Abstract
Therapeutic hypothermia or targeted temperature management has been used after cardiac arrest to improve neurological outcomes and mortality. However, a side effect of temperature modulation is a centrally mediated shivering response. The Columbia Anti-Shivering Protocol sets up a systematic method of intravenous (IV) and oral medication escalation to suppress this response and preserve the benefits of this therapy. We present the case of a 59-year-old male who began shivering after therapeutic hypothermia for cardiac arrest, leading to a persistent rise in core temperature despite adequate sedation. He was also found to have gastric contents similar to coffee grounds through nasogastric tube suction. The shivering was effectively suppressed and the rising core temperature plateaued using rectal acetaminophen and buspirone administered by means of a novel device, the Macy Catheter. Also, when used in conjunction with other protocol-driven medications, the patient was able to achieve a core temperature of 33°C. The Macy Catheter appears to be a useful approach to rectally administer buspirone and acetaminophen, using an easy-to-place, nonsterile atraumatic device that requires no radiographic confirmation of placement.
Hospitals are treating an increasing number of cardiac arrest patients after return of spontaneous circulation (ROSC) with therapeutic hypothermia or targeted temperature management (TTM). Multiple studies have shown benefits of this approach such as improved neurological outcomes and decreased mortality (Bernard et al., 2002; HACA, 2002; Arrich et al., 2009; Nielsen et al., 2014). One of the common complications of temperature reduction during TTM is the shivering response. Shivering is a centrally mediated response, which has the potential to reverse many of the theoretical benefits of the cooling. The detriments of shivering include increased extracranial oxygen consumption and blood flow, ultimately depriving the brain of the benefits the cooling was meant to provide. In 2011, the Columbia Anti-Shivering Protocol (Table 1) was published to provide guidance on the stepwise pharmacological suppression of this shivering response, with the aim to decrease the need for prolonged sedation and unnecessarily lengthy ICU stays (Choi et al., 2011).
Table 1.
Comparison of the Advocate Christ Medical Center Therapeutic Hypothermia Protocol Followed in the Treatment of This Patient to the Columbia Anti-Shivering Protocol
| Shivering assessment | Advocate Christ Medical Center Therapeutic Hypothermia Protocol | Columbia Anti-Shivering Protocol |
|---|---|---|
| BSAS (Bedside Shivering Assessment Scale) = 0 No shiver |
□ Ensure sedation is optimized (two agents if needed) | □ Acetaminophen 650–1000 mg Q4–6h |
| (1) Fentanyl | □ Buspirone 30 mg Q8h | |
| (2) Propofol, midazolam, or lorazepam | □ Magnesium sulfate 0.5–1 mg/h IV | |
| □ Acetaminophen 650 mg PO Q6h | Goal serum magnesium level of 3–4 mg/dL | |
| □ Buspirone 30 mg PO once then 15 mg PO Q8h | □ Skin counterwarming of 43°C/MAX Temp | |
| BSAS = 1 Mild Shivering localized to the neck and/or thorax; or fine artifact on cardiac rhythm; or fine artifact on BIS tracing; or unexplained, significant increase in BIS value |
□ All interventions above, then add: | □ Choose one: |
| □ Meperidine 25 mg IV push once, then 12.5 mg IV bolus Q30min PRN | □ Dexmedetomidine 0.2–1.5 μg/(kg·h) | |
| (For GFR <50 mL/min/1.73 m2) Meperidine 6.25 mg IV push Q30min PRN | □ Opioid: Meperidine 50–100 mg IM or IV | |
| BSAS = 2 Moderate Shivering involves gross movement of the upper extremities (in addition to neck and thorax) |
□ All interventions above, then add: | □ Use both: |
| □ Meperidine 25 mg IV push Q30min PRN | Dexmedetomidine 0.2–1.5 μg/(kg·h) | |
| (For GFR <50 mL/min/1.73 m2) Meperidine 12.5 mg IV push Q30min PRN | Opioid: Meperidine 50–100 mg IM or IV | |
| □ Magnesium 0.5 g/h continuous IV infusion | ||
| Titrate by 0.5 g/h to maintain serum magnesium level of 3–4 mg/dL | ||
| BSAS = 3 Severe Shivering involves gross movements of the trunk and upper and lower extremities |
□ All interventions above, then add: | □ Propofol 50–75 μg/(kg·min) |
| □ Neuromuscular blockade | □ Neuromuscular blockade: Vecuronium 0.1 mg/kg IV |
Choi et al., 2011.
