Abstract
Post-mortem studies reveal a high rate of cavum septi pellucidi (CSP) in chronic traumatic encephalopathy (CTE). It remains, however, to be determined whether or not the presence of CSP may be a potential in vivo imaging marker in populations at high risk to develop CTE. The aim of this study was to evaluate CSP in former professional American football players presenting with cognitive and behavioral symptoms compared with noncontact sports athletes. Seventy-two symptomatic former professional football players (mean age 54.53 years, standard deviation [SD] 7.97) as well as 14 former professional noncontact sports athletes (mean age 57.14 years, SD 7.35) underwent high-resolution structural 3T magnetic resonance imaging. Two raters independently evaluated the CSP, and interrater reliability was calculated. Within National Football League players, an association of CSP measures with cognitive and behavioral functioning was evaluated using a multivariate mixed effects model. The measurements of the two raters were highly correlated (CSP length: rho = 0.98; Intraclass Correlation Coefficient [ICC] 0.99; p < 0.0001; septum length: rho = 0.93; ICC 0.96; p < 0.0001). For presence versus absence of CSP, there was high agreement (Cohen kappa = 0.83, p < 0.0001). A higher rate of CSP, a greater length of CSP, as well as a greater ratio of CSP length to septum length was found in symptomatic former professional football players compared with athlete controls. In addition, a greater length of CSP was associated with decreased performance on a list learning task (Neuropsychological Assessment Battery List A Immediate Recall, p = 0.04) and decreased test scores on a measure of estimate verbal intelligence (Wide Range Achievement Test Fourth Edition Reading Test, p = 0.02). Given the high prevalence of CSP in neuropathologically confirmed CTE in addition to the results of this study, CSP may serve as a potential early in vivo imaging marker to identify those at high risk for CTE. Future research is needed to investigate the pathomechanism underlying the development of CSP after repetitive head impacts, and its potential association with neuropathologically confirmed CTE.
Key words: : biomarkers, head trauma, MRI, neurodegenerative disorders, traumatic brain injury
Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease with widespread accumulation of hyperphosphorylated tau (p-tau) and regional brain atrophy.1,2 To date, CTE can only be diagnosed post-mortem.1–3 Evidence from a selected case series of those with neuropathologically confirmed CTE suggests that the symptoms of CTE include changes in cognition (e.g., memory, executive dysfunction), mood (e.g., depression, apathy, suicidality), and behavior (e.g., impulsivity, aggression).4,5
CTE has been most often observed in professional contact sports athletes (e.g., boxing, American football6) with a history of repetitive head impacts, including mild traumatic brain injuries (TBIs) or even asymptomatic, repetitive subconcussive brain trauma.3,7 Moreover, CTE has been found in nonathletes who have experienced repetitive head impacts, including military service members,2,8,9 persons with medical conditions such as epilepsy and autism, and victims of physical abuse.2,10
All patients with neuropathologically confirmed CTE reported to date have had a history of brain trauma, typically from repetitive mild or subconcussive head impacts. This suggests that this head impact exposure is a necessary, but not sufficient, condition for the initiation of the pathogenetic cascade that eventually leads to neurodegeneration.3,7
The incidence and prevalence of CTE are unknown, although the number of those who may be afflicted may be large, because each year between 1.6 and 3.8 million persons experience a sports-related mild TBI in the United States.11,12 The incidence of repetitive subconcussive blows is even higher.13
One of the main problems in diagnosing CTE in vivo is the current lack of clinical biomarkers.14,15 A noninvasive imaging biomarker may, however, make it possible to diagnose CTE during life, which could pave the way for developing therapeutic interventions to prevent further neurodegenerative changes and/or to selectively treat the symptoms of this disease.
In addition to the p-tau aggregation and regional brain atrophy, post-mortem studies also reveal a high rate of cavum septi pellucidi (CSP) in CTE: the greater severity of neuropathological findings of CTE, the more often CSP was observed.2 In addition, a review article in 2013 by Smith and associates16 reported a CSP to be present in 64 of 99 (65%) cases of neuropathologically confirmed CTE. The CSP is formed by the two leaves of the septum pellucidum (lateral), the corpus callosum (CC) (superior), and the fornix (posterior).17 The CSP typically closes during brain development, but it can also persist. The overall prevalence of CSP in the population varies greatly in the literature, depending on the cohort and technique used.18,19 Moreover, a higher prevalence of CSP has been linked to neurodevelopmental diseases such as schizophrenia20–23 as well as to genetic conditions.24
Increased rates of CSP have also been reported among boxers and participants of other contact-sports exposed to repetitive head impacts.15,25 In addition, recent studies suggest a possible causal relationship between repetitive head impacts and CSP. Aviv and colleagues,26 for example, studied 164 active boxers who underwent annual magnetic resonance imaging (MRI) for a boxing board license renewal. CSP was present in 49% of boxers but, most noteworthy, in 8 boxers who did not exhibit CSP on their first scan, CSP was present on subsequent scans. Moreover, three boxers showed an increasing extent of CSP in follow-up scans.
It remains to be determined, however, whether or not CSP may be a potential in vivo imaging marker in populations at high risk for development of CTE. The aims of this study are (1) to characterize neuroimaging features of CSP in former professional American football players who present with cognitive, mood, and behavioral symptoms compared with asymptomatic noncontact sports athletes, and (2) to evaluate the association between CSP and cognitive and behavioral functioning in former professional American football players who present with cognitive, mood, and behavioral symptoms.
