Abstract
Accessing cerebrospinal fluid (CSF) from the craniocervical junction through the posterior atlanto-occipital membrane via cerebellomedullary injection (also known as cisternal puncture or cisterna magna injection) has become a standard procedure in preclinical studies. Such delivery provides broader coverage to the central and peripheral nervous system unlike local parenchymal delivery alone. As a clinical application, this approach offers a more reliable method for neurological gene replacement delivery in infants, where skull-mounted devices are not indicated. Here we describe a consistent, precise, and safe method for CSF injection with minimal equipment and technical skills.
Introduction
The blood–brain barrier effectively restricts the transport of most AAV serotypes between the circulation and the central nervous system (CNS). In these cases, systemic administration of therapeutic agents shows few beneficial effects in the CNS. Cerebellomedullary cistern or cisterna magna (CM) puncture method is a rapid and simple procedure that allows researchers to collect or inject into the cerebrospinal fluid (CSF) space with minimal invasiveness. Its apparent simplicity encourages researchers to perform the procedure without the need of stereotactic instruments or head-restraints. Based on our experience, off-target administration, especially during free-hand injection, can lead to a high risk for partial or total medullar injection causing an incomplete CSF delivery, but more importantly, causing medullary injury that can cause many sensory, often fatal, complications.
Here we describe an effective way to perform CSF injections in nonhuman primates. This method ensures precision and reproducibility with only minor equipment and technical skills. This method has been used in current studies and has been briefly described in several peer-reviewed articles.1–4
Experimental Procedure
1. Material
1.1. Reagents
| Reagents | Commercial name, supplier (cat. number) | Specific handling | Storage conditions |
|---|---|---|---|
| Animal sedation | |||
| Ketamine | Ketathesia, Henry Schein (#11695-0707-1) | 5 mg/kg | RT |
| Atropine | AtroJect SA, Henry Schein (#002452) | 0.04 mg/kg | RT |
| Isofluorane | IsoThesia, Henry Schein (#1169-0500-2) | —a | RT |
| Surgical procedure | |||
| Disinfectant | Zoetis, Nolvasan solution | 10% dilutionb | RT |
| Postsurgical care | |||
| NSAID | Meloxicam, EloxiJect, Henry Schein (#11695-6925-2) | 0.2 mg/kg | RT |
| Buprenorphine | Buprenex, Reckitt Benckiser (#12496-0757-1) | 0.01 mg/kg | RT |
RT, room temperature.
Inhalation anesthetic. Follow specific manufacturer's handling recommendations.
Toxic disinfectant. Follow manufacturer's handling and safety recommendations.
1.2. Equipment
| Equipment | Supplier | Specific handling |
|---|---|---|
| Animal sedation | ||
| Venous catheter | Sur-Vet 22Gx1″ (ref: SROX2225V) | Sterile |
| Surgical procedure | ||
| Stereotactic frame | David Kopf Instruments (model: 1430) | Bleached |
| Stereotactic manipulator arm | David Kopf Instruments (model: 1760) | Cidexa |
| Large probe holder | Stoelting (model: 51633) | Cidexa |
| Three-way stopcock | Intralock, Abbott Labs (ref: 42384-01) | Sterile |
| 3 ml Luer lock syringe | Becton Dickinson | Sterile |
| Min-volume extension set (37″ 0.2 ml priming volume) | Smiths Medical (model: 533640) | Sterile |
| Medfusion pump | Smiths Medical (model: 3500) | Bleached |
| 22G 1″ spinal needle (0.70 mm ×38 mm) | BD Medical (ref: 405161) | Sterile |
Clean the instruments by immersion on Cidex for 1 hour. Then let it air-dry, and use.
2. Animal sedation and surgical setup
Animals are sedated in the home cage with an intramuscular injection of ketamine and atropine, and transported to the preparation area. There, animals are intubated, and a venous line is established with a 22-gauge 1-inch catheter in the saphenous vein to deliver isotonic fluids manually at a rate of 5–10 ml/kg/hr. The back of the neck is shaved with a hair clipper and cleaned with 10% Nolvasan solution and isopropyl alcohol. After induction, isoflurane inhalation anesthesia is delivered at 1–3% to maintain a stable plane of anesthesia. Vital signs are monitored continuously, and when pupil and toe-pinch reflexes are absent, along with low jaw-tone, the animal's head is placed in a stereotactic frame and the neck is flexed with the animal in a prone position.