BSAS, Bedside Shivering Assessment Scale; PO, per os; PRN, pro re nata; IV, intravenous; IM, intramuscular; GFR, glomerular filtration rate; BIS, bispectral index.
Although effective, the first-stage medications utilized in this protocol are not generally available in intravenous formulation. A new rectal administration device, the Macy Catheter (Hospi Corp.), has recently become available, offering an easy route to quickly provide first-stage antishivering medications such as acetaminophen and buspirone even before placement of nasogastric (NG) or orogastric (OG) tubes (Lyons et al., 2015). In cases in which an NG or OG tube cannot be placed, or contraindications to NG/OG tube medication administration (such as upper gastrointestinal [GI] bleeding or a need for continuous suction) exist, the Macy Catheter may offer the only practical method of administration.
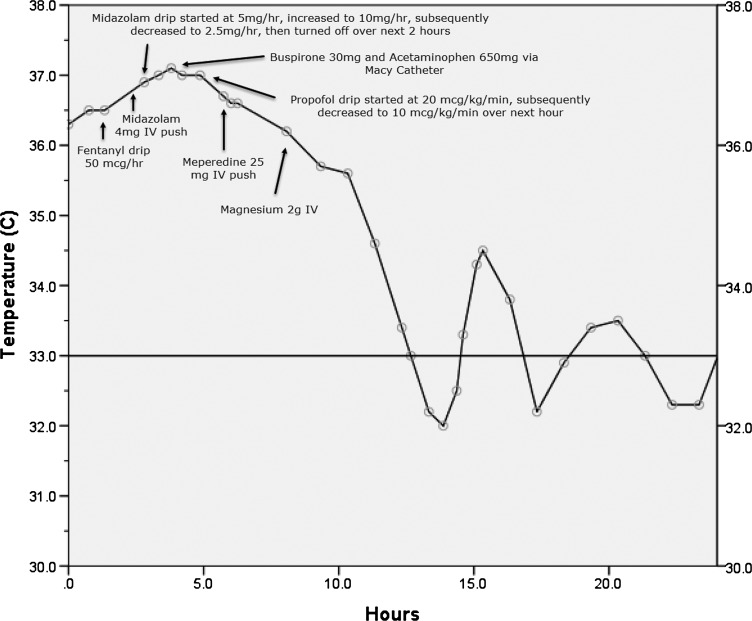
We present the case of a 59-year-old male brought into the emergency department (ED) as a full cardiac arrest outside of the hospital. The patient had ROSC in the ED after multiple rounds of epinephrine and defibrillation. After this, the patient was intubated and placed in an Arctic Sun Cooling System to institute therapeutic hypothermia at 33°C. Temperature feedback was monitored with a continuous bladder thermometer, and his treatment followed the Advocate Christ Medical Center Therapeutic Hypothermia Protocol (Table 1), which closely resembles the Columbia Anti-Shivering Protocol. An NG tube was also placed to suction, which revealed the gastric contents to be similar in appearance to coffee grounds without a clear etiology. In addition, a fecal occult blood test was performed and found to be positive. The source of the bleeding was felt to be GI in nature rather than secondary to a traumatic airway placement. In accordance with the hypothermia protocol, the patient was initially sedated on fentanyl with a core temperature of 36.5°C (Fig. 1). Despite the addition of midazolam and a later increased dose of the midazolam drip, the patient's core temperature continued to rise. After about 4 hours with the Arctic Sun Cooling System set to 33°C and despite adequate doses of sedation, the patient's core temperature curve demonstrated a steady upslope, and the patient began to shiver. In accordance with the hypothermia protocol (Table 1), a step 0 baseline medication administration was initiated to control the shivering. The initial medications selected included liquid acetaminophen 650 mg and buspirone 30 mg. Due to the evidence of upper GI bleeding, these medications were administered rectally using a Macy Catheter. In addition, aspirin 325 mg was administered rectally for its antiplatelet effect, the benefits of which were felt to outweigh risks (e.g., antiprostaglandin effects on the stomach) based on the patient's elevated troponin.
