Abstract
Aim: Oxidative stress is a key contributor to endothelial dysfunction and associated cardiovascular pathogenesis. Hydrogen sulfide (H2S) is an antioxidant gasotransmitter that protects endothelial cells against oxidative stress. Sirtuin3 (SIRT3), which belongs to the silent information regulator 2 (SIR2) family, is an important deacetylase under oxidative stress. H2S is able to regulate the activity of several sirtuins. The present study aims to investigate the role of SIRT3 in the antioxidant effect of H2S in endothelial cells. Results: Cultured EA.hy926 endothelial cells were exposed to hydrogen peroxide (H2O2) as a model of oxidative stress-induced cell injury. GYY4137, a slow-releasing H2S donor, improved cell viability, reduced oxidative stress and apoptosis, and improved mitochondrial function following H2O2 treatment. H2S reversed the stimulation of MAPK phosphorylation, downregulation of SIRT3 mRNA and reduction of the superoxide dismutase 2 and isocitrate dehydrogenase 2 expression which were induced by H2O2. H2S also increased activator protein 1 (AP-1) binding activity with SIRT3 promoter and this effect was absent in the presence of the specific AP-1 inhibitor, SR11302 or curcumin. Paraquat administration to mice induced a defected endothelium-dependent aortic vasodilatation and increased oxidative stress in both mouse aorta and small mesenteric artery, which were alleviated by GYY4137 treatment. This vasoprotective effect of H2S was absent in SIRT3 knockout mice. Innovation: The present results highlight a novel role for SIRT3 in the protective effect of H2S against oxidant damage in the endothelium both in vitro and in vivo. Conclusion: H2S enhances AP-1 binding activity with the SIRT3 promoter, thereby upregulating SIRT3 expression and ultimately reducing oxidant-provoked vascular endothelial dysfunction. Antioxid. Redox Signal. 24, 329–343.
Introduction
Cardiovascular disease is one of the leading causes of death worldwide. A number of factors, including hypertension, high blood sugar, and/or cholesterol, as well as smoking, are known to impair vascular endothelial cell function leading to a plethora of deleterious cardiovascular pathogenesis, including vasoconstriction, proliferation of vascular smooth muscle, vascular remodeling, and changes in platelet function. These changes in turn contribute to the etiology of hypertension, myocardial infarction, and stroke (29). Accordingly, the protection of vascular endothelial cells from damage, for example, due to oxidative stress, has long been considered as the key to the treatment and/or prevention of cardiovascular disease.
Innovation.
Hydrogen sulfide (H2S) is known to protect endothelial cells against oxidative stress, although the underlying molecular mechanism is only partially understood. The present study demonstrates that H2S protects endothelial cells against oxidative damage by enhancing activator protein 1 binding activity with the sirtuin3 (SIRT3) promoter, thereby upregulating the expression of SIRT3 and its downstream genes, SOD2 and IDH2, as well as improving vascular endothelial function. The present study sheds new light on the molecular mechanism responsible for the cytoprotective effect of H2S through SIRT3 activation in endothelial biology.
The silent information regulator 2 (SIR2) family is functionally important in endothelial cells under oxidative stress (45). Sirtuin3 (SIRT3), one deacetylase belonging to SIR2, increases reactive oxygen species (ROS)-scavenging capacity by enhancing antioxidant enzyme (superoxide dismutase, SOD) activity (6). SIRT3−/− mice showed increased mitochondrial matrix oxidant stress without augmentation of intermembrane space or cytosolic oxidant signaling during sustained hypoxia (43).
Hydrogen sulfide (H2S) is not only a potent antioxidant (19), vasodilator (52), and inhibitor of both vascular smooth muscle proliferation (49) and myocardial apoptosis (8) but also synthesized endogenously in a wide array of cell types either from L-cysteine by cystathionine γ-lyase (CSE) and/or cystathionine β-synthase (CBS) or from cysteine and 3-mercaptopyruvate by cysteine aminotransferase and 3-mercaptopyruvate sulfurtransferase (3-MST) (19). Wen et al. reported that H2S protected endothelial cells against oxidative stress by acting first as an antioxidant and second by maintaining mitochondrial structure and function (44). Several studies suggest that H2S is able to regulate the activity of the sirtuin family, such as upregulation of sirtuin1 (SIRT1) in human PC12 cells (18) and human umbilical vein endothelial cells (HUVECs) (36, 53) and increase of SIRT3 (4) and sirtuin 6 (SIRT6) (12), to exert either physiological or pathophysiological effects. Nevertheless, the precise mechanisms of the antioxidant effect of H2S in endothelial cells remain unclear.
In the present study, we used a slow-releasing H2S donor drug, GYY4137 (17), to examine the antioxidant effect of H2S in endothelial cells and to investigate the downstream signal mechanisms involved. We have identified a completely novel role for SIRT3 in regulating the endothelial response to H2S, thereby raising the possibility that H2S interfering with SIRT3 may be of value in the treatment of cardiovascular diseases, which are underpinned by oxidative stress.
Results
The effect of GYY4137 on H2S concentration, survival, and apoptosis of endothelial cells exposed to H2O2
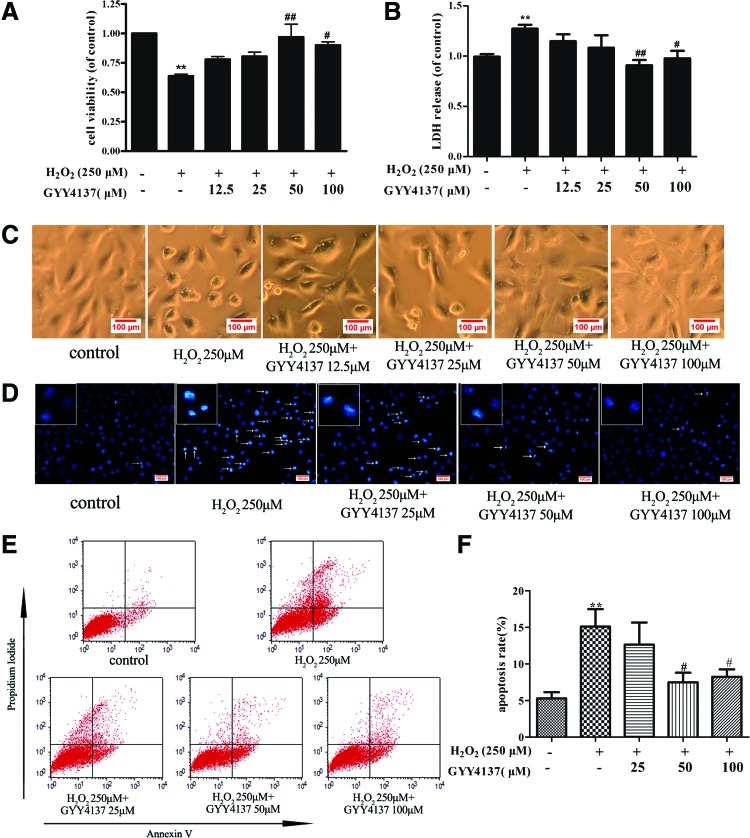
Assessment of H2S release by amperometry showed that exposure of endothelial cells to H2O2 has no significant influence on H2S concentration in the medium (1.56 ± 0.13 μM vs. 1.37 ± 0.09 μM). Exposure of endothelial cells to GYY4137 (12.5, 25, 50, and 100 μM) before H2O2 treatment enhanced H2S concentration (2.15 ± 0.11, 3.14 ± 0.11, 5.46 ± 2.56, and 6.96 ± 0.38 μM, respectively). Meanwhile, H2O2 caused 36% ± 4% of cell death, and GYY4137 (50 and 100 μM) protected endothelial cells against the toxic effect of H2O2 (Fig. 1A), which were confirmed by lactate dehydrogenase (LDH) assay (Fig. 1B). Similar results were obtained when endothelial cells were visualized microscopically. Exposure to H2O2 caused cells to lose their typical fusiform shape as cobblestones and a rounded appearance, which suggests that a number of cells were dead after H2O2 treatment, while H2S preadministration partially restored cell structure and decreased cell death (Fig. 1C). Apoptosis was also measured by Hoechst 33342 staining and Annexin V/PI-positive staining. H2O2 triggered apoptosis in endothelial cells, as evidenced by the condensation and fragmentation of nuclei, and this apoptotic effect was significantly reduced by H2S (representative photomicrographs are shown in Fig. 1D). Likewise, Annexin V/PI-positive staining, as assessed by flow cytometry, showed greater staining in the presence of H2O2, indicative of apoptosis, which effect was reduced in cells pretreated with GYY4137 (Fig. 1E, F). Collectively, the present results demonstrate that exogenous H2S improves cell viability and decreases apoptosis in H2O2-treated endothelial cells.
