Abstract
Emerging evidence suggests that a history of sports-related concussions can lead to long-term neuroanatomical changes. The extent to which similar changes are present in young athletes is undetermined at this time. Here, we tested the hypothesis that collegiate football athletes with (n = 25) and without (n = 24) a self-reported history of concussion would have cortical thickness differences and altered white matter integrity relative to healthy controls (n = 27) in fronto-temporal regions that appear particularly susceptible to traumatic brain injury. Freesurfer software was used to estimate cortical thickness, fractional anisotropy was calculated in a priori white matter tracts, and behavior was assessed using a concussion behavioral battery. Groups did not differ in self-reported symptoms (p > 0.10) or cognitive performance (p > 0.10). Healthy controls reported significantly higher happiness levels than both football groups (all p < 0.01). Contrary to our hypothesis, no differences in fractional anisotropy were observed between our groups (p > 0.10). However, football athletes with a history of concussion had significantly thinner cortex in the left anterior cingulate cortex, orbital frontal cortex, and medial superior frontal cortex relative to healthy controls (p = 0.02, d = −0.69). Further, football athletes with a history of concussion had significantly thinner cortex in the right central sulcus and precentral gyrus relative to football athletes without a history of concussion (p = 0.03, d = −0.71). No differences were observed between football athletes without a history of concussion and healthy controls. These results suggest that previous concussions, but not necessarily football exposure, may be associated with cortical thickness differences in collegiate football athletes.
Key words: : anterior cingulate, concussion, cortical thickness, football, fractional anisotropy
Introduction
There has been a dramatic increase in research aimed towards identifying the effects of sports-related concussion on brain structure and function. The recent observation of reduced hippocampal volume in otherwise healthy collegiate football players,1 a finding also observed post-mortem in retired collision sport athletes with chronic traumatic encephalopathy,2 suggests that observable structural changes may be present in young athletes with high exposure to concussive and sub-concussive head hits. Other structural changes, including altered white matter and advanced cortical thinning, also have been observed in retired athletes with a history of concussion.3–5 However, the extent to which similar structural changes occur in young healthy athletes and whether changes result from concussions or simply exposure to repetitive head hits remains unresolved.
Several studies have identified white matter changes as a result of mild traumatic brain injury (mTBI), including sports-related concussion.6 Consistent with previous observations,7–9 a recent meta-analysis illustrated that increased fractional anisotropy (FA) is typically observed at the acute and sub-acute phases of mTBI (i.e., days to weeks post-concussion), while decreased FA is typically observed at more chronic stages (i.e., months to years) and in chronically symptomatic patients.10 Importantly, chronically symptomatic patients represent a small minority of all mTBI patients and results from this cohort might not be generalizable to more typical cases of mTBI that are associated with more rapid recovery.11 Further, there is emerging evidence that exposure to sports with a risk for repetitive head injuries can result in altered white matter in the absence of concussion.12 Therefore, well-controlled studies are needed to differentiate the relative effects of previous concussions and football exposure on white matter abnormalities.
In contrast to white matter, few studies have investigated changes in gray matter structure in young athletes with a history of sports-related concussion. Research with moderate-to-severe TBI suggests that the lateral frontotemporal cortices represent a common injury site secondary to the morphology of the skull,13 and the frontotemporal cortices are a frequent site of tau pathology in patients with suspected chronic traumatic encephalopathy.2 Although reduced gray matter volume and widespread cortical thinning have been documented in severe and moderate TBI patients,14–16 results from mTBI patients have been inconsistent.17–20 Current research suggests that evidence of atrophy following mTBI may be limited to patients with lesions (i.e., complicated mTBI),21 may be prevalent only in chronically symptomatic patients,22 or may be present only during the late chronic injury phases.17,20 To our knowledge, there have been no studies examining whether cortical thickness differences are observable in asymptomatic athletes with a self-reported history concussion or in athletes with exposure to repetitive head impacts without a self-reported history of concussion.
The current investigation therefore had two major aims. First, we tested the hypothesis that collegiate-aged football players with a self-reported history of concussion would have thinner cortex and disrupted white matter integrity relative to either football players without a self-reported history of concussion or non-football athlete/non-athlete healthy controls. Second, we tested whether exposure to football even without a self-reported history of concussion would be associated with thinner cortex and disrupted white matter relative to non-football athlete/non-athlete healthy controls. We hypothesized that football players with and without a history of concussion would have thinner cortex and lower FA in frontal and temporal regions relative to the control group, but would not differ from each other.
Methods
Participants
A targeted sample of male collegiate athletes (n = 49) from a single National Collegiate Athletic Association Division I American rules football program and non–football athletes/non-athletes without a history of self-reported concussion or football experience (n = 27; HC) were recruited for this study. All individuals reported no past or current history of mood disorders, anxiety disorders, alcohol abuse, or substance abuse. All participants provided written informed consent approved by local institutional review boards. Participant interviews based on retrospective recall were used to establish concussion history, defined as concussions that were medically diagnosed by a clinician at the time of injury, as well as the number of years of tackle football experience. Twenty-four football athletes self-reported no clinically diagnosed concussions (Ath). Twenty-five current or former football athletes self-reported at least one clinician-diagnosed concussion (Ath-mTBI). This group included one track-and-field athlete with extensive football experience (nine years), including experience at the collegiate level. All football athletes with a history of concussion were currently cleared for competition (i.e., no residual concussion symptoms) at the time of scan by clinicians trained in sports medicine.
