Abstract
Objectives: To evaluate the methodologic quality of the evidence for the use of spinal manipulative therapy (SMT) with and without other therapies in the management of chronic obstructive pulmonary disease (COPD).
Design: A systematic review of the literature.
Participants: Any participant of a primary research study that investigated the effect of SMT on COPD. Only studies with participants older than age 18 years with an existing diagnosis of COPD were included.
Interventions: Interventions included any form of high-velocity, low-amplitude spinal manipulation with or without other forms of manual therapy, exercise, and/or pharmacologic intervention.
Outcome measures: Six-minute walking test, forced expiratory volume in 1 second, forced vital capacity, residual volume, total lung capacity, Chronic Respiratory Questionnaire, St George's Respiratory Questionnaire, and the Hospital Anxiety and Depression Scale.
Results: Six articles met all of the inclusion criteria and were included in the review: three randomized controlled trials (RCTs), one pre–post observational study, one case series, and one single case study. Sample sizes varied from 1 to 33 participants ranging in age from 55 to 85 years. Risk of bias was low for the three RCTs and high for the other studies. All three RCTs used SMT in conjunction with exercise from a pulmonary rehabilitation program. Five of the six studies reported improvements in lung function and exercise performance following SMT intervention.
Conclusions: This review provides a methodologic evaluation of the evidence for using SMT with and without other therapies in the management of COPD. While the quality of the evidence provided by three RCTs was high, they were all conducted on small sample sizes. These results highlight the need for further research into the use of SMT in conjunction with exercise on people with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by progressive airflow limitation.1 It is ranked sixth among the common causes of death globally for both men and women and currently affects approximately 14% of adults older than age 40 years.1,2 Extrapulmonary effects, such as skeletal muscle dysfunction, affect the severity of the disease and provide a potential target for therapeutic intervention.1 An estimated 18%–36% of people with COPD experience skeletal muscle dysfunction at a level that affects exercise capacity and dyspnea levels, both predictors of mortality in COPD.3 Because exercise capacity is a measure of the amount of exercise that can be performed before the onset of leg fatigue or exercise-limiting dyspnea, a decrease in capacity has been associated with poorer quality of life and higher hospitalization rates.4,5
Nonpharmacologic interventions benefit people with COPD.6–10 For example, pulmonary rehabilitation (PR) is considered to be a well-developed, multidisciplinary approach to managing many extrapulmonary effects associated with COPD.6–8 However, PR has little clinical effect on lung function. Similarly, research into the effect of acupuncture has shown that this modality has little effect on long-term lung function despite helping improve dyspnea levels and exercise tolerance.9,10
One intervention that has the potential to address some of the changes in respiratory mechanics associated with declining lung function in COPD is manual therapy (MT). This intervention covers a range of techniques, including soft tissue therapy and joint mobilization/manipulation. Some evidence suggests that MT has the potential to alter respiratory mechanics in certain chronic respiratory diseases, such as chronic asthma and COPD. These changes include an increase in flexibility of the chest wall and thoracic excursion, which can indirectly lead to an improvement in exercise capacity and lung function.11,12
A recent systematic review on the use of MT for COPD reported a higher incidence of musculoskeletal pain in patients with COPD compared with the general population (45% and 34%, respectively).13 While the authors of this review considered the use of MT to increase thoracic mobility, reduce the work of breathing, and manage musculoskeletal pain a reasonable approach to consider for people with COPD, they concluded little evidence supported or refuted its use in the management of COPD.13 While the review incorporated a range of manual therapies, it specifically excluded studies that used MT intervention with other interventions, such as exercise.
One form of MT is spinal manipulative therapy (SMT), which uses a high-velocity, low-amplitude force to move a joint complex. The technique is commonly used by osteopaths, chiropractors, physiotherapists, and physical therapists to decrease pain and increase joint range of motion.14–17 However, SMT is often used in conjunction with other interventions. Therefore, excluding studies that combined SMT with another intervention may have altered the outcome of the previous systematic review.