FIG. 1.
Patient's core body temperature after return of spontaneous circulation and placement of Arctic Sun Cooling System set to 33°C with subsequent anti-shivering interventions noted directly on the graph. IV, intravenous.
The Macy Catheter used is a thin silicone tube, 14F in diameter, with a 15 mL balloon at the tip that is sized to allow secure retention in the rectum, yet allow for easy passage in the event of defecation. Placement does not require any sterile technique. Multiple exit ports exist at the distal catheter tip for fluid and medication passage, and an internal one-way check valve is present in the proximal administration port to prevent backflow of fluids. The buspirone was crushed using the pill crusher supplied with the Macy Catheter kit, mixed with normal tap water, and administered alongside the acetaminophen and aspirin. Subsequently, the Macy Catheter was flushed with normal tap water. Shortly after the administration of these medications, the patient's previously rising core temperature effectively plateaued at 36.9°C and he clinically stopped shivering. Further acceleration in temperature reduction was then achieved in accordance with the hypothermia protocol (Table 1) by switching sedation agents to a low-dose propofol drip, then administering meperidine 25 mg IV push, and later use of magnesium 2 g IV (Fig. 1).
Absorption of fluids and medications by the rectal mucosa is well described, and has a long history of use, dating back to at least the early 1900s (Murphy, 1909; Trout, 1912; Nabil et al., 1982; Bruera et al., 1994; Needham, 1995; Zweig et al., 2006; Fouad et al., 2010; Pasero, 2010; Chiang et al., 2011). Because no sterility, complex training, or follow-up X-ray confirmation is required, this approach has also been utilized in both emergent and remote settings (Tremayne, 2010). Practical use of this route has likely been limited by the lack of a simple, effective means of administration and catheter retention, which has been addressed now by the Macy Catheter.
There are no previous studies or guidelines on the rectal administration of buspirone for shivering suppression after therapeutic hypothermia. Buspirone is a 5-HT agonist that has been used effectively to suppress shivering secondary to therapeutic hypothermia, by lowering the shivering threshold without providing overt sedation and paralysis. Acetaminophen acts through a centrally mediated prostaglandin inhibition to decrease the hypothalamic temperature set point (Choi et al., 2011). Buspirone is typically administered orally or using the NG/OG tube, however, given the presence of GI bleeding in this case, this method of administration was felt to be unadvisable. Buspirone has an extensive first-pass metabolism through hepatic oxidation when taken orally, with a mean systemic availability of about 4% and the main metabolite, 1-pyrimidinylpiperazine, at most 25% as potent as buspirone (Gammans et al., 1986). Research on the pharmacokinetics of rectal buspirone is limited; however, the avoidance of the first-pass metabolism may increase the serum concentration over the expected range when compared with oral administration. Clinically, the rectal administration through the Macy Catheter of the combined acetaminophen and buspirone effectively stopped the patient's shivering and plateaued his previously rising core temperature. This combined with other hypothermia protocol-driven medications led to a sustained and continuous decline in the patient's core temperature.