Behavioral assessment
A common concussion behavioral battery was used to aid in interpretation of any potential neuroanatomical differences. Specifically, the Automated Neuropsychological Assessment Metrics 4 Sports Medicine Battery (ANAM)23 was administered to a subset of football athletes (37/49) and control participants (22/27) in a confidential research setting. Behavioral data was only available in this subset because behavioral data collection began at a later stage than neuroimaging data collection. The ANAM is an automated, computerized assessment of self-reported concussion symptoms, mood scales, and cognitive tests.23 Construct validity and test–retest reliability for ANAM measures can be found elsewhere.24,25 Throughput, which is the number of correct responses per minute, was used to measure performance on each cognitive task.26 D′, a measure of signal detection, was used for the go-no-go task. Task scores were standardized relative to a normative sample of male collegiate athletes available as part of the ANAM software. Raw scores were used for the go-no-go and mathematical processing task as the normative group does not include scores for these tests. The standardized throughput score from the two simple reaction time tests were averaged and used as a single measure.
Several steps were taken to ensure data quality. Three athletes with a history of concussion responded with zeros for all self-reported symptom and mood scales, including positive symptoms, indicating low effort. As a result, self-reported scores for these participants were excluded from further analyses. The ANAM Validity Indicator Report was used to exclude participants with questionable effort on the cognitive battery and individual subtests with suboptimal performance indicative of poor effort or misunderstanding of subset instructions.27
Imaging parameters
MRI was performed using a GE Discovery MR750 3-Tesla whole body MRI scanner (General Electric Healthcare, Milwaukee, WI) and brain-dedicated receive-only 32-element coil array optimized for parallel imaging (Nova Medical, Inc., Wilmington, MA). T1-weighted anatomical images were collected using a parallelized magnetization-prepared rapid gradient-echo sequence with sensitivity encoding (field of view [FOV] = 240 mm; 130 axial slices; slice thickness = 1.1 mm; image matrix = 256 × 256; repetition time [TR]/echo time [TE] = 5/1.948 msec; acceleration factor R = 2 in the phase encoding direction; flip angle = 8°; inversion time = 725 msec; sampling bandwidth = 31.25 kHz; voxel size = 0.9375 × 0.9375 ×1.1 mm). Diffusion tensor imaging (DTI) was performed in a subset of football athletes (46/49) and control participants (22/27) with the following parameters: 30 non-collinear directions; b-value =1000 sec/mm2; TR = 8800 msec; TE = minimum; acquisition matrix = 96 × 96 interpolated to 256 × 256; FOV = 256 × 256 mm; slice thickness = 2 mm; inter-slice spacing 0.2 mm; slices = 69; acceleration factor R = 2 in the phase encoding direction; and original voxel size 2.7 × 2.7 × 2.2 mm.
DTI processing
DTI analyses were performed in the Analysis of Functional NeuroImages analysis suite.28 Gradients images were co-registered to the b0 volume using a 12-parameter affine alignment to correct for subject motion and eddy currents, with resultant rotational parameters also applied to the gradient table. Similar to previous publications,29 a 3.5 mm Gaussian full-width at half-maximum smoothing kernel was applied to each gradient to reduce Gibb's ringing artifact. A non-linear algorithm was used to calculate the diffusion tensors, followed by scalar measures of axial diffusivity, radial diffusivity, and FA. Separate 12-parameter affine alignments were used to register each participant's b0 volume to their anatomical T1 image in native space and the native T1 anatomical image to the N27 brain template in Talairach space.30 These alignment matrices were subsequently concatenated to create a single transformation matrix for each participant.
Region of interest (ROI) analyses were conducted to examine a priori white matter tracts defined from the Johns Hopkins University white matter atlas.31 Selected a priori ROIs included the genu, body, and splenium of the corpus callosum, as well as the left and right anterior corona radiata, superior corona radiata, combined anterior and posterior internal capsule, and the cingulum of hippocampus. ROIs were subsequently transformed to each participant's native b0 image using the inverse of the transformation matrix. The intersection between each ROI and a binary FA map (FA > 0.25) created for each participant was calculated to restrict analyses to white matter only.
DTI quality assurance
A previously described binary classification scheme was used to flag individual gradients with excessive motion or other artifacts.32 One participant with six flagged gradients was excluded from DTI analyses. No other participant had more than three flagged gradients. Head motion during DTI scan acquisition was calculated as the Euclidean norm of the temporal derivatives of the translational and rotational head motion estimated using a six-parameter affine registration of gradient images to each participant's b0 image.33
Cortical thickness estimation
Cortical reconstruction and volume segmentation was performed using the FreeSurfer image analysis suite version 5.1.34,35 The result from each step was inspected for every subject as part of a quality assurance protocol, and corrections were made when necessary. Cortical thickness was measured as the distance between the gray–white matter boundary and the pial surface at each point of the cortical surface.36 Participant's cortical thickness estimates were resampled to an averaged surface template and smoothed with a 20 mm full-width half-maximum Gaussian kernel. Kernel size was determined using previously published algorithms to maximize power based on estimated effect sizes and our sample numbers, which indicated a smoothing kernel of 20 mm was required for an effect size of 0.3 with 25 participants in each group.37
Statistical analysis
All statistical analyses except for the analysis of cortical thickness were conducted in the Systat Software, version 13 (Systat Software, Inc., San Jose, CA). One-way analyses of variance (ANOVA) were performed to determine if age and education differed between groups. For DTI quality assessment, the null hypothesis for group differences in the number of flagged diffusion gradients was evaluated with Mann-Whitney tests due to expected violations of normality. Differences in head motion were evaluated with ANOVA.
Multivariate analyses of covariance (MANCOVAs) were used when appropriate to reduce the number of tests and control for false positives given moderate covariance structure between data. Age was included as a covariate due to the significant differences among groups (see results). A priori multivariate comparisons of athletes with a history of concussion versus athletes without a history of concussion, and both football groups versus healthy control participants were performed for FA ROI analyses and considered significant at p < 0.05. Follow-up univariate contrasts were performed following significant multivariate effects and considered significant at p < 0.05.