Methods
This study is part of the Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT) study, funded by the National Institutes of Health, to develop biomarkers for the in vivo diagnosis of CTE. Study participants underwent extensive testing, which included neuroimaging, neuropsychological testing, interview on medical history, neurological and psychiatric evaluation, as well as genetic testing and lumbar puncture for cerebrospinal fluid (CSF) protein analysis.
Recruitment for DETECT was started in November 2011 by means of e-mails to a mailing list of the National Football League (NFL) Players Association and NFL Alumni Associations, and also a post on the Boston University CTE Center website, presentations held at NFL alumni meetings, and by word of mouth. Details of the study protocol can also be found elsewhere.27,28 The study protocol was approved by the Boston University Medical Center Institutional Review Board, and by the Partners Institutional Review Board. Written informed consent was obtained from all study participants before enrollment into the study.
Participants
The DETECT inclusion criteria for former NFL players were: male, age 40–69 years, English as primary language, more than 12 years of organized football experience, including a minimum of 2 years in the NFL, and a self-report of mood, cognitive, and behavioral symptoms ongoing for at least 6 months before enrollment. Athletes with contraindication for MRI or lumbar puncture, as well as other diagnosed diseases of the central nervous system, were excluded from the study. At the time of this study, 74 former NFL players were included in DETECT, and 2 had no imaging data. Thus, a total of 72 imaging data sets of former NFL players were included in this study.
The DETECT inclusion criteria for controls were: male, age 40–69 years, and English as the primary language. In addition, healthy controls had to have participated in organized noncontact sports for at least 4 years, of which 2 years had to have been at the college level or beyond. Control subjects were excluded if they had ever served in the military or had ever participated in any of the following organized sports: football, hockey, rugby, soccer, lacrosse, wrestling, boxing, basketball, gymnastics, martial arts, kickboxing, cycling, or distance running. Medical conditions that led to the exclusion of controls included self-reported history of concussion,29 diagnosis of dementia, or medication that would make lumbar puncture unsafe.
Evaluation of mood, behavior, and cognition
All subjects were administered the following tests: Neuropsychological Assessment Battery (NAB) List Learning; NAB Map Reading; NAB Naming, Rey-Osterrieth Complex Figure (scored using the Boston Qualitative Scoring System); Trailmaking Test, Parts A and B; Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Symbol; Wide Range Achievement Test Fourth Edition (WRAT-4) Reading Test; and the Wisconsin Card Sort Test (WCST). The Hamilton Depression Rating Scale (HAM-D), Brown-Goodwin Lifetime History of Aggression, Barratt Impulsivity Scale (BIS), and Modified Scale for Suicidal Ideation (MSSI) are assessed as part of the psychiatric interview, while the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A), Beck Depression Inventory (BDI), Beck Hopelessness Inventory (BHI), and Buss-Durkee Hostility Inventory (BDHI) were self-completed by the subject in paper form.
Data acquisition
All subjects underwent MRI at Brigham and Women's Hospital using a 3T scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) equipped with a 32-channel head coil. A T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence was acquired with the following sequence parameters: TE = 3.36 msec, TR = 1800 msec, inversion time 1100 msec, flip angle 7 degrees, acquisition matrix of 256 × 256 × 176 voxels with a voxel size of 1 × 1 × 1 mm.
Of the initial 15 controls screened, one control was excluded because of motion artifacts, resulting in 14 controls included in this study (Table 1).
Table 1.
Demographic Data of Study Participants
| NFL players | Controls | t test | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p value | |
| Age (y) | 54.53 (8.03) | 57.14 (7.62) | 0.264 |
| BMI | 32.04 (4.47) | 28.49 (3.60) | 0.007 |
| Years of education | 16.39 (0.87) | 17.64 (2.10) | 0.045* |
| Years of football total | 18.11 (3.49) | ||
| Years in NFL | 7.80 (2.67) | ||
| History of concussion** | 446.9 (1077.4) | 0.5 (1.53) | 0.001* |
SD, standard deviation; BMI, body mass index; NFL, National Football League.
Satterthwaite correction for unequal variance.
Based on self-report after being given a modern definition of concussion using the methods described by Robbins and associates.28
Post-processing
Data sets were visually inspected for distortion and motion artifacts. All images were reoriented using 3D Slicer version 3.6 (Surgical Planning Laboratory, Brigham and Women's Hospital, Boston, MA) after visually identifying the anterior and posterior commissure and the midsagittal plane as landmarks.
Rating of CSP
CSP was defined as CSF being visible between two leaflets of the septum pellucidum on coronal slices of the T1-weighted MRI. The length of the cavum was determined by counting the number of coronal slices where CSP was visible and then multiplying the number of slices by the slice thickness of 1 mm to calculate the overall length of the CSP.23,26 Raters also evaluated the presence and length of cavum vergae, a posterior variant of CSP. The average amount of time to obtain all measurements was approximately 3 min per data set.