FIG. 1.
Protective effect of H2S on H2O2-induced cell injury in endothelial cells. EA.hy926 endothelial cells were pretreated with GYY4137 (12.5, 25, 50, and 100 μM) for 4 h before H2O2 (250 μM, 4 h). (A) Cell viability in EA.hy926 endothelial cells was measured by the CCK-8 kit. (B) Lactate dehydrogenase (LDH) release in cell culture medium was assayed by a commercial LDH kit. (C) Shape of EA.hy926 cells was detected with a light microscope. (D) Cells were stained with Hoechst 33342 and the images were taken under a fluorescence microscope, white arrows indicate the apoptotic cells. (E, F) Cells were stained with Annexin V/PI and apoptotic rates were analyzed by flow cytometry. **p < 0.01 versus control; #p < 0.05, ##p < 0.01 versus the H2O2-treated group, n = 5–7. H2O2, hydrogen peroxide; LDH, lactate dehydrogenase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The effect of H2S on oxidative stress, mitochondrial function, and mitochondrial permeability potential in endothelial cells exposed to H2O2
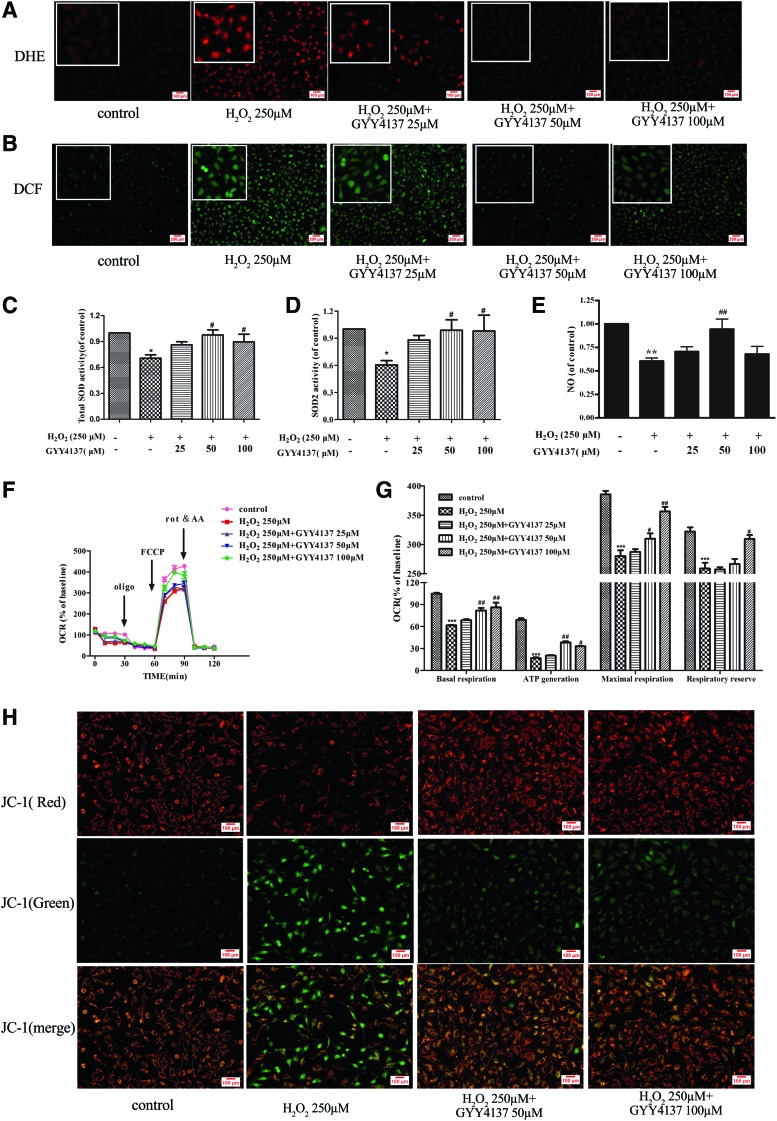
Oxidative stress plays an important role in the pathogenesis of cell death. To determine whether the protective role of H2S against apoptosis is related to reduction of ROS, redox status was monitored by dihydroethidium (DHE) oxidative fluorescence microtopography and the dichloro-dihydro-fluorescein diacetate (DCFH-DA) method. Endothelial cells responded to H2O2 with a significant rise in ROS formation and this rise was reduced by pretreatment of cells with GYY4137 (50 and 100 μM) (Fig. 2A, B). In addition, redox status is also related to the activity of antioxidant enzymes because H2O2 also reduced endothelial total SOD and Mn-SOD (SOD2) enzyme activity, while these effects were again reversed by H2S (Fig. 2C, D). In addition, H2S also elevated nitric oxide (NO) content to improve endothelial function after H2O2 treatment (Fig. 2E).
FIG. 2.
Effect of H2S on ROS accumulation, SOD activity, mitochondrial function, and NO synthesis in endothelial cells with H2O2 treatment. EA.hy926 endothelial cells were pretreated with GYY4137 (25–100 μM) for 4 h before H2O2 (250 μM, 4 h). (A, B) Cellular ROS production was detected by DHE and DCFH-DA staining. (C, D) Total SOD and Mn-SOD (SOD2) enzyme activities were assessed using an SOD kit. (E) NO production was measured with an NO sensor. (F) The bioenergetic profiles of EA.hy926 cells were detected by a Seahorse Extracellular Flux Analyzer, oxygen consumption rate (OCR) in cells treated with oligomycin (2 μg/ml), FCCP (2 μM), and antimycin A and rotenone (rot and AA, respectively, 4 μM). (G) Basal respiration, ATP generation, maximal respiratory, and respiratory reserve capacity are shown. (H) Mitochondrial permeability transition (ΔΨm) was determined by JC-1 staining. *p < 0.05, **P < 0.01, ***p < 0.001 versus control, #p < 0.05, ##p < 0.01 versus the H2O2-treated group, n = 3–8. ROS, reactive oxygen species; SOD, superoxide dismutase; NO, nitric oxide; DHE, dihydroethidium; DCFH-DA: dichlorodihydrofluorescein diacetate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The decreased cellular ROS in H2S-treated cells led us to postulate that mitochondrial function could be impaired by H2O2. To test this hypothesis, we measured the oxygen consumption rate (OCR) in the presence and absence of H2S using the Seahorse XF analyzer. Figure 2F and G shows that cellular respiration and the response to modifiers of mitochondrial function were significantly decreased in H2O2-treated cells. We then performed the MitoStress test to quantify several bioenergetic parameters and we found that H2O2 significantly suppressed the basal respiration and oxygen consumption due to ATP turnover. Maximal respiration and respiratory reserve capacity were also significantly reduced by H2O2, indicating a generally depressed mitochondrial activity. Notably, H2S prevented these effects of H2O2 (Fig. 2F, G). In addition, mitochondrial permeability transition (Δψm) also was determined by JC-1 staining, as depicted in Figure 2H. Treatment of H2O2 resulted in the increase of green fluorescence intensity, but the decrease of red fluorescence intensity, indicating that the Δψm of the cells was significantly decreased. Pretreatment with H2S attenuated H2O2-induced collapse of Δψm in endothelial cells (Fig. 2H). These results suggest that H2S can improve mitochondrial function to limit oxidative stress in H2O2-injured endothelial cells.
