Abstract
Cessation of highly active antiretroviral therapy (HAART) in HIV-infected individual leads to a rebound of viral replication due to reactivation of a viral reservoir composed largely of latently infected memory CD4+ T cells. Efforts to deplete this reservoir have focused on reactivation of transcriptionally silent latent proviruses. HIV provirus transcription depends critically on the positive transcription elongation factor b (P-TEFb), whose core components are cyclin-dependent kinase 9 (CDK9) and cyclin T1. In resting CD4+ cells, the functional levels of P-TEFb are extremely low. Cellular activation upregulates cyclin T1 protein levels and CDK9 T-loop (T186) phosphorylation. The broad-spectrum histone deacetylase inhibitors (HDACis) vorinostat and panobinostat have been shown to reactivate latent virus in vivo in HAART-treated individuals. In this study, we have found that vorinostat and panobinostat activate P-TEFb in resting primary CD4+ T cells through induction of CDK9 T-loop phosphorylation. In contrast, tacedinaline and romidepsin, HDAC 1 and 2 inhibitors, were unable to activate CDK9 T-loop phosphorylation. We used a CCL19 primary CD4+ T-cell model HIV latency to assess the correlation between induction of CDK9 T-loop phosphorylation and reactivation of latent HIV virus by HDACis. Vorinostat and panobinostat treatment of cells harboring latent HIV increased CDK9 T-loop phosphorylation and reactivation of latent virus, whereas tacedinaline and romidepsin failed to induce T-loop phosphorylation or reactivate latent virus. We conclude that the ability of vorinostat and panobinostat to induce latent HIV is, in part, likely due to the ability of the broad-spectrum HDACis to upregulate P-TEFb through increased CDK9 T-loop phosphorylation.
Processive elongation of HIV RNA directed by the viral 5′ LTR promoter is dependent upon activation of RNA polymerase II (RNAP II) by the viral Tat protein. Following transcription initiation from the HIV-1 LTR, RNAP II pauses due to the action of two negative elongation factors (NELFs), DRB sensitivity inducing factors (DSIF) and the NELF, which are associated with the RNAP II complex.1,2 To activate elongation, Tat recruits positive transcription elongation factor b (P-TEFb) to the TAR RNA element at the 5′ end of the nascent viral transcript. Core P-TEFb consists of the cyclin-dependent kinase 9 (CDK9) and the regulatory subunit cyclin T1 or cyclin T2.3,4 Tat binds directly to cyclin T1 and therefore only targets cyclin T1-containing P-TEFb. P-TEFb stimulates processive transcriptional elongation by phosphorylating the Ser2 residues of the carboxyl terminal domain of RNA pol II, as well as the Spt5 subunit of DSIF and the RD subunit of NELF, thereby abrogating their inhibition of RNAP II elongation.2 Importantly, the catalytic activity of P-TEFb is dependent on phosphorylation of Thr186 in the CDK9 T-loop. In primary resting CD4+ T cells and monocytes, P-TEFb is downregulated due to low expression levels of cyclin T1 and repression of CDK9 T-loop phosphorylation.5–9 In contrast to resting cells, P-TEFb exists in distinct complexes in growing cells. In active cells, a portion of core P-TEFb is found on active chromatin in association with BRD4, specific transcription factors such as NF-κB or steroid hormone receptors, or a larger protein complex termed the super-elongation complex composed of three pairs of homologous proteins: ELL1/ELL2, AFF1/AFF4, and ENL/AF9.2,10–12 In these active cells, a large portion of core P-TEFb is sequestered in a catalytically inactive complex termed the 7SK RNP, which is composed of the noncoding 7SK snRNA, hexamethylene bisacetamide (HMBA)-induced proteins 1 or 2 (HEXIM1/2), La-related protein 7 (LARP7), and methyl phosphate-capping enzyme (MeCPE).2,4
Compounds of diverse chemical nature such as the differentiation agent HMBA, histone deacetylase inhibitors (HDACis), bromodomain and extraterminal bromodomain inhibitors, and protein kinase c (PKC) agonists can activate HIV-1 transcription from either latent cell lines or from resting primary CD4+ T cells.13–17 Treatment of resting CD4+ T cells with 335 nM of the broad-spectrum HDACi, vorinostat, which is equivalent to a single in vivo dose of 400 mg, activated latent HIV-1 in patients' cells ex vivo.18 Vorinostat was also evaluated for its ability to reactive the latent HIV-1 in vivo and it was shown to reactivate latent HIV-1 in vivo in patients in two studies.18,19 Despite activation of latent virus by vorinostat, no reduction in the size of the latent HIV reservoir was observed in patients in either of these two studies. In addition, the broad-spectrum HDACi panobinostat has been shown to reactivate latent HIV in patients in vivo, but like vorinostat, panobinostat failed to reduce the level of the latent HIV reservoir.20
