Abstract
We report that the addition of an host paracaspase MALT1 inhibitor, MI-2, to HIV latently infected ACH-2, Jurkat E4, and J-LAT cells accelerated cell death in the presence of cell stimuli or the protein kinase C agonist, bryostatin 1. MI-2-mediated cell death correlated with the induction of the cellular RNase MCPIP1 and requires the presence of viral component(s). Altogether, the combination of MI-2 and bryostatin 1 displays selective killing of HIV latently infected CD4+ T cells.
Human immunodeficiency virus 1 (HIV-1) persists even under highly active antiretroviral therapy because of a reservoir of long-lived, quiescent, memory CD4+ T cells, which constitute a fraction of resting CD4+ T cells (rCD4+ T).1–4 These cells harbor chromosomally integrated HIV-1 proviruses that are transcriptionally silent and immunologically unrecognizable, and hence constitute the majority of the latently infected reservoir. The “Shock and kill” strategy envisions that proviral reactivation can be achieved by the addition of small molecules called latency reversing agents (LRAs), which activate HIV-1 transcription in the latent HIV reservoir. Under this theory, a complete (or near-complete) reactivation of HIV-1 will induce de novo virion synthesis, viral cytopathic effect, immune clearance, and the ultimate death of the latent reservoir. Unfortunately, this approach faces serious challenges revealed by many recent findings, including the heterogeneous reservoirs of HIV-1 latency,5 insufficiency for LRAs alone to reactive patient-derived cells,6,7 a very small proportion of replication competent provirus that can be reactivated by any given LRA,8 and the fact that even when virus activation is achieved, the immune system often fails to clear the infected cells.9
We have previously reported that a cellular RNase monocyte chemotactic protein-induced protein 1 (MCPIP1) restricts HIV-1 infection in resting CD4+ T cells.10 Interestingly, MCPIP1 is rapidly degraded in activated primary T cells.10 We11 and others12 subsequently demonstrated that MCPIP1 was cleaved in activated human and mouse CD4+ T cells by the mucosa-associated lymphoid-tissue lymphoma-translocation gene 1 (MALT1), a paracaspase whose activity is critically important for activation of T and B lymphocytes.13,14 MALT1 cleaves MCPIP1 at the C-terminal side of an arginine residue of the PEST sequence found in its substrates, including Bcl10, CYLD, and A20.15 Of note, MCPIP1 knockout mice displayed hyperactivation of CD4+ T cells, including memory CD4+ T cells.12,16
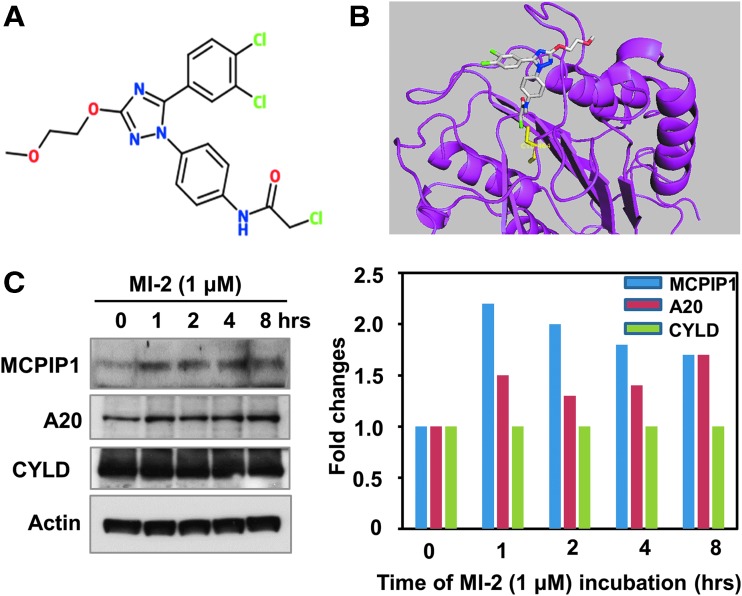
Based on these findings, we postulated that blocking MALT1-dependant MCPIP1 cleavage in activated CD4+ T cells may restore MCPIP1 levels and confer resistance to HIV-1. Among several reported MALT1 inhibitors, MI-2 was shown to selectively bind to and inhibit the cleavage activity of MATL1.17 MI-2 contains a reactive chloromethyl amide and covalently binds to and irreversibly blocks MALT1 cleavage activity (Fig. 1A, B).17 To examine the effect of MI-2 on MALT1-mediated MCPIP1 cleavage, we treated Jurkat T cells with MI-2 and found that MCPIP1 is rapidly upregulated on addition of MI-2 (Fig. 1C). Interestingly, the protein levels of another two MALT1 substrates, A20 and CYLD, either modestly changed or did not change at all following MI-2 treatment.