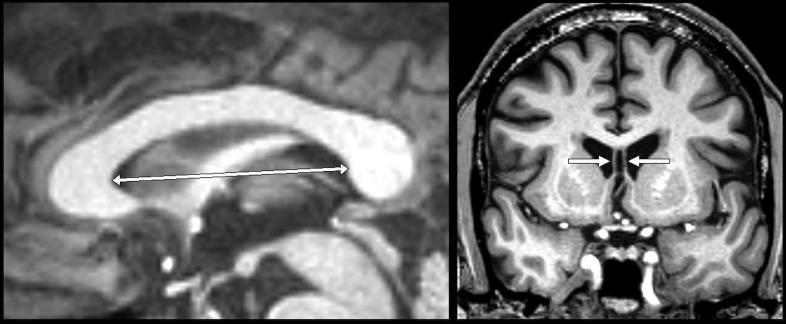
To account for possible bias of individual head size and ventricular enlargement, the length of the entire septum pellucidum was measured. Septum length was measured in 3D Slicer version 4.3.0 (Surgical Planning Laboratory) by visually defining the borders of the septum at the genu and splenium of the CC on the midsagittal plane, and measuring the longest distance between these two (Fig. 1). These landmarks were selected because the development of the CC is closely associated with the development of the septum pellucidum.30 To adjust for individual septum length, the ratio of CSP length to septum length was calculated. Ratings of CSP length and septal length were performed in random order by two different raters for each subject after both agreed on the definitions and procedures described above. Both raters were blinded to the subjects' group and to the rating of the other rater.
FIG. 1.
Left: Illustration of measurement of septum length on a T1-weighted magnetic resonance image in the midsagittal plane; Right: Example of cavum septi pellucidi visible on a coronal slice.
Statistical analyses
Statistical analyses were performed using SAS (Version 9.4). Results with a p value below 0.05 are reported as statistically significant. Interrater reliability was tested using the Intraclass Correlation Coefficient (ICC) of the two raters for continuous outcomes and the Cohen kappa for binary outcomes, and a paired t test for the difference between raters. Mean measurement discrepancies between raters by group (NFL-Control) were tested using analysis of variance (ANOVA), as well as for potential association with body mass index (BMI) and age using simple linear regression. A group comparison between the NFL players and controls was performed using a Wilcoxon two-sample test.
For estimating the effect of CSP on neuropsychological scores, the group of former professional NFL players was divided in those with CSP ≤2 mm and those with CSP ≥6 mm based on the literature.31 Multivariate linear mixed effects models were run using the vector of neuropsychological scores from each subject as the dependent variable, and CSP as the main predictor of interest adjusted for BMI, age, years of education, and years playing football. Hence, we model two levels of correlations in our outcomes: (1) correlations of all test scores from the same subjects, and (2) correlations of all scores from the same domain (e.g., Trailmaking A and Digit Symbol in the Attention and Psychomotor Speed Domain).
Results
Demographical data, cognitive and behavioral functioning
Information on demographics of former professional football players and controls is summarized in Table 1. Of the 14 controls, 11 (79%) reported swimming as their primary sport, 2 (14%) reported baseball, and 1 (7%) reported rowing.
Cognitive and behavioral functioning
Former professional NFL players demonstrated significantly lower outcome scores in most tests of cognitive functioning and higher scores in behavioral evaluations. Table 2 summarizes test scores of cognitive and behavioral evaluation of both groups.
Table 2.
Test Scores of Cognitive and Behavioral Evaluation
| Outcome measures | Control (n = 14) | NFL (n = 72) | |||
|---|---|---|---|---|---|
| Scale | Mean | SD | Mean | SD | P value |
| BRI | 45.57 | 9.05 | 61.85 | 12.07 | <0.0001 |
| Baratt Total | 49.73 | 8.27 | 64.60 | 14.28 | <0.0001 |
| Beck-Depression | 2.36 | 3.86 | 15.18 | 11.50 | <0.0001 |
| Beck-Hopelessnes | 1.21 | 2.67 | 4.39 | 5.13 | 0.0017 |
| Brown-Goodwin Adult Sum | 12.14 | 1.35 | 16.48 | 3.29 | <0.0001 |
| Brown-Goodwin Max Aggression Scale | 12.93 | 1.94 | 18.27 | 4.54 | <0.0001 |
| Buss-Durkee total | 16.57 | 9.52 | 33.44 | 13.09 | <0.0001 |
| Hamilton Total | 0.93 | 1.86 | 8.26 | 7.28 | <0.0001 |
| List A Long Delay | 48.36 | 12.43 | 41.86 | 14.43 | 0.1196 |
| List A Short Delay | 51.50 | 11.02 | 45.00 | 13.28 | 0.0895 |
| List A(1 + 2 + 3)Immediate Recall | 51.21 | 6.28 | 44.04 | 9.22 | 0.0067 |
| List B Immediate Recall | 51.93 | 8.90 | 46.31 | 10.43 | 0.0627 |
| MSSI | 0.00 | 0.00 | 0.55 | 2.58 | 0.0808 |
| Meta-Cognition Index | 47.57 | 11.29 | 62.69 | 13.20 | 0.0001 |
| NAB Map Reading | 52.79 | 6.00 | 46.09 | 10.15 | 0.0023 |
| NAB naming | 51.43 | 5.37 | 44.46 | 11.38 | 0.0011 |
| ROCF Delayed Presence & Accuracy | 55.71 | 7.59 | 48.36 | 11.53 | 0.0248 |
| ROCF Immediate Presence & Accuracy | 54.93 | 7.21 | 47.61 | 10.24 | 0.0126 |
| ROCF Organization | 50.50 | 7.18 | 42.61 | 16.08 | 0.0055 |
| Trailmaking A | 52.64 | 8.79 | 48.89 | 12.02 | 0.2705 |
| Trailmaking B | 50.07 | 16.42 | 44.24 | 16.33 | 0.2268 |
| WAIS-R Digit Symbol | 52.14 | 9.80 | 46.97 | 9.41 | 0.0655 |
| WRAT-4 Reading | 111.93 | 14.69 | 98.07 | 10.70 | <0.0001 |
| Wisconsin Percent Errors | 44.57 | 10.63 | 39.96 | 9.84 | 0.1192 |
SD, standard deviation; BRI, Behavioral Regulation Index; MSSI, Modified Scale for Suicidal Ideation; NAB, Neuropsychological Assessment Battery; ROCF, Rey-Osterrieth Complex Figure; WAIS-R, Wechsler Adult Intelligence Scale-Revised; WRAT-4, Wide Range Achievement Test Fourth Edition.