Vertex-based analysis of covariance (ANCOVA) with age as covariate was performed with a priori comparisons of cortical thickness between athletes with a history of concussion versus athletes without a history of concussion, and both football groups versus healthy control participants. A cluster-wise multiple comparison correction was performed using a Monte Carlo simulation with a vertex-wise threshold of p < 0.05, Bonferroni corrected for contrasts across two hemispheres.
Results
Demographics and behavior
Demographic information can be found in Table 1. An ANOVA indicated a significant difference in age among groups (F[2,73] = 6.01; p = 0.004). Planned contrasts showed that HC were significantly older than athletes without a history of concussion (p = 0.001), and older than athletes with a history of concussion at a trend level (p = 0.08). Athletes with and without a history of concussion did not differ in age (p = 0.10). There was no significant difference in education among groups (F[2,73] = 1.30; p = 0.28).
Table 1.
Demographic Information
| Ath-mTBI | Ath | HC | |
|---|---|---|---|
| mean (SD; n) | mean (SD; n) | mean (SD; n) | |
| Age* | 21.0 (1.5; 25) | 20.2 (1.2; 24) | 21.9 (2.2; 27) |
| Education | 13.4 (1.3; 25) | 13.1 (1.2; 24) | 13.7 (1.6; 27) |
| Years football experience | 11.6 (3.8) | 10.6 (2.7) | 0 |
| Diagnosed concussions | 2.1 (1.0) | 0 | 0 |
| Months since last concussion | 10.0 (12.0) | ||
| DTI quality assurance | |||
| Flagged gradients | 0.65 (0.94; 23) | 0.82 (0.73; 22) | 0.59 (0.85; 22) |
| Head motion | 0.26 (0.07; 23) | 0.25 (0.05; 22) | 0.28 (0.06; 22) |
| Football position | |||
| Quarterback | 0 | 1 | |
| Running back | 4 | 2 | |
| Tight end | 0 | 1 | |
| Wide receiver | 7 | 3 | |
| Offensive line | 1 | 3 | |
| Defensive tackle | 1 | 4 | |
| Defensive end | 1 | 2 | |
| Linebacker | 6 | 4 | |
| Defensive back | 5 | 4 | |
| Other sports healthy controls | |||
| Non-athlete | 20 | ||
| Cross Country/track | 6 | ||
| Basketball | 1 | ||
| Medication/health | |||
| Acetaminophen, antibiotic, Mucinex | 0 | 1 | 0 |
| Adderall | 1 | 0 | 0 |
| Antacid | 0 | 0 | 1 |
| Antibiotic | 0 | 0 | 1 |
| Loratadine | 0 | 0 | 1 |
| Protriptyline | 0 | 0 | 1^ |
| Self-reported ADHD | 3 | 0 | 0 |
Indicates significant group differences at p < 0.01. ^Reported taking for headaches. Ath-mTBI, football athletes with concussion history; SD, standard deviation; Ath, football athletes without concussion history; HC, healthy controls; DTI, diffusion tensor imaging; ADHD, attention-deficit hyperactivity disorder.
Behavioral measures
The mean, standard deviation, and the final number of participants with data for each ANAM measure are listed in Table 2. There was varying but largely moderate correlation structures among ANAM measures, including self-reported symptom metrics (r's, 0.81-0.94), cognitive measures (r's, 0.0-0.69), and mood measures (r's, 0.01-0.70); thus, three separate MANCOVAs were performed. The multivariate effect of group was not significant for self-reported symptoms (Wilk's λ = 0.84; F[8,98] = 1.11; p = 0.36) or cognitive measures (Wilk's λ = 0.62; F[18,84] = 1.23; p = 0.25). There was a significant multivariate effect of group on ANAM mood measures (Wilk's λ = 0.59; F[14,92] = 1.98; p = 0.03). Follow up ANCOVAS showed there was a significant effect of group on self-reported happiness (F[2,52] = 5.65; p = 0.006), with HC self-reporting more happiness than both athletes with (p = 0.008) and without (p = 0.004) a history of concussion. Football athlete groups did not differ in self-reported happiness (p = 0.75).
Table 2.
