kaestner K. H., Geiman D. E., Lane M. D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Yang V. W., Ntambi J. M., Geiman D. E., Landschulz W. H., Friedman A. D., Nakabeppu Y., Kelly T. J., Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989 Sep;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., Lane M. D. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jan;87(1):251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lin F. T., Lane M. D. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992 Apr;6(4):533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Samuelsson L., Strömberg K., Vikman K., Bjursell G., Enerbäck S. The CCAAT/enhancer binding protein and its role in adipocyte differentiation: evidence for direct involvement in terminal adipocyte development. EMBO J. 1991 Dec;10(12):3787–3793. doi: 10.1002/j.1460-2075.1991.tb04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Swick A. G., Blake M. C., Kahn J. W., Azizkhan J. C. Functional analysis of GC element binding and transcription in the hamster dihydrofolate reductase gene promoter. Nucleic Acids Res. 1989 Nov 25;17(22):9291–9304. doi: 10.1093/nar/17.22.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Vasseur-Cognet M., Lane M. D. Trans-acting factors involved in adipogenic differentiation. Curr Opin Genet Dev. 1993 Apr;3(2):238–245. doi: 10.1016/0959-437x(93)90029-o. [DOI] [PubMed] [Google Scholar]
- Yang V. W., Christy R. J., Cook J. S., Kelly T. J., Lane M. D. Mechanism of regulation of the 422(aP2) gene by cAMP during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1989 May;86(10):3629–3633. doi: 10.1073/pnas.86.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]