The effect of H2S on the MAPK signaling pathway and caspase-3
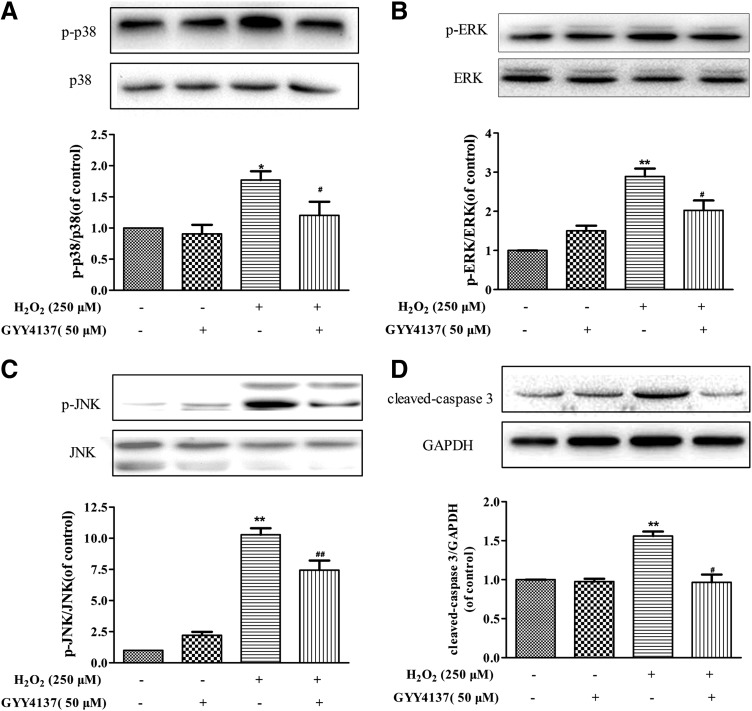
Mitochondrial ROS are known to activate the mitogen-activated protein kinase (MAPK) pathway, which in turn participates in cell apoptosis. As shown in Figure 3A–C, H2S pretreatment attenuated H2O2-induced phosphorylation of the MAPK family, including p38 MAPK, ERK1/2, and Jun N-terminal kinase 1/2(JNK1/2). Caspases are crucial mediators of apoptosis. Among them, caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins (26). As expected, the H2O2-induced expression of cleaved caspase-3 was reversed by H2S (Fig. 3D).
FIG. 3.
Effect of H2S on MAPK signal pathway and caspase-3. EA.hy926 endothelial cells were pretreated with GYY4137 (50 μM) for 4 h before H2O2 (250 μM, 4 h). (A–C) Representative Western blots and quantification of p-p38 MAPK, p-ERK, and p-JNK protein expression. (D) Representative examples of Western blots and quantification of cleaved capase-3 protein expression. *p < 0.05, **p < 0.01 versus control, #p < 0.05, ##p < 0.01 versus the H2O2-treated group, n = 4–6. MAPK, mitogen-activated protein kinase.
SIRT3 is involved in the protective effect of H2S in endothelial cells
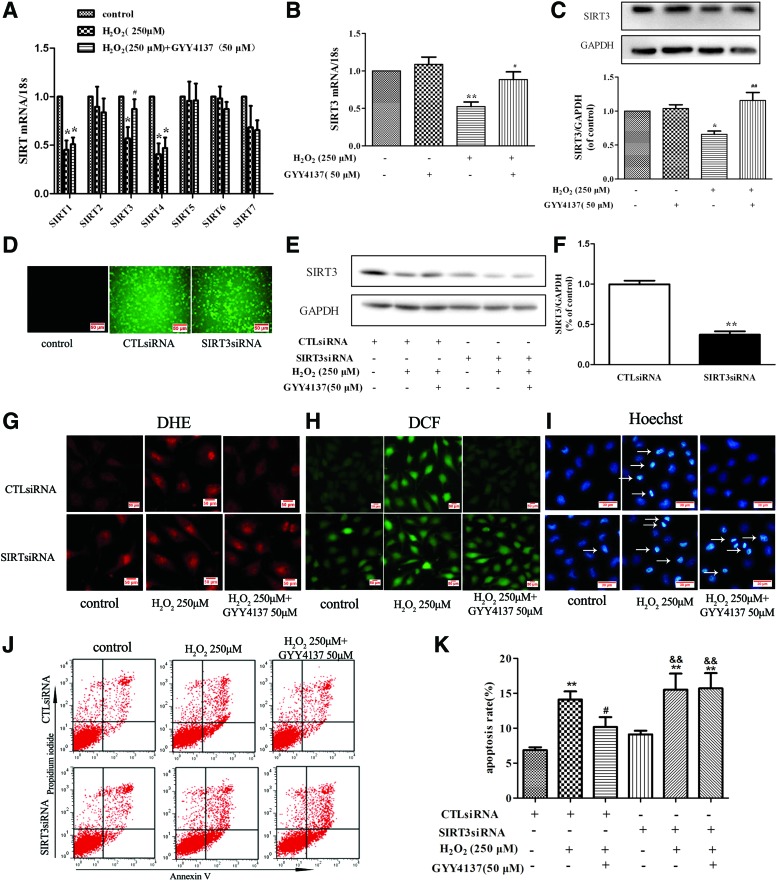
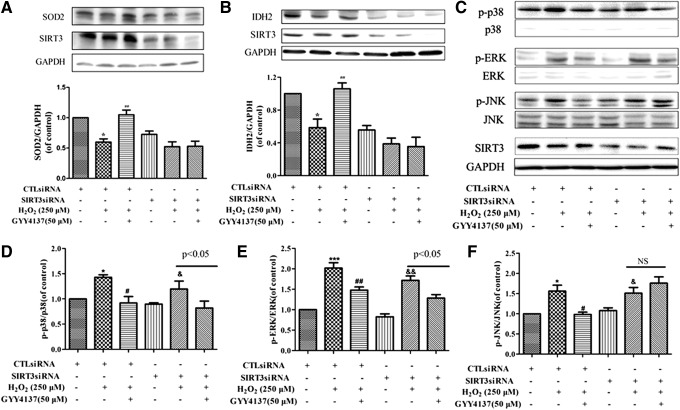
SIR2 is a family of highly conserved NAD-dependent histone deacetylases that act as cellular sensors to detect energy availability and thus modulate metabolic processes, including mitochondrial function (45). SIR2 exists widely in mammals and consists of seven members (SIRT1-SIRT7), which vary in their cellular targeting and location and play a significant role in metabolism, carcinomatosis, cell survival, and other physiological and pathological processes (1). To examine the involvement of SIR2 in the antioxidant effect of H2S in endothelial cells, we examined mRNA expression of SIRT1-7 after exposure to H2O2. The mRNA expression of SIRT1, SIRT3, and SIRT4 was reduced in H2O2-treated cells, while expression of SIRT2, SIRT5, SIRT6, and SIRT7 was unchanged. Expression of SIRT3 (but not SIRT1 or SIRT4) mRNA level was restored to near baseline in cells incubated with GYY4137 (50 μM, 4 h) (Fig. 4A). Moreover, H2S alone did not alter mRNA and protein expression of SIRT3, but it reversed H2O2-induced reduction in SIRT3 expression in endothelial cells exposed to H2O2 (Fig. 4B, C). The positive role of SIRT3 in endothelial cells treated with H2S was further supported by silencing experiments. SIRT3-specific siRNA was transfected into endothelial cells (Fig. 4D) to reduce expression of SIRT3 (37% ±10% vs. 100% ±11%, Fig. 4E, F). SIRT3 silencing abolished the ability of H2S to reverse the H2O2-induced oxidant stress (Fig. 4G, H) and apoptosis in endothelial cells (Fig. 4I–K).
FIG. 4.
Role of SIRT3 in H2S-mediated protection of endothelial cell injury by H2O2. (A, B) EA.hy926 endothelial cells were pretreated with GYY4137 (50 μM) for 4 h before H2O2 (250 μM, 4 h). Quantification of SIRT family (SIRT1 to SIRT7) mRNA expression was determined by real-time PCR. *p < 0.05 versus control, #p < 0.05 versus the H2O2-treated group, n = 6–7. (C) Representative examples of Western blots and quantification of SIRT3 protein. *p < 0.05 versus control, ##p < 0.01 versus the H2O2-treated group, n = 5. (D) EA.hy926 endothelial cells were transfected with SIRT3-specific siRNA (SIRT3siRNA) or a nonspecific control siRNA (CTLsiRNA). Transfection efficiency was assessed by immunofluorescence. (E, F) Representative examples of Western blots and quantification of SIRT3 after transfection. **p < 0.01 versus CTLsiRNA, n = 6. Transfected with CTLsiRNA or SIRT3siRNA for 24 h, EA.hy926 endothelial cells were exposed to H2O2 (250 μM, 4 h) after pretreatment with GYY4137 (50 μM) for 4 h, (G, H) ROS in endothelial cells was examined by DHE and DCFH-DA staining, and (I) apoptosis in endothelial cells was detected with Hoechst 33342 staining, white arrows indicate the apoptotic cells. (J, K) Cells were stained with Annexin V/PI and apoptotic rates were analyzed by flow cytometry. **p < 0.01 versus CTLsiRNA transfection, #p < 0.05, ##p < 0.01 versus the H2O2-treated group with CTLsiRNA transfection, &&p < 0.01 versus SIRT3siRNA transfection, n = 5. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The effect of H2S on SIRT3-regulated signaling in endothelial cells
Two direct mechanisms linking SIRT3 with reduced ROS have been proposed following the identification of isocitrate dehydrogenase 2 (IDH2) and SOD2 as direct targets for SIRT3. SIRT3, as a major mitochondrial NAD+-dependent deacetylase, directly deacetylates and activates mitochondrial IDH2 and leads to increased NADPH levels and thereby an increased ratio of reduced to oxidized glutathione in mitochondria (34). A pivotal mitochondrial ROS-scavenging enzyme, SOD2, can be activated by SIRT3-mediated deacetylation to reduce levels of superoxide anions (6, 28, 38). Therefore, the expression of IDH2 and SOD2 was measured. Exposure of endothelial cells to H2O2 reduced expression of both SOD2 and IDH2, and this effect was reversed by pretreatment with GYY4137 (50 μM, 4 h). H2S failed to reverse this effect of H2O2 in cells transfected with SIRT3 siRNA (Fig. 5A, B). In addition, to test whether the effect of H2S on MAPKs is mediated by SIRT3 in H2O2-injured endothelial cells, we determined the expression of p-JNK, p- p38 MAPK, p-ERK, and total MAPK (p38 MAPK, ERK, JNK) in SIRT3 knockdown cells. As shown in Figure 5C–F, after transfection with SIRT3 siRNA, H2S failed to decrease the phosphorylation of JNK, but it was still able to attenuate the levels of p-p38 MAPK and p-ERK. These results suggest that H2S protects endothelial cells against oxidative damage not only by augmenting SIRT3-mediated IDH2, SOD2, and JNK pathways but also by impacting p38 MAPK and ERK pathways.