Mean, Standard Deviation, and n for ANAM Data for Each Group
| Ath-mTBI | Ath | HC | |
|---|---|---|---|
| mean (SD; n) | mean (SD; n) | mean (SD; n) | |
| Symp. Freq. [0-21] | 2.6 (2.2; 16) | 3.7 (2.9; 18) | 3.0 (2.5; 22) |
| Symp. Sev. [0-126] | 4.1 (3.5; 16) | 6.5 (5.4; 18) | 5.1 (6.0; 22) |
| CSI Freq. [0-12] | 1.5 (1.8; 16) | 2.3 (2.1; 18) | 2.1 (2.0; 22) |
| CSI Sev. [0-72] | 1.9 (2.1; 16) | 3.6 (4.0; 18) | 3.5 (4.2; 22) |
| Anger [0-100] | 2.6 (8.4; 16) | 1.4 (4.2; 18) | 1.0 (3.2; 22) |
| Anxiety [0-100] | 1.9 (3.4; 16) | 3.2 (4.8; 18) | 2.6 (4.6; 22) |
| Depression# [0-100] | 0.4 (1.5; 16) | 1.9 (3.7; 18) | 0.3 (0.9; 22) |
| Fatigue [0-100] | 7.9 (7.9; 16) | 11.9 (10.2; 18) | 16.1 (15.2; 22) |
| Happiness* [0-100] | 64.8 (27.1; 16) | 63.9 (17.2; 18) | 81.2 (15.3; 22) |
| Restlessness [0-100] | 3.8 (8.5; 16) | 4.8 (6.8; 18) | 1.8 (2.6; 22) |
| Vigor [0-100] | 53.9 (20.7; 16) | 47.4 (21.3; 18) | 53.5 (23.4; 22) |
| Code Subs. | 111 (14.4; 19) | 114 (11.8; 16) | 107 (15.9; 22) |
| Procedural RT | 108 (14.0; 19) | 108 (13.6; 16) | 105 (12.4; 21) |
| Spatial Proc. | 107 (12.3; 19) | 98 (12.6; 16) | 100 (12.7; 21) |
| Match to Sample | 105 (15.7; 18) | 101 (11.2; 16) | 108 (13.0; 22) |
| Del. Code Subs. | 112 (14.9; 18) | 107 (14.9; 16) | 107 (19.7; 22) |
| Memory Search | 109 (16.1; 19) | 107 (15.2; 16) | 106 (9.9; 21) |
| Go-no-go | 3.2 (1.4; 18) | 4.1 (1.3; 16) | 3.9 (1.7; 22) |
| Math. Proc. | 24.4 (8.5; 19) | 23.5 (6.2; 16) | 23.4 (6.5; 22) |
| Simple RT | 108 (9.8; 19) | 108 (7.8; 16) | 103 (11.4; 22) |
p < 0.01; #p < 0.10. Numbers in brackets indicate the minimum and maximum possible scores on self-report scales. All cognitive scores are expressed as standard scores with mean = 100.
ANAM, Automated Neuropsychological Assessment Metrics 4 Sports Medicine Battery; Ath-mTBI, football athletes with concussion history; SD, standard deviation; Ath, football athletes without concussion history; HC, healthy controls; Symp., symptom; Freq., frequency; CSI, concussion symptom inventory; Subs., substitution; RT, reaction time; Del., delayed; Math., Mathematical; Proc., processing.
Though it did not reach significance, there was a statistical trend for differences in self-reported depression (F[2,52] = 2.46; p = 0.095), with planned contrasts demonstrating that athletes without a history of concussion had higher scores than either athletes with a history of concussion (p = 0.07) or healthy controls (p = 0.05). Football athletes with a history of concussion did not differ from healthy controls in self-reported depression (p = 0.90). It is noteworthy that overall depression responses were low across all groups (Table 2). Groups did not significantly differ in anger (F[2,52] = 0.37; p = 0.69), anxiety (F[2,52] = 0.29; p = 0.75), restlessness (F[2,52] = 1.44; p = 0.25), vigor (F[2,52] = 0.78; p = 0.46), or fatigue (F[2,52] = 2.21; p = 0.12) mood scores.
DTI analyses
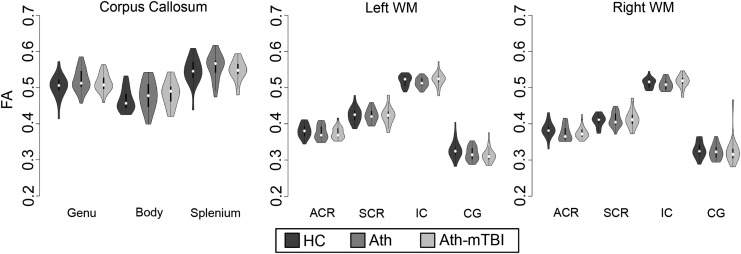
There were no significant differences among groups in the number of flagged gradients (χ2(2) = 1.88; p = 0.39) or in overall head motion during DTI acquisition (F[2,64] = 1.97; p = 0.15). Three separate MANCOVAs were performed for a priori ROIs in the right hemisphere (r's, 0.06-0.43), left hemisphere (r's, 0.02-0.39), and corpus callosum (r's, 0.41-0.73). Contrary to a priori hypotheses, there were no multivariate differences in FA between athletes with a history of concussion relative to healthy controls in corpus callosum ROIs (Wilk's λ = 0.97; F[3,61] = 0.70; p = 0.55; Fig. 1), left hemisphere ROIs (Wilk's λ = 0.91; F[4,60] = 1.55, p = 0.20), or right hemisphere ROIs (Wilk's λ = 0.93; F[4,60] = 1.55; p = 0.36). Similarly, there were no multivariate differences in FA between athletes without a history of concussion relative to healthy controls in corpus callosum ROIs (Wilk's λ = 0.94; F[3,61] = 1.37; p = 0.26), left hemisphere ROIs (Wilk's λ = 0.93; F[4,60] = 1.11; p = 0.34), or right hemisphere ROIs (Wilk's λ = 0.90; F[4,60] = 1.58; p = 0.19). Finally, athletes with and without a history of concussion did not differ in FA in corpus callosum ROIs (Wilk's λ = 0.93; F[3,61] = 1.59; p = 0.20), left hemisphere ROIs (Wilk's λ = 0.93; F[4,60] = 1.11; p = 0.36), or right hemisphere ROIs (Wilk's λ = 0.95; F[4,60] = 0.80; p = 0.53).
FIG. 1.
Violin plots containing box and whisker and kernel density plots are displayed for fractional anisotropy (FA) in a priori regions of interest for healthy controls (HC; dark gray), football athletes without a history of concussion (Ath; gray), and football players with a history of concussion (Ath-mTBI; light gray). WM, white matter; ACR, anterior corona radiate; SCR, superior corona radiate; IC, internal capsule; CG, cingulum of the hippocampus. No significant differences existed among groups.