The aim of the current review is to evaluate the methodologic quality of the evidence for the use of SMT in the management of COPD as well as updating the previous systematic review. This review focuses on the effect of SMT when used alone or in combination with other therapies.
Materials and Methods
Design
This article reports on a systematic review of the literature.
Study eligibility criteria
To be included in this review, a study must have been conducted on participants older than age 18 years who had a diagnosis of COPD. MT intervention had to include some form of SMT administered as a stand-alone intervention or in conjunction with another intervention.
Trials had to include at least one lung function measure, such as forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), residual volume (RV), or total lung capacity (TLC). Trials could also include other outcome measures, such as chest wall movement, dyspnea level, exercise capacity, or a patient-reported quality-of-life measure.
All quantitative study designs were accepted for review, including randomized controlled trials (RCTs), observational studies, case series, and case studies.
Search
The search was conducted in December 2014 and updated in July 2015. The Medical Subject Heading terms listed in Table 1 were entered into the following databases: MEDLINE/Ovid, ScienceDirect, PubMed, Web of Science, and Scopus. There was no limit on date of publication, and full texts were required. Citations and reference lists were also used to search for articles.
Table 1.
Search Terms and Medical Subject Headings Used for Literature Search
| Medical Subject Headings | Chronic obstructive pulmonary disease, chronic respiratory disease, respiratory disease, dyspnoea, chronic asthma, chronic bronchitis, emphysema, manual therapy, manipulative therapy, physical therapy, chiropractic, osteopathy, physiotherapy, spinal manipulative therapy, and/or exercise/pulmonary rehabilitation |
Study selection
Studies were excluded according to the eligibility criteria listed in Table 2. All articles that met the eligibility criteria were included in this review. Two researchers (C.W. and B.B.) conducted the initial eligibility review.
Table 2.
Eligibility Criteria for Systematic Review
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Peer-reviewed journal articles • RCTs, case-control studies, crossover studies, case series, case studies, clinical trials, pilot trials, preliminary trials (primary research) • Articles reported in English • Respiratory disease measured with spirometry • Participants age ≥18 y • Spinal manipulation or manipulative manual therapy (high-velocity, low-amplitude) only, or in conjunction with other therapies (e.g., medications, exercise) • Intervention on respiratory disease |
• Books, reference views, systematic reviews, literature reviews (secondary research) • Participants age <18 y • Manual therapy not including spinal manipulation (e.g., massage only, exercise only, TENS) • Lung cancer, other cancers affecting the lung/airways |
RCT, randomized controlled trial; TENS, transcutaneous electrical nerve stimulation.
Data collection
Using an adapted Cochrane Review Group standardized data collection form,18 three reviewers (C.W., S.B., and D.F.) independently extrapolated data from the included trials, such as study design, interventions, randomization and concealment, participant characteristics, methods, statistical analyses used, types of outcome measures, and results. The data were tabulated, and any discrepancies were resolved by discussion at a meeting of these reviewers.
Risk of bias in individual studies
The Cochrane risk of bias tool19 was applied to the trials included in the review. Each element was assessed as being present, not present, or unclear for each trial. Because of the inclusion of case series, case studies, and pre–post studies, the revised risk of bias table by Viswanathan et al.20 was also used to evaluate the risk of bias in studies other than RCTs.
Statistical analysis
The homogeneity of the studies was assessed to determine whether pooling of data and meta-analysis would be appropriate.