FIG. 1.
MI-2 induces MCPIP1 expression in Jurkat T cells. (A) Chemical structure of MI-2. (B) MI-2 binds to the catalytic pocket of MALT1, which is shown in magenta with C464 in yellow. MI-2 (in stick model) is shown with carbons in gray, oxygens in red, nitrogens in blue, and chlorines in green. (C) 1 × 106 Jurkat T cells were treated with MI-2 (1 μM) for indicated periods of time. The cells were harvested and the whole cell lysates were subjected to analysis by Western blot with MCPIP1 (GeneTex GTX110807), A20 and CYLD antibodies (left panel). Actin was probed as loading control. Right panel: quantification of the blot by ImageJ.
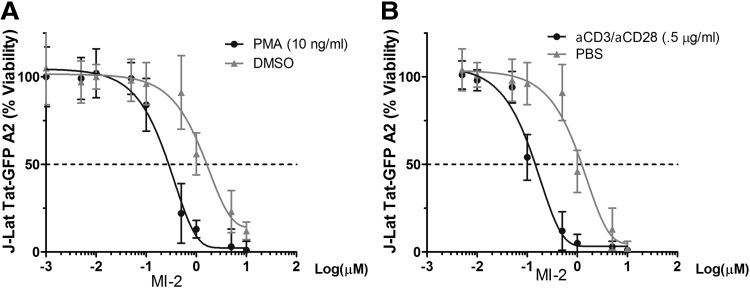
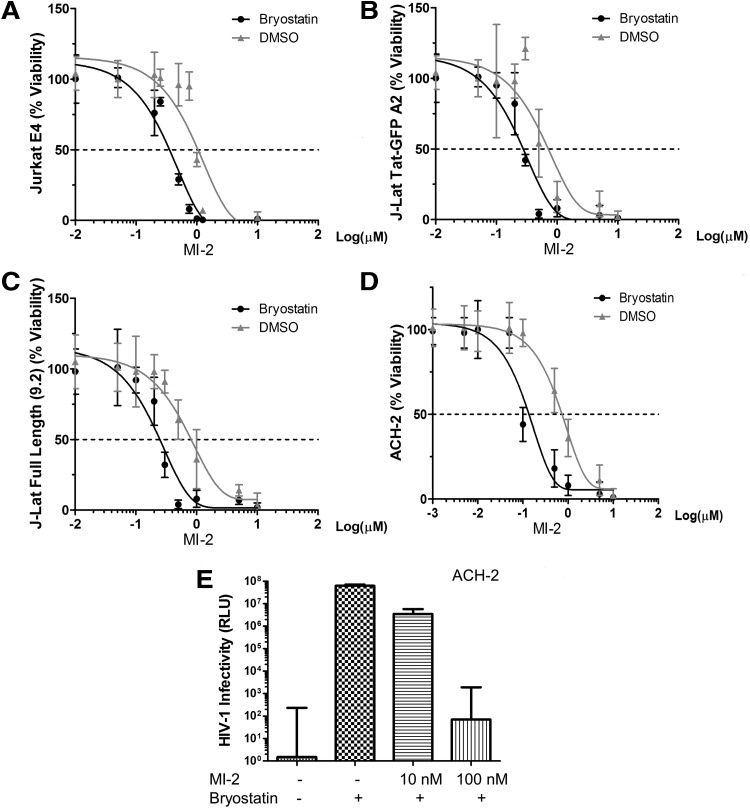
Next, we sought to test whether MI-2 treatment confers resistance to HIV in reactivated HIV latently infected cells. We chose to work with three cell line-based HIV latency models, including the J-Lat Tat-GFP (A2 clone) and J-Lat full length 9.2 clone from Verdin's laboratory,18 the Jurkat E4 clone from Karn's group,19 and the ACH-2 clone,20 which all harbor a latent HIV provirus in various forms. To our surprise, although MI-2 is nontoxic to animals, it induced massive cell death in cell line-based HIV latency models when cell activation signals were supplied (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). Measured by an ATP-based metabolic luminescent assay, the half-maximum toxicity (CC50) of MI-2 on J-Lat Tat-GFP (A2 clone) ranges between 1 and 2 μM (Fig. 2). Addition of phorbol 12-myristate 13-acetate (PMA) or anti-CD3/anti-CD28 antibody combo, however, accelerated MI-2-mediated cell death by shifting the curve to the left (CC50 ∼100–200 nM). To examine whether a specific LRA will have similar effect, bryostatin 1, a potent modulator of protein kinase C,21 was added to the cells. As shown in Figure 3A–D, bryostatin 1 significantly enhanced the MI-2-mediated cytotoxicity in all three latency models by an order of magnitude. Consequently, the production of infectious HIV from the bryostatin 1-treated ACH-2 cells was reduced by 4 logs (Fig. 3E). At 10 nM, bryostatin 1 alone strongly activated the proviral transcription without causing any cell death (Supplementary Fig. S2).