Cortical thickness
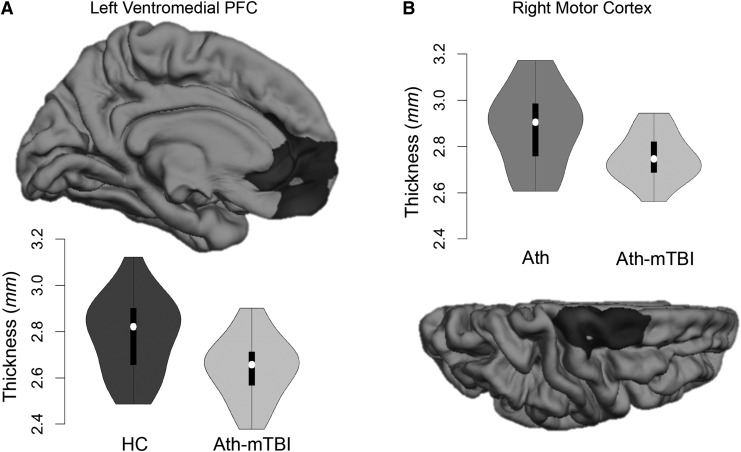
Significantly thinner cortex was observed for athletes with a history of concussion, compared with healthy controls (p = 0.02; d = −0.69; Fig. 2A), in a 2583 mm2 cluster covering the left rostral anterior cingulate cortex, medial orbitofrontal cortex, and medial superior frontal cortex (i.e., ventromedial prefrontal cortex). Additionally, athletes with a history of concussion had significantly thinner cortex in a 2362 mm2 cluster in the right central sulcus and precentral gyrus (i.e., motor cortex), compared with football athletes without a history of concussion (p = 0.03; d = -0.71; Fig. 2B). There was no difference in cortical thickness between athletes without a history of concussion and healthy controls.
FIG. 2.
Football athletes with a history of concussion (Ath-mTBI) had cortical thinning in the left ventromedial prefrontal cortex (PFC) relative to healthy controls (HC; in A) and in the right motor cortex relative to football athletes without a history of concussion (Ath; in B). Violin plots containing box and whisker and kernel density plots are displayed for each cluster.
Supplementary analyses were performed to confirm that the three athletes with a history of concussion that self-reported previous diagnoses of attention-deficit hyperactivity disorder (ADHD) did not drive cortical thickness results. As in the main analysis, the right central sulcus was significantly thinner in athletes with a history of concussion than in athletes without a history of concussion (2736 mm2; p = 0.01; d = −0.76). In contrast, there was no difference in cortical thickness between athletes with a history concussion relative to healthy controls. However, the effect size of the original cluster covering the medial frontal cortex calculated in this subset of participants was still medium to large (d = −0.67), suggesting that the lack of significance reflects loss of power.
Exploratory analyses
Exploratory analyses were conducted in football athletes with a history of concussion to determine whether cortical thickness in the right motor cortex or medial frontal cortex was associated with the number of self-reported concussions or years of tackle football experience. Spearman correlations revealed no significant correlations between right motor cortex thickness and self-reported concussion history (rs = −0.09; p = 0.68) or tackle football experience (rs = 0.24; p = 0.24). Similarly, there was no significant relationship between medial frontal cortex thickness and self-reported concussion history (rs = −0.24; p = 0.26) or tackle football experience (rs = −0.08; p = 0.72).
Discussion
To our knowledge, current results provide the first evidence of thinner cortex in collegiate football athletes with, but not without, a history of concussion. Relative to healthy controls, athletes with a concussion history had significantly thinner cortex in the left ventromedial prefrontal cortex. In addition, athletes with a history of concussion also had significantly thinner cortex in the right motor cortex relative to athletes without a concussion history. These results demonstrate that structural changes may be present in asymptomatic, collegiate-aged football players with a history of concussion.
No differences were observed among groups in cognitive performance or concussion symptoms, confirming that football athletes with and without a history of concussion neither self-reported chronic post-concussive symptoms nor demonstrated observable deficits in cognitive functioning on brief computerized measures. Thus, current results fail to replicate previous research suggesting that a history of concussion is associated with worse baseline cognitive performance38 and increased baseline symptom reporting.39 In contrast to the relatively modest sample size of the present study, these studies had large sample sizes (n > 100-1000), which are likely needed to observe such small behavioral effects. The one significant finding was that athletes with and without a history of concussion self-reported lower happiness relative to healthy controls. A loss of pleasure, along with a variety of other mood symptoms, has been reported in retired National Football League players with a history of concussion,40 though the high levels of depressive symptoms reported in retired players was not observed in the current sample of collegiate athletes. Nevertheless, the clinical significance of this finding is uncertain at this time and requires independent replication.
Contrary to our hypotheses, we found no differences in FA in football athletes relative to controls. This included football athletes with a self-reported history of concussion, which occurred on average 10 months prior to the current study. Studies of mTBI at the chronic phase (operationally defined here as months to years post-injury) have typically reported decreased FA.10 However, these studies have primarily focused on chronically symptomatic patients, which only represent a minority of all concussions due to the fact that symptoms typically recover within a few weeks post-injury.41 Previous prospective work has documented partial normalization of FA by the early chronic stage (three to five months) following a single mTBI incident,42 with additional evidence that mTBI patients with good outcome have no observable white matter differences at the same time period (approximately three to five months).43 Thus, the fact that football athletes with a concussion history were asymptomatic, with the most recent concussion occurring on average 10 months prior, could explain the lack of observable FA differences in the current study. However, increased FA has been observed in hockey players with a history of concussion at several months post-injury relative to those without,44 indicating that additional studies are required to clarify the long-term effects of concussion on white matter.
