Abstract
Brain hypothermia has been considered as a promising alternative to whole-body hypothermia in treating acute neurological disease, for example, traumatic brain injury. Previously, we demonstrated that 2-hours selective brain cooling (SBC) effectively mitigated acute (≤24 hours postinjury) neurophysiological dysfunction induced by a penetrating ballistic-like brain injury (PBBI) in rats. This study evaluated neuroprotective effects of extended SBC (4 or 8 hours in duration) on sub-acute secondary injuries between 3 and 21 days postinjury (DPI). SBC (34°C) was achieved via extraluminal cooling of rats' bilateral common carotid arteries (CCA). Depending on the experimental design, SBC was introduced either immediately or with a 2- or 4-hour delay after PBBI and maintained for 4 or 8 hours. Neuroprotective effects of SBC were evaluated by measuring brain lesion volume, axonal injury, neuroinflammation, motor and cognitive functions, and post-traumatic seizures. Compared to untreated PBBI animals, 4 or 8 hours SBC treatment initiated immediately following PBBI produced comparable neuroprotective benefits against PBBI-induced early histopathology at 3 DPI as evidenced by significant reductions in brain lesion volume, axonal pathology (beta-amyloid precursor protein staining), neuroinflammation (glial fibrillary acetic protein stained-activated astrocytes and rat major histocompatibility complex class I stained activated microglial cell), and post-traumatic nonconvulsive seizures. In the later phase of the injury (7–21 DPI), significant improvement on motor function (rotarod test) was observed under most SBC protocols, including the 2-hour delay in SBC initiation. However, SBC treatment failed to improve cognitive performance (Morris water maze test) measured 13–17 DPI. The protective effects of SBC on delayed axonal injury (silver staining) were evident out to 14 DPI. In conclusion, the CCA cooling method of SBC produced neuroprotection measured across multiple domains that were evident days/weeks beyond the cooling duration and in the absence of overt adverse effects. These “proof-of-concept” results suggest that SBC may provide an attractive neuroprotective approach for clinical considerations.
Introduction
Preclinical research has consistently demonstrated that therapeutic hypothermia is a promising neuroprotective strategy for treating traumatic brain injury (TBI) by effectively reducing injury-induced increases in intracranial pressure, cellular damage, and improving neurological outcomes (Fingas et al., 2007; Doll et al., 2009; Dietrich and Bramlett, 2010; Wei et al., 2011). In contrast, clinical studies have yielded less consistent results and failed to establish a standardized hypothermia strategy for treating severe TBI patients (McIntyre et al., 2003; Hutchison et al., 2008; Grande et al., 2009; Adelson et al., 2013).
In clinical settings, whole-body hypothermia is usually induced by skin surface cooling or intravascular cooling (Hemmen and Lyden, 2007; Holzer, 2008). Potential adverse effects of whole-body cooling include increased risk of coagulopathy (Watts et al., 1998; Polderman and Herold, 2009), hypotension (Shiozaki et al., 2001; Milhaud et al., 2005), and infectious pneumonia (Shiozaki et al., 2001; Alderson et al., 2004). Critically, whole-body hypothermia would be contraindicated for treating TBI casualties who suffer polytrauma (Cobb and Pridgen, 2008; O'Connell et al., 2012) as it causes increased platelet dysfunction and inhibits the coagulation cascade, which promote coagulopathy as it may exacerbate the coagulation cascade and contribute to the lethal triad (Hess and Lawson, 2006; Misgav and Martinowitz, 2011).
To harness the potential therapeutic benefits of induced hypothermia while mitigating adverse effects, more recent preclinical research efforts have been directed toward techniques designed to selectively cool the brain while maintaining the normal body temperature. In keeping with this, we have developed a selective brain cooling (SBC) method in rats achieved by extraluminal cooling of the bilateral common carotid arteries (CCA) that can effectively reduce rat's brain temperature within 30 minutes without causing detectible pathology of the CCA or coagulopathy measured by simple tail bleeding time (Wei et al., 2008, 2011). Previously, we demonstrated that when initiated immediately following a penetrating ballistic-like brain injury (PBBI) in rats, SBC (2-hours duration) effectively mitigated PBBI-induced acute (≤24 hours postinjury) increases in intracranial pressure, neuropathological, and neurofunctional deficits (Wei et al., 2011; Yao et al., 2011).
The focus of this study was to evaluate the sub-acute neuroprotective effects of extended SBC duration (e.g., 4 or 8 hours), initiated either immediately or after a 2–4 hours delay, using comprehensive outcome measures performed between 3 and 21 days postinjury (DPI) in the rat PBBI model.
Materials and Methods
Animals
Experiments were conducted using male Sprague Dawley rats (275–325 g; Charles River Laboratories, Raleigh, VA). All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Walter Reed Army Institute of Research (WRAIR) and conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the “Guide for the Care and Use of Laboratory Animals.” The animals housing facility was accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Surgical procedures
All surgical procedures were conducted in anesthetized rats using aseptic techniques approved by WRAIR IACUC. The anesthesia was induced with 5% isoflurane delivered in a mixture of oxygen and air, and then maintained at ∼1.5–2%.
Penetrating ballistic-like brain injury
PBBI was performed as previously described (Shear et al., 2010). Briefly, the rat was placed in a stereotaxic device. A craniotomy was made over the right frontal cortex (AP: 4.5 mm and ML: +2.0 mm to bregma). A PBBI probe was inserted at a fixed angle through the right frontal cortex to a depth of 12 mm. A computer-controlled pulse generator was then activated to rapidly inflate the silastic tubing on the PBBI probe to an elliptical balloon with a volume equal to ∼10% of the total brain volume. Upon deflation of the balloon, the probe was withdrawn and the cranial wound was closed. Sham control rats received only craniotomy without the insertion of the PBBI probe and matching duration of anesthesia.
Selective brain cooling
Both CCAs were exposed and isolated. A custom-made cooling cuff was placed around the exposed segment of each CCA (∼0.5 cm below the bifurcation of internal and external carotid artery) and secured by a piece of silk suture. The SBC (34°C) was induced by continuously pumping ice-cold water through the cooling cuffs to cool the arterial blood as it entered the brain. Rewarming was achieved by terminating cold-water circulation to allow the brain temperature to normalize spontaneously. Brain temperature was monitored via a temperature probe positioned into the left cerebral hemisphere. Core body temperature was maintained at ∼37°C using a heating blanket and monitored via a rectal temperature probe. Fraction of inspired oxygen (FiO2) was maintained at 0.26–0.27 (Murakami et al., 2012).
EEG electrode implantation
Four epidural electroencephalogram (EEG) electrodes (a piece of Nichrome wire soldered to a 080 stainless steel screw) were symmetrically implanted on the rat skull over the bilateral frontal and parietal regions of the brain (AP: +1 and −4 mm; ML: ±3.5 mm to bregma). A reference electrode (R) was placed posterior to the lambda. The free end of each electrode wire was then soldered to a multi-pin connector (March Electronics, West Hempstead, NY) in the sequence of a (left frontal), b (left parietal), c (right frontal), and d (right parietal), which was individually referenced to the electrode R to create a four-channel mono-referential EEG recording montage (Lu et al., 2013). The screw electrodes were secured on the rat skull with dental acrylic. All rats were allowed at least 5 days of recovery before receiving the PBBI surgery.