Interrater reliability and bias analysis of CSP
The measurements of the two raters were highly correlated (CSP length, rho = 0.98; ICC 0.99; p < 0.0001; septum length, rho = 0.93; ICC 0.96; p < 0.0001; CSP length to septum length ratio, rho = 0.98; ICC 0.97; p < 0.0001). Measurements of septum length by rater 1 were on average significantly higher than those by rater 2 (average difference of measurements 0.6 mm; SD = 1.8). The mean measurement discrepancies between raters, however, were not significantly different between groups (t test = 0.53; p = 0.5992) and showed no statistically significant correlation with BMI (t test = −1.18; p = 0.2424) or age (t test = 0.83; p = 0.4114) (Table 3). Therefore, all further analyses were calculated based on the mean of the two raters' measurements.
Table 3.
Length of Septum Pellucidum and Cavum Septi Pellucidi and Interrater Reliability
| Rater 1 | Rater 2 | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean of raters (SD) | |||||
| NFL n = 72 | Controls n = 14 | NFL n = 72 | Controls n = 14 | Correlation of ratings | NFL | Controls | |
| CSP present (N [%]) | 65 (90%) | 8 (57%) | 64 (89%) | 7 (50%) | Kappa = 0.83 p < 0.0001 |
66 92% | 8 (57%) |
| Septum length (mm) | 53.4 (4.6) | 55.7 (5.2) | 52.9 (4.7) | 54.8 (5.0) | rho = 0.93 p < 0.0001 |
53.2 (4.6) | 55.3 (5.1) |
| CSP length (mm) | 7.75 (7.7) | 4.3 (6.2) | 7.7 (8.0) | 4.5 (6.9) | rho = 0.98 p < 0.0001 |
7.7 (7.8) | 4.4 (6.8) |
| Ratio CSP/septum length |
0.15 (0.14) | 0.08 (0.1) | 0.15 (0.15) | 0.08 (0.13) | rho = 0.98 p = 0.0001 |
0.15 (0.14) | 0.08 (0.12) |
SD, standard deviation; NFL, National Football League; CSP, cavum septi pellucidi.
Length of septum pellucidum
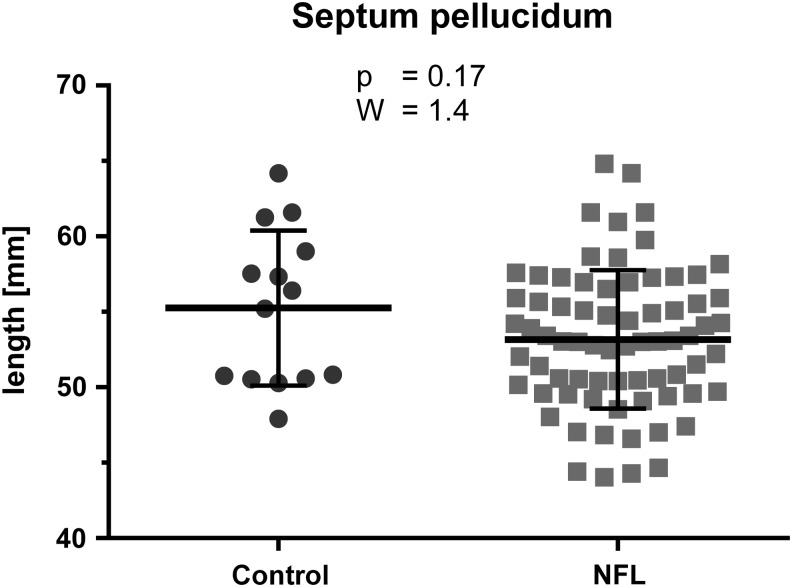
Length of the septum pellucidum was not significantly different (W = 1.40; p = 0.17) between NFL players (mean = 53.2 mm; SD = 4.6) and controls (mean = 55.3 mm; SD = 5.1) (Fig. 2).
FIG. 2.