In contrast to null findings in white matter, reduced cortical thickness was observed within the left ventromedial prefrontal cortex in athletes with a history of concussion relative to healthy controls. This observation is consistent with longitudinal evidence of atrophy in the anterior cingulate cortex in mTBI patients over the course of one year.17 The ventromedial prefrontal cortex was also one of several regions in which thinner cortex was associated with baseline symptomology in high school and collegiate ice hockey athletes in a recent study; although no direct relationship between concussion history and cortical thickness was observed.45 The ventromedial frontal cortex is associated with several behavioral and cognitive domains, including decision-making and emotional regulation,46,47 which overlap common symptoms reported by both active and retired athletes with a history of concussion.40,48 Thinner cortex in the medial frontal regions in football athletes with a self-reported history of concussion relative to healthy controls, but not other football athletes, suggests that this may be due to a combination of previous concussions and high exposure to repetitive head hits associated with football.
In addition to the left ventromedial prefrontal cortex, athletes with a history of concussion also had thinner cortex in the right motor cortex relative to athletes without a history of concussion. Previous work has shown that asymptomatic collegiate football athletes with a history of concussion have impairments of synaptic plasticity in the primary motor cortex and postural control relative to football athletes with no concussion history.49,50 However, contrary to our hypothesis, we observed no cortical thickness differences in football athletes without a self-reported concussion history relative to healthy controls. This hypothesis was based on our previous finding of smaller hippocampal volumes in both football athletes with and without a history of concussion.1 It is possible that thinner cortex is associated only with concussive head injuries, in contrast to the hippocampal volume, which also appears to be sensitive to football exposure in general. If so, this would suggest that these structural changes are associated with different cellular mechanisms of volume loss, such as suppression of neurogenesis or excitotoxicity.
The observed cortical thickness differences in football athletes with a history of concussion must be interpreted with the caveat that three of these athletes also self-reported ADHD. Cortical thickness differences, including differences in the frontal cortex, have been observed in children with ADHD51,52 and in adults with a history of childhood ADHD.53 In addition, previous retrospective work has demonstrated that children with ADHD and collegiate athletes with ADHD are more likely to self-report a history of concussion.54,55 However, the fact that the effect sizes were generally equivalent for analyses with and without athletes with self-reported ADHD suggests that the observed differences in cortical thickness were not principally driven by ADHD. However, the interaction between ADHD, cortical thickness, and concussion history requires future investigation in larger sample sizes.
Our finding of thinner cortex in asymptomatic football athletes with a concussion history suggests that structural changes may occur in young athletes without obvious symptomology. These results highlight the need to identify thresholds of exposure, such as diagnosed concussions or repetitive sub-concussive head injuries, which might be associated with an increased risk of neuroanatomical abnormalities in contact sport athletes. The effects of repetitive head injuries during key periods of neurodevelopment are of particular interest due to the large number of youths that participate in contact sports. A more thorough understanding of the neuroanatomical consequences of sports-related concussion in young athletes might help elucidate specific risk factors for the development of behavioral deficits that have been associated with a history of concussions.39,40
Limitations
This work should be interpreted within the limitations of the present dataset. First, our groups differed in age. Although age was used as a covariate, it is possibly that age confounded some of the observed results. Second, as previously mentioned, we did not specifically exclude participants with a self-reported history of ADHD, although the observed effect sizes for our regions with thinner cortex were similar with or without inclusion of these subjects. Third, previous work has demonstrated that the operational definition of concussion can influence athletes' self-report of previous concussions.56 The reliance on self-reported medically diagnosed concussions in the current work may have led to under-estimation, as many concussions go unreported.57 Although we observed no significant relationship between the number of concussions and cortical thickness in football athletes with a history of concussion, alternative cut-off points (e.g., three or more concussions) and larger sample sizes should be used in future studies to investigate potential exposure-dependent relationships with neuroanatomical measures. In addition, the ROI approach used to compare FA between groups in the current study was reliant on a priori region selection and may be insensitive to smaller differences in FA due to volume averaging. Finally, the healthy control group included a combination of both non-football athletes and non-athlete healthy controls, preventing us from adequately controlling for certain confounds associated with being a collegiate student-athlete, such as high levels of exercise and academic stressors. These facts, together with the cross-sectional nature of the current study, prevent any conclusions about causality.
Conclusions
Current results suggest that a history of concussion in collegiate football athletes is associated with thinner cortex in the ventromedial prefrontal cortex and motor cortex. These results add to the growing body of literature showing that repeated exposure to concussive head injuries might result in structural brain changes in young, collegiate football players without overt symptomology. Large-scale, longitudinal studies are required to assess the effects of concussive and sub-concussive head hits on normal brain development and the long-term clinical consequences of these structural changes.