Histopathology
At the termination of respective experiments (defined below), rats were anesthetized with ketamine/xylazine (70/6 mg/kg) mixture and perfused with cold saline followed by 0.1 M phosphate buffer (pH 7.4) containing 4% paraformaldehyde. Serial coronal sections (40 μm) were cut through the whole cerebrum.
Hematoxylin and eosin (H&E) staining was used for quantifying brain lesion volume. Immunohistochemical staining of glial fibrillary acetic protein (GFAP) and rat major histocompatibility complex class I (OX-18) were used to detect activated astrocytes and microglia, respectively, in selected brain sections in the peri-lesional regions as a measure of neuroinflammation. Axonal injury was detected by human beta-amyloid precursor protein (β-APP) immunoreactivity in the peri-lesional regions and silver staining in the thalamus.
Acute postinjury lesion volume (3 DPI) was measured by tracing the perimeter of the lesion area on each affected brain section. Delayed brain damage (21 DPI) was quantified as the % tissue loss in the injured versus the contralateral hemispheres. The lesion volume or % tissue volume loss was calculated using the Cavalieri formula applied to the brain areas of the sequential brain sections (ImageJ software, Version 1.44; NIH, Bethesda, MD).
Immunoreactivity of GFAP, OX-18, and β-APP was quantified by threshold analysis within the regions of interest using the ImageJ software (Version 1.44; NIH) at 20× magnification as described previously (Cunningham et al., 2014). The threshold value of immunoreactivity (i.e., grey level index) for each stain was set to consistently detect maximal positive staining with minimal artifacts. All pixel measurements were converted into area measurements (mm2) according to the resolution scale (pixels/mm) and expressed as the percent area (% area) for a given image. The threshold analysis was also applied to the silver stained images at 40× magnification.
Neurobehavioral tests
Neurological test
Neurological deficits were evaluated at 3 DPI using a modified clinical examination. Neurological scores (neuroscore) were calculated based on a 12-point scale comprised of four neurological exams: (1) contralateral forelimb flexion during tail suspension, (2) shoulder adduction (body upward curling) during tail suspension, (3) impaired resistance to lateral push, and (4) abnormal circling behavior. Each exam was scored from 0 (normal) to 3 (severely impaired).
Rotarod task
The Rotamex-5 rotarod apparatus (Columbus Instruments, Columbus, OH) was used to measure animals' motor coordination and balance (Shear et al., 2010). Before PBBI surgery, rats were trained to criteria on a fixed-speed version of the rotarod task. Preinjury baseline measures were taken 1 day before PBBI. Animals that failed to achieve preinjury baseline criteria (i.e., unable to maintain their balance on the rotarod for ≥50 seconds at 10 rpm) were excluded from the subsequent experiments. After PBBI, the animals' ability to remain balanced on the rotating rod was assessed at fixed-speed increments of 10, 15, and 20 rpm for a maximum of 60 s/trial and 2 trials/speed with a 60-seconds inter-trail interval (ITI) between 7 and 21 DPI. Average latency to fall off the rotating rod was used to evaluate the treatment effects of SBC.
Morris water maze task
The Morris water maze (MWM) with a video-tracking system (Noldus EthoVision XT) was used to assess spatial learning performance (Shear et al., 2010, 2011). The MWM apparatus consisted of a circular pool (75 cm deep, 175 cm diameter) filled with clear water (22°C ± 1°C) to a depth of 60 cm. A clear Plexiglas platform was submerged 1 inch below the water surface and located in the center of the northwest quadrant. Animals were tested for a maximum 90 s/trial, 4 trials/day (30 minutes ITI) for 5 consecutive days between 13 and 17 DPI. Latency to locate the hidden platform was used to evaluate the treatment effect of SBC.
EEG detection of nonconvulsive seizures
Video EEG recordings were collected continuously in unanesthetized rats housed individually in EEG recording chambers via flexible shield cables connected to swivel commutators, which were interfaced with an EEG amplifier and a digital signal acquisition system (Stellate Harmonie software; Natus Medical Incorporated, San Carlos, CA). The primary seizure outcome measures included the following: (1) nonconvulsive seizures (NCS) incidence (the number of animals displaying NCS events, expressed as the percentage of the respective group), (2) NCS frequency (the number of NCS events experienced by a given animal), (3) NCS episode duration (seconds), (4) NCS cumulative duration (the sum of NCS episode duration for a given animal) (seconds), and (5) NCS onset latency (hours).
Experimental design
A total of 302 rats were used in the analysis of this study, not including those dropped due to mortality (∼20%) and justified exclusions (∼15%), for example, statistical outliers, failure to meet preinjury behavioral training criteria, and so on. All animals were randomly assigned to the experimental groups described below.
Experiment 1. Physiological responses to prolonged isoflurane exposure in naïve rats
Before conducting experiments using an extended SBC treatment protocol, the safety profiles of prolonged isoflurane exposure were examined in naïve rats without mechanical ventilation. For this purpose, rats (n = 4) were anesthetized with 1.5–2% isoflurane for 9 hours (the maximum anesthesia duration required for PBBI+SBC procedures). FiO2 was maintained at 0.26–0.27. The body and brain temperatures were maintained at ∼37°C. Regional cerebral blood flow (rCBF) was continuously monitored using a laser Doppler probe (Moor Instruments, Devon, England) placed on the parietal cortex (AP: −6 mm and ML: 4 mm to the bregma). Mean arterial blood pressure (MABP) was measured via a femoral artery catheter connected to a pressure transducer (Harvard Apparatus, Holliston, MA). Arterial blood gases were measured at 0.5, 2.5, 5, 7, and 9 hours after the onset of anesthesia using a blood gas analyzer (Radiometer America, Inc., Brea, CA).
Experiment 2. Effects of 4 hours SBC treatment initiated immediately after PBBI
In this experiment, the experimental conditions included sham control (craniotomy followed by sham SBC procedures, that is, cooling cuff insertion and 4 hours isoflurane anesthesia), PBBI alone (PBBI followed by sham SBC), and PBBI+SBC (PBBI followed by 4 hours SBC). The SBC or sham SBC procedures were initiated immediately (within 30 minutes) after PBBI or craniotomy.
Before examining the effects of SBC on axonal injury, the time course of PBBI-induced axonal injury was determined in PBBI alone rats using β-APP staining at 2, 6 hours, 1, 3, 7, and 21 DPI and silver staining at 1, 3, 7, and 21 DPI (N = 6 rats/group/time point). Subsequently, the effects of 4 hours SBC on early axonal pathology was examined at 3 DPI using β-APP staining (Sham: N = 6; PBBI alone: N = 12; PBBI+SBC: N = 13), whereas the effects of SBC on delayed axonal pathology was examined at 21 DPI using silver staining (on the brain tissues generated from neurobehavioral studies described below).
The effects of SBC on H&E stained brain lesion in addition to GFAP and OX-18 stained activation of astrocytes and microglia, respectively, were also evaluated at 3 DPI using different series of brain sections from the respective groups of animals used for β-APP staining. In addition, the effects of SBC on delayed brain tissue loss were examined at 21 DPI on the brain tissues generated from neurobehavioral studies described below.