Results of group comparison of the length of the septum pellucidum: Wilcoxon two-sample test of National Football League (NFL) players (mean = 53.2 mm; standard deviation [SD] = 4.6) and controls (mean = 55.3 mm; SD = 5.1); No significant difference was found (W = 1.40; p = 0.17);
Presence and length of CSP
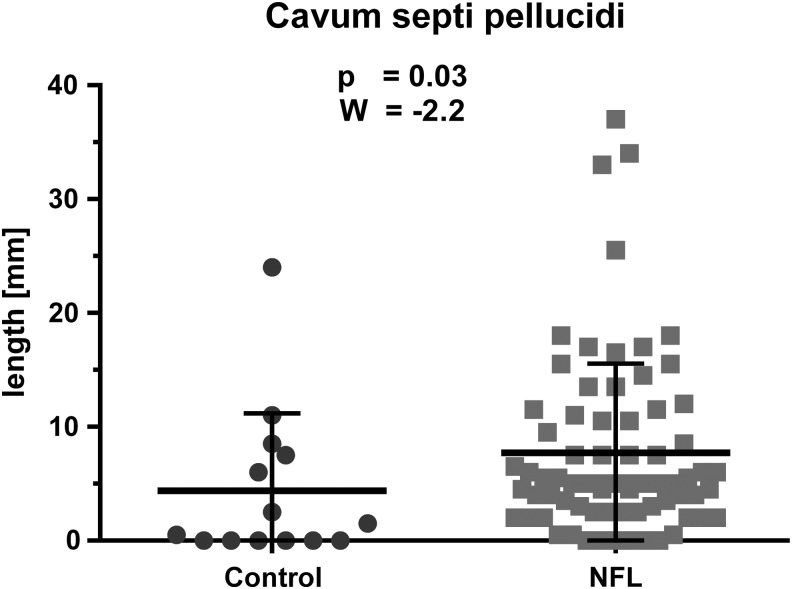
CSP was present in 66 (92%) football players and in 8 (57%) controls (p = 0.0006). Length of the CSP was significantly (W = −2.2; p = 0.03) greater in NFL players (mean = 7.7 mm; SD = 7.8) than in controls (mean = 4.4 mm; SD = 6.8). A threshold of 2 mm had a sensitivity of 87.5%, specificity of 57%, positive predictive value (PPV) of 91.3%, and a negative predictive value (NPV) of 47.1% (Fig. 3).
FIG. 3.
Results of group comparison of the length of the cavum septi pellucidi (CSP): Wilcoxon two-sample test of National Football League (NFL) players (mean = 7.7 mm; SD = 7.8) and controls (mean = 4.4 mm; SD = 6.8); length of the CSP was significantly (W = −2.2; p = 0.03) greater in NFL players.
Ratio of length of CSP and length of septum pellucidum
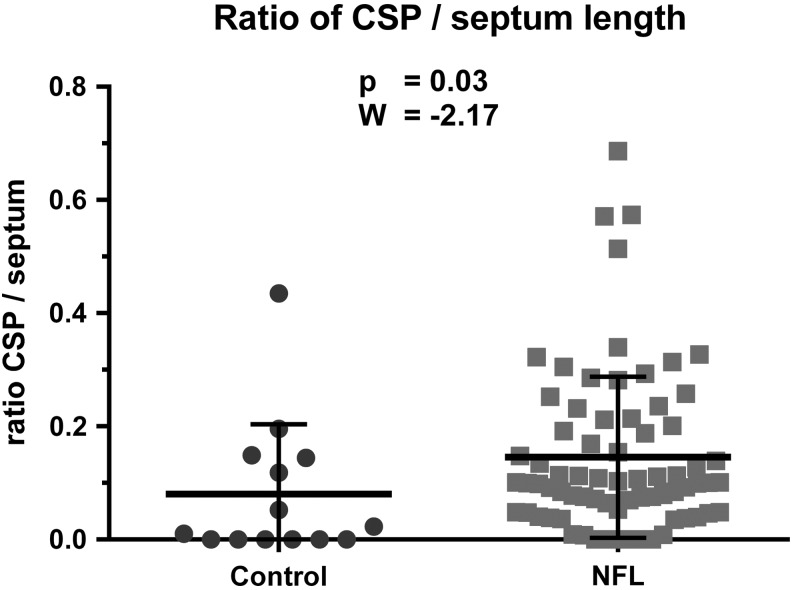
The ratio of CSP length and septum length was significantly higher (W = −2.17; p = 0.03) in football players (mean = 0.15; SD = 0.14) than in controls (mean = 0.08; SD = 0.12) (Fig. 4).
FIG. 4.
Results of group comparison of the ratio of cavum septi pellucidi (CSP) length to septum length: Wilcoxon two-sample test of National Football League (NFL) players (mean = 0.15; SD = 0.14) and controls (mean = 0.08; SD = 0.12); ratio of CSP/septum length was significantly (W = −2.17; p = 0.03) higher in NFL players.
Association of CSP with cognitive and behavioral measures
Table 4 summarizes the results of the association between cognitive and behavioral measures with length of CSP within the group of former professional NFL players. A CSP of 6 mm and greater (n = 35) was associated with lower scores on NAB List Learning List A Immediate Recall and lower scores on WRAT-4 Reading compared with players with CSP of 2 mm and smaller (n = 23). (Note: the remaining 14 patients had a CSP >2 mm and <6 mm.)
Table 4.