FIG. 2.
Cell activation increases MI-2-mediated cell death in HIV latently infected cells. (A) J-Lat Tat-GFP A2 cells were seeded at 0.25 × 106/ml and were treated with dimethyl sulfoxide (DMSO) or stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng/ml) and increasing concentrations of MI-2. (B) Anti-CD3/anti-CD28 antibody combo was added to stimulate cells. n = 3, error bars represent standard deviations.
FIG. 3.
Bryostatin 1 increases MI-2-mediated cell death in HIV latently infected cells. (A) Jurkat E4 clone was seeded at 0.25 × 106/ml and was treated with DMSO or stimulated with bryostatin 1 (10 nM) and increasing concentrations of MI-2. (B) Bryostatin 1 (10 nM) was added to stimulate J-Lat Tat-GFP A2 cells and increasing concentrations of MI-2. (C) Bryostatin 1 (10 nM) was added to stimulate J-Lat Full length 9.2 cells and increasing concentrations of MI-2. (D) Bryostatin 1 increases MI-2-mediated cell death in HIV latently infected ACH-2 cells. cART (Lamivudine, Emitricitabine, Indinavir, and Tenofovir, 10 μM of each) was added to prevent spread and the contribution of unintegrated viral species. (E) HIV produced from MI-2-treated ACH-2 cells was titered on TZM-bl reporter cells. Equal number of ACH-2 cells were treated as indicated for 2 days. Cells were then pelleted down and then resuspended in fresh medium to ensure no carryover of compounds. After 24 h, supernatants were collected and titered on TZM-bl cells.
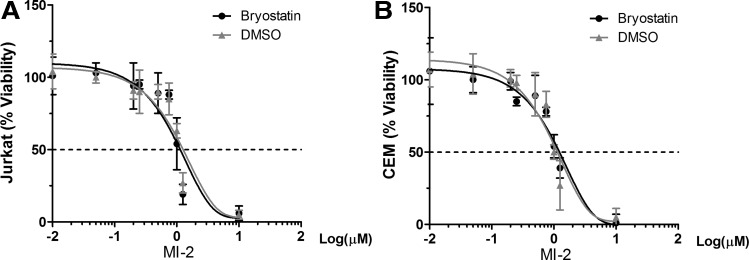
Finally, we determined whether MI-2 in combination with bryostatin 1 selectively targets reservoir with lethal effect. As shown in Figure 4A and B, MI-2 in combination with bryostatin 1 does not accelerate cell death of uninfected Jurkat T nor CEM cells, suggesting that the accelerated cell death achieved in the HIV latently infected cell lines involves viral components. The question is whether the observed cell death is exclusively dependent on MCPIP1 induction. Previously we have shown that elevated MCPIP1 enhanced stress-induced apoptosis in RAW264.7 cells.22 We speculate that HIV latently infected CD4+ T cells are more prone to MCPIP1-mediated cell death on reactivation. Since MCPIP1 is an RNase and shown by others to directly bind viral RNA,23 newly transcribed HIV mRNA species in the reactivated cells may trigger MCPIP1-mediated cell death. In other words, elevated MCPIP1 on MI-2 treatment detects HIV mRNA species and causes cell death. If true, such a mechanism may only require the transcriptional reactivation of proviruses, which can be easily achieved by LRAs. Ongoing experiments are underway to test this possibility.
FIG. 4.
Bryostatin 1 does not increase MI-2-mediated cell death in uninfected Jurkat T (A) or CEM T cells (B). Cells were seeded at 0.25 × 106/ml and were treated with DMSO or stimulated with Bryostatin 1 (10 nM) and increasing concentrations of MI-2.