Results
Study selection
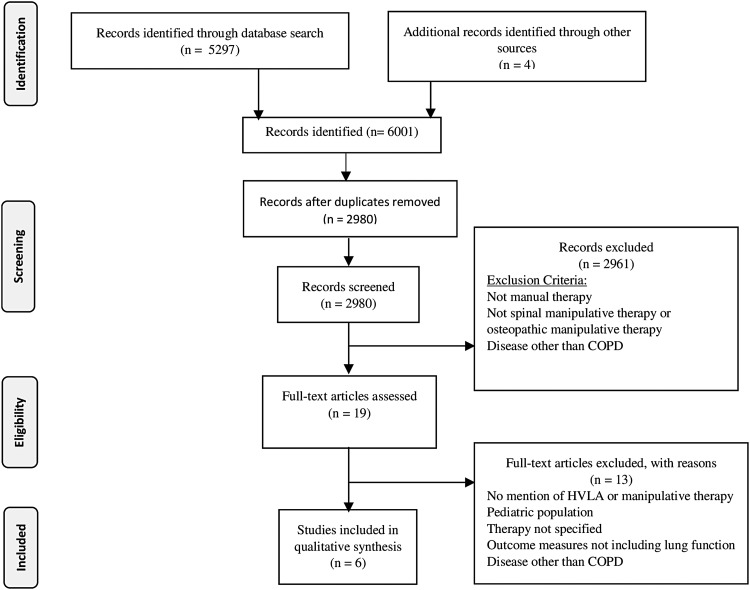
A search of the literature returned 5297 articles, and an additional four articles were recovered from citation tracking of the reference lists. After application of the eligibility criteria, 5291 articles were rejected for the following reasons: They were duplicates, SMT was not an intervention, COPD was not diagnosed, or only the abstract was available. Six articles were retained for review: three RCTs,21–23 one pre–post observational study,24 one case series,25 and one single case study.26 All three RCTs were preliminary or pilot trials.21–23 Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for article selection.27
FIG. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection for review of manual therapy and chronic obstructive pulmonary disease (COPD).21 HVLA, high-velocity, low-amplitude.
Participant characteristics
Results of the data extraction form are reported in Table 3. The sample sizes from the included trials ranged from 1 to 33. Inclusion and exclusion criteria were adequately reported in the three RCTs and case series but not in the observational and single case studies. Only one RCT matched participants at baseline for age and lung function.23 Three of the studies21,22,25 reported adverse events. The age of the participants ranged from 40 to 90 years, and one study did not report age.24 There were more men in each of the six studies. All three RCTs excluded participants if they could not complete a 6-minute walking test (6MWT).
Table 3.
Results from Data Extraction Form Reporting Study Design, Participant Characteristics, and Intervention and Control Groups
| Author/year/country | Title | Study design | Inclusion/exclusion criteria | Participant characteristics | Intervention | Comparator |
|---|---|---|---|---|---|---|
| Howell et al., 1975, USA USA | The influence of osteopathic manipulative therapy in the management of patients with chronic obstructive lung disease | Pre–post | Exclusion: Bronchial asthma, pulmonary fibrosis, restrictive lung disease Inclusion: COPD |
n = 17 Sex: NR Age: NR 11/17 included in study 9-mo duration |
Osteopathic manipulative therapy, following manual palpation of spinal segments for presence of hypomobility and edematous reported segments T2 and T3 | No comparator |
| Engel et al., 2014, Australia | Medium term effects of including manual therapy in a pulmonary rehabilitation program for chronic obstructive pulmonary disease (COPD): a randomized controlled pilot trial | RCT, pilot | Exclusion: osteoporosis, current smokers, contraindications to SMT Inclusion: age 55–70 y, diagnosis of COPD, nonsmoker for preceding 12 mo, ability to complete a 6MWT |
n = 33; 31/33 assessed Mean age: 65.5 ± 4 y Groups were similar at baseline except for sex and anxiety |
Pulmonary rehabilitation consisted of a 24-wk program. The intervention phase consisted of two stages: an 8-wk “introductory” stage, followed by an 8-wk “maintenance” stage. Manual therapy protocol, including soft tissue therapy and manipulation of thoracic segments. | 3 groups: group 1 (PR) group 2 (ST + PR) group 3 (ST + SM + PR) |
| Dougherty et al., 2011, USA | Spinal manipulative therapy for elderly patients with chronic obstructive pulmonary disease: a case series | Case series | Exclusion: severe dementia, severe osteoporosis, history of fracture, cauda equina syndrome, spinal neoplasia, destructive joint pathology, spinal surgery Inclusion: age ≥65 y, COPD |