Association Between Cognitive and Behavioral Measures with Length of Cavum Septi Pellucidi in Former National Football League Players
| Outcome measures | Difference ≤2 (n = 23) ≥6 (n = 35) | Standard error | t value | p value |
|---|---|---|---|---|
| BRI | 2.1797 | 3.4929 | 0.62 | 0.5328 |
| Baratt Total | 0.07251 | 4.2767 | 0.02 | 0.9865 |
| Beck-Depression | 2.2415 | 3.2237 | 0.7 | 0.487 |
| Beck-Hopelessnes | 1.5988 | 1.5995 | 1 | 0.3178 |
| Brown-Goodwin Adult Sum | −0.02043 | 0.9933 | −0.02 | 0.9836 |
| Brown-Goodwin Max Aggression S | 0.07394 | 1.4843 | 0.05 | 0.9603 |
| Buss-Durkee total | −0.152 | 4.1246 | −0.04 | 0.9706 |
| Hamilton Total | 2.0301 | 2.0197 | 1.01 | 0.3151 |
| List A Long Delay | −5.1681 | 4.5248 | −1.14 | 0.2537 |
| List A Short Delay | −2.0992 | 4.1832 | −0.5 | 0.6159 |
| List A(1+2+3)Immediate Recall | −5.6609 | 2.8171 | −2.01 | 0.0448 |
| List B Immediate Recall | −4.2249 | 3.2249 | −1.31 | 0.1905 |
| MSSI | 0.6676 | 1.028 | 0.65 | 0.5162 |
| Meta-Cognition Index | 3.7021 | 3.7869 | 0.98 | 0.3285 |
| NAB Map Reading | −2.9307 | 3.333 | −0.88 | 0.3795 |
| NAB naming | −0.9737 | 3.6733 | −0.27 | 0.791 |
| ROCF Delayed Presence & Accura | −2.0983 | 3.3465 | −0.63 | 0.5308 |
| ROCF Immediate Presence & Accu | −0.5896 | 3.0608 | −0.19 | 0.8473 |
| ROCF Organization | 2.0236 | 5.543 | 0.37 | 0.7151 |
| Trails A | 1.2368 | 3.9888 | 0.31 | 0.7566 |
| Trails B | 0.4247 | 5.0576 | 0.08 | 0.9331 |
| WAIS-R Digit Symbol | −2.4132 | 2.7587 | −0.87 | 0.3819 |
| WRAT-4 Reading | −7.8474 | 3.4267 | −2.29 | 0.0222 |
| Wisconsin Percent Errors | −3.9538 | 2.8514 | −1.39 | 0.1659 |
Bolded terms indicate statistically significant findings.
BRI, Behavioral Regulation Index; MSSI, Modified Scale for Suicidal Ideation; NAB, Neuropsychological Assessment Battery; ROCF, Rey-Osterrieth Complex Figure; WAIS-R, Wechsler Adult Intelligence Scale-Revised; WRAT-4, Wide Range Achievement Test Fourth Edition.
Discussion
This study reports a higher rate of CSP, a greater length of CSP, and a greater ratio of CSP length versus septum length in symptomatic former professional American football players compared with same age noncontact sport athlete controls. Within the group of symptomatic former professional NFL players, a CSP of 6 mm and greater was associated with lower scores on measures of verbal learning and word pronunciation. In the clinical setting, a CSP of 6 mm and greater may provide valuable information regarding the risk for having or developing CTE, although we note that CTE can only be diagnosed post-mortem at this time.
Ninety-two percent of the former professional football players showed presence of CSP. High rates of CSP have been reported in neuropathologically confirmed cases of CTE2,32 as well as in living contact sports athletes with a history of exposure to repetitive head impacts (CSP in 43%),25 and in boxers in particular (CSP in 57%).33 High rates of CSP have also been reported in post-mortem studies (CSP in 69% of cadavers with history of head trauma)34 and in vivo studies of persons with exposure to brain trauma (67% of those with CSP had history of head trauma).35
CSP was found in 54% of victims of fatal road accidents compared with 38% in a control group. This study also reported an association between severity of axonal injury and shape of CSP.36 CSP was found to be larger in 98 children with a history of TBI compared with a control group.37 Of note, that study also reported a correlation between CSP volume and atrophy of the right entorhinal cortex and the bilateral hippocampi, structures that are also reported to be affected in CTE.2,38
Rates of CSP, in both former professional NFL players and controls, were higher in this study compared with most previous studies. Hagino and coworkers,39 however, similar to our study, counted the coronal slices in which CSP was present and reported a prevalence of 74.7% in a control group of 79 subjects. In our control group, CSP was present in 57%, which is well within the broad range previously described in the literature. Further, because we used high-resolution MRI with slice thickness of 1 mm, our method was more sensitive in detecting CSP than most of previously published imaging studies. Finally, we note, as above, that presence versus absence of CSP was not as important in distinguishing between groups as was the number of slices involved, or length of the CSP.
A study by Aviv and colleagues26 investigated longitudinal brain changes in 79 boxers scanned for annual boxing license renewal. They reported the formation of CSP in eight athletes over the time. In addition, three boxers showed an increased volume of CSP over time. Both findings support the hypothesis of a causative association between repetitive head impacts and CSP. The same study reports only a trend toward a higher CSP prevalence in boxers (CSP in 49% versus in 40% in controls; p = 0.099). One explanation for a missing significant group difference is that Aviv and associates26 classified the CSP in three categories: (1) anterior to the fornix; (2) extending up to the fornix; (3) extending into the cavum vergae) instead of measuring the absolute length of CSP. Notably, none of their controls had a type 2 or 3 extension (p < 0.0009) in contrast to 30% and 16% prevalence in boxers.
Although links to both the immediate mechanical shearing injury as well as brain atrophy and ventricular enlargement after brain trauma have been reported,1,16 the exact pathomechanism of the development or enlargement of a CSP after brain trauma remains unknown. While CSP is also seen in patients with neurodevelopmental disorders,20–23 and is therefore not specific to CTE, we believe that in those who experience repetitive head impacts, CSP is likely the result of trauma, which is also thought to be one of the risk factors for development of CTE or other possible neurodegenerative disorders.
In this study, a CSP of 6 mm and greater was associated with lower test scores in a memory test and a test of word pronunciation within the group of NFL players. Longitudinal studies are, however, needed to determine whether a greater CSP length is merely a reflection of exposure to repetitive head trauma or may serve as an early indicator and biomarker to identify those at highest risk for development of CTE.