The neurofunctional measures (neurological, rotarod, and MWM tests) were performed in a separate cohort of animals, which were randomly assigned to sham control (N = 6), PBBI alone (N = 16), and PBBI+SBC (N = 17) conditions. The neurological functions were assessed at 3 DPI, the rotarod performance was assessed at 7, 10, 14, and 21 DPI, and the MWM performance was assessed from 13 to 17 DPI.
Experiment 3. Effects of 8 hours SBC treatment initiated immediately after PBBI
In this experiment, the procedures of Experiment 2 were replicated in a separate cohort of animals except that the SBC duration and the corresponding anesthesia duration in the control animals were extended to 8 hours. These animals were randomly divided into three groups for the 3 DPI histo- and immunohistopathology studies (Sham N = 6; PBBI N = 14; PBBI+SBC N = 13) and three groups for the neurofunctional studies (Sham N = 11; PBBI N = 8; PBBI+SBC N = 13). The brains of these animals were also used for H&E and silver staining at the end of the study (21 DPI).
Experiment 4. Effects of 4 hours SBC treatment initiated 2 or 4 hours after PBBI
In this experiment, the initiation of SBC treatment (4 hours in duration) was delayed until either 2 hours (N = 9) or 4 hours (N = 12) after PBBI. Matched sham control groups (n = 8/condition) and PBBI alone groups (N = 14/condition) were also included. Neuroprotective efficacy was measured on the fixed-speed rotarod task to evaluate motor coordination and balance from 7 to 14 DPI. Lesion volume (H&E staining) and axonal pathology (silver staining) were assessed at 14 DPI.
Experiment 5. Effects of SBC treatment (4 hours) on post-traumatic NCS
In this experiment, rats received either PBBI alone (N = 16) or PBBI+SBC (N = 22) as described above. Continuous video EEG recordings were collected for 72 hours initiated 4 hours post-PBBI (immediately after the termination of anesthesia in each group). The NCS were identified from offline review of EEG records and verified by synchronized video recordings.
Data analysis
One-way ANOVA, repeated measures ANOVA followed by Student–Newman–Keuls multiple comparison test and Student's t-test, or nonparametric Mann–Whitney test were used when appropriate. A p ≤ 0.05 was considered statistically significant. Data are presented as mean ± standard error of the mean. The analyses of histopathology, neurobehavior, and NCS were conducted by experienced personnel who were blinded to the treatment conditions of individual animals.
Results
Experiment 1. Physiological responses to prolonged isoflurane exposure in naïve rats
Prolonged (9 hours) isoflurane anesthesia did not have significant effects on MABP and rCBF of naïve animals. The pH, partial pressure of carbon dioxide (PaCO2), and partial pressure of oxygen (PaO2) in arterial blood were also normal throughout the entire procedure (Table 1).
Table 1.
Physiological Parameters in Isoflurane-Anesthetized Naive Rats
| Baseline | 2.5 h | 5 h | 7 h | 9 h | |
|---|---|---|---|---|---|
| MABP (mmHg) | 90.5 ± 0.9 | 87.4 ± 3.7 | 95.1 ± 3.7 | 95.6 ± 9.4 | 99.2 ± 11.1 |
| rCBF (flux) | 323.2 ± 60.8 | 328.9 ± 69.4 | 317.7 ± 61.5 | 305.5 ± 47.8 | 281.6 ± 52.5 |
| pH | 7.41 ± 0.02 | 7.42 ± 0.01 | 7.42 ± 0.01 | 7.43 ± 0.01 | 7.43 ± 0.01 |
| PaCO2 | 44.0 ± 0.91 | 41.0 ± 1.82 | 40.5 ± 1.32 | 38.5 ± 1.25 | 37.3 ± 1.31 |
| PaO2 (mmHg) | 110.2 ± 9.8 | 102.0 ± 10.8 | 105.8 ± 0.4 | 106.5 ± 1.4 | 104.1 ± 10.4 |
Data are expressed as mean ± SEM; n = 4.
MABP, mean arterial blood pressure; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; rCBF, regional cerebral blood flow.
Experiments 2 and 3. Effects of 4 or 8 hours SBC treatment initiated immediately after PBBI
Brain temperature profile
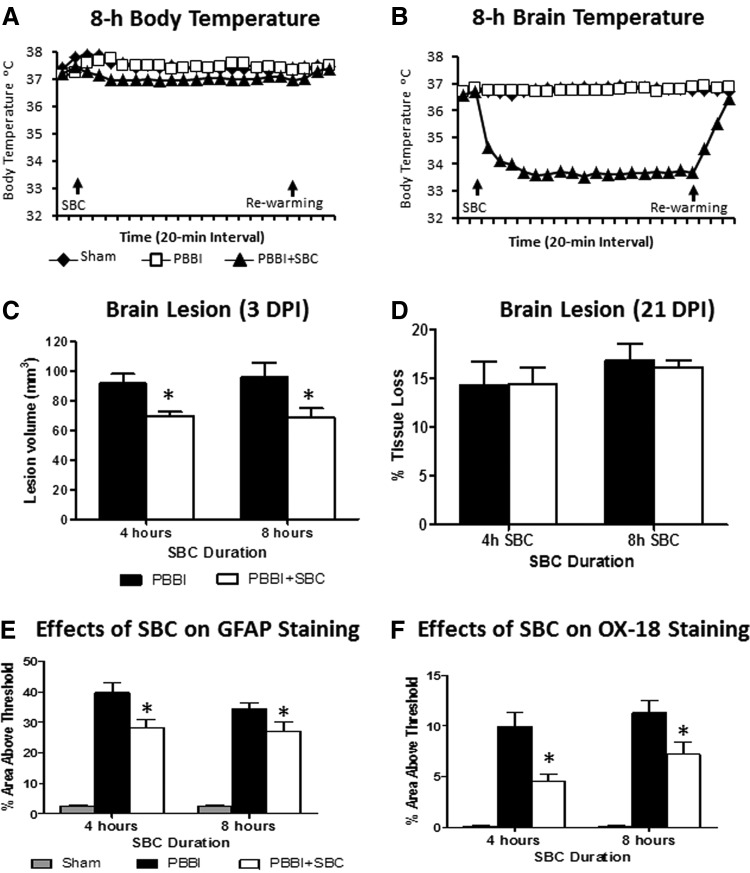
Similar to our previous report, bilateral CCA cooling reduced brain temperature by ∼3°C within 30 minutes after the initiation of SBC. Throughout the 4 hours SBC duration, the brain temperature was maintained at 34.5°C ± 0.3°C and body temperature was maintained at 37.2°C ± 0.03°C via use of a warming blanket (Harvard Apparatus). At the end of each cooling period, spontaneous rewarming allowed the brain temperature to return to baseline levels within 30 minutes. Similar body (37.1°C ± 0.03°C, Fig. 1A) and brain (34.1°C ± 0.17°C, Fig. 1B) temperatures were also achieved/maintained during 8 hours SBC durations.
FIG. 1.