n = 6 Sex: 1 man, 5 women Age: Range, 68–89 y; mean, 79.1 y 3 stages of COPD |
12 SMT sessions over 4 weeks: 3 sessions per week. 2 manual spinal manipulations at T6/7 and 9/10, supine, and one instrument-assisted spinal manipulation at T4/5 | No comparator |
| Zanetti et al., 2012, Italy | Osteopathic manipulative treatment effectiveness in severe chronic obstructive pulmonary disease: a pilot study | RCT, pilot | Exclusion: acute exacerbation, neurologic disease or joint disease Inclusion: stable COPD, GOLD stage III (severe) |
n = 20 Sex: 5 women Age: 60 y GOLD stage III (severe) |
PR: 1 session on cyclette and 1 on cycle ergometer for 5 d/wk for 4 wk OMT 1 time/wk for 4 wk No description of exact OMT techniques |
Group 1: PR + sham OMT Group 2: OMT + PR |
| Masarsky and Weber, 1988, USA | Chiropractic management of chronic obstructive pulmonary disease | Case study | No inclusion/exclusion criteria |
n = 1 Sex: male Age: 53 y |
Two baseline phases, 2-wk duration: no treatment Two treatment phases, 3- to 4-mo duration: mixed treatment with SMT |
No comparator |
| Engel et al., 2013, Australia | Short term effects of a course of manual therapy and exercise in people with moderate chronic obstructive pulmonary disease: a preliminary clinical trial | RCT, preliminary | Exclusion: inability to walk unassisted, contraindications for SMT Inclusion: age 40–65 y, moderate stage COPD |
n = 15, 14/15 completed Sex: 60% men and 40% women Age: 56.1 y Moderate COPD Groups matched for age |
PR twice/wk for 4 wk Manual therapy protocol Exercise: walking on a flat surface for 6 min |
3 groups: ST ST + MT ST + MT + exercise |
COPD, chronic obstructive pulmonary disease; NR, not reported; T2, second thoracic vertebrae; T3, third thoracic vertebrae; SMT, spinal manipulative therapy; 6MWT, 6-minute walking test; PR, pulmonary rehabilitation; ST, soft tissue therapy; SM, spinal manipulation; GOLD, Global Initiative for Obstructive Lung Disease; OMT, osteopathic manipulative therapy.
Intervention and control groups
Interventions varied across the six included studies. Two studies23,24 did not report details about intervention other than to refer to it in general terms as “spinal manipulation” or “osteopathic manipulative therapy.” Two of the RCTs21,22 described the use of a standardized MT protocol that included soft tissue therapy and thoracic SMT. One study25 included instrument-assisted spinal manipulation. The single case study26 reported therapy that varied in type and frequency over time. All three RCTs included a control group of sham manipulation or exercise only. The pre–post observational study, case series, and single case study did not include a comparator.
Outcome measures
Outcome measures used and results reported from each of the six studies included in this review are shown in Table 4. All of the trials included FEV1 and FVC as primary outcome measures; two studies23,24 also included RV and TLC. All three RCTs included a 6MWT to evaluate exercise capacity. Standardized quality-of-life questionnaires, such as the Chronic Respiratory Questionnaire, St George's Respiratory Questionnaire, or Hospital Anxiety and Depression scale were used in two of the RCTs,21,22 while the single case study26 reported patient subjective comments on fatigue and breathlessness over time.
Table 4.