FIG. 5.
Effect of H2S on the SIRT3-regulated SOD2, IDH2, and MAPK signaling pathways in endothelial cells. Transfected with CTLsiRNA or SIRT3siRNA for 24 h, EA.hy926 endothelial cells were exposed to H2O2 (250 μM, 4 h) after pretreatment with GYY4137 (50 μM) for 4 h. (A, B) Representative Western blots and quantification of SOD2 and IDH2 protein expression. (C–F) Representative Western blots and quantification of p-p38 MAPK, p-ERK, and p-JNK protein expression. *p < 0.05, ***p < 0.001 versus CTLsiRNA transfection, #p < 0.05, ##p < 0.01 versus the H2O2-treated group with CTLsiRNA transfection, &p < 0.05, &&p < 0.01 versus SIRT3siRNA transfection, n = 3–4. IDH, isocitrate dehydrogenase; NS, no statistical significance.
The effect of H2S on SIRT3 gene transcription
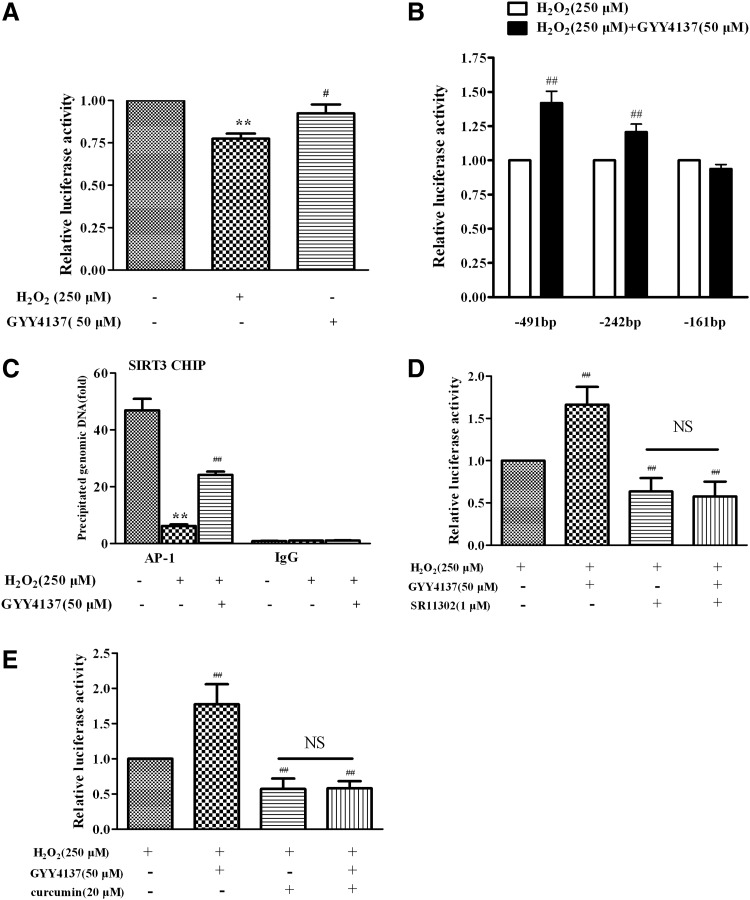
To investigate the mechanism by which H2S regulated the transcription of the SIRT3 gene in response to oxidative stress triggered by H2O2, a number of luciferase reporter plasmids containing a series SIRT3 promoter constructs with various lengths were constructed. EA.hy926 endothelial cells were transiently transfected with luciferase reporter plasmids containing the SIRT3 promoter (−491/+146). The reporter assays revealed a diminished SIRT3 promoter activity in endothelial cells exposed to H2O2, which was reversed by H2S (Fig. 6A). With a series of deletion constructs, the stimulatory effects of H2S on SIRT3 promoter activity were observed in −491 Luc and −242 Luc, of which the 5′ ends correspond to 491 bp and 242 bp from the transcription start site, respectively. However, H2S-induced enhancement of SIRT3 promoter activity was abolished in −161 Luc (Fig. 6B), suggesting that the presence of a critical site between 242 bp and 161 bp on the upstream of the SIRT3 promoter was responsible for the effect of H2S on SIRT3 transcription. The putative AP-1 binding site is present in this region of the SIRT3 promoter and the ChIP assay showed that H2S increased AP-1 binding activity with the SIRT3 promoter, which had been decreased in endothelial cells treated with H2O2 (Fig. 6C). The enhanced effect on SIRT3 promoter activity in the presence of H2S was absent when specific AP-1 inhibitor, SR11302 (1 μM) or curcumin (20 μM), was present (Fig. 6D, E). Collectively, these results suggest that H2S upregulated the SIRT3 gene expression via increasing the AP-1 binding activity with the SIRT3 promoter.
FIG. 6.
Effect of H2S on SIRT3 gene transcript activity. (A) EA.hy926 endothelial cells were transfected with SIRT3 promoter (−491/+146) luciferase fusion plasmid and pRL-TK plasmids. Twenty-four hours later, cells were pretreated with GYY4137 (50 μM) for 4 h before H2O2 (250 μM, 4 h). SIRT3 promoter luciferase activity was determined using a dual-luciferase reporter assay system. (B) Cells were transfected with plasmids contain the SIRT3 promoter region up to −491, −242, and −161, respectively. The 3′ end of the promoter of all of these constructs corresponds to +146. Transfected cells were treated and promoter luciferase activities were measured as described above. **p < 0.01 versus control, #p < 0.05, ##p < 0.01 versus the H2O2-treated group, n = 5–7. (C) Cells were pretreated with GYY4137 (50 μM) for 4 h before H2O2 (250 μM, 4 h). Chromatin fragments for ChIP assays were immunoprecipitated with anti-AP-1 antibody. Precipitated DNA was amplified by real-time PCR with primers spanning the SIRT3 promoter region. IgG as a negative control. **p < 0.01 versus control, ##p < 0.01 versus the H2O2-treated group, n = 4. (D, E) Cells were transfected with SIRT3 promoter (−491/+146) luciferase fusion plasmid. Twenty-four hours later, cells were treated with specific AP-1 inhibitor, SR11302 (1 μM) or curcumin (20 μM), for 4 h. Then, GYY4137 (50 μM) was pretreated for 4 h before H2O2, and then luciferase activities were determined. ##p < 0.01 versus the H2O2-treated group, n = 4. AP-1, activator protein 1; NS, no statistical significance.