Acknowledgments
This research was conducted using internal funds from the Laureate Institute for Brain Research, which is supported by the William K. Warren Foundation. The funding sponsor had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; nor the preparation, review, or approval of the manuscript. This article was prepared while Patrick S. F. Bellgowan was employed at the National Institute of Neurological Disorders and Stroke. The opinions expressed in this article are the authors' own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. The authors would like to thank the psychiatric assessment team and magnetic resonance technicians at the Laureate Institute for Brain Research for their assistance in data collection, and Christopher Nerio, M.S., ATC, and David Polanski, M.S., ATC, LAT, at the University of Tulsa for athlete referral.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D., and Bellgowan P.S. (2014). Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 311, 1883–1888 [DOI] [PubMed] [Google Scholar]
- 2.McKee A.C., Daneshvar D.H., Alvarez V.E., and Stein T.D. (2014). The neuropathology of sport. Acta Neuropathol. 127, 29–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart J., Jr., Kraut M.A., Womack K.B., Strain J., Didehbani N., Bartz E., Conover H., Mansinghani S., Lu H., and Cullum C.M. (2013). Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 70, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremblay S., De Beaumont L., Henry L.C., Boulanger Y., Evans A.C., Bourgouin P., Poirier J., Theoret H., and Lassonde M. (2013). Sports concussions and aging: a neuroimaging investigation. Cereb. Cortex 23, 1159–1166 [DOI] [PubMed] [Google Scholar]
- 5.Strain J., Didehbani N., Cullum C.M., Mansinghani S., Conover H., Kraut M.A., Hart J., Jr., and Womack K.B. (2013). Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 81, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner A., Kay-Lambkin F., Stanwell P., Donnelly J., Williams W.H., Hiles A., Schofield P., Levi C., and Jones D.K. (2012). A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma 29, 2521–2538 [DOI] [PubMed] [Google Scholar]
- 7.Ling J.M., Pena A., Yeo R.A., Merideth F.L., Klimaj S., Gasparovic C., and Mayer A.R. (2012). Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 135, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 29, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipton M.L., Gellella E., Lo C., Gold T., Ardekani B.A., Shifteh K., Bello J.A., and Branch C.A. (2008). Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 10.Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., and LaConte S.M. (2014). Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer A.R., Bellgowan P.S., and Hanlon F.M. (2015). Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci. Biobehav. Rev. 49, 8–18 [DOI] [PubMed] [Google Scholar]
- 12.McAllister T.W., Ford J.C., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Bolander R.P., Tosteson T.D., Turco J.H., Raman R., and Jain S. (2014). Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 82, 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigler E.D., Abildskov T.J., Petrie J., Farrer T.J., Dennis M., Simic N., Taylor H.G., Rubin K.H., Vannatta K., Gerhardt C.A., Stancin T., and Owen Yeates K. (2013). Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology 27, 438–451 [DOI] [PubMed] [Google Scholar]
- 14.Gale S.D., Baxter L., Roundy N., and Johnson S.C. (2005). Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry 76, 984–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porto L., Jurcoane A., Magerkurth J., Althaus J., Zanella F., Hattingen E., and Kieslich M. (2011). Morphometry and diffusion MR imaging years after childhood traumatic brain injury. Eur. J. Paediatr. Neurol. 15, 493–501 [DOI] [PubMed] [Google Scholar]
- 16.Merkley T.L., Bigler E.D., Wilde E.A., McCauley S.R., Hunter J.V., and Levin H.S. (2008). Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. J. Neurotrauma 25, 1343–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Kierans A., Kenul D., Ge Y., Rath J., Reaume J., Grossman R.I., and Lui Y.W. (2013). Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology 267, 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tate D.F., York G.E., Reid M.W., Cooper D.B., Jones L., Robin D.A., Kennedy J.E., and Lewis J. (2014). Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 8, 102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay S., Beaule V., Proulx S., Tremblay S., Marjanska M., Doyon J., Lassonde M., and Theoret H. (2014). Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin. Neurophysiol. 125, 1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling J.M., Klimaj S., Toulouse T., and Mayer A.R. (2013). A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology 81, 212–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofman P.A., Stapert S.Z., van Kroonenburgh M.J., Jolles J., de Kruijk J., and Wilmink J.T. (2001). MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 22, 441–449 [PMC free article] [PubMed] [Google Scholar]
- 22.Ross D.E., Ochs A.L., Seabaugh J.M., Demark M.F., Shrader C.R., Marwitz J.H., and Havranek M.D. (2012). Progressive brain atrophy in patients with chronic neuropsychiatric symptoms after mild traumatic brain injury: a preliminary study. Brain Inj. 26, 1500–1509 [DOI] [PubMed] [Google Scholar]
- 23.Cernich A., Reeves D., Sun W., and Bleiberg J. (2007). Automated Neuropsychological Assessment Metrics sports medicine battery. Arch. Clin. Neuropsychol. 22 Suppl 1, S101–S114 [DOI] [PubMed] [Google Scholar]
- 24.Johnson D.R., Vincent A.S., Johnson A.E., Gilliland K., and Schlegel R.E. (2008). Reliability and construct validity of the Automated Neuropsychological Assessment Metrics (ANAM) mood scale. Arch. Clin. Neuropsychol. 23, 73–85 [DOI] [PubMed] [Google Scholar]
- 25.Short P., Cernich A., Wilken J.A., and Kane R.L. (2007). Initial construct validation of frequently employed ANAM measures through structural equation modeling. Arch. Clin. Neuropsychol. 22 Suppl 1, S63–S77 [DOI] [PubMed] [Google Scholar]
- 26.Thorne D.R. (2006). Throughput: a simple performance index with desirable characteristics. Behav. Res. Methods 38, 569–573 [DOI] [PubMed] [Google Scholar]
- 27.Roebuck-Spencer T.M., Vincent A.S., Gilliland K., Johnson D.R., and Cooper D.B. (2013). Initial clinical validation of an embedded performance validity measure within the automated neuropsychological metrics (ANAM). Arch. Clin. Neuropsychol. 28, 700–710 [DOI] [PubMed] [Google Scholar]