(A, B) Time course profiles of body (A) and brain (B) temperature during an 8-hour selective brain cooling (SBC) period followed by spontaneous rewarming in anesthetized rats. (C) SBC significantly reduced brain lesion volume (mm3) measured at 3 days postinjury (DPI). (D) SBC had no effect on delayed lesion cavity measured at 21 DPI (expressed as % tissue loss relative to the contralateral hemisphere). (E) SBC significantly reduced glial fibrillary acetic protein (GFAP) stained activation of astrocytes measured at 3 DPI. (F) SBC significantly reduced OX-18 stained activation of microglial cells measured at 3 DPI. * indicates p < 0.05 (penetrating ballistic-like brain injury [PBBI] vs. PBBI+SBC).
Brain tissue damage
The analysis of histopathology at 3 DPI showed that 4 or 8 hours SBC treatment significantly reduced PBBI-induced lesion size by 24% (p < 0.01) and 25% (p < 0.05), respectively (Fig. 1C). However, by 21 DPI the brain tissue damage was manifested as an enlarged cavity owing to the significant loss of injurious tissue. Under such conditions, the early protective effects on brain lesion size were diminished in both 4 hours (p > 0.05) and 8 hours (p > 0.05) SBC treated animals (Fig. 1D).
Neuroinflammation
At 3 DPI, significant increases in GFAP and OX-18 positive staining were evident in the perilesion regions in the PBBI alone animals. SBC treatment reduced PBBI-induced activation of GFAP by 23% (4 hours SBC, p < 0.01) and 26% (8 hours SBC, p < 0.05) (Fig. 1E). Likewise, SBC treatment resulted in significant reductions in OX-18 positive staining by 58% (4 hours SBC, p < 0.01) and 33% (8 hours SBC, p < 0.05) in the peri-lesional regions (Fig. 1F).
Axonal injury
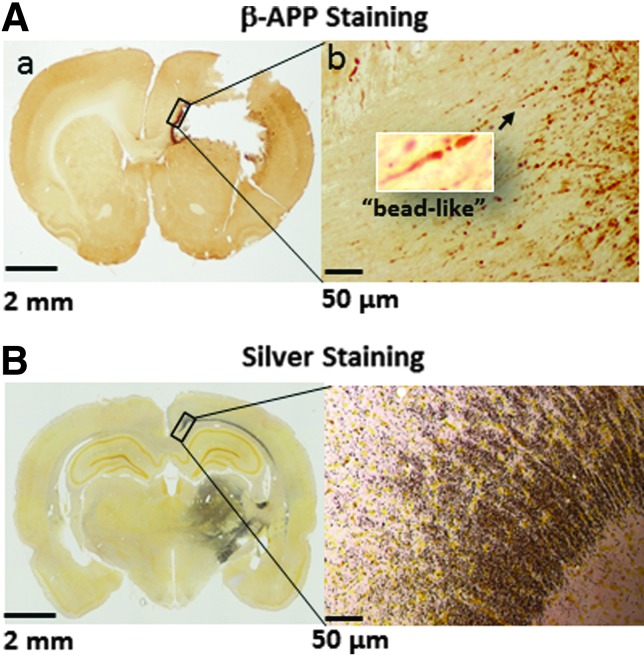
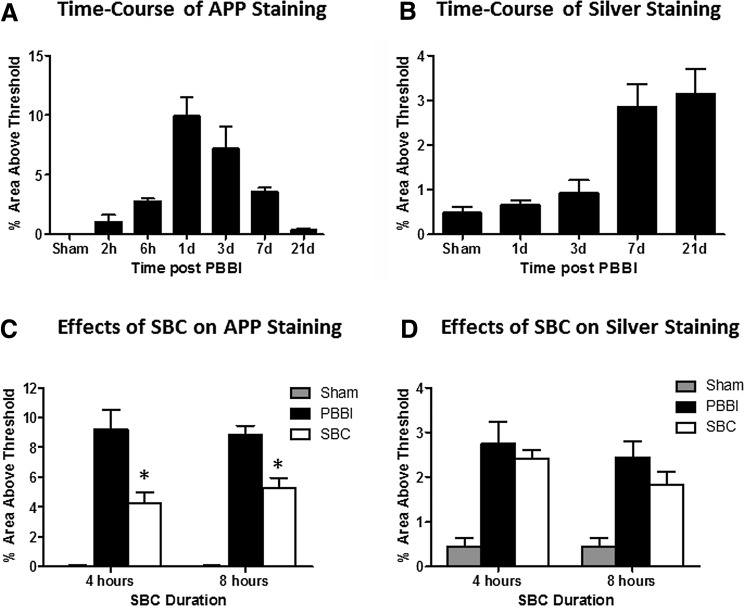
The majority of PBBI-induced axonal injury labeled by β-APP immunohistochemistry staining was detected in the anterior corpus callosum and caudate putamen adjacent to the injury core, showing typical “bead-like” or “bulb-like” features at 20× magnification (Fig. 2Aa, the insert). The time course analysis showed that β-APP immunoreactivity significantly increased between 1 and 3 DPI, but was resolved by 7 DPI (Fig. 3A). In contrast, axonal degeneration indicated by silver staining was detected in areas more distal from the injury core, for example, posterior corpus callosum and the thalamus (Fig. 2B) and increased between 7 and 21 DPI, concomitantly with the decrease in β-APP staining (Fig. 3B).
FIG. 2.
(A) Representative images of a β-amyloid precursor protein (β-APP) stained brain section associated with brain lesions. The left image (a) was taken at 4× magnification. The right image was the enlargement (10× magnification) of the area marked by the rectangle in (a). The insert in image (b) was taken at 20×. (B) Representative images of a silver stained brain section posterior to brain lesions. The left image was taken at 4× magnification and right image was the enlargement (10× magnification) of the area marked by the rectangle in the image to the left.
FIG. 3.
The time course analysis of PBBI-induced axonal injury stained by β-APP (A) or silver (B) showed different temporal profiles. SBC treatment (either 4 or 8 hours duration) significantly reduced β-APP-stained early axonal injury at 3 DPI (C), but not silver stained delayed axonal injury at 21 DPI (D). * indicates p < 0.05 (PBBI vs. PBBI+SBC).
Treatment with SBC significantly reduced β-APP detected axonal injury at 3 DPI by 53% (4 hours SBC, p < 0.01) and 41% (8 hours SBC, p < 0.01) (Fig. 3C). The effects of SBC on the delayed axonal injury measured by silver staining at 21 DPI were also reduced, although not significantly, yielding 6% (4 hours SBC, p = 0.54) and 29% (8 hours SBC, p = 0.21) (Fig. 3D) reductions in quantified silver staining.