Collectively, our data suggest that the MALT1 inhibitor in combination with LRA represents a novel approach to kill HIV latently infected T cells. The therapeutic index (TI, ∼10) of MI-2 warrants in-depth follow-up analysis. Interestingly, two studies of MALT1 inhibitors revealed that MALT1 cleavage activities can also be achieved through reversible binding of MALT1 by one MI-2 analog, MI-2A3,17 and another small molecule named mepazine.24 These compounds may offer wider therapeutic window because reversible inhibition of MALT1 is expected to cause less death to bystander cells. Further investigations of these compounds using primary latency model and ex vivo HIV-1 latency model will confirm the validity of this novel approach.
Supplementary Material
Acknowledgments
This study was sponsored by the National Institute of Health Grant R01DK088787 and R56DK088787 (to T.T.W.) and by the Natural Science Foundation of Heilongjiang Province grant QC2012C094 (to H.L.). M.F was supported by the National Institute of Health Grant R21AI103618. H.L. is a recipient of the “Reserve Talents of Universities Overseas Research Program of Heilongjiang Education Department.” The funders had no role in the study design, data collection, and interpretation, or the decision to submit the work for publication. The authors wish to thank Dr. Fatah Kashanchi for providing reagents and helpful advice. The J-Lat and ACH-2 clones were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: J-Lat Full Length GFP Cells from Dr. Eric Verdin and Dr. Thomas Folks.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS: HIV-1 entry into quiescent primary lymphocytes: Molecular analysis reveals a labile, latent viral structure. Cell 1990;61:213–222 [DOI] [PubMed] [Google Scholar]
- 2.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M: Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 1991;254:423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDougal JS, Mawle A, Cort SP, et al. : Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol 1985;135:3151–3162 [PubMed] [Google Scholar]
- 4.Ganesh L, Burstein E, Guha-Niyogi A, et al. : The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature 2003;426:853–857 [DOI] [PubMed] [Google Scholar]
- 5.Dahabieh MS, Battivelli E, Verdin E: Understanding HIV latency: The road to an HIV cure. Annu Rev Med 2015;66:407–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF: New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014;20:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cillo AR, Sobolewski MD, Bosch RJ, et al. : Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2014;111:7078–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho YC, Shan L, Hosmane NN, et al. : Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013;155:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan L, Deng K, Shroff NS, et al. : Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Qiu C, Miao R, et al. : MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells. Proc Natl Acad Sci U S A 2013;110:19083–19088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeltsch KM, Hu D, Brenner S, et al. : Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol 2014;15:1079–1089 [DOI] [PubMed] [Google Scholar]
- 12.Uehata T, Iwasaki H, Vandenbon A, et al. : Malt1-induced cleavage of regnase-1 in CD4(+) helper T cells regulates immune activation. Cell 2013;153:1036–1049 [DOI] [PubMed] [Google Scholar]
- 13.Ruefli-Brasse AA, French DM, Dixit VM: Regulation of NF-kappaB-dependent lymphocyte activation and development by paracaspase. Science 2003;302:1581–1584 [DOI] [PubMed] [Google Scholar]
- 14.Ruland J, Duncan GS, Wakeham A, Mak TW: Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 2003;19:749–758 [DOI] [PubMed] [Google Scholar]
- 15.Rebeaud F, Hailfinger S, Posevitz-Fejfar A, et al. : The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol 2008;9:272–281 [DOI] [PubMed] [Google Scholar]
- 16.Liang J, Saad Y, Lei T, et al. : MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med 2010;207:2959–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontan L, Yang C, Kabaleeswaran V, et al. : MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell 2012;22:812–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan A, Bisgrove D, Verdin E: HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 2003;22:1868–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson R, Kim YK, Hokello J, et al. : Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J Virol 2008;82:12291–12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clouse KA, Powell D, Washington I, et al. : Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol 1989;142:431–438 [PubMed] [Google Scholar]
- 21.Kinter AL, Poli G, Maury W, Folks TM, Fauci AS: Direct and cytokine-mediated activation of protein kinase C induces human immunodeficiency virus expression in chronically infected promonocytic cells. J Virol 1990;64:4306–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi D, Huang S, Miao R, et al. : Monocyte chemotactic protein-induced protein 1 (MCPIP1) suppresses stress granule formation and determines apoptosis under stress. J Biol Chem 2011;286:41692–41700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Peng W, Sun Y, et al. : Structural study of MCPIP1 N-terminal conserved domain reveals a PIN-like RNase. Nucleic Acids Res 2012;40:6957–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagel D, Spranger S, Vincendeau M, et al. : Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell 2012;22:825–837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.