- 28.Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 [DOI] [PubMed] [Google Scholar]
- 29.Turken A.U., Herron T.J., Kang X., O'Connor L.E., Sorenson D.J., Baldo J.V., and Woods D.L. (2009). Multimodal surface-based morphometry reveals diffuse cortical atrophy in traumatic brain injury. BMC Med. Imaging 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talairach J. and Tournoux P. (1988). Co-planar Stereotaxic Atlas of the Human Brain. Thieme: New York [Google Scholar]
- 31.Mori S. and van Zijl P. (2007). Human white matter atlas. Am. J. Psychiatry 164, 1005. [DOI] [PubMed] [Google Scholar]
- 32.Ling J., Merideth F., Caprihan A., Pena A., Teshiba T., and Mayer A.R. (2012). Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum. Brain Mapp. 33, 50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones T.B., Bandettini P.A., Kenworthy L., Case L.K., Milleville S.C., Martin A., and Birn R.M. (2010). Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. NeuroImage 49, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischl B., Sereno M.I., and Dale A.M. (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207 [DOI] [PubMed] [Google Scholar]
- 35.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., and Dale A.M. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 [DOI] [PubMed] [Google Scholar]
- 36.Fischl B. and Dale A.M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 97, 11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardoe H.R., Abbott D.F., and Jackson G.D.; Alzheimer's Disease Neuroimaging Initiative. (2013). Sample size estimates for well-powered cross-sectional cortical thickness studies. Hum. Brain Mapp. 34, 3000–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Covassin T., Elbin R., Kontos A., and Larson E. (2010). Investigating baseline neurocognitive performance between male and female athletes with a history of multiple concussion. J. Neurol. Neurosurg. Psychiatry 81, 597–601 [DOI] [PubMed] [Google Scholar]
- 39.Mannix R., Iverson G.L., Maxwell B., Atkins J.E., Zafonte R., and Berkner P.D. (2014). Multiple prior concussions are associated with symptoms in high school athletes. Ann. Clin. Transl. Neurol. 1, 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Didehbani N., Munro Cullum C., Mansinghani S., Conover H., and Hart J., Jr (2013). Depressive symptoms and concussions in aging retired NFL players. Arch. Clin. Neuropsychol. 28, 418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C., Onate J.A., Yang J., and Kelly J.P. (2003). Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290, 2556–2563 [DOI] [PubMed] [Google Scholar]
- 42.Mayer A.R., Ling J., Mannell M.V., Gasparovic C., Phillips J.P., Doezema D., Reichard R., and Yeo R.A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messe A., Caplain S., Paradot G., Garrigue D., Mineo J.F., Soto Ares G., Ducreux D., Vignaud F., Rozec G., Desal H., Pelegrini-Issac M., Montreuil M., Benali H., and Lehericy S. (2011). Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. 32, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki T., Pasternak O., Mayinger M., Muehlmann M., Savadjiev P., Bouix S., Kubicki M., Fredman E., Dahlben B., Helmer K.G., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., Echlin P.S., and Koerte I.K. (2014). Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: a diffusion tensor imaging study. J. Neurosurg. 120, 882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albaugh M.D., Orr C., Nickerson J.P., Zweber C., Slauterbeck J.R., Hipko S., Gonyea J., Andrews T., Brackenbury J.C., Watts R., and Hudziak J.J. (2014). Postconcussive symptoms are associated with cerebral cortical thickness in healthy collegiate and preparatory school ice hockey players. J. Pediatr. 166, 394–400 [DOI] [PubMed] [Google Scholar]
- 46.Euston D.R., Gruber A.J., and McNaughton B.L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etkin A., Egner T., and Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seichepine D.R., Stamm J.M., Daneshvar D.H., Riley D.O., Baugh C.M., Gavett B.E., Tripodis Y., Martin B., Chaisson C., McKee A.C., Cantu R.C., Nowinski C.J., and Stern R.A. (2013). Profile of self-reported problems with executive functioning in college and professional football players. J. Neurotrauma 30, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Beaumont L., Tremblay S., Poirier J., Lassonde M., and Theoret H. (2012). Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb. Cortex 22, 112–121 [DOI] [PubMed] [Google Scholar]
- 50.De Beaumont L., Mongeon D., Tremblay S., Messier J., Prince F., Leclerc S., Lassonde M., and Theoret H. (2011). Persistent motor system abnormalities in formerly concussed athletes. J. Athl. Training 46, 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw P., Lerch J., Greenstein D., Sharp W., Clasen L., Evans A., Giedd J., Castellanos F.X., and Rapoport J. (2006). Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 63, 540–549 [DOI] [PubMed] [Google Scholar]
- 52.Narr K.L., Woods R.P., Lin J., Kim J., Phillips O.R., Del'Homme M., Caplan R., Toga A.W., McCracken J.T., and Levitt J.G. (2009). Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 48, 1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proal E., Reiss P.T., Klein R.G., Mannuzza S., Gotimer K., Ramos-Olazagasti M.A., Lerch J.P., He Y., Zijdenbos A., Kelly C., Milham M.P., and Castellanos F.X. (2011). Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch. Gen. Psychiatry 68, 1122–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alosco M.L., Fedor A.F., and Gunstad J. (2014). Attention deficit hyperactivity disorder as a risk factor for concussions in NCAA division-I athletes. Brain Inj. 28, 472–474 [DOI] [PubMed] [Google Scholar]
- 55.Iverson G.L., Atkins J.E., Zafonte R., and Berkner P.D. (2014). Concussion history in adolescent athletes with attention-deficit hyperactivity disorder. J. Neurotrauma 2014. November 6; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Robbins C.A., Daneshvar D.H., Picano J.D., Gavett B.E., Baugh C.M., Riley D.O., Nowinski C.J., McKee A.C., Cantu R.C., and Stern R.A. (2014). Self-reported concussion history: impact of providing a definition of concussion. Open Access J. Sports Med. 5, 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCrea M., Hammeke T., Olsen G., Leo P., and Guskiewicz K. (2004). Unreported concussion in high school football players: implications for prevention. Clin. J. Sports Med. 14, 13–17 [DOI] [PubMed] [Google Scholar]