This novel method of medication administration using the Macy Catheter has the potential to change current practices. The large first-pass hepatic metabolism of buspirone may initially be avoided as a result of rectal administration. This may allow for less escalation of protocol-driven shivering suppression leading to quicker hypothermia induction and fewer sedating agents used. Further research into this matter is needed. This method also has the ability to ease medication administration issues (e.g., nausea and emesis) in patients with difficulty taking oral medications, a common problem in patients suffering from acute coronary syndrome.
Limitations in this case report include the concurrent administration of acetaminophen and aspirin alongside the buspirone, which may alone have been enough to cause the cessation of shivering. Shivering was assessed clinically and thought to be the only reason delaying the patient's achievement of goal temperature. We did not perform pharmacokinetic measurements of the medications administered and relied solely on clinical response to guide our treatment plans.
In summary, rectal administration of buspirone and acetaminophen through a new medical device, the Macy Catheter, was effective and offered an easy to use first-line method of shivering suppression in the setting of therapeutic hypothermia.
Author Disclosure Statement
No competing financial interests exist.
References
- Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009;CD004128. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Bruera E, Schoeller T, Pruvost M. Proctoclysis for hydration of terminal cancer patients. Lancet 1994;344:1699. [DOI] [PubMed] [Google Scholar]
- Chiang LM, Wang HS, Shen HH, Deng ST, Tseng CH, Chen YI, Chou ML, Hung PC, Lin KL. Rectal diazepam solution is as good as rectal administration of intravenous diazepam in the first-aid cessation of seizures in children with intractable epilepsy. Pediatr Neonatol 2011;52:30–33 [DOI] [PubMed] [Google Scholar]
- Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, Gilmore E, Malhotra R, Mayer SA, Lee K, Claassen J, Schmidt JM, Badjatia N. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care 2011;14:389–394 [DOI] [PubMed] [Google Scholar]
- Fouad EA, El-Badry M, Alanazi FK, Arafah MM, Al-Ashban R, Alsarra IA. Preparation and investigation of acetyl salicylic acid-caffeine complex for rectal administration. Pharm Dev Technol 2010;15:249–257 [DOI] [PubMed] [Google Scholar]
- Gammans RE, Mayol RF, LaBudde JA. Metabolism and disposition of buspirone. Am J Med 1986;80:41–51 [DOI] [PubMed] [Google Scholar]
- HACA. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Lyons N, Nejak D, Lomotan N, Mokszycki R, Jamieson S, McDowell M, Kulstad E. An alternative for rapid administration of medication and fluids in the emergency setting using a novel device. Am J Emerg Med 2015;33:1113.e1115–e1116 [DOI] [PubMed] [Google Scholar]
- Murphy JB. Proctoclysis in the treatment of peritonitis. JAMA 1909;LII:1248–1250 [Google Scholar]
- Nabil N, Miner DJ, Amatruda JM. Methimazole: an alternative route of administration. J Clin Endocrinol Metab 1982;54:180–181 [DOI] [PubMed] [Google Scholar]
- Needham D. Proctoclysis for hydration. Lancet 1995;345:596. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Friberg H. Targeted temperature management after cardiac arrest. N Engl J Med 2014;370:1360. [DOI] [PubMed] [Google Scholar]
- Pasero C. Perioperative rectal administration of nonopioid analgesics. J Perianesth Nurs 2010;25:5–6 [DOI] [PubMed] [Google Scholar]
- Tremayne V. Emergency rectal infusion of fluid in rural or remote settings. Emerg Nurse 2010;17:26–28 [DOI] [PubMed] [Google Scholar]
- Trout HH. Proctoclysis: some clinical and experimental observations. JAMA 1912;LVIII:1352–1354 [Google Scholar]
- Zweig SB, Schlosser JR, Thomas SA, Levy CJ, Fleckman AM. Rectal administration of propylthiouracil in suppository form in patients with thyrotoxicosis and critical illness: case report and review of literature. Endocr Pract 2006;12:43–47 [DOI] [PubMed] [Google Scholar]