Neurobehavioral deficits
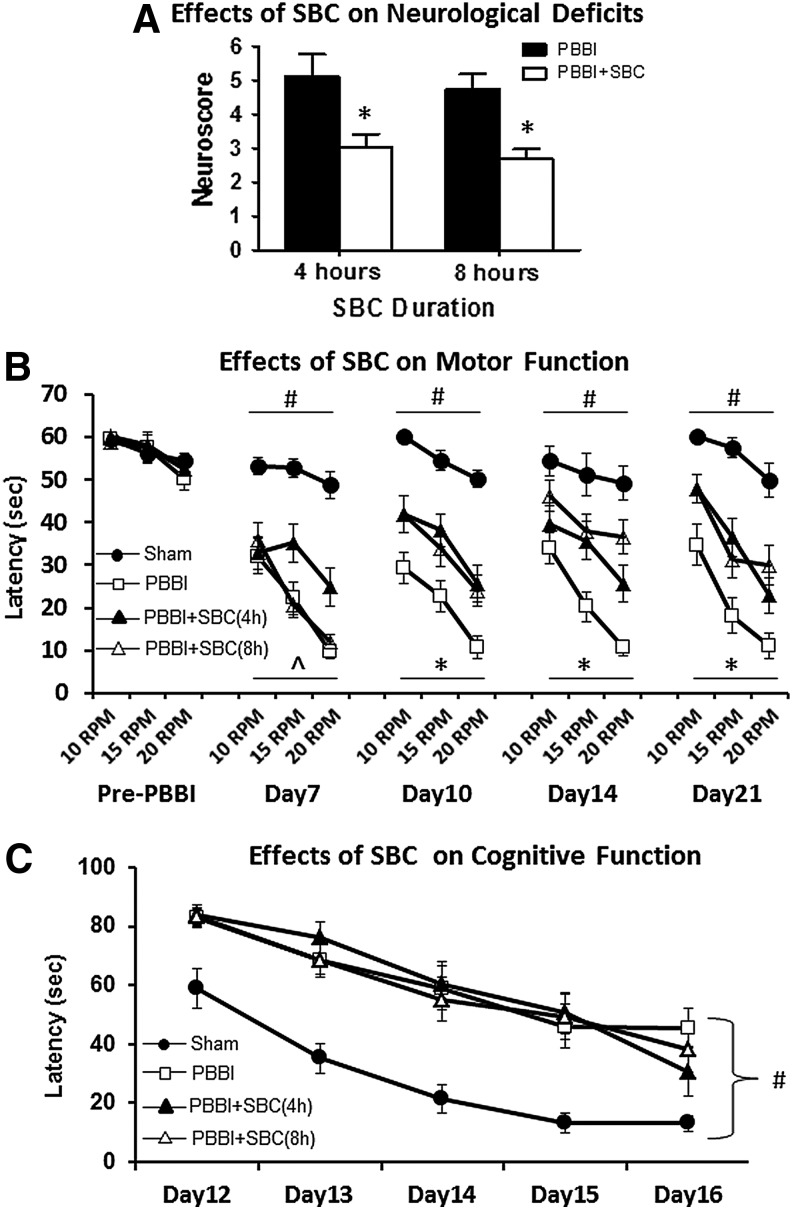
Compared to sham control animals, all PBBI alone animals displayed comparable impairment on neurological (neuroscore), motor (rotarod test), and cognitive (MWM test) functions regardless of 4 or 8 hours exposure of anesthesia. Thus, the sham control animals with 4 and 8 hours anesthesia were pooled together for motor and cognitive data analysis. This same approach was applied to the 4 and 8 hours PBBI alone animals (Fig. 4B, C).
FIG. 4.
SBC treatment significantly improved neurological (A) and motor (B) functions measured by neuroscores at 3 DPI and rotarod test between 7 and 21 DPI, respectively. SBC treatment failed to improve cognitive performance measured by Morris water maze (MWM) test between 13 and 17 DPI (C). # indicates p < 0.05, Sham vs. PBBI (average three speeds in (C)). ^ indicates p < 0.05, PBBI vs. 4 hours SBC. * indicates p < 0.05, PBBI vs. both 4 and 8 hours PBBI+SBC.
Compared with PBBI alone group, SBC treatment produced significant, 41% (4 hours SBC, p < 0.05) and 44% (8 hours SBC, p < 0.005), improvement in neurological deficits measured by the neuroscore at 3 DPI (Fig. 4A). Significant improvement in motor function measured by the rotarod task was apparent 7 DPI following 4 hours SBC treatment (PBBI vs. PBBI+SBC(4 hours): p < 0.05, average of three speeds) and 10 DPI following 8 hours SBC treatment, (p < 0.005, Fig. 4B). Such improvement was maintained throughout 21 DPI (p < 0.05 at 14 and 21 DPI for both SBC treatment protocols).
SBC treatment failed to improve cognitive outcome on the MWM task regardless of the treatment duration. While there appeared to be a trend toward improved spatial learning performance on the last day of MWM testing, this was not significant following either 4 hours (p = 0.11) or 8 hours (p = 0.24) of SBC exposure (Fig. 4C).
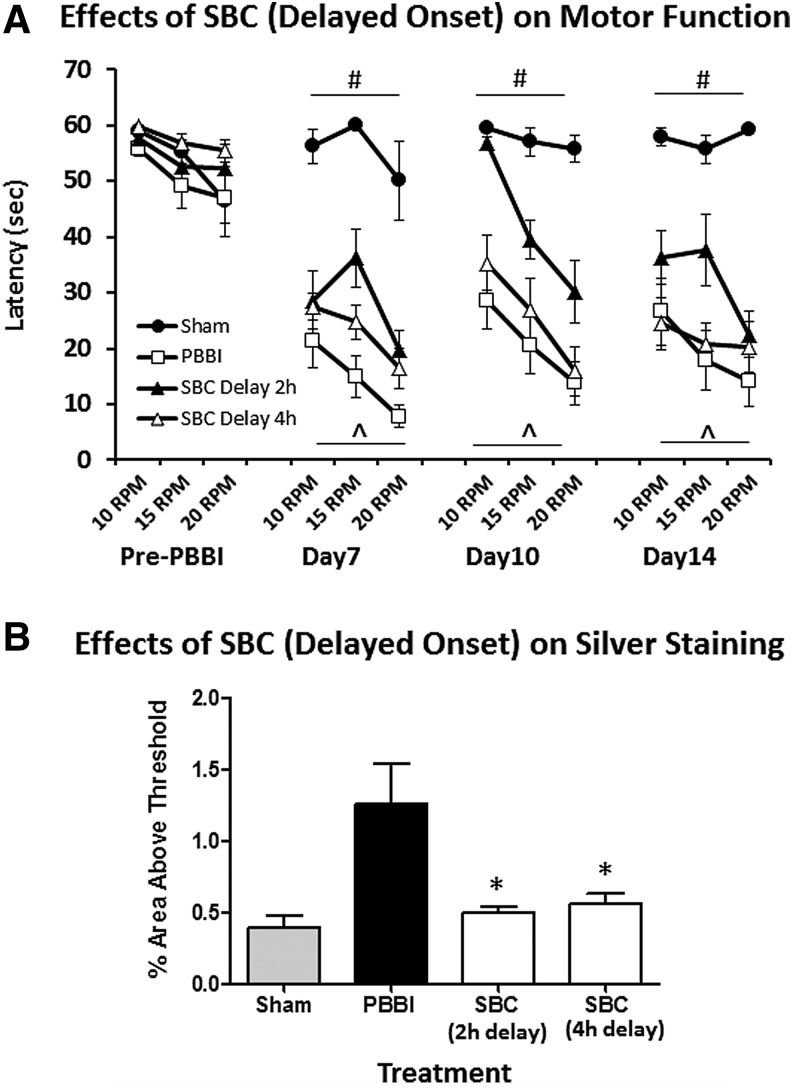
Experiment 4. Effects of SBC (4 hours) initiated 2 or 4 hours after PBBI
Both 4 and 8 hours SBC initiated immediately after PBBI produced comparable benefits. Thus, the effects of delayed SBC treatment was only evaluated using the 4 hours SBC duration protocol. Results showed that a 2-hour delay in SBC onset still provided significant improvement on motor function measured by the rotarod test at 7, 10, and 14 DPI (p < 0.01, p < 0.005, and p < 0.05, vs. PBBI, respectively), but a 4-hour delay was not effective (p > 0.05 vs. PBBI; Fig. 5A). At 14 DPI both the 2- and 4-hour delayed SBC treatment significantly reduced silver stained axonal pathology by 60% (p < 0.05 vs. PBBI) and 55% (p < 0.05 vs. PBBI), respectively (Fig. 5B).