Outcome Measures Reported in the Six Studies Included in the Systematic Review
| Author/year/country | Study design and intervention | Outcome measures | Results | Comments |
|---|---|---|---|---|
| Howell et al., 1975, USA | Pre–post OMT |
RV, FVC, FEV1, TLC, arterial gases, disease severity score | Progressive decline in severity score: 10.7 Average decrease: Pco2 (p = 0.005), O2 saturation (p = 0.05), TLC (p = 0.001), RV (p = 0.05) |
Disease severity score |
| Engel et al., 2014, Australia | RCT MTP and/or exercise |
FEV1, FVC, blood pressure, SGRQ, HAD, 6MWT6MWT | FVC at 24 wk (p = 0.04) (ST + SM + PR group vs. PR only) Significant increase in FVC at 24 wk (p = 0.03) Difference between groups for distance walked (6MWT) at 16 wk (p = 0.01) and 24 wk (p = 0.03) No differences between groups for SGRQ or HAD |
Adverse events reported: small number of minor adverse events |
| Dougherty et al., 2011, USA | Case series SM, IASM |
FVC, FEV1 | No clinically significant change in FEV1 at 2 or 4 wk | Adverse events reported: small number of minor adverse events |
| Zanotti et al., 2012, Italy | RCT OMT |
VC, FVC, FEV1, RV, 6MWD, modified Borg scale | PR and OMT gain in 6MWD (p = 0.01) PR and OMT (p = 0.05) reduction in RV, 11% |
|
| Masarsky and Weber, 1988, USA | Case study Chiropractic techniques (including MT) |
FEV1, FVC, dyspnea, fatigue | FVC greater in phase 1 vs. baseline (6 mo); p < 0.005 FVC greater in phase 2 vs. baseline (3 mo); p < 0.005 Mean subjective coughing score during phase 2 less than baseline in phase 2 (3 mo); p < 0.005 No other changes in outcome measures were statistically significant |
Most outcome measures vs. baseline and phases 1–3 |
| Engel et al., 2013, Australia | RCT MTPs and/or exercise |
FEV1, FVC, 6MWT | FVC increased in ST + SM + exercise group at 4 wk; p = 0001 6MWT increased in ST + MT + exercise and ST + MT groups vs. ST only; p = 0.0001 Dyspnea improved in ST + MT + exercise and ST + MT groups vs. ST only; p = 0.0001 |
Adverse events reported: small number of minor adverse events |
RV, residual volume; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; Pco2, partial pressure of CO2; O2, oxygen level; MTP, manual therapy protocol; SGRQ, St George's Respiratory Questionnaire; HAD, Hospital Anxiety and Depression Scale; IASM, instrument-assisted spinal manipulation; VC, vital capacity MT, manual therapy.
Risk of bias
Results from the risk of bias analysis are reported in Table 5. The three RCTs21–23 had a low risk of bias. The other three studies24–26 had a high risk of bias primarily associated with the study design. There was performance bias in all trials, including the RCTs.
Table 5.
Risk of Bias of Included Studies
| Author/year/country | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Level of bias |
|---|---|---|---|---|---|---|
| Howell et al., 1975, USA | Present | Present | Unclear | Present | Unclear | High |
| Engel et al., 2014, Australia | Not present | Present | Not present | Not present | Not present | Low |
| Dougherty et al., 2011, USA | Unclear | Present | Present | Present | Not present | High |
| Zanotti et al., 2012, Italy | Not present | Present | Not present | Not present | Unclear | Low |
| Masarsky and Weber, 1988, USA | Present | Present | Present | Present | Present | High |
| Engel et al., 2013, Australia | Not present | Present | Not present | Not present | Not present | Low |
Synthesis of results
Because of the heterogeneous nature of the included trials, pooling of data and meta-analysis of the results were not possible.
Reported results of studies
One RCT plus the observational study23,24 reported a decrease in RV and an increase in TLC following MT. Two of the RCTs21,22 reported an increase in FVC in the short and medium term, respectively, in the group receiving MT. The single case study26 reported similar increases but only in the short term. The three RCTs reported an increase in distance walked (6MWT) in the groups receiving MT, with one22 also reporting an improvement in dyspnea scores. Quality-of-life scores changed little across all trials. Three studies21,22,25 reported on adverse events following SMT, describing a small number of minor adverse events and no moderate or severe adverse events. Minor adverse events are described as muscle soreness up to 24 hours after treatment that resolves with no further intervention.