Recent data from our laboratory demonstrated that vorinostat activates P-TEFb in primary resting CD4+ T cells through an increase in CDK9 T-loop phosphorylation.21 In this present study, we examined whether other HDACis can also activate P-TEFb and if there exists a relationship between P-TEFb activation and increased histone H3 acetylation. To determine whether vorinostat and the additional broad-spectrum HDACi panobinostat can activate P-TEFb through an increased phosphorylation of CDK9 and whether this effect is dose dependent, resting CD4+ T cells were incubated with increasing concentrations of different HDACis for 24 h, cell extracts were prepared as previously described,8 and CDK9 was analyzed in immunoblots (Fig. 1). Vorinostat and panobinostat treatment of CD4+ T cells increased the CDK9 T-loop phosphorylation in a dose-dependent manner. We did not observe a reproducible increase in the basal expression level of cyclin T1 by either vorinostat or panobinostat (data not shown). Vorinostat and panobinostat treatment also increased the acetylation histone H3 in a dose-dependent manner compared to untreated cells. In contrast, treatment of CD4+ T cells with either tacedinaline or romidepsin, both of which are HDAC 1 and 2 inhibitors, failed to increase of CDK9 T-loop phosphorylation or increase cyclin T1 levels (Fig. 1). Although romidepsin failed to increase the phosphorylation of CDK9, we found a dose-dependent increase of histone H3 acetylation in CD4+ T cells. Only a very modest increase in histone H3 acetylation was observed for tacedinaline; there are reports that tacedinaline can cause hyperacetylation of histone H3 in HCT-8 colon carcinoma cells, but histone H3 acetylation occurred only at high concentrations (750 nM) in the previous study22; the IC50 of tacedinaline is 0.57 μM for HDAC1. Thus, our data show that the broad-spectrum HDACis, vorinostat and panobinostat, induced CDK9 T-loop phosphorylation in resting CD4+ T cells, while the HDAC1 and HDAC2 inhibitors, tacedinaline and romidepsin, did not.
FIG. 1.
Vorinostat (SAHA) and panobinostat induce CDK9 Thr-186 T-loop phosphorylation in resting CD4+ T cells. Resting CD4+ T cells were isolated by negative selection from peripheral blood of healthy donors by the Rosettesep CD4+ cells isolation kit (STEMCELL Technologies, Inc.). Flow cytometry analysis confirmed that the CD4+ cell populations were routinely >98% (data not shown). Cells were treated with various concentrations of vorinostat, panobinostat, tacedinaline, or romidepsin for 24 h and lysates were prepared in EBCD buffer [50 mM Tris-HCl (pH 8.0), 120 mM NaCl, 0.5% Nonidet P-40, 5 mM dithiothreitol, 4 mM MgCl2 buffer containing protease inhibitor cocktail] and analyzed for the expression of indicated proteins in immunoblots. Experiments were repeated at least three times and representative figures are shown. CDK9, cyclin-dependent kinase 9.
Next, we examined the time course of CDK9 T-loop phosphorylation in resting CD4+ T cells by either vorinostat (335 nM) or panobinostat (10 nM). Cell lysates were prepared at different time points of HDACi treatment, and lysates were examined in immunoblots. As shown in Figure 2, increased CDK9 Thr-186 phosphorylation by both vorinostat and panobinostat was first observed at 12 h post-treatment. CDK9 T-loop phosphorylation increased at 18 h and remained high at 24 h of HDACi treatment. Interestingly, increased acetylation of histone H3 was observed at 3 h by panobinostat but not vorinostat; increased H3 acetylation by vorinostat was first observed at 12 h post-treatment, similar to the kinetics of CDK9 T-loop phosphorylation by vorinostat. Thus, vorinostat and panobinostat induce significant levels of CDK9 T-loop in resting CD4+ T cells at 12 h post-treatment. The kinetics of increased T-loop phosphorylation and histone H3 acetylation is similar for vorinostat, whereas for panobinostat, histone H3 acetylation is more rapid than T-loop phosphorylation.
FIG. 2.
Time course of CDK9 T-loop phosphorylation in HDACi-treated CD4+ T cells. CD4+ T cells prepared as described in Figure 1 were treated with either vorinostat or panobinostat for the indicated time periods. Cell lysates were prepared at the indicated times as described in Figure 1 and lysates were examined in immunoblots. HDACi, histone deacetylase inhibitor.