Limitations
This study is limited by the fact that CTE is a post-mortem diagnosis. Given the fact that CSP is evident at post-mortem examination, however, its presence in vivo is likely suggestive of those with presumed CTE. A further limitation is that the study does not include asymptomatic former NFL players or others believed to be at high risk for CTE because of significant exposure to repetitive head impacts as well as those with a single major TBI (and no history of additional repetitive head impacts). Future studies including such cohorts would make it possible to investigate the relationship among presence and length of CSP, history of brain trauma, and clinical correlates of CTE.
Conclusion
This study reports a higher prevalence of CSP, a greater length of CSP, and a greater ratio of CSP length to septum length, in symptomatic former professional football players compared with athlete controls. Greater length of CSP was also associated with lower verbal memory and word pronunciation. Given the high prevalence of CSP in neuropathologically confirmed CTE, CSP may serve as a potential early in vivo imaging marker to identify those at high risk for CTE. Future research is needed to investigate the pathomechanism underlying the development of CSP after repetitive head impacts and its potential association with neuropathologically confirmed CTE.
Acknowledgments
We thank all study participants for taking the time to contribute to our research. This study was supported by the NIH (R01 NS 078337; F31 NS 081957 (JMS); P30 AG13846; UL1-TR000157) and participant travel was funded by gifts from JetBlue Airlines, the National Football League (NFL), and the NFL Players Association. This study was also partly supported by the Else Kröner-Fresenius Foundation, Germany (IK), and by a VA Merit Award (MES, MC). This study was part of the doctoral thesis of Jakob Hufschmidt.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montenigro P.H., Corp D.T., Stein T.D., Cantu R.C., and Stern R.A. (2015). Chronic traumatic encephalopathy: historical origins and current perspective. Ann. Rev. Clin. Psychol. 11, 309–330 [DOI] [PubMed] [Google Scholar]
- 4.Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O., Fritts N.G., Stamm J.M., Robbins C.A., McHale L., Simkin I., Stein T.D., Alvarez V.E., Goldstein L.E., Budson A.E., Kowall N.W., Nowinski C.J., Cantu R.C., and McKee A.C. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montenigro P.H., Baugh C.M., Daneshvar D.H., Mez J., Budson A.E., Au R., Katz D.I., Cantu R.C., and Stern R.A. (2014). Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res. Ther. 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner R.C., Hess C.P., Brus-Ramer M.C., Possin K.C., Cohn-Sheehy B.I., Kramer J.H., Berger M., Yaffe K., Miller B., and Rabinovici G.D. (2015). Cavum septum pellucidum in retired American pro-football players. J. Neurotrauma. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., and Stern R.A. (2012). Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 6, 244–254 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A., Upreti C., Kracht J.M., Ericsson M., Wojnarowicz M.W., Goletiani C.J., Maglakelidze G.M., Casey N., Moncaster J.A., Minaeva O., Moir R.D., Nowinski C.J., Stern R.A., Cantu R.C., Geiling J., Blusztajn J.K., Wolozin B.L., Ikezu T., Stein T.D., Budson A.E., Kowall N.W., Chargin D., Sharon A., Saman S., Hall G.F., Moss W.C., Cleveland R.O., Tanzi R.E., Stanton P.K., and McKee A.C. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omalu B., Hammers J.L., Bailes J., Hamilton R.L., Kamboh M.I., Webster G., and Fitzsimmons R.P. (2011). Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg. Focus 31, E3. [DOI] [PubMed] [Google Scholar]
- 10.Stern R.A., Riley D.O., Daneshvar D.H., Nowinski C.J., Cantu R.C., and McKee A.C. (2011). Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 3, Suppl 2, S460–S467 [DOI] [PubMed] [Google Scholar]
- 11.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 12.Clay M.B., Glover K.L., and Lowe D.T. (2013). Epidemiology of concussion in sport: a literature review. J. Chiropr. Med. 12, 230–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini D., Eckner J., Kutcher J., and Broglio S.P. (2013). Subconcussive head impact biomechanics: comparing differing offensive schemes. Med. Sci. Sports Exerc. 45, 755–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koerte I.K., Lin A.P., Willems A., Muehlmann M., Hufschmidt J., Coleman M.J., Green I., Liao H., Tate D.F., Wilde E.A., Posternak O., Bouix S., Rathi Y., Bigler E.D., Stern R.A., Shenton M.E. (2015). A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 25, 318–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng T.S., Lin A.P., Koerte I.K., Pasternak O., Liao H., Merugumala S., Bouix S. and Shenton M.E. (2014). Neuroimaging in repetitive brain trauma. Alzheimers Res. Ther. 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith D.H., Johnson V.E., and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handratta V., Hsu E., Vento J., Yang C., and Tanev K. (2010). Neuroimaging findings and brain-behavioral correlates in a former boxer with chronic traumatic brain injury. Neurocase 16, 125–134 [DOI] [PubMed] [Google Scholar]