3. Cerebellomedullary cistern delivery
3.1. Cisterna magna
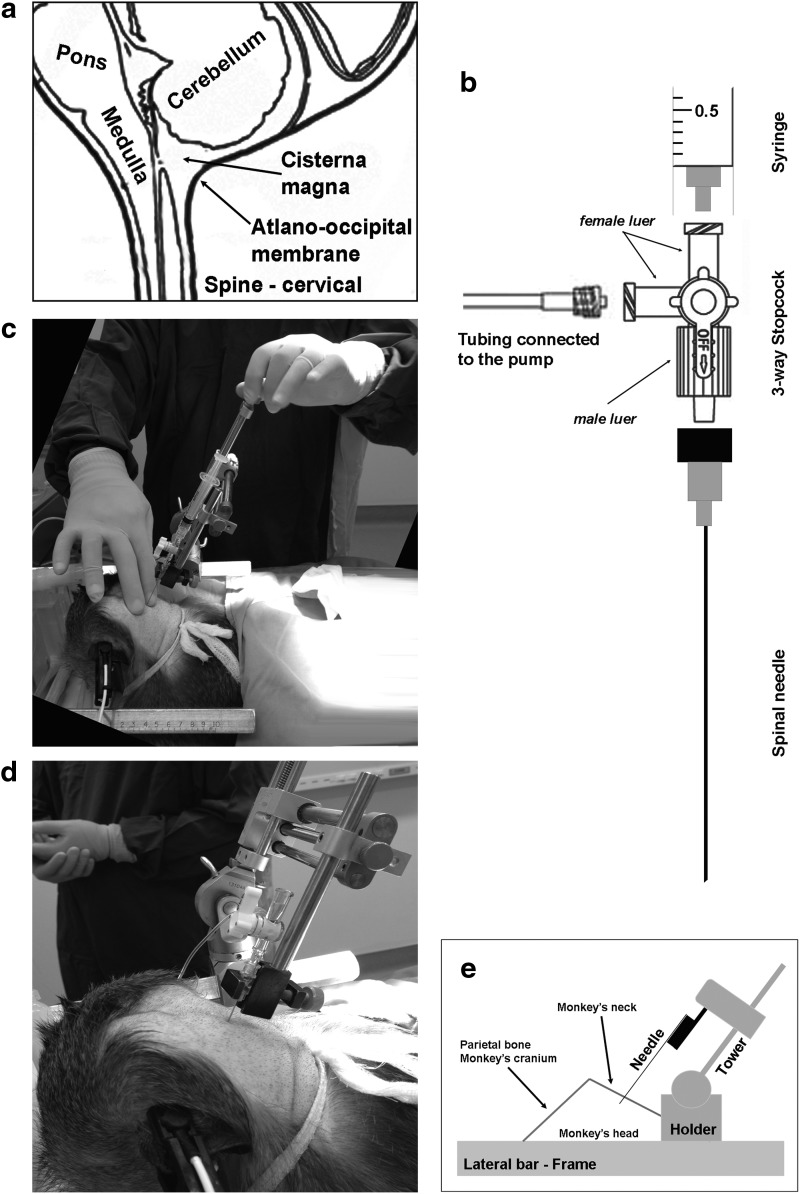
The cisterna magna (CM), or cerebellomedullary cistern, is part of the subarachnoid space below cerebellum, and dorsal to the medulla oblongata. CSF is produced by the choroid plexus in the fourth ventricle and drains mainly through the medial aperture filling the CM. In mammals, the atlanto-occipital membrane ensures integrity and preserves CM flux (Fig. 1a).
Figure 1.
Stereotactic-guided injection into the cerebrospinal fluid (CSF) through cerebellomedullary cistern. (a) Sagittal axis illustration showing the main anatomical structures that surround cisterna magna. (b) Schematic representation of all components that comprise the injection port used during cisterna magna delivery. (c) Once the injection port is secured in the holder, the atlanto-occipital gap in the craniocervical junction is identified by palpation and the needle is manually guided to the target. (d) Needle is slowly introduced through the atlanto-occipital membrane until clear CSF comes up to the syringe. Then, the 3-way stopcock is opened to the loading line to allow vector infusion by means of a pump. (e) Based on monkey's head position in the frame, stereotactic arm might be repositioned. Gently flexed neck in prone position is recommended, and will require an angle (∼33° to ∼45°) to reach a perpendicular plane to the back of the monkey's head.