FIG. 5.
(A) The neuroprotective effects of SBC on motor function were maintained when the initiation of the treatment was delayed to 2 hours but not 4 hours postinjury. (B) Delayed SBC treatment (either by 2 or 4 hours) significantly reduced silver stained axonal injury measured at 14 DPI. # indicates p < 0.05 (Sham vs. PBBI). ^ indicates p < 0.05 PBBI vs. PBBI+SBC (2-hours delay only), * indicates a p < 0.05 (PBBI vs. PBBI+SBC).
Experiment 5. Effects of SBC treatment (4 hours) on post-traumatic NCS
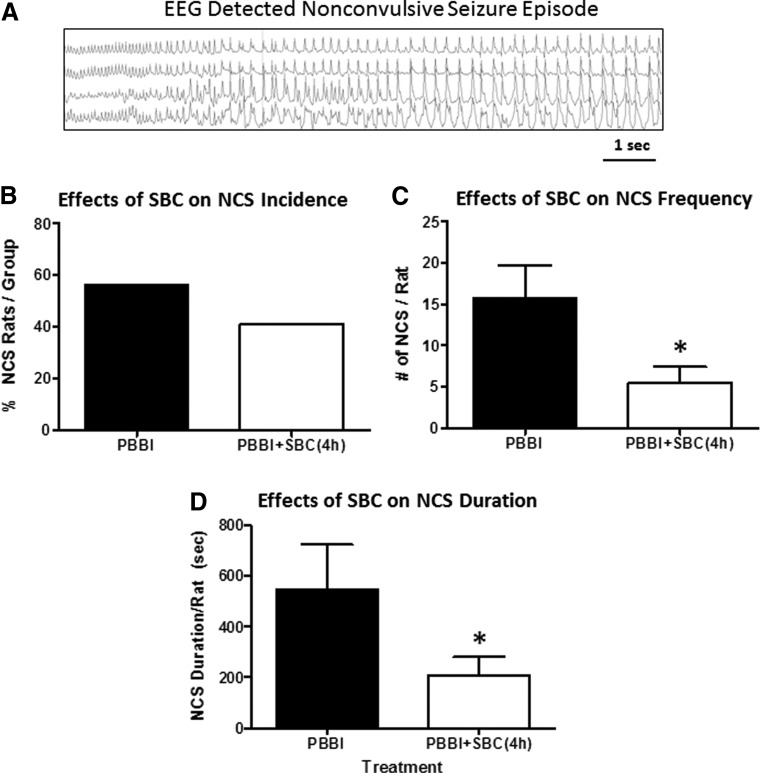
Following PBBI, 56% of PBBI alone animals experienced NCS with an onset latency of 24 hours, whereas 41% of PBBI+SBC animals experienced NCS and their onset latency was delayed to 38 hours postinjury. Neither of these measures reached statistical significance (p = 0.51 and p = 0.0.21 vs. PBBI for NCS incidence and latency, respectively, Fig. 6B). Importantly, compared to PBBI alone animals, SBC treatment significantly reduced NCS frequency by 65% (p < 0.05, Fig. 6C), yielding a 59% reduction in cumulative NCS duration (p < 0.05, Fig. 6D), although the NCS episode duration was not affected (PBBI = 33.39 ± 6.01 s/episode; PBBI+SBC = 28.73 ± 7.44 s/episode, p > 0.05, data not shown).
FIG. 6.
(A) Representative EEG detected nonconvulsive seizure (NCS). SBC treatment (initiated immediately post-PBBI and maintained for 4 hours) reduced NCS incidence (B) and significantly attenuated NCS frequency (C), and total duration (D) compared with untreated PBBI group. * indicates a p < 0.05.
Discussion
This study demonstrated comprehensive neuroprotective benefits of SBC (34°C) via the CCA cooling method in amelioration of brain tissue damage, axonal injury, neuroinflammation, and post-traumatic NCS during the sub-acute postinjury period (3 DPI). The protective effects of SBC on improving animals' motor functions persisted throughout 14–21 DPI, even when the SBC treatment was delayed by 2 hours after PBBI. However, SBC failed to protect against cognitive dysfunction or mitigate brain tissue damage out to 21 DPI, suggesting that enduring protection may require more prolonged exposure to SBC. In keeping with this, studies of ischemic brain injury have shown that an even 12-hours brain cooling duration failed to mitigate behavioral impairment and infarct size measured at 1 month after permanent middle cerebral artery occlusion, whereas significant beneficial effects were achieved by 48 hours brain cooling (Clark et al., 2009).
The use of β-APP and silver staining in this study demonstrated a temporal profile of axonal injury following PBBI, which appeared to respond to the immediate SBC treatments differently. The early axonal injury near the injury core was likely caused by the acute axonal cytoskeletal disruption, which inhibited axoplasmic flow and resulted in β-APP accumulation (Maxwell et al., 1997, 1999). The protective effects of the immediate SBC on reducing PBBI-induced early axonal injury were likely associated with hypothermic protection against axonal cytoskeletal damage via inhibiting calpain-mediated spectrin proteolysis (Buki et al., 1999; Buki and Povlishock, 2006) and ameliorating the loss of axonal microtubules and compaction of neurofilaments (Maxwell et al., 1999) as evidenced in other studies using different traumatic axonal injury models (Buki et al., 1999; Maxwell et al., 1999).
On the other hand, the silver stained axonal injury was detected in areas remote to the injury core, for example, thalamus, in the late phase of PBBI. SBC treatment, even delayed by 2–4 hours postinjury, reduced silver stained axonal injury at 14 DPI, but such effects were diminished by 21 DPI. Admittedly, silver stain is considered less specific for axonal degeneration because it also stains neuronal damage. Thus, the intense silver staining in regions more distant from the injury core may not solely represent axonal injury, but could be indicative of neurodegeneration as a result of the loss of connections between thalamus and its input and output areas of the brain. In fact, by 21 DPI a large lesion cavity developed in the PBBI core region, which became unsalvageable by the SBC treatment. This could be responsible for the lack of SBC effects on silver stained axonal and neuronal injury at 21 DPI.
In contrast to the benefits of SBC on acute versus chronic injury-induced histopathology, SBC improvement of motor function (rotarod performance) manifested by 7–10 DPI was sustained through 21 DPI. This discrepancy in the time course of SBC neuroprotection measured for histopathology versus neurobehavioral deficits is intriguing, but not necessarily counterintuitive. Studies of brain trauma and ischemia have repeatedly shown that neurofunctional recovery may involve integrated compensatory abilities of the brain in the absence of focal neuronal survival (Dixon et al., 1998; Lu et al., 2009). In this study, the protective effects of SBC on acute postinjury histopathology, neuroinflammation, and post-traumatic seizures might have played important roles in facilitating the brain's compensatory mechanisms to promote motor recovery.