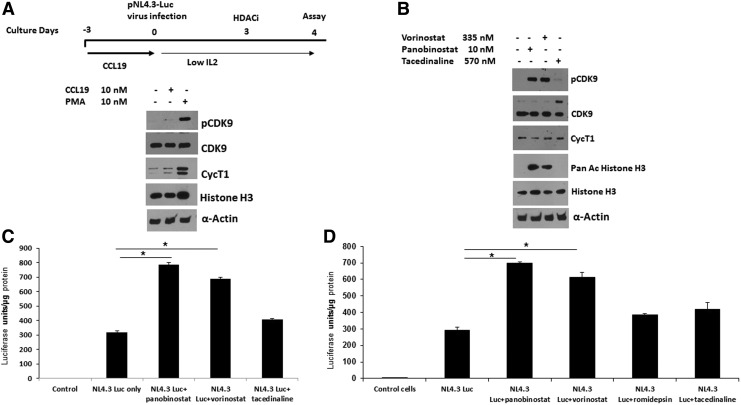
We next determined whether the induction of CDK9 T-loop phosphorylation by vorinostat and panobinostat might correlate with reactivation of latent HIV-1 in a primary CD4+ T-cell model. We therefore used the CCL19 chemokine latency model.23,24 In this model, resting CD4+ T cells are treated with CCL19, which establishes conditions in which the cells support high levels of latent infection that can be subsequently reactivated by T-cell activation. We generated stocks of vesicular stomatitis virus (VSV) pseudotyped HIV-1 reporter virus, which contain the Luciferase coding sequences in place of the nef gene and inactivating mutations in the env and vpr genes (obtained from the NIH AIDS Reagent program). Resting CD4+ T cells were treated with the chemokine CCL19 for 72 h followed by infection with the reporter virus for another 5 days. As shown in Figure 3A and 3B, CCL19 treatment alone did not induce T-loop phosphorylation or cyclin T1 levels. In contrast, phorbol 12-myristate 13-acetate (PMA) plus ionomycin treatment increased T-loop phosphorylation and the level of cyclin T1. Both vorinostat and panobinostat, but not tacedinaline, increased CDK9 T-loop phosphorylation in CCL19-treated cells (Fig. 3B) compared to control cells. Vorinostat and panobinostat, but not tacedinaline, also increased histone H3 acetylation in CCl19-treated cells. In agreement with the induction of CDK9 T-loop phosphorylation, both vorinostat treatment and panobinostat treatment, but not tacedinaline, were able to significantly reactivate latent virus as determined by induction of Luciferase expression (Fig. 3C). In an additional experiment shown in Figure 3D, tacedinaline and romidepsin failed to demonstrate a significant reactivation of latent HIV, while vorinostat and panobinostat demonstrated a significant reactivation of latent HIV similar to that observed in the experiment shown in Figure 3C. We note that tacedinaline treatment resulted in an increase in the minor 55 kDa form of CDK9 in the experiment shown in Figure 3D (band above majo 42 kDa form of CDK9).25,26 The significance of this increase is unclear. We conclude from these data that vorinostat and panobinostat can reactivate latent virus in the CCL19 model of latency and this correlates with an induction of CDK9 T-loop phosphorylation.
FIG. 3.
Vorinostat and panobinostat activate HIV-1 in CCL19-mediated HIV-1 latency model in resting CD4+ T cells. (A) Purified resting CD4+ T cells were cultured for 3 days in the presence of CCL19 (10 nM; PeproTech, NJ) or were left inactivated. As a positive control, CD4+ cells were treated with PMA+ionomycin for 16 h. Cell lysates were prepared and examined in immunoblots. (B) Resting CD4+ T cells were cultured for 3 days in the presence of CCL19 and then infected for 5 days with the HIV-1 NL4.3-Luc reporter virus, which contains a deletion in the nef gene (Δnef) and replaced with luciferase gene (HIV-1 NL4.3 Δnef luc). Cells were treated with vorinostat, panobinostat, or tacedinaline for 24 h at the indicated concentrations. Cell lysates were prepared and examined in immunoblots (B), and luciferase activity was measured (C, D) as per the manufacturer's recommendations (Luciferase assay system with reporter lysis buffer, cat E4030; Promega Corporation). *p < .02. PMA, phorbol 12-myristate 13-acetate.