3.2. Needle assembly and tubing setup
An in-line three-way stopcock permits both collection and injection during the procedure, preserves sample sterility, and minimizes CSF–vector cross-contamination. To confirm CM access and perform a baseline CSF collection, a sterile 3 ml syringe is connected to the female Luer fitting of the 3-way stopcock. On the opposite end of the 3-way stopcock, a 22-gauge 1-inch spinal needle is attached to the male Luer lock fitting and placed into a large probe holder that is mounted onto a stereotactic manipulator arm. To control the administration into the CM space, a syringe containing test article or vehicle solution is locked into an infusion pump and connected to primed 36-inch high-pressure intravenous tubing. The other end of the tubing is connected to the second female Luer fitting of the 3-way stopcock (Fig. 1b).
Critical step: The investigator must ensure that there are no air bubbles in the loading line and should prime the side-port (female Luer fitting) of the 3-way stopcock with infusate. Access between the 3 ml syringe and spinal needle should be in the open position.
3.3. Cisternal injection
After the needle is assembled and infusion system is prepared, the atlanto-occipital gap in the craniocervical junction is identified by palpation (Fig. 1c). Then, once the needle is aligned with the cervical gap, the needle is manually advanced with the manipulator through the skin, atlanto-occipital membrane, and into the cerebellomedullary cistern space (Fig. 1d). To verify the correct depth and to avoid damage to the medulla oblongata, negative pressure is applied during needle insertion by slightly retracting the plunger of the attached syringe. It is important to emphasize that the negative pressure has to be maintained during all the needle insertion until CSF starts to flow inside the syringe. Intermittent peaks of negative pressure while needle is being inserted should be avoided. Constant negative pressure while needle is inserted will ensure to locate needle inside the CM as deep as practicable to avoid medullary injury. Once the needle has passed the atlanto-occipital membrane and entered the CM space, a small volume of CSF (∼1 ml) may be aspirated into the 3 ml syringe and stored at −20°C as a baseline sample. Then, the 3-way stopcock is opened to the loading line to allow pumped infusion of the vector at a rate of 0.5 ml/min. After vector infusion, the line is flushed with 0.5 ml of saline buffer. After a 5 min resting period, the needle is slowly retracted out of the CM space with the manipulator. Hemostasis is achieved by direct pressure after the needle is completely removed. Finally, the head is taken out of the frame, the animal is maintained on a warming blanket, and vital signs are monitored until full recovery from anesthesia. Total time for the procedure is approximately 60 min.
Critical step: Depending on animal's head position in the frame, the investigator may vary the angle of the stereotactic arm to accommodate perpendicular incision into the CM (Fig. 1e). Based on our experience, correct position of the head in the frame and needle alignment will avoid vessel bleeding that would contaminate the CSF sample and case injury.
Critical step: Because the brain is enclosed in a rigid compartment, vascular pulsations related to cardiac cycle also induce pulsations in flow and pressure in the brain and spinal cord, causing minor tissue movement that the investigator has to take into account when inserting the spinal needle through the atlanto-occipital membrane. Hence, it is strongly recommended that the needle be inserted slowly with the manipulator, locking the position as soon as CSF appears in the syringe in order to locate the needle as far as practicable from the medullary tissue.
4. Provisions to minimize discomfort, distress, pain, and injury
Animals receive an intramuscular injection of Meloxicam (NSAID) during cisternal injection and twice the day after the injection. Once the animal is returned to its cage, it is evaluated twice daily for 5 days by veterinary staff. Detailed, standardized forms are completed for each animal that include evaluations of the surgical-site integrity, edema, infection, balance, locomotion, attitude, food intake, and fecal and urine output. Any abnormalities or signs of discomfort are promptly reported to veterinary staff for evaluation and appropriate treatment.
Acknowledgment
This study was supported by a grant to K.B. from NIH-NINDS (R01NS073940).
Author Disclosure
All authors declare no conflict of interest related with this work.
References
- 1.Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012;23:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samaranch L, Salegio EA, San Sebastian W, et al. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Hum Gene Ther 2013;24:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samaranch L, San Sebastian W, Kells A, et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther 2014;22:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salegio EA, Streeter H, Dube N, et al. Distribution of nanoparticles throughout the cerebral cortex of rodents and non-human primates: Implications for gene and drug therapy. Front Neuroanat 2014;8:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]