Discussion
This systematic review updates the results from a previous review and is the first to focus on evidence of the effect of administering SMT in conjunction with other interventions in the management of COPD. Improvements in lung function (increases in FEV1 and FVC; decrease in RV) and exercise capacity (increase in 6MWT) were reported in three RCTs following a combination of SMT and exercise. While these findings were recorded in pilot and preliminary trials, they represent preliminary evidence that the combination of SMT with exercise may be more beneficial to people with COPD than exercise or SMT alone. Furthermore, the results provide additional information to the review by Heneghan and colleagues; however, the findings of this review contrast with the earlier conclusion that no evidence supported or refuted the use of MT on patients with COPD.13
While performance bias was present in all of the studies included in this review, it was the result of the difficulty in blinding the therapists who provided SMT intervention. Thus, it should be interpreted in light of the general difficulty in finding a suitable sham intervention for SMT. Setting this aside, the level of bias in the three RCTs was low, confirming that their designs were satisfactory.
The reduction in RV and increase in FVC resulting from SMT intervention may be the mechanism underlying the delay in the onset of exercise-limiting dyspnea. The suggestion that this leads to the reported improvements in exercise capacity is plausible because the onset of dyspnea is the primary cause for exercise cessation in COPD.21–23 This is supported in principle by Heneghan et al., who stated that using MT to increase thoracic mobility in an effort to reduce the work of breathing was a reasonable approach to consider.13
The three studies that recorded adverse events following MT21,22,25 reported similar rates of mild adverse events. A mild adverse event consisted of muscle soreness that resolved within 48 hours without the need for further medical treatment. This included the two studies that used a predetermined MT protocol consisting of soft tissue therapy and thoracic SMT.21,22 No moderate or severe adverse events following any type of SMT intervention were reported in these studies
This review had several methodologic limitations. These include the small number of studies, the sample size in each of the studies, variation in participant characteristics and SMT intervention across studies, and that the cohorts may not be representative of the general population of people with COPD. These limitations notwithstanding, the review highlights the potential of SMT to benefit people with COPD.
In conclusion, this appears to be the first systematic review to investigate the evidence for administering SMT in conjunction with other modalities, such as exercise, on people with COPD. The exclusion of such combinations may explain the disparity in findings between this review and the review by Heneghan et al., who found no evidence to support or refute the use of MT in the management of COPD. The importance of increasing exercise capacity even by indirect methods such as increasing thoracic mobility should not be underestimated because exercise capacity is a predictor of mortality in COPD. As PR does not improve lung function, the current findings may have wider implications if repeated in a larger cohort. The results of this systematic review support the recommendation for further research in the field, in particular a larger RCT designed to investigate the effect of combining SMT with exercise.
Acknowledgments
The authors thank Ms. Hazel Jenkins for assisting in the design and editing of the project and the Department of Chiropractic at Macquarie University for in-kind support for this project. No funding was required for this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2015. Online document at: www.goldcopd.org Accessed March23, 2015
- 2.Abramson M, Dabscheck E, Frith P, et al. , on behalf of Lung Foundation Australia and the Thoracic Society of Australia and New Zealand. The COPD-X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease. 2015. Online document at: www.copdx.org.au/
- 3.Choudhury G, Rabinovich R, MacNee W. Comorbidities and systemic effects of chronic obstructive pulmonary disease. Clin Chest Med 2014;35:101–130 [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aymerich J, Agustí À, Barberà JA, et al. Phenotypic heterogeneity of chronic obstructive pulmonary disease. Arch Bronconeumol 2009;45:133–142 [DOI] [PubMed] [Google Scholar]