However, limitations of our study are recognized. For example, protective effects measured may have indeed been greater if not for our method of spontaneous rewarming, which returns brain temperature back to 37°C within 30 minutes. This more rapid rewarming might potentially counteract even greater benefits of hypothermia therapy as have been described in other studies (Suehiro and Povlishock, 2001; Suehiro et al., 2003). We should note, however, that by using an identical rewarming method we previously demonstrated acute neuroprotection in both ischemic and TBI without producing overt adverse effects on CCA or other cerebral physiological and histological parameters (Wei et al., 2008, 2011). Consequently, to achieve long-term protective effects across the spectrum of outcome metrics, including cognitive function where only trends were observed in this study, slower rewarming may be required to maximize the measured therapeutic benefits of SBC. These studies are currently being addressed in our SBC model.
Another recognized limitation of this study was the use of male rats only. Considering the gender differences in response to brain injuries and hypothermia therapy, female rats should be evaluated in future studies.
Acute post-traumatic NCSs represent a debilitating consequence of severe TBI. However, treatments of these seizures are particularly difficult due to their sporadic nature, random occurrence, and intractability to standard antiepileptic drugs. Ideal management of post-traumatic seizure would rely on safe and effective method for seizure prophaxis.
The anticonvulsant property of hypothermia has been recognized for decades in clinical and preclinical studies of refractory status epilepticus (Schmitt et al., 2006; Corry et al., 2008) and brain injury-induced seizures (Clifton et al., 1993; Hayes, 2009). In most clinical cases, whole-body hypothermia therapy requires a prolonged period of maintenance, for example, 24–48 hours, to cover the high-risk period of seizure occurrence (McIntyre et al., 2003; Bernard and Rosalion, 2008; Diller and Zhu, 2009). During the whole-body cooling therapy, sedatives used to restrain patients may confound the anticonvulsant effects of sustained hypothermia. By contrast, our CCA cooling method significantly shortened cooling duration to achieve antiseizure effects without the complications of medications. More importantly, since the majority (∼90%) of NCS occurred during 18–48 hours post-PBBI (Lu et al., 2011, 2013), many hours beyond the cessation of SBC treatment, the short-term SBC therapy has in fact achieved seizure prophylaxis.
In general, the mechanisms by which hypothermia is able to blunt seizure events are considered multifactorial with evidence centered in areas that hypothermia counteracts seizure-provoked increases in cerebral metabolic demand (Maeda et al., 1999) and excessive release and accumulation of excitatory neurotransmitters (Busto et al., 1989; Globus et al., 1995). These mechanisms are plausible for situations where sustained hypothermia has direct influence on seizure occurrence during the treatment. But in this study, the effects of seizure protection appeared to extend beyond the treatment duration; thus, other mechanistic actions of SBC might play an important role in its antiseizure activities.
Recently, we demonstrated that following PBBI, there was a positive correlation between PBBI-induced NCS activities and neuroinflammation mediated by activation of astrocytes (Lu et al., 2015). The involvement of brain inflammation in seizures and epilepsy has been well documented in human and animals studies (Choi and Koh, 2008; Vezzani et al., 2013a, 2013b). Activation of astrocytes and microglia has also been shown to facilitate the development of seizures and epilepsy by increasing brain excitability (Devinsky et al., 2013) or promoting induction of major proinflammatory pathways (Vezzani et al., 2013a, 2013b). In this regard, the attenuation of PBBI-induced astrocytes and microglia by SBC treatment measured by the reduction in GFAP and OX-18 immunoreactivity might, in part, account for its anticonvulsant effects.
In summary, the findings of this study extended our previous knowledge on the acute neuroprotective effects of SBC on neurophysiological outcomes achieved by 2 hours CCA cooling and demonstrated a broad spectrum of neuroprotection in the sub-acute phase (3–21 DPI) of TBI following more prolonged SBC durations (4–8 hours). The SBC protocols used in this study were safe and sufficient to achieve multiple beneficial effects days and weeks beyond the cooling duration, which can be a promising therapeutic approach for clinical consideration. Although at this stage the clinical feasibility of CCA cuff method of SBC remains to be evaluated for TBI patients, the results of this study provide a proof-of-principle for the usefulness of SBC as an alternative “hyporthermia” therapy for mitigating the post-traumatic sequelae especially encountered in the TBI/polytrauma casualty (Tortella and Leung, 2015).
Acknowledgments
This work was supported by funding provided by the U.S. Army Medical Research and Material Command, Combat Casualty Care Research Program. We would like to thank Ms. Xiaofang Yang for her excellent technical support on these experiments.
Author Disclosure Statement
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
References
- Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, Okada P, Beers SR, Balasubramani GK, Hirtz D; Paediatric Traumatic Brain Injury Consortium. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol 2013;12:546–553 [DOI] [PubMed] [Google Scholar]
- Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database Syst Rev 2004;4:CD001048. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Rosalion A. Therapeutic hypothermia induced during cardiopulmonary resuscitation using large-volume, ice-cold intravenous fluid. Resuscitation 2008;76:311–313 [DOI] [PubMed] [Google Scholar]
- Buki A, Koizumi H, Povlishock JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol 1999;159:319–328 [DOI] [PubMed] [Google Scholar]
- Buki A, Povlishock JT. All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148:181–193 [DOI] [PubMed] [Google Scholar]
- Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke 1989;20:904–910 [DOI] [PubMed] [Google Scholar]
- Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J 2008;49:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Penner M, Wowk S, Orellana-Jordan I, Colbourne F. Treatments (12 and 48 h) with systemic and brain-selective hypothermia techniques after permanent focal cerebral ischemia in rat. Exp Neurol 2009;220:391–399 [DOI] [PubMed] [Google Scholar]
- Clifton GL, Allen S, Barrodale P, Plenger P, Berry J, Koch S, Fletcher J, Hayes RL, Choi SC. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma 1993;10:263–271 [DOI] [PubMed] [Google Scholar]
- Cobb AM, Pridgen N. Polytrauma care: a delicate balance for the military nurse case manager. J Trauma Nurs 2008;15:192–196 [DOI] [PubMed] [Google Scholar]
- Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care 2008;9:189–197 [DOI] [PubMed] [Google Scholar]