The protective effect of H2S in SIRT3 KO mice
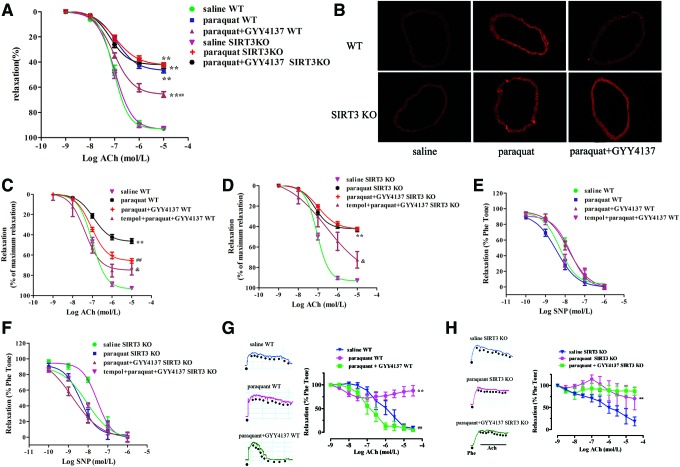
Our results suggest that H2S-regulated SIRT3 is a critical endogenous inhibitor of oxidative damage induced by H2O2 in endothelial cells in vitro. To further explore the pathophysiological significance of H2S-induced SIRT3 expression in vivo, a state of oxidative stress was induced in mice by administration of paraquat (21). Mice treated with paraquat showed impaired endothelium-dependent relaxations and increased oxidative stress in their aortas (Fig. 7A, B). H2S administration improved endothelium-dependent relaxations accompanied by reducing oxidative stress in paraquat-treated mice. More importantly, H2S treatment lost its ability to augment endothelium-dependent relaxations and to lower oxidative stress in the aortas of SIRT3 KO mice (Fig. 7A, B). Although vascular oxidative stress was eliminated by tempol, H2S produced a greater improvement of endothelium-dependent relaxations in tempol preincubated aortas in WT mice, followed by paraquat and GYY4137 administration, than in SIRT3 KO mice (p < 0.05, Fig. 7C, D). In addition, there was no difference in aortic relaxation in response to NO donor (SNP) among all groups, suggesting that the sensitivity of vascular smooth muscle to NO was not altered (Fig. 7E, F). Moreover, H2S treatment augmented endothelium-dependent dilatations in small mesenteric arteries from WT mice, but not SIRT3 KO mice (Fig. 7G, H). These results demonstrate that SIRT3 is most likely required for H2S to inhibit endothelial oxidative damage in vivo, leading to improved endothelial function.
FIG. 7.
Effects of H2S on vasorelaxation in SIRT3 KO mice. (A) WT or SIRT3 KO mice were injected with either paraquat (50 mg/kg, i.p) or saline 1 h before either GYY4137 (133 μM/kg, ip) or saline. Mice were killed and aortic segments were removed 24 h thereafter. Endothelium-dependent vasorelaxation to acetylcholine of precontracted aortic sections was assessed. **p < 0.01 versus the saline-treated group of the same genotype, ##p < 0.01 versus the paraquat-treated group of the same genotype, n = 6. (B) DHE staining of aorta for superoxide production (n = 4). (C, D) Above aortic segments were preincubated with tempol (1 mM) for 30 min, then endothelium-dependent vasorelaxation to acetylcholine of precontracted aortic sections was assessed. **p < 0.01 versus the saline-treated group of the same genotype, ##p < 0.01 versus the paraquat-treated group of the same genotype, &p < 0.05 versus paraquat- and GYY4137-treated groups of the same genotype, n = 6. (E, F) Vasorelaxation to SNP of precontracted aortic sections was assessed. (G, H) Endothelium-dependent vasorelaxation in small mesenteric artery to acetylcholine of precontracted aortic sections was assessed. **p < 0.01 versus the saline-treated group of the same genotype, ##p < 0.01 versus only the paraquat-treated group of the same genotype, n = 4–6. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Discussion
Oxidative stress can be broadly defined as an imbalance of ROS production over antioxidant defenses. The mitochondrial respiratory chain is not only a major source of intracellular ROS generation but also an important target for the damaging effects of ROS (31). Endothelial cells are crucial both for vascular homeostasis and for protecting the vasculature against oxidant species. Endothelial cells are replete with CSE and are endogenous sources of H2S. The latter is a powerful antioxidant and it may defend the endothelium against oxidative stressors.
In the present study, oxidative injury was induced by H2O2 and paraquat, both in vitro and in vivo, and the protective effect of H2S was assessed using the donor, GYY4137. Consistent with previous studies (13, 44), the present study shows that H2S protects endothelial cells from cytotoxicity and improves mitochondrial function against H2O2 insult.
The seven mammalian sirtuin orthologs (Sirt1-7) have been studied in diverse disease models, including insulin resistance and diabetes, inflammation, neurodegenerative disease, and cancer, and more recently in cardiovascular pathologies such as cardiac hypertrophy, heart failure, and atherosclerosis (45). mRNA expression of all SIRT1-7 genes was reduced with advanced passages in several kinds of endothelial cells and such reduction was exaggerated in high-glucose-treated cells (22). Our study shows that mRNA expression of SIRT1, SIRT3, and SIRT4 was reduced in H2O2-treated cells, while the expression of SIRT2, SIRT5, SIRT6, and SIRT7 was unaffected. Other studies show that the expression and activity of SIRT1 can be increased by H2S (18, 36, 53). Zheng et al. found that H2S delayed nicotinamide-induced premature senescence of HUVECs via upregulation of SIRT1 (53). H2S also prevented H2O2-induced senescence of HUVECs through SIRT1 activation (36). The present results indicate that SIRT1 mRNA levels decreased in cultured endothelial cells following H2O2 treatment; however, this effect was not restored by H2S cotreatment. As such, our observation contrasted with a previous report that NaHS and GYY4137 were able to reverse H2O2-induced reduction of SIRT1 mRNA in HUVECs and in hyperoxic lungs (36, 41). This discrepancy may be attributed to different regulatory mechanisms after H2O2 exposure in different cell types, the use of different H2S treatment regiments giving rise to different kinetics of H2S release. The observation of increased SIRT3 mRNA by H2S in H2O2-exposed endothelial cells drove us to focus on SIRT3 in the present study.
We found that the effect of H2S was attenuated when SIRT3 was knocked down, suggesting that the protective effect of H2S against oxidative stress and apoptosis depends, at least in part, on SIRT3. SIRT3 plays a role in the maintenance and regulation of normal physiological function of mitochondria (25), which is achieved via its ability to deacetylate acetylated proteins in mitochondria (9, 10, 14). SIRT3 exists as a soluble protein in mitochondria to regulate the acetylation reaction, including those of acetyl-coenzyme A synthase, glutamate dehydrogenase, Ku70, IDH2, a forkhead protein, FOXO3a, and SOD2 (28, 38, 40). Overexpression of SIRT3 can enhance the binding between FoxO3a and the promoter of SOD2 to strengthen the activity of SOD2 transcription (23, 35). SIRT3 may increase mitochondrial ROS-scavenging capacity by enhancing antioxidant enzyme activity, including SOD2 (6). SIRT3 also deacetylates IDH2, which mediates intermediary metabolism and energy production (34, 39). Mitochondrial oxidative respiration is thus enhanced, maintaining mitochondrial energy metabolism and reducing ROS generation. It has been reported that H2S reduces atherosclerotic plaques and endoplasmic reticulum stress by SOD2 activation (7). Likewise, we found that both SOD2 and IDH2, downstream of SIRT3, were activated by H2S in endothelial cells. Therefore, we demonstrated that the SIRT3-SOD2 and SIRT3-IDH2 pathways were also involved in the protective effect of H2S against endothelial dysfunction.
Little is currently known about the molecular mechanism of the regulation of the SIRT3 gene expression. A previous study showed that peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) activated the mouse SIRT3 promoter, which was mediated by an estrogen-related receptor binding element mapped to the promoter region (15). Our study suggests a potential role of H2S in modulating SIRT3 promoter activity in response to H2O2. This observation agrees with a previous report, indicating that mitochondria, especially during cellular stress or damage, are able to regulate a series of nuclear targeted genes by transcriptional factors acting as mediators of the well-known nucleus–mitochondrion cross talk (3). We also found that H2S could increase AP-1 binding activity with SIRT3 promoter, which was decreased after H2O2 treatment in endothelial cells. The enhanced effect of H2S on SIRT3 promoter activity was absent when specific AP-1 inhibitor, SR11302 or curcumin, was used. This result suggests that H2S can enhance the ability of AP-1 binding into SIRT3 promoter. AP-1 is a multifunctional transcription factor, which regulates gene expression, either positively or negatively, in different types of cells under various physiological and pathological conditions. It is noted that H2S donor inhibits the activation of AP-1, which is bound to the COX-2 gene promoter, in 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin (33). The factors, such as AP-1 and GATA2, have several motif binding sites located within the SIRT3 promoter region (2). Our study is probably the first one to show that H2S increases AP-1 binding activity with the SIRT3 promoter and thus enhances SIRT3 transcription to attenuate endothelial oxidative stress.