- 18.Gur R.E., Kaltman D., Melhem E.R., Ruparel K., Prabhakaran K., Riley M., Yodh E., Hakonarson H., Satterthwaite T., and Gur R.C. (2013). Incidental findings in youths volunteering for brain MRI research. AJNR Am. J. Neuroradiol. 34, 2021–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Born C.M., Meisenzahl E.M., Frodl T., Pfluger T., Reiser M., Moller H.J., and Leinsinger G.L. (2004). The septum pellucidum and its variants. An MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 254, 295–302 [DOI] [PubMed] [Google Scholar]
- 20.Shenton M.E., Dickey C.C., Frumin M., and McCarley R.W. (2001). A review of MRI findings in schizophrenia. Schizophr. Res. 49, 1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarley R.W., Wible C.G., Frumin M., Hirayasu Y., Levitt J.J., Fischer I.A., and Shenton M.E. (1999). MRI anatomy of schizophrenia. Biol. Psychiatry 45, 1099–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brent B.K., Thermenos H.W., Keshavan M.S. and Seidman L.J. (2013). Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: a review of structural MRI findings. Child Adolesc. Psychiatr Clin N. Am. 22, 689–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon J.S., Shenton M.E., Hirayasu Y., Salisbury D.F., Fischer I.A., Dickey C.C., Yurgelun-Todd D., Tohen M., Kikinis R., Jolesz F.A., and McCarley R.W. (1998). MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am. J. Psychiatry 155, 509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaton E.A., Qin Y., Nguyen V., Johnson J., Pinter J.D., and Simon T.J. (2010). Increased incidence and size of cavum septum pellucidum in children with chromosome 22q11.2 deletion syndrome. Psychiatry Res. 181, 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orrison W.W., Hanson E.H., Alamo T., Watson D., Sharma M., Perkins T.G., and Tandy R.D. (2009). Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J. Neurotrauma 26, 689–701 [DOI] [PubMed] [Google Scholar]
- 26.Aviv R.I., Tomlinson G., Kendall B., Thakkar C., and Valentine A. (2010). Cavum septi pellucidi in boxers. Can. Assoc. Radiol. J. 61, 29–32 [DOI] [PubMed] [Google Scholar]
- 27.Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M., Giwerc M.Y., Zhu A., Coleman M.J., Bouix S., Fritts N.G., Martin B., Chaisson C., McClean M.D., Lin A.P., Cantu R.C., Tripodis Y., Stern R., and Shenton M.E. (2015). Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J. Neurotrauma. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamm J.M., Bourlas A.P., Baugh C.M., Fritts N.G., Daneshvar D.H., Martin B.M., McClean M.D., Tripodis Y., and Stern R.A. (2015). Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84, 1114–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins C.A., Daneshvar D.H., Picano J.D., Gavett B.E., Baugh C.M., Riley D.O., Nowinski C.J., McKee A.C., Cantu R.C., and Stern R.A. (2014). Self-reported concussion history: impact of providing a definition of concussion. Open Access J. Sports Med. 5, 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter T.C., Kennedy A.M., Byrne J., and Woodward P.J. (2010). The cavum septi pellucidi: why is it important? J. Ultrasound Med. 29, 427–444 [DOI] [PubMed] [Google Scholar]
- 31.Flashman L.A., Roth R.M., Pixley H.S., Cleavinger H.B., McAllister T.W., Vidaver R., and Saykin A.J. (2007). Cavum septum pellucidum in schizophrenia: clinical and neuropsychological correlates. Psychiatry Res. 154, 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Areza-Fegyveres R., Rosemberg S., Castro R.M., Porto C.S., Bahia V.S., Caramelli P., and Nitrini R. (2007). Dementia pugilistica with clinical features of Alzheimer's disease. Arq, Neuropsiquiatr, 65, 830–833 [DOI] [PubMed] [Google Scholar]
- 33.Hasiloglu Z.I., Albayram S., Selcuk H., Ceyhan E., Delil S., Arkan B., and Baskoy L. (2011). Cerebral microhemorrhages detected by susceptibility-weighted imaging in amateur boxers. AJNR Am. J. Neuroradiol. 32, 99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filipovic B., Prostran M., Ilankovic N., and Filipovic B. (2004). Predictive potential of cavum septi pellucidi (CSP) in schizophrenics, alcoholics and persons with past head trauma. A post-mortem study. Eur. Arch. Psychiatry Clin. Neurosci. 254, 228–230 [DOI] [PubMed] [Google Scholar]
- 35.Bogdanoff B., and Natter H.M. (1989). Incidence of cavum septum pellucidum in adults: a sign of boxer's encephalopathy. Neurology 39, 991–992 [DOI] [PubMed] [Google Scholar]
- 36.Pittella J.E., and Gusmao S. (2005). Cleft cavum of the septum pellucidum in victims of fatal road traffic accidents: a distinct type of cavum associated with severe diffuse axonal injury. Surg. Neurol. 63, Suppl 1, S30–S35 [DOI] [PubMed] [Google Scholar]
- 37.Silk T., Beare R., Crossley L., Rogers K., Emsell L., Catroppa C., Beauchamp M., and Anderson V. (2013). Cavum septum pellucidum in pediatric traumatic brain injury. Psychiatry Res. 213, 186–192 [DOI] [PubMed] [Google Scholar]
- 38.Costanza A., Weber K., Gandy S., Bouras C., Hof P.R., Giannakopoulos P. and Canuto A. (2011). Review: Contact sport-related chronic traumatic encephalopathy in the elderly: clinical expression and structural substrates. Neuropathol. Appl. Neurobiol. 37, 570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagino H., Suzuki M., Kurokawa K., Mori K., Nohara S., Takahashi T., Yamashita I., Yotsutsuji T., Kurachi M., and Seto H. (2001). Magnetic resonance imaging study of the cavum septi pellucidi in patients with schizophrenia. Am. J. Psychiatry 158, 1717–1719 [DOI] [PubMed] [Google Scholar]