- 5.Díaz AA, Morales A, Díaz JC, et al. CT and physiologic determinants of dyspnea and exercise capacity during the six-minute walk test in mild COPD. Respir Med 2013;107:570–579 [DOI] [PubMed] [Google Scholar]
- 6.Lacasse Y, Martin S, Lasserson T, Goldstein R. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eur Medicophys 2007;43:475–485 [PubMed] [Google Scholar]
- 7.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;(4):CD003793. [DOI] [PubMed] [Google Scholar]
- 8.Egan C, Deering BM, Blake C, et al. Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med 2012;106:1671–1679 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Muro S, Ando Y, et al. A randomized, placebo-controlled trial of acupuncture in patients with chronic obstructive pulmonary disease (COPD): the COPD-acupuncture trial (CAT). Arch Intern Med 2012;172:878–886 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Namura K, Ohno Y, et al. The effect of acupuncture in the treatment of chronic obstructive pulmonary disease. J Alternat Complement Med 2008;14:1097–105 [DOI] [PubMed] [Google Scholar]
- 11.Bockenhauer SE, Julliard KN, Lo KS, Huang E, Sheth AM. Quantifiable effects of osteopathic manipulative techniques on patients with chronic asthma. J Am Osteopath Assoc 2002;102:371–375 [PubMed] [Google Scholar]
- 12.Engel R, Vemulpad S. The role of spinal manipulation, soft-tissue therapy, and exercise in chronic obstructive pulmonary disease: a review of the literature and proposal of an anatomical explanation. J Alternat Complement Med 2011;17:797–801 [DOI] [PubMed] [Google Scholar]
- 13.Heneghan NR, Adab P, Balanos GM, Jordan RE. Manual therapy for chronic obstructive airways disease: a systematic review of current evidence. Man Ther 2012;17:507–518 [DOI] [PubMed] [Google Scholar]
- 14.International Federation of Orthopaedic Manipulative Physical Therapists. OMT definition. Online document at: http://www.ifompt.com/About+IFOMPT/OMT+Definition.html Accessed July1, 2015
- 15.National Institutes of Health. Spinal manipulation for low back pain. Online document at:: http://nccam.nih.gov/health/pain/spinemanipulation.htm Accessed July1, 2015
- 16.Maigne J-Y, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine 2003;70:336–341 [DOI] [PubMed] [Google Scholar]
- 17.Clar C, Tsertsvadze A, Court R, Hundt GL, Clarke A, Sutcliffe P. Clinical effectiveness of manual therapy for the management of musculoskeletal and non-musculoskeletal conditions: systematic review and update of UK evidence report. Chiropr Man Ther 2014;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochrane Group for Systematic Reviews. Data extraction form. Oxford, UK: The Cochrane Collaboration; 2014. Online document at http://www.cochrane.org [Google Scholar]
- 19.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Oxford, UK: The Cochrane Collaboration, 2011. Online document at: www.cochrane-handbook.org [Google Scholar]
- 20.Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews. AHRQ Publication No. 12-EHC047-EF. Bethesda, MD; Agency for Healthcare Research and Quality, March 2012. Online document at: www.effectivehealthcare.ahrq.gov [Google Scholar]
- 21.Engel RM, Gonski P, Beath K, Vemulpad S. Medium term effects of including manual therapy in a pulmonary rehabilitation program for chronic obstructive pulmonary disease (COPD): a randomized controlled pilot trial. J Man Manip Ther 2014. doi: 10.1179/2042618614Y.0000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel RM, Vemulpad SR, Beath K. Short-term effects of a course of manual therapy and exercise in people with moderate chronic obstructive pulmonary disease: a preliminary clinical trial. J Manip Physiol Ther 2013;36:490–496 [DOI] [PubMed] [Google Scholar]
- 23.Zanotti E, Berardinelli P, Bizzarri C, et al. Osteopathic manipulative treatment effectiveness in severe chronic obstructive pulmonary disease: a pilot study. Complement Ther Med 2012;20:16–22 [DOI] [PubMed] [Google Scholar]
- 24.Howell R, Allen T, Kappler R. The influence of osteopathic manipulative therapy in the management of patients with chronic obstructive lung disease. J Am Osteopath Assoc 1975;74:757–760 [PubMed] [Google Scholar]
- 25.Dougherty PE, Engel RM, Vemulpad S, Burke J. Spinal manipulative therapy for elderly patients with chronic obstructive pulmonary disease: a case series. J Manip Physiol Ther 2011;34:413–417 [DOI] [PubMed] [Google Scholar]
- 26.Masarsky CS, Weber M. Chiropractic management of chronic obstructive pulmonary disease. J Manip Physiol Ther 1988;11:505–510 [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]