The MAPK family is one of the most important downstream signal pathways of oxidative stress. A previous study found that exposure of endothelial cells to H2S increased the phosphorylation of p38 MAPK (24). H2S also activated ERK1/2 to inhibit angiogenic features of human endothelial cells (46). However, others reported that H2S scavenged particulate matter-induced endothelial cell ROS and inhibited the oxidative activation of p38 MAPK (42). Inhibiting ERK decreased the H2S-induced rise in cell migration rate (50). H2S also decreased the expression of caspase-3 to protect human endothelial cells under a hypoxic condition (32). MAPK, as a double-edged sword, plays a vital role in regulating oxidative stress and other pathophysiological processes. Therefore, H2S plays diverse roles in the MAPK signaling pathway in endothelial cells in response to different stimulations. In the present study, prior treatment of endothelial cells with H2S not only conferred protection against death but also reduced activation of intracellular MAPK signaling and apoptosis. Similar results have been reported by other researchers, although using different types of cells (11, 30, 48). In addition, the present results show that the SIRT3 mediates the effect of H2S on JNK, but not p38 MAPK and ERK, in H2O2-treated endothelial cells. Further study is required to establish whether H2S, SIRT3, MAPK, and oxidative stress will form a complicated regulatory circuit. Nevertheless, this study demonstrates a significant role of SIRT3 activation in the protective effects of H2S against H2O2- and paraquat-induced injury of endothelial cells.
In summary, we provide new evidence that SIRT3 plays an important role in the antioxidant effect of H2S in vascular endothelial cells. In addition to inhibition of p38 MAPK and ERK signaling, H2S also enhances AP-1 binding activity with the SIRT3 promoter, which upregulates SIRT3, and subsequent elevation of SOD2 and IDH2 expression and downregulation of JNK activity. We therefore propose that H2S protects endothelial cells against oxidative damage not only by inhibiting p38 MAPK and ERK pathways but also by increasing the expression and activity of SIRT3 as a new mechanism by which H2S protects endothelial cells. Whether H2S donors or other drugs, which can modify SIRT3 signaling, are of value to diminish endothelial dysfunction, a key early event in the development of cardiovascular and metabolic diseases, warrants further investigation.
Materials and Methods
Materials
Unless otherwise stated, all reagents used were obtained from Sigma-Aldrich.
Cell culture and treatment
The EA.hy926 endothelial cell line was purchased from the American Type Culture Collection. Cells were seeded into six-well plates and cultured until they reached about 80% confluence. Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) with 10% (v/v) fetal bovine serum (FBS; Gibco) was replaced immediately before addition of different concentrations of H2S donor, GYY4137 (12.5–100 μM). After 4 h of treatment, cells were incubated in freshly prepared medium containing H2O2 (250 μM; Sigma-Aldrich) for a further 4 h. Microscopic observation of cells to monitor the differentiation status of cultures and to record any cell changes during treatment was conducted using a converted phase-contrast microscope (Olympus).
Measurement of H2S in culture medium
H2S in culture medium was measured using an H2S-specific microelectrode (ISO-H2S-2; World Precision Instruments) connected to a free radical analyzer (TBR4100; World Precision Instruments) as described previously (51). The sensor was set to the 10-nA range and the poise voltage to +150 mV. Before initiation of the experiments, the sensor was polarized and calibrated by adding 4 aliquots of the Na2S stock solution at final concentrations of 0.5, 1, 2, and 4 μM. Concentrations of H2S in the samples were calculated using a standard curve of Na2S.
Cell viability assay
Cell proliferation was measured by Cell Counting Kit-8 (CCK-8; Beyotime) according to the manufacturer's directions. Briefly, EA.hy926 cells (1 × 104) were seeded in a 96-well plate and cultured overnight, then exposed to H2O2 (250 μM, 4 h) after pretreatment with GYY4137 or vehicle (medium, DMEM) for 4 h, followed by addition of 10 μl of the WST-8 mixture to each well. The cells were then incubated for 1 h at 37°C in the incubator. The absorbance was measured in a microplate reader (Biotek Instruments) at a wavelength of 450 nm.
Cell death was evaluated by the quantification of plasma membrane damage, which resulted in the release of LDH. The level of LDH released in the cell culture medium was detected by the LDH cytotoxicity assay detection kit (Beyotime) following the manufacturer's instructions. The optical density was measured spectrophotometrically at 490 nm on a microplate reader (Biotek Instruments).
Quantification of apoptosis
EA.hy926 endothelial cells were seeded in six-well plates and incubated overnight. Cells were then treated with H2O2 (250 μM, 4 h) after pretreatment with GYY4137 (25–100 μM) or vehicle for 4 h. To visualize nuclear morphology, EA.hy926 endothelial cells were fixed in 4% v/v paraformaldehyde and stained with Hoechst 33342 DNA dye (2.5 μg/ml). Uniformly stained nuclei were scored as healthy and viable cells. Condensed or fragmented nuclei were scored as apoptotic. The percentage of cells undergoing apoptosis was also determined by Annexin V/PI staining using the BU-ANNEXIN V-FITC apoptosis detection kit (Biouniquer Technology Co., Ltd.) following the manufacturer's instructions. Annexin V-FITC binding was detected by flow cytometry (BD Biosciences, FL1 filter for Annexin-V-FITC and FL3 filter for PI). The data were analyzed by Cellquest Pro software.
Measurement of ROS in endothelial cells
Cells were exposed to H2O2 (250 μM, 4 h) after pretreatment with GYY4137 or vehicle (DMEM) for 4 h and washed with phosphate-buffered saline (PBS) twice, then switched to serum-free DMEM containing DHE (5 μM; Vigorous Biotechnology Beijing Co., Ltd.) or DCFH-DA (10 μM; Beyotime) as described previously (37, 47). After that cells were washed with PBS again, and the red or green fluorescence was measured with a Zeiss Inverted Microscope (Carl Zeiss). DHE enters the cells and is oxidized by O2•− to yield ethidium, which binds to DNA to produce bright red fluorescence. DCFH-DA is a lipophilic cell-permeable compound that is deacetylated in the cytoplasm to DCF by cellular esterases. DCF is then oxidized by radicals such as hydroxyl, peroxyl, alkoxyl, nitrate, and carbonate to a fluorescent molecule (excitation 530 nm, emission 485 nm).
Measurement of total SOD and SOD2 activity in endothelial cells
EA.hy926 endothelial cells were treated with H2O2 (250 μM, 4 h) after pretreatment with GYY4137 (25–100 μM) or vehicle (DMEM) for 4 h as described above. Then, cells were washed using PBS and lysed in ice-cold 0.1 M Tris/HCl (pH 7.4) containing 0.5% Triton, 5 mM β-mercaptoethanol, and 0.1 mg/ml phenylmethylsulfonyl fluoride. Lysates were clarified by centrifugation at 14,000 g at 4°C for 5 min and cell debris was discarded. SOD activity was detected using a commercial SOD Assay Kit-WST according to the manufacturer's protocol (Dojindo Molecular Technologies). SOD2 activity was measured in the presence of a CuZnSOD inhibitor (3 mM NaCN) and normalized to total protein content. The highly water-soluble tetrazolium salt, WST-1, which produces a water-soluble formazan dye upon reduction by the superoxide anion, was used to measure SOD2 activity. Absorbance values at 450 nm were measured using a microplate reader (Biotek Instruments).
Measurement of NO
The production of NO in cultured supernatants was measured using an Apollo 1000 single-channel free radical detector employing an amperometric-type NO probe (ISO-NOPF100H; World Precision Instruments), as described previously (27).
Analysis of mitochondrial respiration
EA.hy926 endothelial cells were plated (1.2 × 103 cells/well) in an XF 96-well plate (Seahorse Bioscience) and 18 h thereafter cells were treated with GYY4137 (25–100 μM, 4 h), followed by addition of the medium or H2O2 (250 μM, 4 h) immediately before the medium was changed to unbuffered DMEM containing glucose (10 mM), pyruvate (10 mM), and GlutaMAX (2 mM; Invitrogen). Mitochondrial respiration OCR was measured by a Seahorse Extracellular Flux Analyzer XF96 (Seahorse Bioscience) (5). Briefly, a classical MitoStress test was performed according to the following procedure: (1) basal respiration was measured in unbuffered medium; (2) oligomycin (2 μg/ml final concentration), an inhibitor of ATP synthesis, was injected to determine respiration linked to ATP production; (3) the uncoupler, carbonyl cyanide 4-(trifluoromethoxy) phenylhy-drazone (FCCP, 2 μM), was added to measure maximal respiration; and (4) antimycin A (4 μM) plus rotenone(4 μM) was applied in combination to block respiration due to simultaneous inhibition of complexes III and I, respectively.