- Cunningham TL, Cartagena CM, Lu XC, Konopko M, Dave JR, Tortella FC, Shear DA. Correlations between blood-brain barrier disruption and neuroinflammation in an experimental model of penetrating ballistic-like brain injury. J Neurotrauma 2014;31:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci 2013;36:174–184 [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics 2010;7:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller KR, Zhu L. Hypothermia therapy for brain injury. Annu Rev Biomed Eng 2009;11:135–162 [DOI] [PubMed] [Google Scholar]
- Dixon CE, Markgraf CG, Angileri F, Pike BR, Wolfson B, Newcomb JK, Bismar MM, Blanco AJ, Clifton GL, Hayes RL. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J Neurotrauma 1998;15:95–103 [DOI] [PubMed] [Google Scholar]
- Doll H, Truebel H, Kipfmueller F, Schaefer U, Neugebauer EA, Wirth S, Maegele M. Pharyngeal selective brain cooling improves neurofunctional and neurocognitive outcome after fluid percussion brain injury in rats. J Neurotrauma 2009;26:235–242 [DOI] [PubMed] [Google Scholar]
- Fingas M, Clark DL, Colbourne F. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol 2007;208:277–284 [DOI] [PubMed] [Google Scholar]
- Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem 1995;65:1704–1711 [DOI] [PubMed] [Google Scholar]
- Grande PO, Reinstrup P, Romner B. Active cooling in traumatic brain-injured patients: a questionable therapy? Acta Anaesthesiol Scand 2009;53:1233–1238 [DOI] [PubMed] [Google Scholar]
- Hayes GM. Severe seizures associated with traumatic brain injury managed by controlled hypothermia, pharmacologic coma, and mechanical ventilation in a dog. J Vet Emerg Crit Care (San Antonio) 2009;19:629–634 [DOI] [PubMed] [Google Scholar]
- Hemmen TM, Lyden PD. Induced hypothermia for acute stroke. Stroke 2007;38:794–799 [DOI] [PubMed] [Google Scholar]
- Hess JR, Lawson JH. The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma 2006;60:S12–S19 [DOI] [PubMed] [Google Scholar]
- Holzer M. Devices for rapid induction of hypothermia. Eur J Anaesthesiol Suppl 2008;42:31–38 [DOI] [PubMed] [Google Scholar]
- Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R, Joffe AR, Kirpalani HM, Meyer PG, Morris KP, Moher D, Singh RN, Skippen PW; Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group. Hypothermia therapy after traumatic brain injury in children. N Engl J Med 2008;358:2447–2456 [DOI] [PubMed] [Google Scholar]
- Lu XC, Chen RW, Yao C, Wei H, Yang X, Liao Z, Dave JR, Tortella FC. NNZ-2566, a glypromate analog, improves functional recovery and attenuates apoptosis and inflammation in a rat model of penetrating ballistic-type brain injury. J Neurotrauma 2009;26:141–154 [DOI] [PubMed] [Google Scholar]
- Lu XC, Hartings JA, Si Y, Balbir A, Cao Y, Tortella FC. Electrocortical pathology in a rat model of penetrating ballistic-like brain injury. J Neurotrauma 2011;28:71–83 [DOI] [PubMed] [Google Scholar]
- Lu XC, Mountney A, Chen Z, Wei G, Cao Y, Leung LY, Khatri V, Cunningham T, Tortella FC. Similarities and differences of acute nonconvulsive seizures and other epileptic activities following penetrating and ischemic brain injuries in rats. J Neurotrauma 2013;30:580–590 [DOI] [PubMed] [Google Scholar]
- Lu XC, Shear DA, Graham PB, Bridson GW, Uttamsingh V, Chen Z, Leung LY, Tortella FC. Dual therapeutic effects of C-10068, a dextromethorphan derivative, against post-traumatic nonconvulsive seizures and neuroinflammation in a rat model of penetrating ballistic-like brain injury. J Neurotrauma 2015;32:1621–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Hashizume K, Tanaka T. Effect of hypothermia on kainic acid-induced limbic seizures: an electroencephalographic and 14C-deoxyglucose autoradiographic study. Brain Res 1999;818:228–235 [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Donnelly S, Sun X, Fenton T, Puri N, Graham DI. Axonal cytoskeletal responses to nondisruptive axonal injury and the short-term effects of posttraumatic hypothermia. J Neurotrauma 1999;16:1225–1234 [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma 1997;14:419–440 [DOI] [PubMed] [Google Scholar]
- McIntyre LA, Fergusson DA, Hebert PC, Moher D, Hutchison JS. Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA 2003;289:2992–2999 [DOI] [PubMed] [Google Scholar]
- Milhaud D, Thouvenot E, Heroum C, Escuret E. Prolonged moderate hypothermia in massive hemispheric infarction: clinical experience. J Neurosurg Anesthesiol 2005;17:49–53 [PubMed] [Google Scholar]
- Misgav M, Martinowitz U. [Trauma-induced coagulopathy—mechanisms and state of the art treatment]. Harefuah 2011;150:99–103, 207 [PubMed] [Google Scholar]
- Murakami Y, Wei G, Yang X, Lu XC, Leung LY, Shear DA, Tortella FC. Brain oxygen tension monitoring following penetrating ballistic-like brain injury in rats. J Neurosci Methods 2012;203:115–121 [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Littleton-Kearney MT, Bridges E, Bibb SC. Evaluating the Joint Theater Trauma Registry as a data source to benchmark casualty care. Mil Med 2012;177:546–552 [DOI] [PubMed] [Google Scholar]
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009;37:1101–1120 [DOI] [PubMed] [Google Scholar]
- Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis 2006;23:689–696 [DOI] [PubMed] [Google Scholar]
- Shear DA, Lu XC, Bombard MC, Pedersen R, Chen Z, Davis A, Tortella FC. Longitudinal characterization of motor and cognitive deficits in a model of penetrating ballistic-like brain injury. J Neurotrauma 2010;27:1911–1923 [DOI] [PubMed] [Google Scholar]
- Shear DA, Lu XC, Pedersen R, Wei G, Chen Z, Davis A, Yao C, Dave J, Tortella FC. Severity profile of penetrating ballistic-like brain injury on neurofunctional outcome, blood-brain barrier permeability, and brain edema formation. J Neurotrauma 2011;28:2185–2195 [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Hayakata T, Taneda M, Nakajima Y, Hashiguchi N, Fujimi S, Nakamori Y, Tanaka H, Shimazu T, Sugimoto H. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg 2001;94:50–54 [DOI] [PubMed] [Google Scholar]
- Suehiro E, Povlishock JT. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J Neurosurg 2001;94:493–498 [DOI] [PubMed] [Google Scholar]
- Suehiro E, Ueda Y, Wei EP, Kontos HA, Povlishock JT. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J Neurotrauma 2003;20:381–390 [DOI] [PubMed] [Google Scholar]
- Tortella FC, Leung LY. Traumaticbrain injury and polytrauma in theaters of combat: the case for neurotrauma resuscitation? Shock 2015;44 Suppl 1:17–26 [DOI] [PubMed] [Google Scholar]
- Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol 2013a;244:11–21 [DOI] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 2013b;69:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma 1998;44:846–854 [DOI] [PubMed] [Google Scholar]
- Wei G, Hartings JA, Yang X, Tortella FC, Lu XC. Extraluminal cooling of bilateral common carotid arteries as a method to achieve selective brain cooling for neuroprotection. J Neurotrauma 2008;25:549–559 [DOI] [PubMed] [Google Scholar]
- Wei G, Lu XC, Shear DA, Yang X, Tortella FC. Neuroprotection of selective brain cooling after penetrating ballistic-like brain injury in rats. Ther Hypothermia Temp Manag 2011;1:33–42 [DOI] [PubMed] [Google Scholar]
- Yao C, Wei G, Lu XC, Yang W, Tortella FC, Dave JR. Selective brain cooling in rats ameliorates intracerebral hemorrhage and edema caused by penetrating brain injury: possible involvement of heme oxygenase-1 expression. J Neurotrauma 2011;28:1237–1245 [DOI] [PubMed] [Google Scholar]