In summary, this study demonstrates that the broad-spectrum HDAC inhibitors vorinostat and panobinostat can reactivate latent HIV-1 to some extent and this is likely due to counteracting repressive chromatin through increased histone acetylation as well as an induction of P-TEFb activity through an increase in CDK9 T-loop phosphorylation. However, the data to date from the field indicate that single agents such as vorinostat or panobinostat will not be effective when used alone in reactivating latent HIV in vivo.27 An effective reactivation strategy will require multiple agents that act through distinct mechanisms to relieve a repressive chromatin environment for latent viruses and induce limiting levels of cellular transcription factors, especially P-TEFb. In addition, reduction of the reservoir will require strategies to enhance the immune system's ability to recognize and clear cells in which latent viruses have been reactivated.28 Further studies on the mechanism through which vorinostat and panobinostat induced CDK9 T-loop phosphorylation may provide clues for potent latency reactivation strategies.
Acknowledgment
This work was supported by the National Institutes of Health grants AI110263 and AI116173 (to APR) and P30AI1036211 (Baylor-UT-Houston CFAR).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Karn J, Stoltzfus CM: Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb Perspect Med 2012;2:a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterlin BM, Price DH: Controlling the elongation phase of transcription with P-TEFb. Mol Cell 2006;23:297–305 [DOI] [PubMed] [Google Scholar]
- 3.Mbonye U, Karn J: Transcriptional control of HIV latency: Cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology 2014;454–455:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott M, Geyer M, Zhou Q: The control of HIV transcription: Keeping RNA polymerase II on track. Cell Host Microbe 2011;10:426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budhiraja S, Famiglietti M, Bosque A, Planelles V, Rice AP: Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J Virol 2013;87:1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung TL, Rice AP: miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog 2009;5:e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang K, Sung TL, Rice AP: Regulation of cyclin T1 and HIV-1 replication by microRNAs in resting CD4+ T lymphocytes. J Virol 2012;86:3244–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramakrishnan R, Dow EC, Rice AP: Characterization of Cdk9 T-loop phosphorylation in resting and activated CD4(+) T lymphocytes. J Leukoc Biol 2009;86:1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong C, Kwas C, Wu L: Transcriptional restriction of human immunodeficiency virus type 1 gene expression in undifferentiated primary monocytes. J Virol 2009;83:3518–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobhian B, Laguette N, Yatim A, et al. : HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol Cell 2010;38:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C, Smith ER, Takahashi H, et al. : AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell 2010;37:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He N, Liu M, Hsu J, et al. : HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol Cell 2010;38:428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM: Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem 2012;287:36609–36616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archin NM, Espeseth A, Parker D, et al. : Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses 2009;25:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras X, Barboric M, Lenasi T, Peterlin BM: HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog 2007;3:1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei DG, Chiang V, Fyne E, et al. : Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog 2014;10:e1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying H, Zhang Y, Lin S, Han Y, Zhu HZ: Histone deacetylase inhibitor Scriptaid reactivates latent HIV-1 promoter by inducing histone modification in in vitro latency cell lines. Int J Mol Med 2010;26:265–272 [DOI] [PubMed] [Google Scholar]
- 18.Archin NM, Liberty AL, Kashuba AD, et al. : Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012;487:482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott JH, Wightman F, Solomon A, et al. : Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014;10:e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen TA, Tolstrup M, Brinkmann CR, et al. : Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV 2014;1:e13–e21 [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Liu H, Rice AP: Short communication: SAHA (vorinostat) induces CDK9 Thr-186 (T-loop) phosphorylation in resting CD4+ T cells: Implications for reactivation of latent HIV. AIDS Res Hum Retroviruses 2015;31:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraker AJ, Mizzen CA, Hartl BG, et al. : Modulation of histone acetylation by [4-(acetylamino)-N-(2-amino-phenyl) benzamide] in HCT-8 colon carcinoma. Mol Cancer Ther 2003;2:401–408 [PubMed] [Google Scholar]
- 23.Cameron PU, Saleh S, Sallmann G, et al. : Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U S A 2010;107:16934–16939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh S, Solomon A, Wightman F, et al. : CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: A novel model of HIV-1 latency. Blood 2007;110:4161–4164 [DOI] [PubMed] [Google Scholar]
- 25.Shore SM, Byers SA, Maury W, Price DH: Identification of a novel isoform of Cdk9. Gene 2003;307:175–182 [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Herrmann CH: Differential localization and expression of the Cdk9 42k and 55k isoforms. J Cell Physiol 2005;203:251–260 [DOI] [PubMed] [Google Scholar]
- 27.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF: New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014;20:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan L, Deng K, Shroff NS, et al. : Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]