Assessment of mitochondrial membrane potential
Mitochondrial membrane potential (Δψm) was measured by a commercial JC-1 kit (Beyotime) following the manufacturer's instructions. Briefly, EA.hy926 were grown on a six-well plate and were treated with GYY4137 (25–100 μM, 4 h), followed by addition of the medium or H2O2 (250 μM, 4 h), then confluent cells were rinsed with PBS and incubated in 1 ml JC-1 staining solution at 37°C for 20 min. Cells were then rinsed twice with JC-1 washing solution and digital pictures for JC monomers (Green fluorescence; 535 nm) and JC aggregates (Red fluorescence; 570 nm) were captured with a Zeiss Inverted Microscope (Carl Zeiss). The ratio of red (J-aggregate)/green (monomeric JC-1) emission is directly proportional to the Δψm.
RNA interference
The following double-stranded RNA oligos specific for SIRT3 (sense, 5′-GCCCAACGTCACTCACTACTT-3′, antisense, 5′-GUAGUGAGUGACGUUGGGCTT-3′) were synthesized by Shanghai GenePharma. Commercially available siRNA to random noncoding sequences were used for control transductions (Shanghai GenePharma). To obtain SIRT3 knockdown cells with transient transfection, cells were transfected with siRNA duplexes at the final concentration of 100 nM using Lipofectamine 2000 reagent (Invitrogen). The cells were used for experiments in 24 h after transfection, and gene silencing was detected by analysis of SIRT3 protein expression with Western blot. Transfected with control siRNA or SIRT3 siRNA for 24 h, EA.hy926 cells were exposed to H2O2 (250 μM, 4 h) after pretreatment with GYY4137 or vehicle (DMEM) for 4 h; ROS and apoptosis were measured as described earlier.
Western blot analysis
Cytoplasmic protein samples were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane (Millipore), and then immunoblotted with primary anti-IDH2 (1:500; Santa Cruz Biotechnology), anti-SIRT3, anti-p38 MAPK, anti-p-p38 MAPK, anti-ERK, anti-p-ERK, anti-JNK, anti-p-JNK, anti-cleaved caspase-3 (1:1000; Cell Signaling Technology), anti-SOD2 (1:500; Abcam), and anti-GAPDH (1:6000; Sigma). Proteins were visualized by enhanced chemiluminescence substrate (Thermo Fisher Scientific, Inc.).
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Takara). RNA (500 ng) was added as a template to reverse transcriptase reactions carried out using the PrimeScript™ RT Master Mix Kit (Takara). Quantitative real-time PCR (qRT-PCR) was carried out with the resulting cDNAs in triplicate using SYBR Green remix (Takara) and the ABI 7500 Real-Time PCR System (Applied Biosystems ABI). Experimental Ct values were normalized to 18 s and relative mRNA expression was calculated versus a reference sample. Each sample was run and analyzed in triplicate (Structures of all primers used are listed in Table 1).
Table 1.
Sequences of Primers
| Human gene | Sequences(5′-3′) |
|---|---|
| SIRT1-Reverse | CTTCATCTTTGTCATACTTCATGGCTCTATG |
| SIRT1-Forward | TGCGGGAATCCAAAGGATAATTCAGTGTC |
| SIRT2-Reverse | AAGGTCCTCCAGCTCCTTCTTC |
| SIRT2-Forward | CAGAACATAGATACCCTGGAGCGAA |
| SIRT3-Reverse | CAGCGGCTCCCCAAAGAACAC |
| SIRT3-Forward | CGGCTCTACACGCAGAACATC |
| SIRT4-Reverse | TTCCCCACAATCCAAGCAC |
| SIRT4-Forward | ACCCTGAGAAGGTCAAAGAGTTAC |
| SIRT5-Reverse | ACTCTTGTAATTCTCAGCCACAACTCCAC |
| SIRT5-Forward | CGAGTCGTGGTCATCACCCAGAACATC |
| SIRT6-Reverse | GGCCAGACCTCGCTCCTCCATGG |
| SIRT6-Forward | GAGGAGCTGGAGCGGAAGGTGTG |
| SIRT7-Reverse | CACAGTTCTGAGACACCACATGCT |
| SIRT7-Forward | TGTGGACACTGCTTCAGAAAGGGA |
Plasmids and luciferase reporter assays
The mSIRT3 promoter (−491 to +146) and pGL3-Basic were provided by Professor Yongsheng Chang of the Chinese Academy of Medical Sciences and Peking Union Medical College. Various fragments of the 5′ flanking promoter region of mSIRT3 were generated by PCR amplification of Luc-491, followed by cloning into pGL3-Basic using the Acc65 I and Xho I sites, as described previously (15).
EA.hy926 endothelial cells were cultured in 24-well plates and cotransfected with luciferase reporter construct (0.2 mg/well) and pRL-TK reporter plasmid (control reporter, 0.006 mg/well) using Lipofectamine 2000 reagent, according to the manufacturer's protocol (Invitrogen). After transfection (24 h), cells were treated with GYY4137 or DMEM as vehicle for 4 h and then exposed to H2O2 (250 μM) for a further 4 h. Cells were harvested in lysis buffer and luciferase activity was measured using the dual-luciferase reporter assay system (Promega). Firefly luciferase activity was normalized to that of control reporter.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed using the Pierce Agarose ChIP Kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's recommendations. In brief, EA.hy926 endothelial cells were treated with H2S (GYY4137 50 μM) for 4 h before H2O2 (250 μM) treatment for 4 h. Protein samples were then precleared with protein A-agarose/salmon sperm DNA (30 min, 4°C), followed by overnight incubation at 4°C with antibodies specific for AP-1 or normal rabbit IgG (as a negative control). The immune complexes were precipitated with protein A-agarose for 1 h. Precipitated genomic DNA was amplified by real-time PCR with primers. Potential AP-1 binding sites on SIRT3 promoter region were amplified with the following primer pairs: 5′-AATCTCCCGGTTTGGCTTCC-3′ (sense) and 5′-CCCGCACGATAACCCGAAGT-3′ (antisense).
Animals and treatment
SIRT3+/− mice were the gift of Professor Hongliang Li (Wuhan University, China). These mice were intercrossed to produce homozygous SIRT3−/− and wild-type 129S1/SvImJ animals. Male SIRT3−/− mice and wild-type (WT) control animals (8–10 weeks) were randomly treated with either GYY4137 (133 μM/kg, i.p.) or vehicle (saline, i.p.) 1 h after paraquat (50 mg/kg, i.p.) or vehicle (saline, i.p.) injection. The doses of both GYY4137 and paraquat used were based upon prior reports in the literature (5, 16). Endothelium-dependent vasorelaxation of mouse aorta and small mesenteric artery was assessed after paraquat injection for 24 h. All animal experiments were conformed to the Guide for the Care and Use of Laboratory Animals published by NIH and also approved by the Committee on Animal Care of Nanjing Medical University (NJMU-ERLAUA-20100112).
Measurement of superoxide formation in mouse aorta
Superoxide production in tissue sections of mouse aorta was detected by fluorescence microtopography using the fluorescent probe, DHE, as previously described (20).
Assessment of endothelium-dependent relaxations
Vasorelaxation of isolated aortic ring segments was determined in organ baths containing oxygenated Krebs' solution. After an equilibration period of 60 min, aortic rings were precontracted with norepinephrine or phenylephrine (Phe, 0.1 μM). Endothelium-dependent or independent relaxation was then assessed in response to cumulative addition of acetylcholine (Ach, 10−9–10−5 M) or sodium nitroprusside (SNP, 10−10–10−6 M) with or without superoxide scavenger tempol (1 mM) preincubation for 60 min. Relaxation at each concentration was measured and expressed as the percentage of force generated in response to norepinephrine.
Statistical analysis
All data are presented as mean ± SEM. Statistical analysis was performed by one-way ANOVA, followed by the Newman–Keuls multiple comparison test as appropriate (Stata13.0 software). p < 0.05 was considered to be statistically significant.
Abbreviations Used
- Δψm
mitochondrial membrane potential
- AP1
activator protein 1
- CBS
cystathionine β-synthase
- ChIP
chromatin immunoprecipitation
- CSE
cystathionine γ-lyase
- DCFH-DA
dichlorodihydrofluorescein diacetate
- DHE
dihydroethidium
- DMEM
Dulbecco's modified Eagle's medium
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HUVECs
human umbilical vein endothelial cells
- IDH
isocitrate dehydrogenase
- LDH
lactate dehydrogenase
- MAPK
mitogen-activated protein kinase
- MST
mercaptopyruvate sulfurtransferase
- NO
nitric oxide
- OCR
oxygen consumption rate
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time PCR
- ROS
reactive oxygen species
- SIRT3
sirtuin3
- SOD
superoxide dismutase
Acknowledgments
This work was supported by grants to Y.J. from the National Basic Research Program of China (973) (2011CB503903), grants to Y.J. (81170083, 81330004, 31371156), to L.X. (81200197), to Y.H. (81200196), and to G.M. (81400203) from the National Natural Science Foundation of China, and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. Additional support was provided by a grant to M.X. from NIH (R01HL116571), a grant to P.K.M. from the Biomedical Research Council (BMRC) of Singapore, and an operating grant to R.W. from Canadian Institutes of Health Research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Anderson KA. and Hirschey MD. Mitochondrial protein acetylation regulates metabolism. Essays Biochem 52: 23–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellizzi D, Covello G, Di Cianni F, Tong Q, and De Benedictis G. Identification of GATA2 and AP-1 Activator elements within the enhancer VNTR occurring in intron 5 of the human SIRT3 gene. Mol Cells 28: 87–92, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Biswas G, Guha M, and Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene 354: 132–139, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calenic B, Yaegaki K, Ishkitiev N, Kumazawa Y, Imai T, and Tanaka T. p53-Pathway activity and apoptosis in hydrogen sulfide-exposed stem cells separated from human gingival epithelium. J Periodontal Res 48: 322–330, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Nie YC, Luo YL, Lin F, Zheng YF, Cheng GH, Wu H, Zhang KJ, Su WW, Shen JG, and Li PB. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol 58: 133–140, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, and Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 12: 534–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ZF, Zhao B, Tang XY, Li W, Zhu LL, Tang CS, DU JB, and Jin HF. Hydrogen sulfide regulates vascular endoplasmic reticulum stress in apolipoprotein E knockout mice. Chin Med J (Engl) 124: 3460–3467, 2011 [PubMed] [Google Scholar]
- 8.Gao Y, Yao X, Zhang Y, Li W, Kang K, Sun L, and Sun X. The protective role of hydrogen sulfide in myocardial ischemia-reperfusion-induced injury in diabetic rats. Int J Cardiol 152: 177–183, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RJ, Alt FW, Kahn CR, and Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RJ, Kahn CR, and Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44: 177–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu LF, Lu M, Wu ZY, Wong PT, and Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol 75: 27–34, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Li R, Yang H, Luo H, and Chen Z. Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells. J Stroke Cerebrovasc Dis 24: 601–609, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Jeney V, Komodi E, Nagy E, Zarjou A, Vercellotti GM, Eaton JW, Balla G, and Balla J. Supression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H(2)S). Free Radic Biol Med 46: 616–623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin L, Wei W, Jiang Y, Peng H, Cai J, Mao C, Dai H, Choy W, Bemis JE, Jirousek MR, Milne JC, Westphal CH, and Perni RB. Crystal structures of human SIRT3 displaying substrate-induced conformational changes. J Biol Chem 284: 24394–24405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, and Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5: e11707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Fox B, Keeble J, Salto-Tellez M, Winyard PG, Wood ME, Moore PK, and Whiteman M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J Cell Mol Med 17: 365–376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, and Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117: 2351–2360, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Zhang KY, Zhang P, Chen L X, Wang L, Xie M, Wang CY, and Tang XQ. Hydrogen sulfide inhibits formaldehyde-induced endoplasmic reticulum stress in PC12 cells by upregulation of SIRT-1. PLoS One 9: e89856, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YH, Lu M, Hu LF, Wong PT, Webb GD, and Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal 17: 141–185, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, Wang X, Chen Q, Dai W, Tan CH, Pan S, Moore PK, and Ji Y. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(-/-) mice. Br J Pharmacol 169: 1795–1809, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, and Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Mortuza R, Chen S, Feng B, Sen S, and Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One 8: e54514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, and Monsalve M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem 284: 14476–14484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, and Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, and Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter AG. and Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6: 99–104, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Purwantini E. and Mukhopadhyay B. Conversion of NO2 to NO by reduced coenzyme F420 protects mycobacteria from nitrosative damage. Proc Natl Acad Sci U S A 106: 6333–6338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu X, Brown K, Hirschey MD, Verdin E, and Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, and Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci 9: 1057–1069, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, and Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 86: 391–397, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Rocha M, Apostolova N, Hernandez-Mijares A, Herance R, and Victor VM. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr Med Chem 17: 3827–3841, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Shen Y, Guo W, Wang Z, Zhang Y, Zhong L, and Zhu Y. Protective effects of hydrogen sulfide in hypoxic human umbilical vein endothelial cells: a possible mitochondria-dependent pathway. Int J Mol Sci 14: 13093–13108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrotriya S, Kundu JK, Na HK, and Surh YJ. Diallyl trisulfide inhibits phorbol ester-induced tumor promotion, activation of AP-1, and expression of COX-2 in mouse skin by blocking JNK and Akt signaling. Cancer Res 70: 1932–1940, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, and Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, and Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suo R, Zhao ZZ, Tang ZH, Ren Z, Liu X, Liu LS, Wang Z, Tang CK, Wei DH, and Jiang Z S. Hydrogen sulfide prevents H(2)O(2)-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep 7: 1865–1870, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Tampo Y, Kotamraju S, Chitambar CR, Kalivendi SV, Keszler A, Joseph J, and Kalyanaraman B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res 92: 56–63, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes MW, Olivier AK, Spitz DR, and Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian G, Sawashita J, Kubo H, Nishio S Y, Hashimoto S, Suzuki N, Yoshimura H, Tsuruoka M, Wang Y, Liu Y, Luo H, Xu Z, Mori M, Kitano M, Hosoe K, Takeda T, Usami S, and Higuchi K. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid Redox Signal 20: 2606–2620, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng AH, Shieh SS, and Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 63: 222–234, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Vadivel A, Alphonse RS, Ionescu L, Machado DS, O'Reilly M, Eaton F, Haromy A, Michelakis ED, and Thebaud B. Exogenous hydrogen sulfide (H2S) protects alveolar growth in experimental O2-induced neonatal lung injury. PLoS One 9: e90965, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T, Wang L, Zaidi SR, Sammani S, Siegler J, Moreno-Vinasco L, Mathew B, Natarajan V, and Garcia JG. Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol 47: 491–496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waypa GB, Osborne SW, Marks JD, Berkelhamer SK, Kondapalli J, and Schumacker PT. Sirtuin 3 deficiency does not augment hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 49: 885–891, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, and Zhu YZ. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One 8: e53147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winnik S, Auwerx J, Sinclair DA, and Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J, 2015. [Epub ahead of print]; DOI: http://dx.doi.org/10.1093/eurheart/ehv290 [DOI] [PMC free article] [PubMed]
- 46.Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, and Singh SV. Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer 55: 94–107, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Xie L, Liu Z, Lu H, Zhang W, Mi Q, Li X, Tang Y, Chen Q, Ferro A, and Ji Y. Pyridoxine inhibits endothelial NOS uncoupling induced by oxidized low-density lipoprotein via the PKCalpha signalling pathway in human umbilical vein endothelial cells. Br J Pharmacol 165: 754–764, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu ZS, Wang XY, Xiao DM, Hu L F, Lu M, Wu ZY, and Bian JS. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic Biol Med 50: 1314–1323, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Yang G, Wu L, Bryan S, Khaper N, Mani S, and Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res 86: 487–495, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Zhang LJ, Tao BB, Wang MJ, Jin HM, and Zhu YC. PI3K p110alpha isoform-dependent Rho GTPase Rac1 activation mediates H2S-promoted endothelial cell migration via actin cytoskeleton reorganization. PLoS One 7: e44590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao D, Liu TZ, Chan EC, Fein H, and Zhang X. A novel enzymatic method for determination of homocysteine using electrochemical hydrogen sulfide sensor. Front Biosci 12: 3774–3780, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Zhao W, Zhang J, Lu Y, and Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng M, Qiao W, Cui J, Liu L, Liu H, Wang Z, and Yan C. Hydrogen sulfide delays nicotinamide-induced premature senescence via upregulation of SIRT1 in human umbilical vein endothelial cells. Mol Cell Biochem 393: 59–67, 2014 [DOI] [PubMed] [Google Scholar]